Abstract
The risk of cardiovascular complications is increased in patients with obstructive sleep apnea (OSA). Continuous positive airway pressure (CPAP) is the most effective way to treat clinically significant OSA. We hypothesized that the concentrations of the cardiac risk markers N-terminal brain natriuretic peptide (NT-proBNP) and high-sensitive troponin T (hs-TropT) correlate with the effectiveness of CPAP therapy in patients with OSA and coexisting coronary artery disease (CAD). Twenty-one patients with severe OSA and coexisting CAD (group 1) and 20 control patients with severe OSA alone (group 2) were treated with CPAP and monitored by laboratory-based polysomnography. NT-proBNP and hs-TropT levels were measured before and after CPAP. Apnea–hypopnea index (AHI) and oxygen desaturation were similar in both groups. In group 1, hs-TropT levels correlated with AHI and oxygen desaturation upon CPAP. Elevated NT-proBNP levels in group 1 were significantly reduced by CPAP. NT-proBNP levels correlated with AHI and showed negative correlation with ST-segment depression. No such correlations were found in group 2. CPAP has the potential to normalize elevated NT-proBNP serum levels in patients with severe OSA and coexisting CAD. Levels of NT-proBNP and hs-TropT correlated with AHI and oxygen desaturation.
Keywords: obstructive sleep apnea, myocardial ischemia, coronary artery disease, CPAP, NT-pro BNP, troponin
Introduction
Obstructive sleep apnea (OSA) is linked to known cardiovascular risk factors, including obesity, insulin resistance, and dyslipidemia, and shows an association with adverse long-term outcome.1–3 OSA is characterized by repetitive interruption of ventilation during sleep with a fall in oxygen saturation. OSA is an independent risk factor for hypertension and has also been implicated in the pathogenesis of congestive cardiac failure.4 Repetitive apneas leading to intermittent nocturnal hypoxemia and sympathetic hyperactivity may result in sub-clinical myocardial injury.5
High-sensitive troponin T (hs-TropT) is a sensitive marker of myocardial damage and a clinical outcome predictor.6–8 Ventricular myocytes are also the major source of synthesis and secretion of cardiac N-terminal brain natriuretic peptide (NT-proBNP). In response to increased myocardial wall stress due to volume- or pressure-overload states, NT-proBNP levels are elevated in cardiac failure.9,10 Natriuretic peptide production is also upregulated in the area adjacent to ischemic myocardium.11
Other studies have shown higher hs-TropT and NT-proBNP serum concentrations associated with frequent apneas or hypoxemia in sleep apnea patients.5,12–14 Nasal continuous positive airway pressure (CPAP) is the gold standard therapy for clinically significant OSA.15,16
We hypothesized that the serum concentrations of the cardiac risk markers NT-proBNP and hs-TropT correlate with the effectiveness of CPAP therapy in OSA patients with coexist ing coronary artery disease (CAD).
Materials and Methods
The study obtained ethics approval from the ethics committee of the State Medical Association (FF 6/2012). All participants provided written informed consent. All aspects of the study were performed according to the principles of the Declaration of Helsinki.
Study participants
Initially, 80 patients were screened for clinically significant OSA by polygraphy. After a subsequent laboratory-based polysomnography, 39 participants did not satisfy the study criteria (Fig. 1). Twenty-one patients with severe OSA and coexisting CAD proven by coronary angiography (group 1) were recruited for this study. Twenty patients with OSA alone served as a control group (group 2).
Figure 1.
Study flow diagram.
Abbreviations: OSA, obstructive sleep apnea; AHI, apnea–hypopnea index; CAD, coronary artery disease; CPAP, continuous positive airway pressure; hs-Trop, high-sensitive troponin T; NT-proBNP, N-terminal brain natriuretic peptide.
Inclusion criteria were age ≥18 years, apnea–hypopnea index (AHI) >15/h, oxygen saturation ≤80% during apnea, and CAD (group 1). Exclusion criteria were heart failure [Left ventricular ejection fraction (LVEF) <40% quantified by echocardiography] and renal failure (glomerular filtration rate <50 mL/min).
