Abstract
The late phase of long-term potentiation (LTP) requires activation of the mammalian target of rapamycin (mTOR) pathway and synthesis of new proteins. mTOR regulates protein synthesis via phosphorylation of 4E-binding proteins (4E-BPs) and S6K, and via selective up-regulation of 5′ terminal oligopyrimidine (5′ TOP) mRNAs that encode components of the translational machinery. In this study, we explored the regulation of 5′ TOP mRNAs during late-LTP (L-LTP). Synaptic plasticity was studied at Schaffer collateral – CA1 pyramidal cell synapses in rat organotypic hippocampal slices. Forskolin, an adenylate cyclase activator, induced L-LTP in organotypic slices that was mTOR-dependent. To determine if 5′ TOP mRNAs are specifically up-regulated during L-LTP, we generated a 5′ TOP-myr-dYFP reporter to selectively monitor 5′ TOP translation. Confocal imaging experiments in cultured slices revealed an increase in somatic and dendritic fluorescence after forskolin treatment. This up-regulation was dependent on an intact TOP sequence and was mTOR, extracellular signal-regulated kinase (ERK), and phosphatidylinositol 3-kinase (PI3K)-dependent. Our findings indicate that forskolin induces L-LTP in hippocampal neurons and up-regulates 5′ TOP mRNAs translation via mTOR, suggesting that up-regulation of the translational machinery is a candidate mechanism for the stabilization of LTP.
Keywords: 5′ terminal oligopyrimidine tract mRNAs, mammalian target of rapamycin, synaptic plasticity, translation
Long-term potentiation (LTP), a candidate for the cellular basis of memory, requires a complex cascade of molecular and cellular events. Classically, LTP can be divided into two stages: an early stage that depends on phosphorylation and modification of pre-existing proteins (early-LTP, lasting minutes to hours) and a late stage that requires transcription and translation of new proteins [late-LTP (L-LTP), lasting several hours]. While protein synthesis can take place both in the cell body and the dendrites of hippocampal neurons, local protein synthesis is thought to play an important role for the reinforcement of specific synaptic connections during late-phase LTP (Kelleher et al. 2004b; Sutton and Schuman 2006). In the hippocampus, cAMP and mammalian target of rapamycin (mTOR) pathways have both been shown to be required for the late-form of LTP (Wong et al. 1999; Tang et al. 2002; Cammalleri et al. 2003; Wang et al. 2004) but it is still unclear whether these two pathways interact with each other or represent independent inputs.
Mammalian target of rapamycin activation regulates protein synthesis via phosphorylation of 4E-BPs and S6K, and via selective translation of 5′ terminal oligopyrimidine tract (5′ TOP) mRNAs, a subset of mRNAs encoding components of the translational machinery (Gingras et al. 2001; Hay and Sonenberg 2004). The 5′ TOP mRNA family is characterized by the presence of a 5′ TOP and comprises all the ribosomal proteins and several elongation factors (Meyuhas 2000; Meyuhas and Hornstein 2000). Translation of 5′ TOP mRNAs is regulated by the status of the cell: they are selectively stimulated during cell growth and specifically repressed upon growth arrest (Meyuhas 2000; Meyuhas and Hornstein 2000).
Recently, it has been suggested that regulating the synthesis of the translational machinery via mTOR in dendrites might play an important role for the maintenance of L-LTP and the reinforcement of specific synaptic connections (Tsokas et al. 2005, 2007; Poon et al. 2006; Liao et al. 2007). However, while the levels of several 5′ TOP mRNAs including elongation factor 1A (eEF1α), S6, eEF2, and poly-A binding protein (PABP) have been shown to be up-regulated during LTP in an mTOR-dependent manner (Tsokas et al. 2005, 2007), it is still unknown if this is because of their TOP sequence or other translational regulatory pathways.
