Abstract
Animal and human studies suggest fish oil and green tea may have protective effect on prostate cancer. Fatty acid synthase (FAS) has been hypothesized to be linked to chemoprotective effects of both compounds. This study evaluated the independent and joint effects of fish oil (FO) and green tea supplement (epigallocatechin-3-gallate, EGCG) on FAS and Ki-67 levels in prostate tissue. Through a double-blinded, randomized controlled trial with 2x2 factorial design, 89 men scheduled for repeat prostate biopsy following an initial negative prostate biopsy were randomized into either FO alone (1.9g DHA+EPA/day), EGCG alone (600 mg/day), a combination of FO and EGCG, or placebo. We used linear mixed effects models to test the differences of prostate tissue FAS and Ki-67 by immunohistochemistry between pre- and post-intervention within each group, as well as between treatment groups. Results did not show significant difference among treatment groups in pre-to-post-intervention changes of FAS (p=0.69) or Ki-67 (p=0.26). Comparing placebo group with any of the treatment groups, we did not find significant difference in FAS or Ki-67 changes (all p>0.05). Results indicate FO or EGCG supplementation for a short duration may not be sufficient to produce biologically meaningful changes in FAS or Ki-67 levels in prostate tissue.
Keywords: Catechins, ω-3 fatty acids, fatty acid synthase, Ki-67, prostate
INTRODUCTION
Prostate cancer is the most common non-skin cancer among men in the U.S. Men at high risk for prostate cancer may also have increased awareness of prostate cancer and a strong desire to identify methods to reduce the likelihood of ultimately developing this disease. Unfortunately, within traditional Western medicine, there is little to offer these men. Thus, many men may seek out complementary and alternative medicines (CAM) or a change in their lifestyles in an attempt to modify their risk of prostate cancer.
A number of nutritional and CAM supplements, including fish oil and green tea, have been hypothesized to reduce prostate cancer risk. The major components in fish oil are docosahexaenoic acid (DHA) and eicosapentanoic acid (EPA) - part of the omega-3 polyunsaturated fatty acids (ω-3 PUFA) family. The most common ω-3 PUFA in the U.S. diet is short-chain α-linolenic acid (ALA) (1) which can be metabolized by Δ-6 desaturase and elongase to long-chain EPA or DHA. ALA is commonly found in plants while EPA and DHA are commonly found in fatty fish and fish oil (2). As compared to ALA, in human prostate cancer cell lines, EPA and DHA have greater anti-cancer effects through inhibition of cell proliferation and induction of apoptosis (3). Tea is the second most commonly consumed beverage in the world (4) and around 20% of tea production is green tea (5). Green tea contains an abundant bioactive component known as catechins which have been demonstrated to induce cell-cycle arrest and apoptosis in multiple prostate cancer cell lines (6–9). Catechins include (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin (EC), (−)-epigallocatechin (EGC) and (−)-epicatechin-3-gallate (ECG) with EGCG accounting for about two-thirds of catechins (5).
Human studies on the association between ω-3 PUFA or green tea and prostate cancer are inconsistent. Meta-analyses (10–16) and prospective studies (17–20) investigating the association between ω-3 PUFA and prostate cancer generated mixed results with inverse, null or positive associations reported. For the association between green tea and prostate cancer, a meta-analysis pooling thirteen cohort or case-control studies showed green tea’s protective effect on prostate cancer was more apparent among Asian populations (21); yet, a more recent meta-analysis including twenty-one cohort or case-control studies reported no association (22). Despite these mixed results from population-based studies, evidence from animal and in vitro work suggest a plausible common biologic mechanism whereby both ω-3 PUFA and EGCG have been demonstrated to decrease prostate cancer risk through altering regulation and activity of fatty acid synthase (FAS) (3, 23–25).
FAS is a lipogenic multienzyme that catalyzes the final step in de novo fatty acid synthesis (26). In normal cells, FAS is expressed at low levels and its transcription is regulated primarily by the sterol regulatory element binding protein-1 (SREBP-1) in response to variations in nutritional status (27); the primary purpose for the synthesis of fatty acids would be to provide an energy source via β-oxidation. In tumor cells, SREBP-1 gene transcription is reported to be constitutively upregulated by growth factors (GF) and GF receptors (GFR) and/or steroid hormones (SHs) and SH receptors (SHRs) signaling, resulting in the overexpression of FAS and excess production of free fatty acids (26). The rate of fatty acid formation in tumor cells is clearly greater than the cellular needs for energy, suggesting there may be alternative reasons for fatty acid synthesis. Furuta et al. found that FAS gene was upregulated by hypoxia in tumor cells, and both FAS and SREBP-1 expression were localized in hypoxic regions of tumor tissue (28). Due to the highly anaerobic cancer cell environment there may be excess production of acetyl-CoA from lactate and pyruvate, resulting in lactic acidosis. Overexpression of FAS in tumor cells may be in response to the acidic and hypoxic microenvironment of solid tumors. Alternatively, Hochachka proposes that the significance of enhanced FAS expression and fatty acid synthesis is to provide oxidizing power and thus improve the redox balance found in the malignant prostate cell (29). Thus, the FAS overexpression or dysregulation of the FAS appear to play an important role in allowing for continued prostate tumor cell growth.
It has been demonstrated that ω-3 PUFA downregulates FAS mRNA expression (3) and that EGCG inhibits transcribed FAS activity in prostate cancer cells (24). The primary objective of this double-blind, randomized, placebo-controlled clinical trial, is to elucidate, in men at high risk for prostate cancer, a potential biologic mechanism whereby EGCG and ω-3 PUFA, alone or in combination , may alter the expression and activity of FAS and hence reduce cell proliferation, thereby reducing overall risk of prostate cancer. Since there might be molecular abnormalities in histologically normal appearing prostate tissue due to field effect (30, 31), we chose benign tissue to investigate if early dysregulation of normal cells, with the biomarkers FAS and Ki-67 as the primary endpoints, could be modified by natural compounds. FAS is a molecular marker known to be dysregulated early in carcinogenesis (32–34), our study will be the first study to assess whether nutritional intervention could modulate its expression.