Polysomnography and CPAP therapy
Nocturnal, laboratory-based polysomnography (Alice 5; Philips Respironics) was performed in order to confirm the diagnosis and to grade the severity of OSA. Polysomnographic registration and scoring were performed according to the recommendations of the American Academy of Sleep Medicine.17 Bedtime was 11 pm to 6 am. A cessation of airflow for ≥10 seconds was specified as apnea. Hypopnea was characterized as a decrease in airflow lasting ≥10 seconds with a ≥30% reduction in airflow and with at least a 4% oxygen desaturation from baseline. The AHI is the number of apneas or hypopneas recorded during the study per hour of sleep. Based on the AHI, the severity of OSA was classified as follows: mild (AHI 5–14/h), moderate (AHI 15–29/h), and severe (AHI ≥30/h). Optimal CPAP was calibrated in the sleep laboratory during a CPAP titration study in the following night. CPAP was increased until obstructive respiratory events were reduced or eliminated.18 After titration, all patients were monitored under CPAP therapy.
Measurement of cardiac risk markers
The methods of hs-TropT and NT-proBNP measurement and ST-segment analysis have been previously described in detail.19
Briefly, venous blood samples (5 mL) were collected at different time points: before (9 pm) and after (7 am) CPAP. The measurements were performed immediately after the blood samples were drawn. An immunoassay was used for the measurement of hs-TropT (Cobas e 411; Roche Diagnostics Inc.). Within the normal healthy population, 99% of people will have an hs-TropT <14 ng/L. NT-proBNP levels were measured using an immunoassay for the in vitro quantitative determination of NT-proBNP in heparinized venous blood (Cobas Roche Cardiac proBNP+; Roche Diagnostics). The measuring range is 60–9000 pg/mL, with a mean of the variation coefficients below 15% in the range of 60–1200 pg/mL and below 20% in the range of 1200–9000 pg/mL. The reference values of NT-proBNP depend on age and gender. A value of 125 pg/mL has been suggested as a cut point to rule out left ventricular systolic dysfunction. NT-proBNP has a half-life of 120 minutes.
Electrocardiogram analysis
A continuous electrocardiogram (ECG) recording during sleep was screened for nocturnal ST-segment depression. The ECG sign of subendocardial ischemia is ST-segment depression. A horizontal or down-sloping depression of 1 mm (100 µV) or more or up-sloping depression of the same magnitude 80 ms beyond the J point was considered positive signs of ischemia. After CPAP titration, ST-segment depression at the time of lowest oxygen saturation was analyzed and averaged in 10 cardiac cycles.
Statistics
Continuous variables were expressed as mean and standard deviation. For the descriptive statistics, the first, second, and third quartiles from a set of numerical data were calculated. The Mann–Whitney U test was used to compare differences between two independent groups. The Wilcoxon matched pairs test was performed to compare matched subjects. Linear regression analysis was used to investigate the relationship between two parameters. Analysis was done using statistical software (BiAS for Windows 10.12). P values less than 0.05 were reported as statistically significant.
Results
Twenty-one patients with OSA and a history of concomitant CAD were included in group 1: coronary artery bypass surgery in 5 patients, coronary angioplasty with stent implantation in 16 patients, and CAD proven by angiography in 2 patients. Clinical data of both patient groups are shown in Table 1.
Table 1.
Clinical characteristics
| OSA + CAD (n = 21) |
OSA (n = 20) |
P VALUE | |
|---|---|---|---|
| Demographics | |||
| Gender (female/male) | 5/16 | 3/17 | – |
| Age (years) | 61 ± 11 | 54 ± 12 | 0.05 |
| Body mass index (kg/m2) | 35 ± 7 | 33 ± 6 | 0.39 |
| Medical history | |||
| Hypertension | 19 (90%) | 13 (65%) | 0.17 |
| Hypercholesterolemia | 17 (81%) | 5 (25%) | <0.001 |
| Diabetes mellitus | 10 (48%) | 5 (25%) | 0.23 |
| Arrhythmia | 6 (29%) | 1 (5%) | 0.21 |
| Stroke | 2 (10%) | 0 | 0.61 |
| Myocardial infarction | 12 (57%) | 0 | <0.001 |
| COPD | 4 (19%) | 2 (10%) | 0.63 |
| Medication | |||
| Nitrates | 3 (14%) | 0 | 0.45 |
| β-blockers | 19 (90%) | 7 (35%) | <0.01 |
| Renin inhibitors | 0 (0%) | 1 (5%) | 0.81 |
| ACE inhibitors | 14 (67%) | 3 (15%) | <0.01 |
| Angiotensin receptor blockers | 3 (14%) | 5 (25%) | 0.58 |
| Diuretics | 11 (52%) | 5 (25%) | 0.14 |
| Calcium channel blockers | 9 (43%) | 5 (25%) | 0.34 |
| α-blockers | 1 (5%) | 2 (10%) | 0.79 |
| Cardiac and renal function | |||
| Left ventricular ejection fraction (%) | 59 ± 9 | 65 ± 2 | 0.05 |
| Glomerular filtration rate (ml/min) | 121 ± 53 | 137 ± 43 | 0.18 |
Note: Data are presented as mean ± SD or no. (%).