In the present study, we investigated the relationships between cAMP and mTOR pathways in the regulation of 5′ TOP mRNAs and in L-LTP induced by forskolin, an adenylate cyclase activator. Using fluorescent reporters designed to specifically monitor 5′ TOP mRNAs translation, we found that forskolin specifically up-regulates 5′ TOP mRNAs translation in dendrites via activation of the mTOR pathway in a TOP-dependent manner. This suggests that increasing the translational capacity of synapses via the selective synthesis of 5′ TOP mRNAs, could play an important role for the maintenance of L-LTP.
Materials and methods
Electrophysiology
Transverse hippocampal slices (400-μm thick) were obtained from male Sprague–Dawley rats (4–6 weeks old) using a vibratome (Leica VT1000S, Leica Microsystems GmbH, Wetzlar, Germany) as described previously (Costa-Mattioli et al. 2005). Slices were kept submerged at 26–27°C and superfused (1–2 mL/min) with oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose. A bipolar tungsten stimulating electrode was placed in the CA1 stratum radiatum to stimulate the Schaffer collateral and commissural fibers, and extracellular field excitatory post-synaptic potentials (fEPSPs) were recorded with a glass microelectrode (2–3 MΩ, filled with 2 M NaCl) positioned in the stratum radiatum. LTP was induced either electrically using repetitive high frequency stimulation (four trains at 100 Hz, delivered 5 min apart) or chemically using forskolin (50 μM, Calbiochem, La Jolla, CA, USA), an adenylate cyclase activator. Whenever indicated, ACSF was supplemented with rapamycin (200 nM, Sigma, St. Louis, MO, USA) or anisomycin (40 μM, Calbiochem) at least 30 min prior to LTP induction.
Organotypic slice culture
Hippocampal slice cultures were obtained from P7 Sprague–Dawley rats as described previously (Stoppini et al. 1991; Bourdeau et al. 2007). In brief, the brain was removed and dissected in Hanks’ Balanced Salt Solution (Invitrogen, Canada, Burlington, ON, Canada)-based medium. Cortico-hippocampal slices (400-μm thick) were then obtained using a McIlwain tissue chopper (The Mickle Laboratory Engineering Co. Ltd, Gomshall, UK). After dissection, slices were placed on Millicell culture plate inserts (Millipore, Billerica, MA, USA) and allowed to recover in OptiMEM (Invitrogen) kept at 37°C in a humidified atmosphere (95% air, 5% CO2) for 48 h. They were then maintained in a Neurobasal medium (Invitrogen) supplemented with B27 and Glutamax I (Invitrogen) for 7–10 days. Usually, slices were transfected and processed for experiments after 1 week in culture.
Western blotting
Organotypic slice cultures were prepared as described above and acute transverse hippocampal slices (400-μm thick) were obtained from male Sprague–Dawley rats (4–6 weeks old) using a McIlwain tissue chopper. Slices were allowed to recover for 1 h in slice holding chambers containing heated ACSF (32°C) before receiving the indicated treatment. Slices were then processed for blotting as described previously (Topolnik et al. 2006). Briefly, hippocampal extracts were homogenized in ice-cold radioimmunoprecipitation assay buffer containing: 10 mM Tris pH 8, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 0.0001% Chymostatin, leupeptin, antipain, pepstatin (CLAP) protease inhibitors, 200 μM Na3VO4, 200 μM NaF, and 0.2 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 14 000 g for 20 min at 4°C to remove debris, and protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, IL, USA) using bovine serum albumin as a standard. Next, equal sample amounts (50 μg/lane) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% non-fat milk, 0.25% bovine serum albumin dissolved in tris-buffered saline-tween 20 buffer for 1 h at 23°C. After blocking, the membrane was probed with primary antibodies [phospho-S6K Thr389, phospho-S6K Thr421/Ser424, phospho-S6 Ser235/236, and phospho-Akt Ser473, (1 : 1000; Cell Signaling, Beverly, MA, USA) and phospho-extracellular signal-regulated kinases (ERK) Thr202/Tyr204, (1 : 5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)] overnight at 4°C. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, Piscataway, NJ, USA) for 1 h at 23°C. Immunoreactive bands were detected by enhanced chemiluminescence (Perkin Elmer, Waltham, MA, USA). The membrane was then stripped with a buffer containing 0.1 M glycine pH 2.2, 1% sodium dodecyl sulfate and reprobed with antibodies detecting levels of total S6K, S6, Akt, and ERK (Cell Signaling). All immunoreactive bands were scanned with a desktop scanner and quantified with QUANTITY ONE software (Bio-Rad, Hercules, CA, USA).