METHODS
Participants
This study’s participants were recruited from the urology clinics at the Portland VA Medical Center (PVAMC), Oregon Health and Science University’s (OHSU) and Kaiser Permanente Northwest (KPNW) and were scheduled for a repeat biopsy subsequent to an earlier negative, but suspicious, biopsy of the prostate. These men were scheduled for initial biopsy based on elevated PSA (> 4µg/dl), abnormal digital rectal exam (DRE), or suspicious findings by transrectal ultrasound (TRUS). We included men ≥ 21 years of age who signed informed consent. Exclusion criteria included: 1) definitive invasive prostate cancer on initial biopsy; 2) significant active medical illness that in the opinion of the clinician would preclude protocol treatment; 3) history of ventricular tachycardia or ventricular fibrillation; 4) subject reported use of fish oil (greater than 1 gram per day) or green tea supplement within 30 days before day 1 of study treatment or subject reported use ≤ 1 gram per day of fish oil and unwilling to discontinue use for the duration of the study; 5) use of warfarin or need for therapeutic anticoagulation at time of biopsy or at any time during the course of the trial; 6) subject reported allergy or sensitivity to fish oil, olive oil or green tea; 7) subject reported history of hemophilia, van Willebrands disease or other bleeding disorder, except when the subject is evaluated by a hematologist who determines that fish oil supplementation is not contraindicated; 8) total bilirubin greater than institutional upper limit of normal; and 9) PVAMC subjects participating in another greater than minimal risk study. Eligible men met with study coordinators prior to the initiation of any research task to review the study’s purpose and exclusion criteria. The original study protocol was approved by the Institutional Review Boards (IRB) of all participating institutions. At study initiation, the inclusion criteria were as follows: 1) initial negative prostate biopsy result, 2) scheduled repeat biopsy due to a continued elevated PSA, 3) are positive for high grade prostatic intraepithelial neoplasia (PIN) and/or suspicious findings by TRUS or DRE, 4) hemoglobin > 10 g/dL (within 4 weeks), 5) creatinine ≤ 1.5 mg/dL, and 6) Adequate Eastern Cooperative Oncology Group (ECOG) performance status < 2. After 28 subjects were accrued, the Knight Cancer Institute Data & Safety Monitoring Committee conducted an internal audit and found that inclusion criteria were not consistently documented. This was reported to the IRB and the protocol was modified to having only 1 criterion, “clinician recommends repeat biopsy of the prostate”. Of the original 28 patients accrued, 25 met the revised criteria and were included in this analysis; 3 patients recruited prior to the change were deemed ineligible and not included in our final analyses.
Study Design
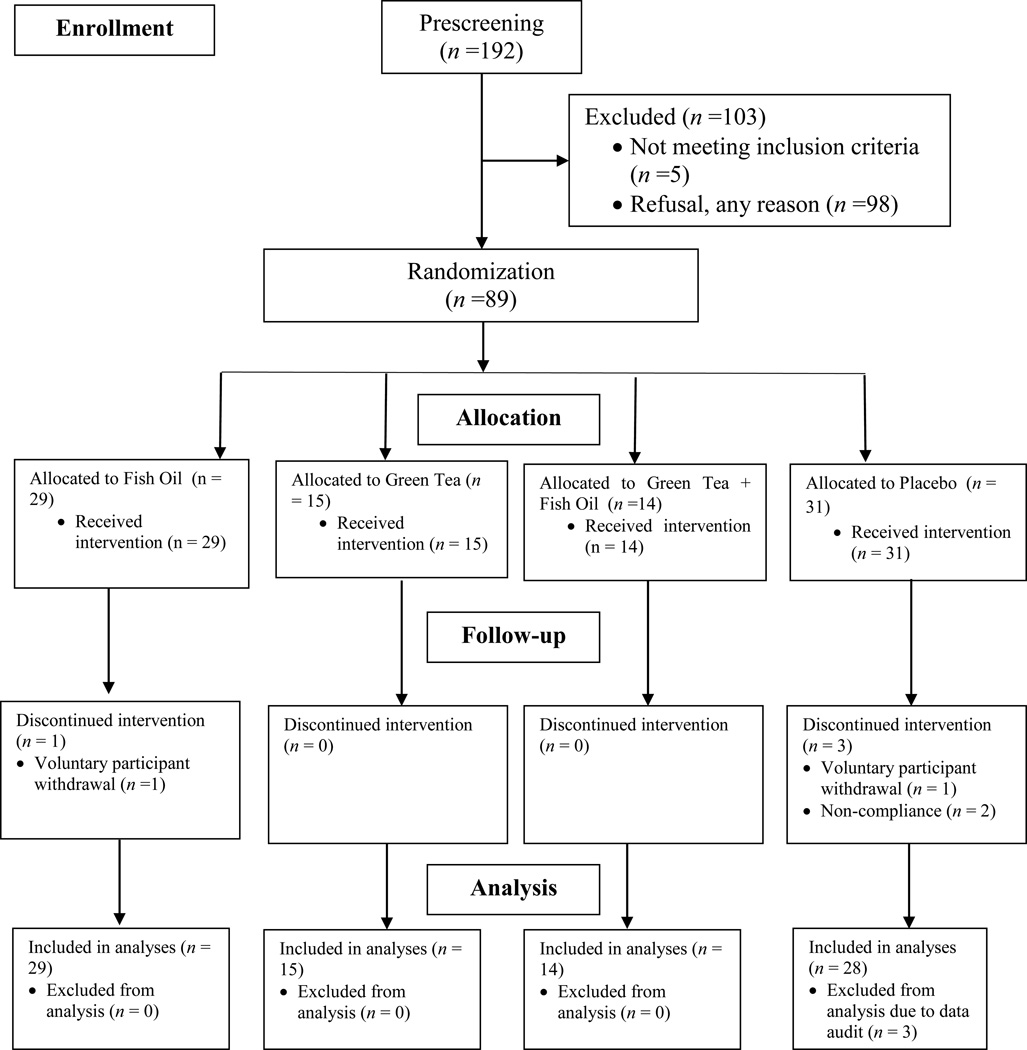
The study sample size flowchart is depicted in Figure 1 following CONsolidated Standards Of Reporting Trials (CONSORT) guidelines (35). The original fish oil and FAS expression study initiated in 2005 was modified in 2006 to add green tea plus fish oil and green tea plus placebo arm. Overall, consented subjects (N =89) were randomized to one of the four groups in a 2 x 2 factorial design: (1) fish oil alone (FO), (2) green tea alone (EGCG), (3) combined fish oil and green tea (EGCG+FO), or (4) placebo group (no fish oil or green tea). Randomization was stratified by age (<65, ≥65 years) and site (VA, OHSU/KPNW). Within each stratum a permuted block randomization with a random block size was used. Study supplements were provided to the study coordinator from the institutional research pharmacies and delivered to the participants. The study coordinator remained blinded to the patient’s study status throughout the intervention. Subjects were enrolled and initiated supplementation at approximately 90 days prior to their scheduled follow up biopsy, ensuring the last day of supplementation would correspond to the date of re-biopsy.
Figure 1.
Randomized controlled trial sample size flow chart
The green tea capsules and matching placebos were donated by Sabinsa Corporation® (Piscataway, NJ). Each green tea capsule was 300 mg and the capsules were considered decaffeinated with less than 2% caffeine per 1000 mg capsule. The composition of the green tea capsules include Epigallocatechin (2.62%), Epicatechin (6.31%), Epigallocatechin gallate (EGCG, 52.67%), Epicatechin gallate (9.02%), catechins (1.32%), gallocatechin gallate (4.23%) and multiple other ingredients. Total identified catechins by HPLC is 76.17%. Total polyphenols by UV method is 82.00%. The placebo for green tea treatment consisted of dicalcium phosphate with a food grade coloring substance. The placebo gelatin capsule matches the active product (green tea) in form and color. Green tea was supplemented at 1 capsule two times a day, providing subjects 600 mg EGCG daily, which is the equivalent of approximately 4–6 regular 8-oz cups of green tea’s EGCG value (5).