CPAP therapy significantly reduced the frequency of obstructive events and improved arterial oxygenation during sleep in both patient groups. The mean AHI and oxygen desaturation under CPAP were similar in groups 1 and 2. Additional polysomnographic variables are shown in Table 2. The data were not different between the two groups except sleep time with oxygen saturation <90%.
Table 2.
Polysomnographic data.
| OSA + CAD (n = 21) |
OSA (n = 20) |
P VALUE | |
|---|---|---|---|
| Baseline | |||
| Apnea–hypopnea index (n/h) | 53 ± 21 | 49 ± 20 | 0.46 |
| Oxygen saturation nadir (%) | 71 ± 12 | 71 ± 15 | 0.58 |
| Sleep time with SaO2 <90% (%) | 18 ± 17 | 18 ± 18 | 0.85 |
| Sleep time with SaO2 <80% (%) | 2 ± 4 | 4 ± 7 | 0.85 |
| Maximal duration of SRBD (sec) | 89 ± 56 | 82 ± 23 | 0.71 |
| CPAP therapy | |||
| Apnea hypopnea index (n/h) | 6 ± 5 | 4 ± 4 | 0.07 |
| Oxygen saturation nadir (%) | 87 ± 6 | 89 ± 5 | 0.29 |
| Sleep time with SaO2 <90% (%) | 10 ± 22 | 0.8 ± 1.5 | <0.05 |
| Sleep time with SaO2 <80% (%) | 0.7 ± 2.8 | 0 | 0.46 |
| Maximal duration of SRBD (sec) | 26 ± 19 | 26 ± 21 | 0.79 |
| CPAP (mbar) | 8.5 ± 1.3 | 7.9 ± 1.3 | 0.10 |
Note: Data are presented as mean ± SD or no. (%).
Abbreviations: SRBD, sleep-related breathing disorders; CPAP, continuous positive airway pressure.
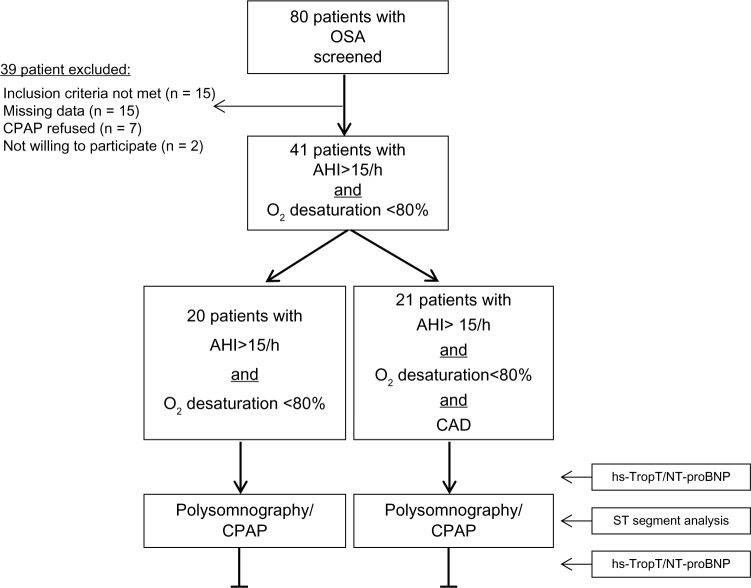
Clinical heart failure symptoms and signs were absent in all patients. Echocardiography showed normal systolic LV function (LVEF ≥50%) in 19 patients and moderately reduced LV function (LVEF 40%) in 2 patients in group 1. All patients in group 2 had normal LV function. Natriuretic peptide concentrations were higher before and after CPAP therapy in group 1 compared to group 2, but the difference between groups was not significant (Fig. 2). Mean NT-proBNP before and after CPAP treatment measured 475 ± 654 and 352 ± 573 pg/mL in group 1 and 105 ± 59 and 93 ± 58 pg/mL in group 2, respectively. In group 1, CPAP significantly reduced NT-proBNP. A moderate but significant correlation was noted between NT-proBNP levels and AHI in group 1 (Fig. 3), while we found no such correlation in group 2.