Constructs
Plasmids encoding pL32 and pL32C-A coupled to green fluorescent protein were a kind gift of Dr. Oded Meyuhas (Tang et al. 2001). Destabilized myristoylated versions of enhanced yellow fluorescent protein were inserted into the pL32 and pL32C-A plasmids, replacing enhanced green fluorescent protein, which gave the 5′ TOP reporter (5′ pL32-myr-dYFP) and the mutated 5′ TOP reporter (5′ pL32C-A-myr-dYFP), respectively. The sufficient and essential 640 bp (Blichenberg et al. 1999) of the dendritic targeting element of the 3′UTR (untranslated region) of microtubule associated protein from Rattus norvegicus was inserted in the 5′ TOP reporter and the mutated 5′ TOP reporter, which gave the 5′-TOP-MAP2-3′UTR reporter (5′-pL32-myr-dYFP-MAP2-3′ UTR) and the mutated 5′-TOP-MAP2-3′ UTR reporter (5′-pL32C-A-myr-dYFP-MAP2 3′ UTR), respectively.
HEK293 cells experiments
Human embryonic kidney cells (HEK293) cells were cultivated in Dulbelcco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, penicillin/streptomycin, and L-glutamine (Wisent Canada, QC) at 37°C in a humidified atmosphere (95% air, 5% CO2). One day prior to transfection, HEK293 cells were plated onto glass coverslips and placed in 35-mm culture dishes. HEK293 cells were transfected by calcium phosphate-DNA precipitation with either the 5′ TOP reporter (5′-pL32-myr-dYFP) or the mutated 5′ TOP reporter (5′-pL32C-A-myr-dYFP). After 24 h, cells were starved overnight before receiving serum and/or 20 nM rapamycin for 4 h. After this point, cells were quickly washed with phosphate-buffered saline, fixed in 4% paraformaldehyde, and processed for fluorescence imaging.
Transfection of hippocampal slice cultures
Gold beads (1.6 μm, Bio-Rad) were coated with 50 μg cDNA of either 5′ TOP reporter (5′-pL32-myr-dYFP), mutated 5′ TOP reporter (5′-pL32C-A-myr-dYFP), 5′-TOP-MAP2-3′ UTR reporter (5′-pL32-myr-dYFP-MAP2-3′ UTR), or mutated 5′-TOP-MAP2-3′ UTR reporter (5′-pL32C-A-myr-dYFP-MAP2-3′ UTR). After 1 week in culture, organotypic slices were transfected using a Helios GeneGun (Bio-Rad) according to the manufacturer’s instructions (150 psi at a distance of 1.5 cm). Slices were then returned to the incubator for 48 h before being treated, fixed, and processed for fluorescence imaging.
Confocal microscopy
After treatment, transfected hippocampal slices were fixed overnight in 4% paraformaldehyde. Slices were then quickly washed with phosphate-buffered saline and mounted in Prolong Antifade kit (Molecular Probes, Invitrogen). Images were acquired in 0.4–0.5 μm sections using a confocal laser scanning microscope LSM 510 (Carl Zeiss, Oberkochen, Germany). Yellow fluorescent protein was excited at 514 nm and emitted light was collected using a long-pass filter (cut-off 530 nm). Acquisition parameters and settings were kept constant for all conditions on a given experimental day. Images were analyzed using either LSM 510 (Carl Zeiss) or IMAGEJ software (NIH, Bethesda, MD, USA). Analysis of mean fluorescence levels was conducted on z-compressed stacks that contained the entire neuron or dendrite of interest.