The fish oil capsules and the placebos were provided by Perfect Source® Natural Products (Fullerton, CA) with fish oil active ingredients manufactured by DSM® Nutritional Products Inc. (Parsippany, NJ). The fish oil supplement is characterized as refined ethyl esters of fish oil; predominantly as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Each fish oil capsule contained a total of around 0.6 grams of ethyl esters of EPA and DHA. The placebo for fish oil treatment consisted of pure extra virgin olive oil, a significant source of oleic acid, for which there had been no described fatty acid synthase effect. The olive oil placebo gelatin capsule matches the active product (fish oil) in form and color. Fish oil was supplemented at 1 pill 3 times per day; this provided subjects 1.9 g EPA+DHA/daily with an EPA/DHA ratio of 1:1.
We stored all the supplements at room temperature (68 – 77° F) in a locked, monitored research pharmacy facility. The quality of the supplements was checked regularly and the study subjects were regularly instructed by the study research coordinator to comply with the intervention protocols.
Research coordinators completed the informed consent, a modified National Cancer Institute (NCI) diet history questionnaire (36), a risk factor questionnaire assessing other potential risk factors for prostate cancer, and an adverse events questionnaire based on known side effects to both supplements. A 10ml blood sample was collected at baseline and prior to the follow-up biopsy visit. Adverse events questionnaires that included common adverse events for EGCG and fish oil were completed at approximately 30 and 60 days during the trial and 30-day follow-up after the trial. For any reported adverse event grade 3 or higher, according to the NCI Common Terminology Criteria for Adverse Events Version 3.0, the responsible clinician was notified; the event was triaged and followed to resolution, depending upon the severity of event. At mid-study, study exit and 30 days post-intervention, subjects were asked about any changes to medications, supplement use or dietary intake over the past one-month. At study exit, blood and flash-frozen prostate biopsy samples were collected. Participant’s capsule containers were returned to the Research Pharmacies and remaining capsules were counted and recorded. Subjects who took ≥80% of the prescribed pills were considered treatment-compliant. If repeat biopsy was delayed for reasons unrelated to study treatment, treatments could extend up to a total of 20 weeks. The last dose of treatment would be given no earlier than 12 hours prior to repeat biopsy.
Biopsy Tissue Specimens Collection and Processing
Prostate biopsies were conducted per standard clinical practice. Tissue was collected, paraffin-embedded and stored as part of the standard prostate biopsy procedure. At the examining physician’s discretion, 6 to 24 biopsy cores were obtained at the initial examination and at the time of the post-intervention examination. Institutional-standard biopsy templates were followed for the biopsy procedure.
All clinical biopsy specimens were immediately placed into 10% neutral buffered formalin. The anatomic location from which each core biopsy was extracted was also recorded by the urology nurse. The specimens were embedded in paraffin at the institution’s histology laboratory. Routine hematoxylin and eosin staining were carried out and the presence of prostate cancer was determined by a pathologist. Reviewed specimens were stored and available for immunohistochemical (IHC) studies. For pre-intervention prostate biopsy tissue, we selected one benign core from each subject for IHC studies; for post-intervention prostate repeat biopsy tissue, we selected one benign core of each subject and one cancer/PIN core if the subject had cancer/PIN. However, only benign cores were used for pre-to-post-intervention comparison.
Immunohistochemical Staining for FAS and Ki-67
The IHC protocol for FAS was adapted from published studies (37, 38). We used published IHC procedures for Ki-67 in paraffin-embedded tissue. Five micron sections of paraffin-embedded tissue from each research core were prepared on Fisher Plus slides and air dried at room temperature over an air vent. Care was taken to orient pre- and post-intervention slides, such that similar locations in the prostate can be compared. Slides were deparaffinized in xylenes, rehydrated with graded alcohols and washed in Tris-buffered saline (TBS). The slides for Ki-67 antigen expression were boiled in a microwave with 0.01 M citrate buffer. Slides were then treated with 3% aqueous solution of hydrogen peroxide and incubated with 3% goat serum for one hour to block nonspecific binding. Slides were incubated at room temperature for one hour with appropriate antibodies FAS (Transduction Laboratories, Lexington KY, dilution 1/20) and Ki-67 (Zy-Med) followed by mouse Envision (Dako, Glostrup, Denmark). Slides were then counterstained with Gill’s hematoxylin and blued in TBS. The slides were finally dehydrated with graded alcohols and xylene, and then coverslipped using Permount.
Stained slides were evaluated by a pathologist on a Leica DMLS microscope. Positive and negative control slides were examined to ensure technical adequacy of staining. Breast cancer cells with known high FAS expression (SKBR-3) served as positive controls for FAS and similarly prepared slides of each subject’s prostate tissue with substitution of normal mouse serum for the primary antibody served as negative controls. Benign, pre-neoplastic (prostatic intraepithelial neoplasia) and cancer tissue were each assessed by our collaborating pathologist and percentage of positive cells was recorded for FAS, average staining intensity [none (0), weak (1), moderate (2), strong (3)] was also recorded for FAS. We used a modified Histo-score (H-score) ranging from 0–300 multiplying percentage of positive cells and intensity for FAS analyses (39); For Ki-67, we used number of positive cells per high-power field (200x, which is 10x objective eyepiece x 20x magnification) for analyses.
Plasma EGCG Measurement
We adapted the method of Masukawa et al (40) using liquid chromatography electrospray tandem mass spectrometry (LC-MS/MS) for plasma EGCG measurement. The internal standard, epicatechin (1 ng), was used. The extracts were analyzed on an ABSciex 4000 QTRAP hybrid/triple quadrupole linear ion trap mass spectrometer (Foster City, CA).) with electrospray ionization (ESI) in negative mode. The mass spectrometer was interfaced to a Shimadzu (Columbia, MD) SIL-20AC XR auto-sampler followed by 2 LC-20AD XR LC pumps. Data were acquired and analyzed using Analyst 1.6.2 software.
Plasma Fatty Acids Measurement
The fatty acids in plasma were analyzed by a modification of the methods described by Langerstedt et al (41). Deuterated fatty acids were added to samples prior to extraction as internal standards. After hydrolysis and extraction, fatty acids were derivatized to the pentafluorobenzyl (PFB)-esters and analyzed by gas chromatography-mass spectroscopy (GC-MS) on a Trace DSQ (Thermoelectron) operating in the negative ion chemical ionization mode with methane as the reagent gas. Each fatty acid was matched to the deuterated internal standard closest in length and retention time. Peak area ratios of known amounts of standard fatty acids and the internal standards were used to generate calibration curves to quantify unknowns using Xcalibur software.
Statistical Methods
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The intent-to-treat analysis was performed for the primary outcomes and included all randomized participants. Baseline characteristics were expressed as means and standard errors (SEs) for continuous variables, and counts (n) and percentages (%) for categorical variables, stratified by treatment group. The comparability of the four treatment groups for baseline characteristics were tested using one-way analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables.