Figure 2.
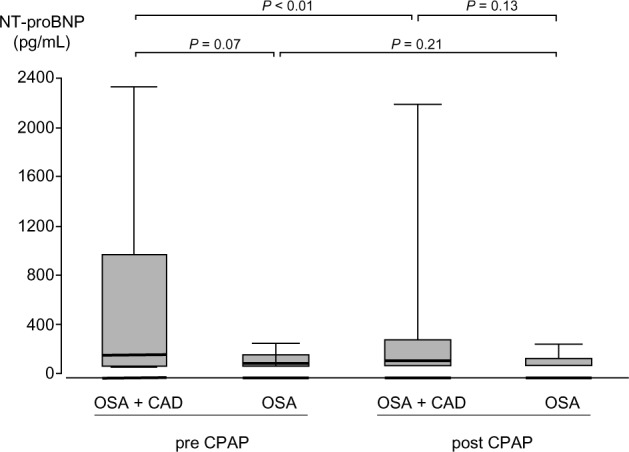
NT-proBNP levels pre and post CPAP therapy. Middle horizontal line inside box indicates median. Bottom and top of the box are 25th and 75th percentiles, and the error bars outside the box represent maximum and minimum values, respectively.
Figure 3.
Correlation between NT-proBNP levels and AHI under CPAP therapy.
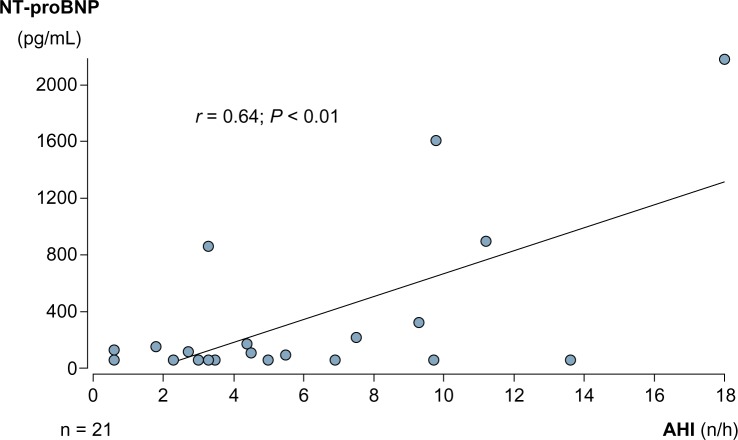
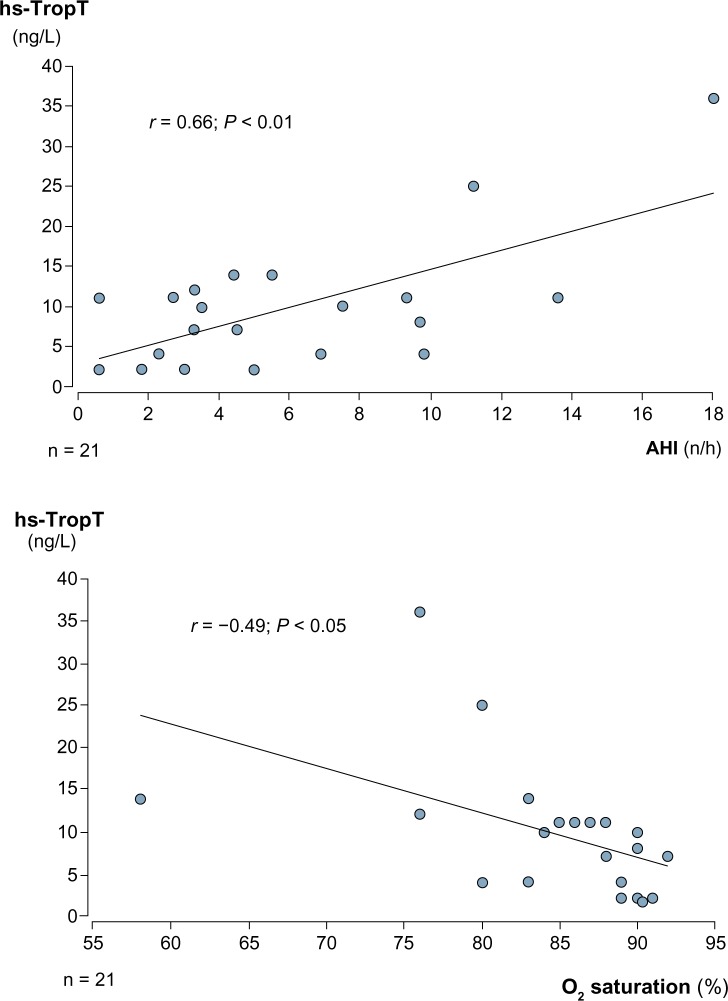
Troponin T levels were not different between the two groups. CPAP had no significant effect on hs-TropT levels. After CPAP therapy, hs-TropT was detectable (≥3 ng/L) in 17 (81% of group 1) and 19 (95% of group 2) patients, respectively. Mean hs-TropT levels before and after CPAP were 9 ± 8 and 10 ± 8 ng/L in group 1 and 7 ± 3 and 7 ± 3 ng/L in group 2. We found a moderate but significant