Statistical analysis
All data are presented as means ± SEM and ‘n’ indicates the number of cells or slices. Statistical significance was determined using two-tailed Student’s t-tests or one-way ANOVAs. Differences were considered significant when p < 0.05.
Results
Forskolin induces L-LTP in organotypic hippocampal slices
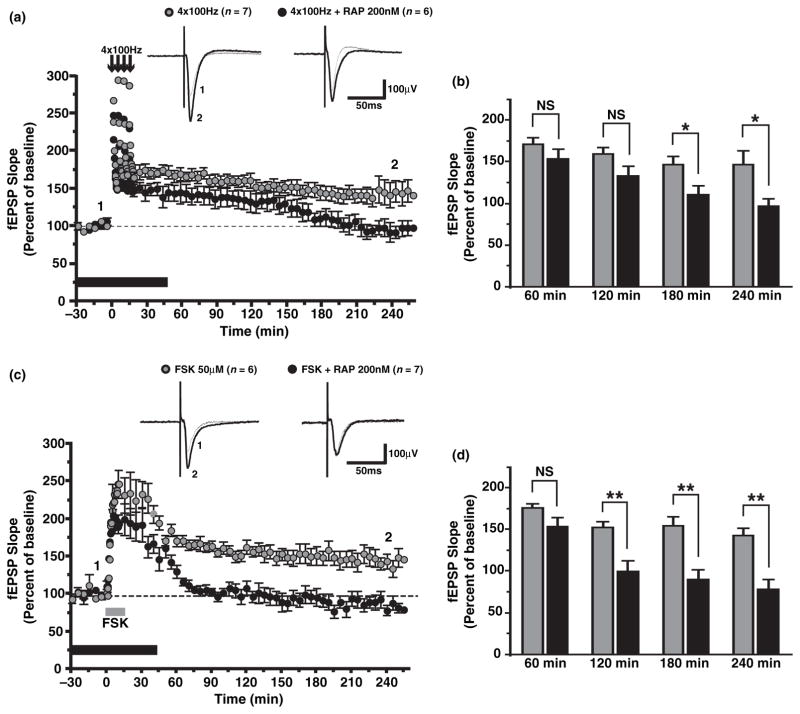
In acute hippocampal slices, high-frequency stimulation (four trains at 100Hz delivered 5 min apart) of Schaffer collateral/commissural fibers generates a sustained potentiation of fEPSP responses (Fig. 1a and b; at 240 min, 146.2 ± 17.5% of baseline for vehicle-treated slices, n = 7). As previously reported (Tang et al. 2002; Cammalleri et al. 2003), rapamycin, an inhibitor of the mTOR, selectively inhibited the late-phase of LTP in acute hippocampal slices (at 240 min, 96.4 ± 9.6% of baseline for rapamycin-treated slices, n = 6; p < 0.05). However, induction protocols such as four trains of high-frequency stimulation or theta-burst stimulation did not reproducibly induce L-LTP of field responses in hippocampal slice cultures (data not shown; Otmakhov et al. 2004; Kopec et al. 2006). This may be because of heavy reorganization of Schaffer collaterals in cultured slices leading to difficulty in achieving sufficient post-synaptic depolarization in a large number of cells to trigger the molecular cascades that induce LTP of field responses or because of the loss of modulatory inputs (such as dopaminergic fibers) that may be required to induce L-LTP (Frey et al. 1991; Matthies et al. 1997). On the other hand, forskolin, an adenylate cyclase activator, could induce a robust and sustained potentiation of fEPSPs responses recorded in CA1 stratum radiatum in slice cultures (Fig. 1c and d; at 240 min, 141.9 ± 9.8% of baseline for forskolin-treated slices, n = 6) (Otmakhov et al. 2004; Kopec et al. 2006). Moreover, forskolin-induced L-LTP was blocked by both anisomycin and rapamycin (at 240 min, 78.6 ± 9.6% of baseline for anisomycin-treated slices, n = 3, p < 0.01; 86.4 ± 9.9% of baseline for rapamycin-treated slices, n = 7, p < 0.01). Therefore, forskolin-induced L-LTP shares similar properties with L-LTP induced by high-frequency stimulation such as dependency on the synthesis of new proteins and activation of the mTOR pathway.