The key primary outcomes are FAS and Ki-67. Our primary interest was to determine whether changes from pre- to post-intervention were significantly different between the four treatment groups. The analysis was conducted separately for FAS and Ki-67. Shapiro-Wilk Normality tests were conducted for all continuous variables.
Both FAS and Ki-67 biomarkers were log2 transformed in order to obtain approximate normality. To assess treatment group differences in the changes in primary outcome biomarkers, linear mixed effects models were conducted separately for each outcome to calculate least-squares means (LSMEANS) and 95% confidence intervals (95% CI), and to test the statistical significance of the difference between pre- and post-intervention within each group, as well as to compare the magnitude of differences among four groups. These comparisons were performed using appropriate statistical contrasts (e.g., green tea vs. placebo, green tea/fish oil vs. placebo, fish oil vs. placebo). We used the false discovery rate (FDR) method to adjust for multiple comparisons of the primary endpoints (42).
Adverse events and compliance between the treatment groups were analyzed using Fisher-Freeman-Halton tests as appropriate. Subjects who took ≥80% of the prescribed pills were considered treatment-compliant. The proportions of the compliant subjects were compared between FO, EGCG, EGCG+FO groups and placebo groups using a chi-square test. To further determine the compliance using biological samples, we also used mixed-effect models to determine whether changes of plasma EGCG from pre- to post-intervention were significant across the four treatment groups. Comparisons of plasma fatty acid levels in FO and EGCG+FO group before and after treatment were determined using pared t-test. Tests of statistical significance were conducted using two-sided tests, and a p-value ≤ 0.05 was considered statistically significant unless otherwise noted.
The sample size and power analyses were initially performed for five primary endpoints and one secondary endpoint using NCSS PASS (43), assuming two-sided 5% overall significance level. It was expected that 144 men would participate in the study with 36 subjects in each treatment arm. Overall, the initially projected sample size of 144 subjects would provide an adequate power to detect a clinically meaningful difference in laboratory-based efficacy parameters for key comparisons. However, we were unable to meet recruitment goals.
RESULTS
Patient Characteristics and Adverse Events
From January 2005 to September 2011, a total of 89 participants aged 50–78 years (63 ± 6.3 years) were randomized into FO group (n=29), EGCG group (n=15), FO+EGCG group (n=14) or placebo group (n=31 with n=28 included in the final analyses). Table 1 describes the baseline characteristics of the 86 subjects by treatment group. There was no statistically significant difference in baseline characteristics including age, BMI, PSA, race, marital status, income, education, smoking, alcohol, family history of prostate cancer or NSAIDs use. Repeat prostate biopsy, post-intervention diagnoses are as follows; 54 (62.8%) were diagnosed as benign, 13 (15.1%) were diagnosed with PIN and 15 (17.4%) subjects had malignant disease. There was no treatment group difference among post-intervention diagnoses (p=0.41).
Table 1.
Basic characteristics of men at baseline by randomization group
| Participant Characteristics | Fish Oil (n = 29) |
Green Tea (n = 15) |
Fish Oil + Green Tea (n=14) |
Placebo (n = 28) |
p1, 2, 3 |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age,yr | 63.3 (5.0) | 64.5 (7.2) | 62.6 (7.5) | 61.8 (6.4) | 0.57 |
| BMI (kg/m2) at baseline | 28.5 (5.0) | 30.1 (3.7) | 29.0 (4.0) | 28.1 (4.1) | 0.54 |
| Prostate Specific Antigen (PSA) Pre- intervention |
6.5 (4.2) | 6.9 (2.6) | 4.8 (2.5) | 6.9 (4.2) | 0.34 |
| Prostate Specific Antigen (PSA) Post-intervention |
6.0 (4.2) | 6.4 (3.3) | 10.8 (18.3) | 7.1 (6.1) | 0.38 |
| FAS H-score Pre-intervention | 138.3 (63.8) | 158.8 (84.6) | 142.7 (102.8) | 114.2 (85.4) | 0.22 |
| FAS H-score Post- intervention | 145.2 (72.2) | 159.3 (75.5) | 140.7 (100.3) | 157.3 (81.5) | 0.79 |
| Ki-67 number of positive cells per high power field Pre-intervention |
13.5 (10.3) | 18.3 (15.9) | 17.9 (12.1) | 15.7 (13.6) | 0.68 |
| Ki-67 number of positive cells per high power field Post- intervention |
11.8 (7.9) | 7.9 (6.6) | 21.8 (17.2) | 13.1 (9.6) | 0.05 |
| n (%) | n (%) | n (%) | n (%) | ||
| Race | 0.45 | ||||
| White | 28 (96.6) | 13 (86.7) | 14 (100.0) | 27 (96.4) | |
| Non-White | 1 (3.4) | 2 (13.3) | 0 (0.00) | 1 (3.6) | |
| Marital status | 0.34 | ||||
| Single, Divorced, Widowed | 3 (10.3) | 3 (20.0) | 0 (0.0) | 4 (10.7) | |
| Married/partner | 26 (89.7) | 11 (73.3) | 14 (100.0) | 21 (75.0) | |
| Missing | 0 (0.0) | 1 (6.7) | 0 (0.0) | 3 (14.3) | |
| Income | 0.74 | ||||
| ≤$50,000 | 5 (17.3) | 3 (20.0) | 1 (7.1) | 2 (7.1) | |
| >$50,000 | 13 (44.8) | 5 (33.3) | 4 (28.6) | 11 (39.3) | |
| Refuse/Don’t know/missing | 11 (37.9) | 7 (46.7) | 9 (64.3) | 15 (53.6) | |
| Education | 0.70 | ||||
| ≤ Some college/technical | 6 (20.7) | 3 (20.0) | 3 (21.4) | 3 (10.7) | |
| ≥ College graduate | 8 (27.6) | 6 (40.0) | 5 (35.7) | 11 (39.3) | |
| Missing | 15 (51.7) | 6 40.0) | 6 (42.9) | 14 (50.0) | |
| Smoking | 0.10 | ||||
| Current | 5 (17.2) | 0 (0.0) | 2 (14.3) | 6 (21.4) | |
| Former | 14 (48.3) | 11 (73.3) | 7 (50.0) | 7 (25.0) | |
| Never | 9 (31.0) | 2 (13.3) | 3 (21.4) | 10 (35.7) | |
| Missing | 1 (3.5) | 2 (13.3) | 2 (14.3) | 5 (17.9) | |
| Alcohol | 0.13 | ||||
| Current | 14 (48.3) | 5 (33.3) | 3 (21.4) | 17 (60.7) | |
| Former | 7 (24.1) | 6 (40.0) | 8 (57.1) | 6 (21.4) | |
| Never | 7 (24.1) | 3 (20.0) | 3 (21.4) | 2 (7.1) | |
| Missing | 1 (3.5) | 1 (6.7) | 0 (7.1) | 3 (10.7) | |
| Family history of Cancer | 0.52 | ||||
| Yes | 9 (31.0) | 2 (13.3) | 5 (35.7) | 7 (25.0) | |
| No | 20 (69.0) | 13 (86.7) | 9 (64.3) | 21 (75.0) | |
| Use of NSAIDs | 0.76 | ||||
| Yes | 17 (58.6) | 6 (40.0) | 7 (50.0) | 14 (50.0) | |
| No | 10 (34.5) | 7 (46.7) | 6 (42.9) | 9 (32.1) | |
| Missing | 2 (6.9) | 2 (13.3) | 1 (7.1) | 5 (17.9) | |
| Repeat Pathology Biopsy Diagnosis4 | 0.41 | ||||
| Benign | 16 (55.2) | 11 (73.3) | 9 (64.3) | 18 (64.3) | |
| Malignant | 6 (20.7) | 1 (6.7) | 2 (14.3) | 6 (21.4) | |
| Prostatic Intraepithelial Neoplasia (PIN) |
6 (20.7) | 3 (20.0) | 3 (21.4) | 1 (3.6) | |
| Missing | 1 (3.4) | 0 (0.0) | 0 (0.0) | 3 (10.7) | |
One-way analysis of variance (ANOVA) tests were conducted for continuous variables (age, BMI, PSA); Kruskal–Wallis test was used for comparing variables without normal distribution (FAS H-score and Ki-67 % positivity), chi-square tests were conducted for categorical variables with expected cell frequencies ≥ 5 (Use of NSAIDs); and Fisher-Freeman-Halton tests were conducted for categorical variables with expected cell frequencies < 5.
