Abstract
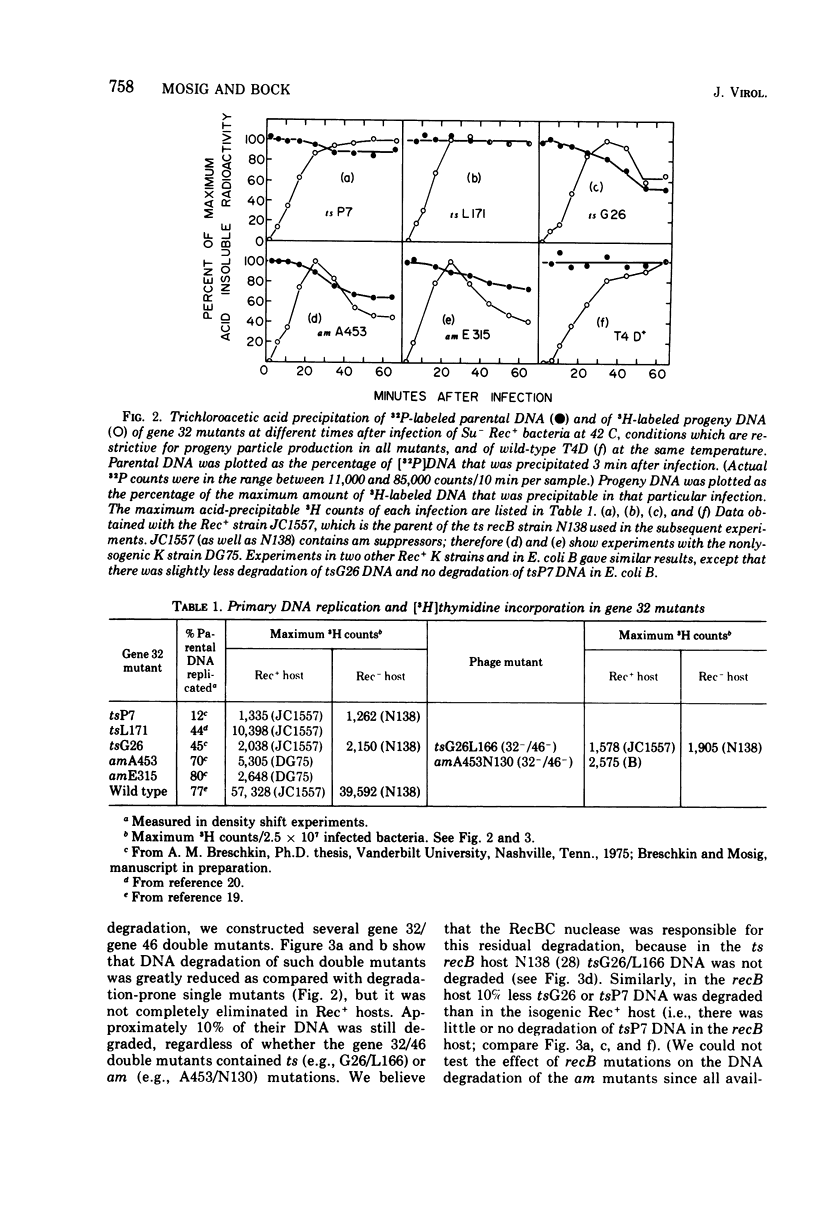
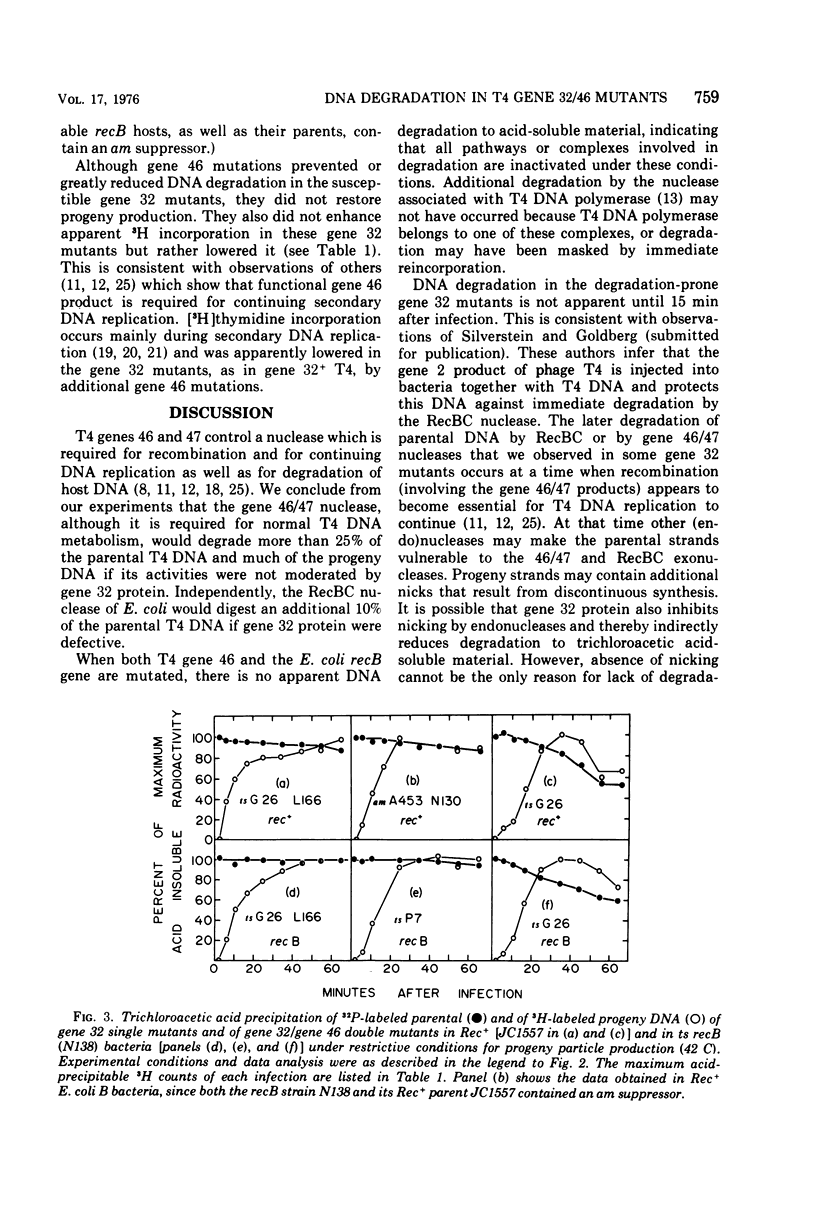
Genes 46 and 47 of phage T4 control a nuclease that is required for genetic recombination and may act similarly to the Escherichia coli RecBC nuclease. In vivo, the nucleolytic activities of both of these nucleases must be moderated so that recombining DNA intermediates are not destroyed. We conclude from our present experiments that the phage T4 gene 32 protein, specifically its C-terminal domain, participates in such moderation. We have investigated DNA degradation in different gene 32 and gene 32/46 mutants under conditions that are completely restrictive for progeny production in all the mutants. Under these conditions, DNA of those gene 32 mutants in which the C-terminal domain of the protein is not synthesized or is modified is degraded to acid-soluble material. T4 gene 46 or E. coli recB mutations reduce such degradation; together they abolish it completely. By contrast, single gene 32 mutants which produce an unaltered C-terminal domain show little or no degradation of their DNA. Residual protection against nucleases is unrelated to residual primary DNA replication or to overproduction of the mutant peptides in the different gene 32 mutants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin C. F. Degradation of T4 DNA synthesized in the absence of phage ligase. Virology. 1973 Apr;52(2):488–501. doi: 10.1016/0042-6822(73)90344-9. [DOI] [PubMed] [Google Scholar]
- Benzinger R., Enquist L. W., Skalka A. Transfection of Escherichia coli spheroplasts. V. Activity of recBC nuclease in rec+ and rec minus spheroplasts measured with different forms of bacteriophage DNA. J Virol. 1975 Apr;15(4):861–871. doi: 10.1128/jvi.15.4.861-871.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. Repair and recombination in phage T4. I. Genes affecting recombination. Cold Spring Harb Symp Quant Biol. 1968;33:325–331. doi: 10.1101/sqb.1968.033.01.037. [DOI] [PubMed] [Google Scholar]