correlation between hs-TropT levels and AHI and a weak negative correlation between hs-TropT levels and oxygen saturation nadir in group 1 (Fig. 4). In group 2, these correlations were not significant.
Figure 4.
Correlation between high-sensitive troponin T levels and AHI and oxygen saturation under CPAP therapy.
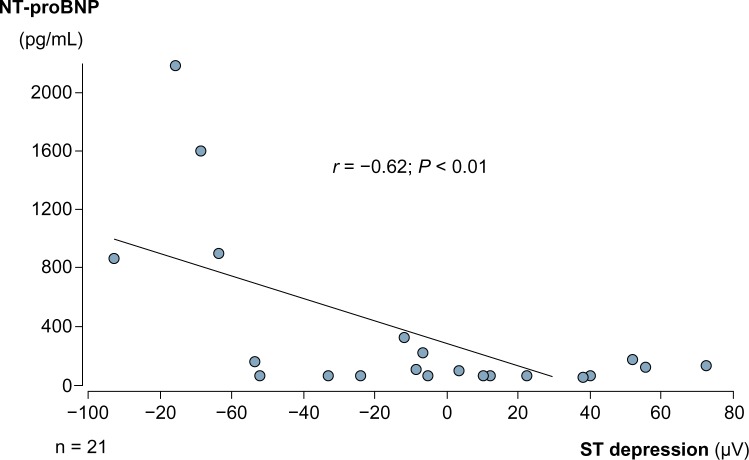
In both groups, ST-segment analysis showed no ST depression ≥100 µV at the time point of lowest oxygen saturation under CPAP treatment. Mean ST depression was −9 ± 47 µV in group 1 and −4 ± 32 µV in group 2. The difference was not significant. In group 1 patients, a moderate negative correlation of ST depression with NT-proBNP concentration was noted (Fig. 5). No such correlation was found in group 2.
Figure 5.
Correlation between NT-proBNP levels and ST-segment depression.