p-value ANOVA or chi-square tests between supplement and placebo groups, *p <0.05. All the p-values were calculated excluding missing category.
Percentages may not add up to 100 due to rounding values.
All the baseline biopsies were diagnosed as benign (n=61, 71%) or PIN (n=25, 29%).
There were no treatment group differences noted for each specific type of adverse event and total number of adverse events (Table 2). No subjects experienced grade ≥ 3 adverse events. One subject in the FO group and one subject in the placebo group withdrew from the study (Figure 1). In addition, no statistically significant difference was observed in terms of compliance to the treatment plan between the four treatment groups (p = 0.86).
Table 2.
Incidence of reported grade 2 adverse events in the fish oil green tea trial (treatment-related). Data represent the number of adverse events (%)
| Adverse Events (AE) | Fish Oil (n = 29) |
Green Tea (n = 15) |
Fish Oil + Green tea (n=14) |
Placebo (n = 28) |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Number (%) | |
| Bloating | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| Burping | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (3.6) |
| Diarrhea | 2 (6.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea/vomiting | 1 (3.4) | 1 (6.7) | 0 (0.0) | 0 (0.0) |
| Bruising | 1 (3.4) | 1 (6.7) | 0 (0.0) | 2 (7.1) |
| Headache | 2 (6.9) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| Upset Stomach | 3 (10.3) | 0 (0.0) | 0 (0.0) | 3 (10.7) |
| Heartburn | 1 (3.4) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| Abdominal Pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Muscle Pain | 0 (0.0) | 1 (6.7) | 1 (7.1) | 1 (3.6) |
| Other1 | 0 (0.0) | 0 (0.0) | 1 (7.1) | 1 (3.6) |
| All2, 3 | 7 (24.1) | 2 (13.3) | 2 (14.3) | 5 (17.9) |
Other changes to health included back/neck pain and mental health issue.
Count of subjects who experienced at least one of the grade 2 adverse events.
p-value for all adverse events comparing the four groups through Fisher-Freeman-Halton test is 0.53.
Immunohistochemistry Biomarkers
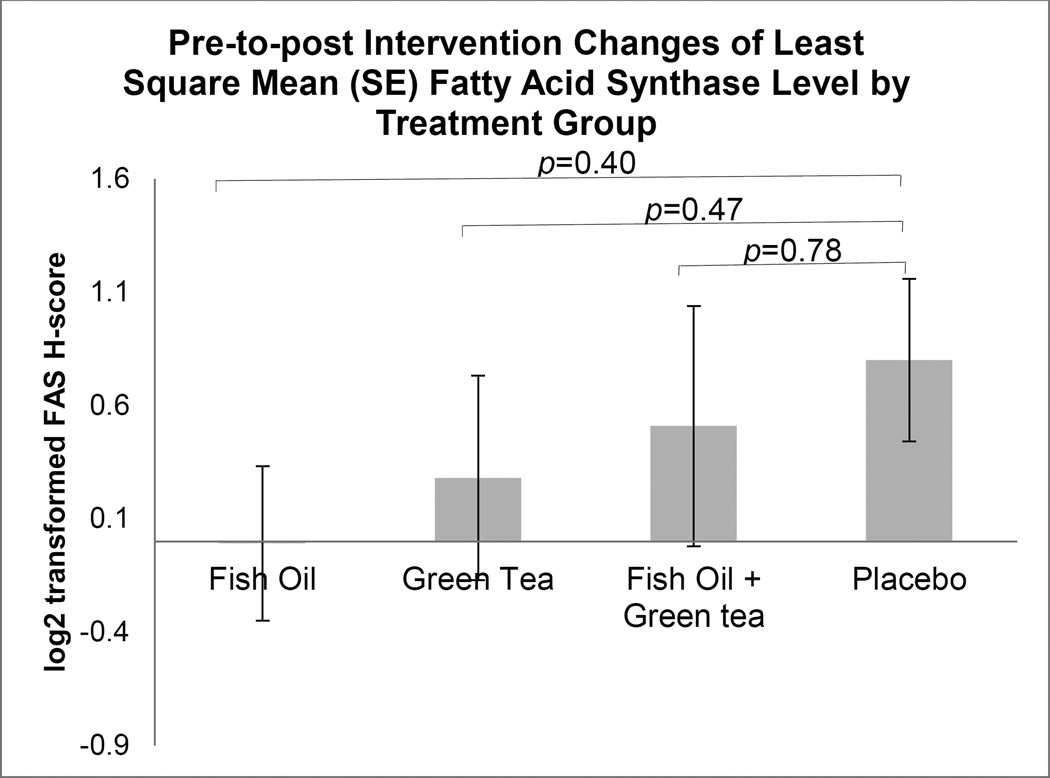
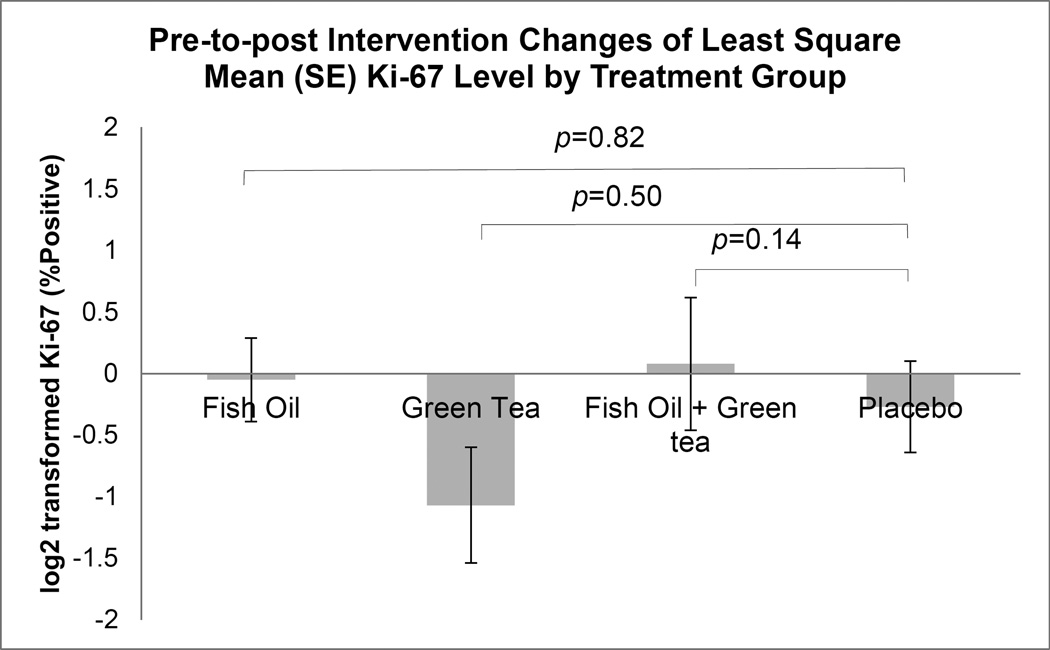
Among the 86 men included in the analysis, 84 (97.7%) had prostate biopsy tissue for IHC analysis. Table 3 shows the log2-transformed LSMEANS of FAS and Ki-67 by treatment groups, the p-values comparing pre- and post-intervention biomarker values within each treatment group, and most importantly, the p-values comparing pre-to-post changes of biomarkers between treatment groups. We also show these results in Figure 2 for FAS and Figure 3 for Ki-67. A significant decrease of Ki-67 was present within EGCG group (p=0.02) and a significant increase of FAS was present within placebo group (p=0.03). After multiple comparisons by utilizing the FDR, the significance disappeared (both p-value=0.12). There was no overall statistical significance among the four treatment groups for pre-to-post changes of both FAS (p=0.69) and Ki-67 (p=0.26).
Table 3.
Log2-transformed least-squares means (LSMEANS) of immunohistochemistry H-score of FAS and Ki-67 positivity in men scheduled for prostate biopsy
| Fish Oil | Green Tea | Fish Oil + Green tea | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-to-Post Change LSMEANS (SE) |
p comparing pre- and post- treatment values |
Pre-to-Post Change LSMEANS (SE) |
p comparing pre- and post- treatment values |
Pre-to-Post Change LSMEANS (SE) |