- Broker T. R. An electron microscopic analysis of pathways for bacteriophage T4 DNA recombination. J Mol Biol. 1973 Nov 25;81(1):1–16. doi: 10.1016/0022-2836(73)90243-x. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., MELECHEN N. E. Synthesis of phage-precursor nucleic acid in the presence of chloramphenicol. Virology. 1957 Feb;3(1):207–236. doi: 10.1016/0042-6822(57)90034-x. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Mathews E. DNA replication in vivo by polynucleotide-ligase defective mutants of T4. II. Effect of chloramphenicol and mutations in other genes. J Mol Biol. 1971 Jan 28;55(2):155–179. doi: 10.1016/0022-2836(71)90189-6. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Mathews E., Jansen B. Role of genes 46 and 47 in bacteriophage T4 reproduction. I. In vivo deoxyribonucleic acid replication. J Virol. 1971 Oct;8(4):372–387. doi: 10.1128/jvi.8.4.372-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the exonuclease activity of phage T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 25;247(10):3139–3146. [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Breschkin A. M., Mosig G. Origin and direction of bacteriophage T4 DNA replication. II. A gradient of marker frequencies in partially replicated T4 DNA as assayed by transformation. J Mol Biol. 1971 Sep 14;60(2):213–233. doi: 10.1016/0022-2836(71)90289-0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Bowden D. W., Bock S. E. coli DNA polymerase I and other host functions participate in T4 DNA replication and recombination. Nat New Biol. 1972 Nov 1;240(96):12–16. doi: 10.1038/newbio240012a0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Breschkin A. M. Genetic evidence for an additional function of phage T4 gene 32 protein: interaction with ligase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1226–1230. doi: 10.1073/pnas.72.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Recombination in bacteriophage T4. Adv Genet. 1970;15:1–53. doi: 10.1016/s0065-2660(08)60070-x. [DOI] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. B., Berger H. Replication of gene 46-47 amber mutants of bacteriophage T4D. J Mol Biol. 1971 Apr 14;57(1):17–34. doi: 10.1016/0022-2836(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Anraku N., Iwama Y. Molecular mechanisms of genetic recombination in bacteriophage. VI. A mutant defective in the joining of DNA molecules. J Mol Biol. 1966 Nov 14;21(2):247–253. doi: 10.1016/0022-2836(66)90095-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]