Discussion
The prevalence of cardiovascular disease is increased in patients with OSA.20 In patients with OSA and CAD, repetitive hypoxia due to sleep-induced apnea and/or hypopnea adversely affects the interaction between myocardial oxygen demand and supply, resulting in nocturnal myocardial ischemia.21 In some studies, insufficient oxygen supply in patients with CAD has been assumed as the main cause for myocardial ischemia, while others postulate a rise in oxygen consumption due to an accelerated heart rate and a sympathetic stimulation following an apnea episode.13,21–24 CPAP therapy is the leading treatment for clinical relevant OSA. CPAP is effective in eliminating hypopnea/apnea and in improving apnea sequelae.15
There is only limited information about the effect of CPAP therapy on the concentrations of hs-TropT and NT-proBNP in individuals with OSA and coexisting CAD. In the current study, we found only slightly elevated hs-TropT (>13 ng/L) in a few patients. CPAP had no effect on hs-TropT levels. Hs-TropT levels were moderately correlated with AHI under CPAP and negatively correlated with oxygen saturation nadir. The results of other studies that investigated the association between OSA and myocardial injury quantified by troponin measurement are not unequivocal. Also the effect of CPAP on troponin levels remains unclear. Several studies found higher troponin concentrations independently associated with AHI in untreated OSA patients free of CAD.5,12,25 Geovanini et al.26 showed that OSA severity was independently associated with hs-TropT levels in patients with refractory angina. In contrast, no significant difference in troponin levels between moderate/severe and mild/no OSA was reported by Maeder et al.27 In this study, short-term CPAP also had no impact on troponin concentrations before and after sleep. Colish et al.28 also showed no significant change of hs-TropT levels from normal baseline values following 12 months of CPAP therapy. Sharafkhaneh et al.29 found that an increase in hs-TropT in unselected patients with OSA was associated with severity of hypoxia but not AHI.29 The effect of CPAP was greatest to those with pronounced hypoxia as assessed by nadir oxygen saturation at baseline. Barceló et al studied patients with OSA without coexistent CAD and assessed the effect of CPAP on hs-TropT levels. In this study, the amount of participants with detectable hs-TropT was higher in patients with OSA than in controls.30 Interestingly, a significant increase in hs-TropT levels was observed after an effective treatment with CPAP. The authors supposed that to a certain extent, CPAP therapy might cause cardiac stress, resulting in higher hs-TropT levels.
We found significantly higher NT-proBNP levels in group 1 compared to group 2. Interestingly, CPAP significantly reduced NT-proBNP serum levels in group 1. NT-proBNP levels were moderately correlated with AHI under CPAP therapy. In line with our findings, a number of studies described elevated NT-proBNP concentrations linked to OSA but the study results are not consistent.5,14,31–34 NT-proBNP is also associated with the extent of CAD in patients with unimpaired LV function.35 N-terminal B-type natriuretic peptide predicts ischemia in patients with stable angina pectoris.36,37 Increase in upper airways resistance in untreated OSA patients can cause high negative intrathoracic pressures and enhanced venous inflow, resulting in intensified right ventricular diastolic filling and secretion of natriuretic peptides. Stimulated sympathetic activity, repetitive rises in blood pressure, and apnea-induced wall stress may also contribute as a trigger to release N-terminal B-type natriuretic peptide in OSA. CPAP treatment eliminates negative intrathoracic pressure fluctuations, and this effect could be the reason for a decline in natriuretic peptides secretion.1 In a meta-analysis, therapy with CPAP was associated with improvements in ejection fraction, diastolic blood pressure, and heart rate in patients with sleep-disordered breathing and congestive heart failure.38 In several other studies, a decrease of elevated natriuretic peptide concentrations was noted as a response to therapy with CPAP.27,32,39–42 A study by Maeder et al.27 found larger relative overnight reduction in BNP concentration in patients with moderate/severe OSA than in those with mild/no OSA. However, a study by Cifci et al in 33 consecutive patients with OSA did not detect any significant difference between severity of OSA syndrome and serum pro-BNP levels. CPAP had no impact on natriuretic peptide serum concentration after six months of CPAP therapy.14 In a prospective study of 47 patients with OSA, levels of NT-proBNP did not change significantly from normal baseline values following one year of CPAP therapy, despite improvement in cardiovascular remodeling.28
In our study, CPAP significantly decreased elevated NT-proBNP levels but had no significant impact on slightly increased hs-TropT levels. The difference might be explained by the fact that NT-proBNP is not only a marker of myocardial ischemia but also relate strongly to changes in the hemodynamic status. CPAP treatment has relevant hemodynamic effects because it eliminates negative intrathoracic pressure fluctuations and therefore causes a reduction in right ventricular preload and wall stress.