p comparing pre- and post- treatment values |
Pre-to-Post Change LSMEANS (SE) |
p comparing pre- and post- treatment values |
p comparing treatment groups1, 2 |
|
| FAS | −0.009 (0.34) | 0.98 | 0.28 (0.45) | 0.54 | 0.51 (0.53) | 0.35 | 0.80 (0.36) | 0.03 | 0.69 |
| Ki-67 | −0.05 (0.34) | 0.89 | −1.07 (0.47) | 0.02 | 0.08 (0.54) | 0.89 | −0.27 (0.37) | 0.47 | 0.26 |
Mixed effect model adjusting treatment and time
Each subject has maximum of 2 observations.
Figure 2.
Comparison of prostate benign tissue fatty acid synthase (FAS) immunohistochemistry H-score level changes between treatment groups. Changes in FAS level from pre- to post-intervention between treatment groups were compared using mixed effect model. Values shown indicate least-squares means of (LSMEANS ± SE) pre-to-post change of FAS level by treatment group.
Figure 3.
Comparison of prostate benign tissue Ki-67 immunohistochemistry number of positive cells/high-power field (200x, which is 10x objective eyepiece x 20x magnification) level changes between treatment groups. Changes in Ki-67 level from pre- to post-intervention between treatment groups were compared using mixed effect model. Values shown indicate least-squares means of (LSMEANS ± SE) pre-to-post change of Ki-67 level by treatment group.
Table 4 demonstrates the pre-to-post treatment LSMEANS difference for each of the intervention treatment compared to placebo groups. For the three groups who received supplementation, there was no significant difference in FAS and Ki-67 level changes (all p>0.05).
Table 4.
Pairwise comparisons of pre-post differences of log2-transformed least-squares means (LSMEANS) of immunohistochemistry H-score of FAS and Ki-67 positivity in men scheduled for prostate biopsy
| Pairwise Comparison | Pre-to-Post Difference Estimate (SE) |
p | |
|---|---|---|---|
| FAS | Fish oil vs. Placebo | 0.27 (0.32) | 0.40 |
| Green tea vs. Placebo | 0.27 (0.37) | 0.47 | |
| Fish oil + Green tea vs. Placebo | −0.12 (0.41) | 0.78 | |
| Ki-67 | Fish oil vs. Placebo | −0.06 (0.26) | 0.82 |
| Green tea vs. Placebo | −0.21 (0.31) | 0.50 | |
| Fish oil + Green tea vs. Placebo | 0.51 (0.34) | 0.14 |
Plasma EGCG and Fatty Acids Levels
Among subjects in green tea group, 11 out of 15 (73%) had EGCG level increased; among subjects in FOGT group, 12 out of 15 (80%) had increased EGCG level; among subjects in fish oil group, 2 out of 29 subjects (6.9%) had increased EGCG level; and in placebo group, none of the subjects (0%) had increased EGCG level. The pre-to-post-intervention changes of plasma EGCG level (least square mean ± standard error) in EGCG (30.71 ± 14.19 ng/mL) and EGCG+FO (48.96 ± 14.19 ng/mL) group were much higher than FO (0.68 ± 11.06 ng/mL) and placebo group (−1.68 ± 11.32 ng/mL) (overall p=0.02). Comparing pre- and post-intervention fatty acids levels within FO and EGCG+FO groups using paired t-test (mean increase ± standard deviation) , the plasma DHA significantly increased in FO (120.9 ± 101.3, p<0.0001) and EGCG+FO group (180.7 ± 113.8, p<0.0001); similarly, EPA also significantly increased in FO (118.0 ± 107.4, p<0.0001) and EGCG+FO group (196.9 ± 102.6, p=0001). All these results indicate the subjects had taken the supplements following the intervention protocols.
DISCUSSION
In this randomized, placebo-controlled trial, we found no significant effect of fish oil or green tea supplement in decreasing prostate tissue FAS or Ki-67 levels among men scheduled for prostate biopsy.
One possible reason that we did not observe an effect may be that the supplementation intervention period was too short or the supplementation doses were too low. In our study, the mean treatment intervention period was 14.4 weeks. Compared to an Italian study which showed a significant chemoprevention effect of green tea catechins (GTC) on prostate cancer (44), our study’s intervention doses (EGCG 600mg) were similar to the GTC study (600 mg); but our intervention period (12–20 weeks) was much short than the GTC study (1 year). Compared to a clinical trial showing a 4–6 week low-fat diet with fish oil supplementation (1g EPA+ 1.8g DHA) intervention could significantly decrease prostate tissue Ki-67 level (45), our fish oil doses (1.9g EPA+DHA) were lower. The shorter duration or lower doses may restrict our ability to discern an effect of supplementation on FAS or Ki-67 expression, especially if the variation in FAS or Ki-67 protein expression caused by fish oil or green tea supplementation is a cumulative effect.
Additionally, not all of the participants were prostate cancer cases. For instance, the green tea group had a lower proportion of malignant cases (6.7%) and the fish oil group had a higher proportion of malignant cases (20.7%). FAS expression is consistently greater in cancer tissue vs. normal tissue (46) and overexpression of FAS is one of the earliest and most common events in prostate cancer development (47). In addition, nuclear localization of FAS captured by IHC analysis correlates with prostate cancer Gleason grade (48). Therefore, the average levels of FAS are lower in our study population as compared to what may have been seen should all study subjects have had confirmed prostate cancer. In a double blinded clinical trial with subjects all diagnosed with high-grade PIN (HGPIN), only 1 out of 30 subjects in the GTC (600 mg/day for 12 months) group vs. 9 out of 30 subjects in the placebo group developed prostate cancer (44). Given this trial included only men diagnosed with HGPIN (a similar disease stage), a future trial should consider including only patients with confirmed HGPIN or prostate cancer diagnosis.
The protective effect of inhibiting FAS level in preventing prostate cancer growth might occur via the inhibition of Ki-67, a marker of cell proliferation. Since most of our study participants were benign cases, cell proliferation Ki-67 level measured from our study participants’ prostate biopsy tissue was relatively low and did not change significantly from pre-to-post-intervention in the entire intervention group after multiple adjustment comparison. Our result on green tea effect is consistent with a clinical trial of ninety-three men who completed the intervention of 6 cups/day brewed green and black tea prior to prostatectomy, which showed no significant effect of tea consumption on Ki-67 level in prostatectomy tissues (49). However, our non-significant finding of fish oil supplementation is not consistent with a clinical trial of low-fat diet with fish oil supplementation among men undergoing radical prostatectomy, which showed a significant decreased tumor tissue Ki-67 level (45) in the intervention group. This suggests that should the alteration of FAS expression slow prostate cancer development or progression it may not function by altering cell proliferation in non-malignant tissue.
Two recent clinical trials evaluating green tea extract and prostate cancer incidence showed promising effect (44, 50). In the Italian study among men with HGPIN mentioned earlier, a statistically significant protective effect of GTC against prostate cancer development was observed after a 1-year intervention (44); after 2-year follow-up in a subset of the original participants, a lasting protective effect against prostate cancer was also found (51). A U.S.-conducted clinical trial detected a statistically significant difference in the cumulative rate of prostate cancer plus atypical small acinar proliferation (ASAP) between a GTC group (3/26) and placebo group (10/25) among men with HGPIN without ASAP (50). In our study, we observed non-statistically significant lower rate of prostate cancer in EGCG group (1/14) compared to placebo group (6/28), the direction of the observation is consistent with the other two trials.
Our study has several strengths. First, to our knowledge, our study is the first to assess in human tissue the joint effect of EGCG and FO on the FAS pathway. Second, we have acquired almost all of the prostate biopsy tissue samples from our study participants (97%); our withdrawn subjects were the only men from whom we did not acquire prostate tissue. Therefore, our FAS and Ki-67 expression IHC analyses were nearly complete. Third, through the 2×2 randomized blinded trial design, we were able to examine two types of supplements together to best utilize the study resources. Despite these strengths, we have identified a few study limitations. First, the sample size is limited, however, we did not observe a trend of effects that are in line with our hypothesis. If we had achieved our recruitment goal, we still likely may not have observed a significant effect given the current intervention dosage and period. Second, our hospital-based design among men scheduled for repeat prostate biopsy may restrict the generalizability of our results. However, our study results are applicable to men with high risk for prostate cancer identified from clinical examination. As we know, up to 75% of men with elevated PSA who undergo prostate biopsy will have no diagnosed cancer. A portion of these men will have suspicious biopsy findings, abnormal digital rectal exams or continue to present with increasing PSA and hence warrant a repeat biopsy. These men, considered at higher risk of having prostate cancer are often left waiting for 4–6 months before a follow up biopsy with little or no option given about how they may reduce their risk for prostate cancer development or progression. More research targeted to this population will contribute toward the gap of literature.
In conclusion, short duration of fish oil or green tea supplements could not reduce FAS or Ki-67 substantially. Future trials with longer treatment durations among a more homogenous study population should be considered.
Acknowledgments
We would like to thank all of the men who contributed their time and effort to join our study (Catechins and omega-3 Fatty Acids Impact on Fatty Acid Synthase Activity in the Prostate: a Randomized Controlled Trial) as well as those who expressed strong interest but who, for many reasons, were not able to participate. Sabinsa Corporation® (Piscataway, NJ) generously donated the green tea and green tea placebo capsules. Fish oil was manufactured by DSM® Nutritional Products Inc. (Parsippany, NJ) and the provision of the fish oil placebo along with the fish oil encapsulation was prepared by Perfect Source® Natural Products Inc. (Fullerton, CA).
We are eternally grateful to our OHSU pathologists, Drs. Christopher Corless and George Thomas who scored immunohistochemistry slides and our OHSU surgical urologists, Drs. Christopher Amling and Mitchell Sokoloff for participant recruitment. Ms. Laura Peters, Portland VA Medical Center urology nurse extraordinaire, has been an invaluable and indispensable honorary member of our study team for the life of this project (and other prostate health projects) and graciously assisted with participant retention and specimen collection. Thanks go to laboratory technical assistance from Dr. Dennis Koop, Lisa Blyele and Karyn Foster at the OHSU BioAnalytical Shared Resource and PharmacoKinetics Core Laboratory as well as tissue staining assistance from Jutta Deininger and Cara Poage at the OHSU Knight Cancer Institute Cancer Pathology Shared Resource. We thank Solange Mongoue-Tchokote for providing statistical analyses assistance. We also appreciate Dr. Shannon’s current and previous research coordinators who spent time with this study’s subjects and worked with OHSU, KPNW and PVAMC surgical urologists; Amy Palma, Gretchen Luhr, Alysia Cox and Courtney Maxcy.
FUNDING
This study was supported by the Department of Defense (W81XWH0410296), the National Center for Complementary and Alternative Medicine (5R21AT003461) and the National Cancer Institute Cancer Center Support Grant (5P30CA069533); Clinical Trial Registration: clinicaltrials.gov identifier: NCT00253643.