In the present study, ECG in group 1 patients revealed only minor ST-segment depression (<100 µV) under CPAP therapy. ST-segment depression showed a significantly negative correlation with NT-proBNP levels. Nocturnal ST-segment depression has been reported in about a third of untreated OSA patients with CAD during apneas with oxygen desaturation.13,21 Treatment with CPAP significantly ameliorated the nocturnal ST depression. Interestingly, Hanley at al also found ST depression during sleep in symptom-free patients with OSA without a history of CAD.43 The exact explanation for nocturnal ST-segment depression in patients with OSA remains unclear. ST depression during sleep in OSA patients may be caused by the combination of increased myocardial oxygen consumption and decreased oxygen supply due to oxygen desaturation and peak hemodynamic changes during the rebreathing phase of the obstructive apnea. Repeated respiratory efforts against an obstructed pharynx cause negative intrathoracic pressure fluctuations and changes in cardiac preload and afterload. This mechanism may also contribute to myocardial ischemia even without hypoxemia.44
Study limitations
The study is limited by the fact that the sample size is relatively small. Factors like sympathetic nervous system overactivity in OSA patients causing accelerated heart rate and increased blood pressure and their impact on myocardial ischemia were not evaluated. The majority of patients of group 1 were on medication (β-blockers, angiotensin converting enzyme [ACE] inhibitors), which may have affected our findings. For analysis of ST-segment depression, we selected the ECG recording at the time of minimal oxygen saturation. However, it is possible that the time of maximum ischemia was during the postapnea tachycardia. Most of the patients in group 1 were treated with coronary interventions before, which could have influenced ischemic thresholds.
Conclusion
Hs-TropT serum concentrations in patients with severe OSA and coexisting CAD were within normal range and similar to individuals with OSA alone. Before sleep, NT-proBNP serum concentrations in patients with OSA and CAD were increased compared to patients with OSA alone. CPAP treatment normalized elevated NT-proBNP serum levels in these patients. Levels of NT-proBNP and hs-TropT correlated with AHI and oxygen desaturation.
Acknowledgments
The authors thank Simone Rack of Krankenhaus Sachsenhausen Sleep Laboratory for her excellent technical assistance.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 523 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Developed the study hypothesis: MV, CT. Acquired data and drafted the manuscript: RS. Conducted the analysis and drafted the manuscript: MV, CT. All authors provided critical revision of the manuscript and have read and approved the final manuscript.
REFERENCES
- 1.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 2.Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep apnea syndrome patients. Mortality. Chest. 1988;94(6):1200–4. doi: 10.1378/chest.94.6.1200. [DOI] [PubMed] [Google Scholar]
- 3.Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4(3):261–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41(9):1429–37. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 5.Querejeta Roca G, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–5. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Christenson E, Christenson RH. Characteristics of cardiac troponin measurements. Coron Artery Dis. 2013;24(8):698–704. doi: 10.1097/MCA.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. N Engl J Med. 2000;343(16):1139–47. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 9.Boerrigter G, Costello-Boerrigter LC, Burnett JC., Jr Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009;5(4):501–14. doi: 10.1016/j.hfc.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagopoulou V, Deftereos S, Kossyvakis C, et al. NTproBNP: an important biomarker in cardiac diseases. Curr Top Med Chem. 2013;13(2):82–94. doi: 10.2174/1568026611313020002. [DOI] [PubMed] [Google Scholar]
- 11.Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004;6(3):257–60. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Einvik G, Rosjo H, Randby A, et al. Severity of obstructive sleep apnea is associated with cardiac troponin I concentrations in a community-based sample: data from the Akershus sleep apnea project. Sleep. 2014;37(6):1116A. doi: 10.5665/sleep.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peled N, Abinader EG, Pillar G, Sharif D, Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol. 1999;34(6):1744–9. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 14.Cifci N, Uyar M, Elbek O, Suyur H, Ekinci E. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath. 2010;14(3):241–4. doi: 10.1007/s11325-009-0306-y. [DOI] [PubMed] [Google Scholar]
- 15.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hukins CA. Obstructive sleep apnea – management update. Neuropsychiatr Dis Treat. 2006;2(3):309–26. doi: 10.2147/nedt.2006.2.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iber CA-IS, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Valo M, Wons A, Moeller A, Teupe C. Markers of myocardial ischemia in patients with obstructive sleep apnea and coronary artery disease. Pulm Med. 2015;2015:621450. doi: 10.1155/2015/621450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoeahypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 21.Schafer H, Koehler U, Ploch T, Peter JH. Sleep-related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest. 1997;111(2):387–93. doi: 10.1378/chest.111.2.387. [DOI] [PubMed] [Google Scholar]
- 22.Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep apnoea and nocturnal angina. Lancet. 1995;345(8957):1085–7. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 23.McNicholas WT, Bonsigore MR, Management Committee of EU COST ACTION B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 24.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 25.Randby A, Namtvedt SK, Einvik G, et al. Obstructive sleep apnea is associated with increased high-sensitivity cardiac troponin T levels. Chest. 2012;142(3):639–46. doi: 10.1378/chest.11-1779. [DOI] [PubMed] [Google Scholar]
- 26.Geovanini GR, Pereira AC, Gowdak LHW, et al. Obstructive sleep apnoea is associated with myocardial injury in patients with refractory angina. Heart. 2016;102(15):1193–9. doi: 10.1136/heartjnl-2015-309009. [DOI] [PubMed] [Google Scholar]
- 27.Maeder MT, Strobel W, Christ M, et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015;48(4–5):340–6. doi: 10.1016/j.clinbiochem.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141(3):674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 29.Sharafkhaneh A, Katigbak J, Hirshkowitz M, et al. Severity of hypoxia modulates effect of CPAP on myocardial stress as measured by highly sensitive troponin T. Respir Res. 2015;16:126. doi: 10.1186/s12931-015-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barceló A, Esquinas C, Bauca JM, et al. Effect of CPAP treatment on plasma high sensitivity troponin levels in patients with obstructive sleep apnea. Respir Med. 2014;108(7):1060–3. doi: 10.1016/j.rmed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest. 2006;130(5):1377–84. doi: 10.1378/chest.130.5.1377. [DOI] [PubMed] [Google Scholar]
- 32.Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7(3):199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 33.Hubner RH, El Mokhtari NE, Freitag S, et al. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med. 2008;102(1):134–42. doi: 10.1016/j.rmed.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Patwardhan AA, Larson MG, Levy D, et al. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29(10):1301–6. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]
- 35.Wu N, Ma F, Guo Y, et al. Association of N-terminal pro-brain natriuretic peptide with the severity of coronary artery disease in patients with normal left ventricular ejection fraction. Chin Med J (Engl) 2014;127(4):627–32. [PubMed] [Google Scholar]
- 36.Weber M, Dill T, Arnold R, et al. N-terminal B-type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J. 2004;148(4):612–20. doi: 10.1016/j.ahj.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108(24):2987–92. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37(1):57–65. doi: 10.1002/clc.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao ZH, Liu ZH, Luo Q, et al. Positive pressure ventilation treatment reduces plasma levels of amino terminal-pro brain natriuretic peptide in congestive heart failure patients with sleep apnea. Circ J. 2006;70(5):572–4. doi: 10.1253/circj.70.572. [DOI] [PubMed] [Google Scholar]
- 40.Tasci S, Manka R, Scholtyssek S, et al. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol. 2006;95(1):23–30. doi: 10.1007/s00392-006-0315-9. [DOI] [PubMed] [Google Scholar]
- 41.Krieger J, Laks L, Wilcox I, et al. Atrial natriuretic peptide release during sleep in patients with obstructive sleep apnoea before and during treatment with nasal continuous positive airway pressure. Clin Sci (Lond) 1989;77(4):407–11. doi: 10.1042/cs0770407. [DOI] [PubMed] [Google Scholar]
- 42.M’Saad S, Grati M, Marrakich R, Gargouri R, Kammoun S, Jamouss K. Impact of CPAP treatment on pro-BNP in obstructive sleep apnea syndrome. Eur Respir J. 2015;46:A2328. [Google Scholar]
- 43.Hanly P, Sasson Z, Zuberi N, Lunn K. ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol. 1993;71(15):1341–5. doi: 10.1016/0002-9149(93)90552-n. [DOI] [PubMed] [Google Scholar]
- 44.Parish JM, Shepard JW., Jr Cardiovascular effects of sleep disorders. Chest. 1990;97(5):1220–6. doi: 10.1378/chest.97.5.1220. [DOI] [PubMed] [Google Scholar]