Abbreviations
- ANOVA
analysis of variance
- ALA
α-linolenic acid
- ASAP
atypical small acinar proliferation
- BMI
body mass index
- CAM
alternative medicine
- CONSORT
CONsolidated Standards Of Reporting Trials
- DHA
docosahexaenoic acid
- DRE
digital rectal exam
- EC
(−)-epicatechin
- ECG
(−)-epicatechin-3-gallate
- ECOG
Eastern Cooperative Oncology Group
- EGC
(−)-epigallocatechin
- EGCG
epigallocatechin-3-gallate
- EPA
eicosapentanoic acid
- FAS
fatty acid synthase
- FDR
false discovery rate
- FO
fish oil
- GF
growth factors
- GFR
growth factor receptors
- GTC
green tea catechins
- HGPIN
high-grade prostatic intraepithelial neoplasia
- IHC
immunohistochemistry
- KPNW
Kaiser Permanente Northwest
- NCI
National Cancer Institute
- LSMEANS
least-squares means
- NSAIDs
nonsteroidal anti-inflammatory drug
- OHSU
Oregon Health & Science University
- PSA
prostate specific antigen
- SE
standard error
- SHs
steroid hormones
- SHRs
steroid hormone receptors
- PIN
prostatic intraepithelial neoplasia
- ω-3 PUFA
omega-3 polyunsaturated acids
- PVAMC
Portland VA Medical Center
- SREBP-1
sterol regulatory element binding protein-1
- TBS
Tris-buffered saline
- TRUS
transrectal ultrasound;
Footnotes
Statements: Our study has not been published elsewhere nor has been submitted simultaneously for publication elsewhere.
Conflicts of Interest: None
REFERENCES
- 1.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 2.Dubnov-Raz G, Finkelstein Y, Koren G. Omega-3 fatty acid supplementation during pregnancy: for mother, baby, or neither? Can Fam Physician. 2007;53:817–818. [PMC free article] [PubMed] [Google Scholar]
- 3.Eser PO, Vanden Heuvel JP, Araujo J, Thompson JT. Marine- and plant-derived omega-3 fatty acids differentially regulate prostate cancer cell proliferation. Mol Clin Oncol. 2013;1:444–452. doi: 10.3892/mco.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 5.Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98:1676S–1681S. doi: 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 7.Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, et al. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001;68:1207–1214. doi: 10.1016/s0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- 8.Yu HN, Yin JJ, Shen SR. Growth inhibition of prostate cancer cells by epigallocatechin gallate in the presence of Cu2+ J Agric Food Chem. 2004;52:462–466. doi: 10.1021/jf035057u. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate. 2001;46:98–107. doi: 10.1002/1097-0045(20010201)46:2<98::aid-pros1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.Chua ME, Sio MC, Sorongon MC, Dy JS. Relationship of dietary intake of omega-3 and omega-6 Fatty acids with risk of prostate cancer development: a meta-analysis of prospective studies and review of literature. Prostate Cancer. 2012;2012:826254. doi: 10.1155/2012/826254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 12.Simon JA, Chen YH, Bent S. The relation of alpha-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89:1558S–1564S. doi: 10.3945/ajcn.2009.26736E. [DOI] [PubMed] [Google Scholar]
- 13.Carleton AJ, Sievenpiper JL, de Souza R, McKeown-Eyssen G, Jenkins DJ. Case-control and prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carayol M, Grosclaude P, Delpierre C. Prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. Cancer Causes Control. 2010;21:347–355. doi: 10.1007/s10552-009-9465-1. [DOI] [PubMed] [Google Scholar]
- 15.Fu YQ, Zheng JS, Yang B, Li D. Effect of individual omega-3 Fatty acids on the risk of prostate cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. J Epidemiol. 2015;25:261–274. doi: 10.2188/jea.JE20140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander DD, Bassett JK, Weed DL, Barrett EC, Watson H, et al. Meta-Analysis of Long-Chain Omega-3 Polyunsaturated Fatty Acids (LComega-3PUFA) and Prostate Cancer. Nutr Cancer. 2015:1–12. doi: 10.1080/01635581.2015.1015745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
- 18.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339–1345. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- 19.Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective study on dietary fat and incidence of prostate cancer (Malmo, Sweden) Cancer Causes Control. 2007;18:1107–1121. doi: 10.1007/s10552-007-9050-4. [DOI] [PubMed] [Google Scholar]
- 20.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J, Yang B, Huang T, Yu Y, Yang J, et al. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 22.Lin YW, Hu ZH, Wang X, Mao QQ, Qin J, et al. Tea consumption and prostate cancer: an updated meta-analysis. World J Surg Oncol. 2014;12:38. doi: 10.1186/1477-7819-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun. 2001;288:1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- 24.Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- 25.Menendez JA, Colomer R, Lupu R. Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med Hypotheses. 2005;64:342–349. doi: 10.1016/j.mehy.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 27.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 29.Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P. Going malignant: the hypoxia-cancer connection in the prostate. Bioessays. 2002;24:749–757. doi: 10.1002/bies.10131. [DOI] [PubMed] [Google Scholar]
- 30.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331–337. [PubMed] [Google Scholar]
- 31.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009;69:1470–1479. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, et al. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 33.Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–622. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- 34.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 35.Bolignano D, Mattace-Raso F, Torino C, D’Arrigo G, Abd ElHafeez S, et al. The quality of reporting in clinical research: the CONSORT and STROBE initiatives. Aging Clin Exp Res. 2013;25:9–15. doi: 10.1007/s40520-013-0007-z. [DOI] [PubMed] [Google Scholar]
- 36.Diet History Quesionnaire, version 1.0. Bethesda, MD. Applied Research Program, National Cancer Institute, National Institute of Health. 2002 [Google Scholar]
- 37.Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 38.Myers RB, Oelschlager DK, Weiss HL, Frost AR, Grizzle WE. Fatty acid synthase: an early molecular marker of progression of prostatic adenocarcinoma to androgen independence. J Urol. 2001;165:1027–1032. [PubMed] [Google Scholar]
- 39.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 40.Masukawa Y, Matsui Y, Shimizu N, Kondou N, Endou H, et al. Determination of green tea catechins in human plasma using liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834:26–34. doi: 10.1016/j.jchromb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, et al. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 43.Hintze J. Power analysis and sample size. Kayesville, Utah: NCSS; 2001. [Google Scholar]
- 44.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 45.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011;4:2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little JL, Kridel SJ. Fatty acid synthase activity in tumor cells. Subcell Biochem. 2008;49:169–194. doi: 10.1007/978-1-4020-8831-5_7. [DOI] [PubMed] [Google Scholar]
- 47.Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 48.Madigan AA, Rycyna KJ, Parwani AV, Datiri YJ, Basudan AM, et al. Novel nuclear localization of fatty acid synthase correlates with prostate cancer aggressiveness. Am J Pathol. 2014;184:2156–2162. doi: 10.1016/j.ajpath.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henning SM, Wang P, Said JW, Huang M, Grogan T, et al. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate. 2015;75:550–559. doi: 10.1002/pros.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, et al. Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention. Cancer Prev Res (Phila) 2015 doi: 10.1158/1940-6207.CAPR-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]