Fig. 1.
Forskolin (Fsk) induces late-long-term potentiation (LTP) in organotypic hippocampal slices. (a) Time plot of field excitatory post-synaptic potential (fEPSP) responses for group data with representative recordings above, showing that four trains of high-frequency stimulation elicit LTP of fEPSP responses that persists for at least 4 hours (grey circles, n = 7) in acute slices. In the presence of 200 nM rapamycin (RAP, black bar), an inhibitor of the mammalian target of rapamycin (mTOR), the late phase of LTP is specifically inhibited (black circles, n = 6). (b) Summary bar graph indicating that LTP in rapamycin (Rap)-treated slices (black bars) is significantly reduced 180–240 min post-tetanus as compared to vehicle-treated slices (grey bars; p < 0.05). (c) Time plot of fEPSP responses showing that the application of 50 μM Fsk (grey bar) produces a robust and sustained LTP (grey circles, n = 6) in cultured slices. Fsk-induced LTP was prevented by the application of 200 nM Rap (black circles, n = 7). (d) Summary bar graph indicating that LTP is significantly reduced 120–240 min post-Fsk (p < 0.01) in Rap-treated slices (black bars) as compared to vehicletreated slices (grey bars). *p < 0.05; **p < 0.01; NS, not significant.
Forskolin increases the activity of several components of the mTOR pathway
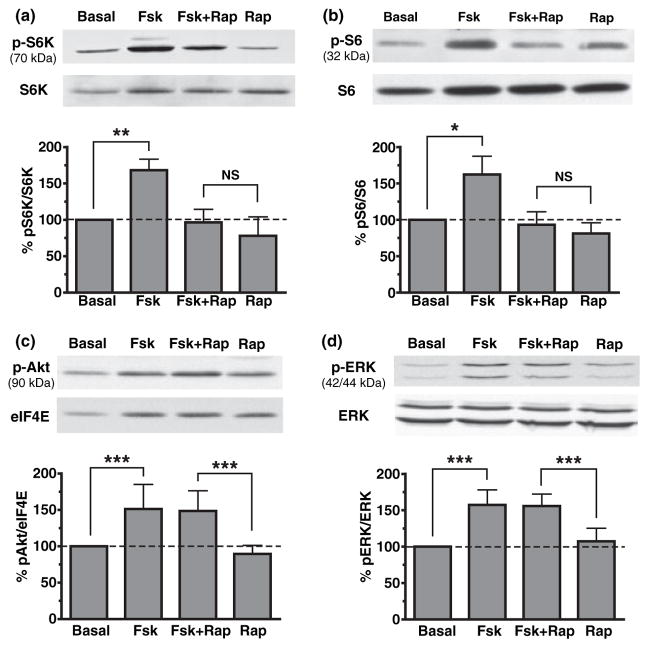
Next, we verified if adenylate cyclase stimulation could indeed activate components of the mTOR pathway in acute slices. Signaling to mTOR pathway is commonly achieved via phosphatidylinositol 3-kinase (PI3K) and Akt/protein kinase B (PKB) (Gingras et al. 2001; Hay and Sonenberg 2004) but it is not known if cAMP can also activate this pathway. Using western blot analysis, we assessed phosphorylation levels of S6K (Thr389) and S6 (Ser235/236) after forskolin treatment as markers of mTOR activation. Application of forskolin to hippocampal slices markedly increased the phosphorylation of S6K and S6 (Fig. 2a and b for phospho-S6K: 168.3 ± 15.2%, n = 5, p < 0.01; for phospho-S6: 162.2 ± 25.1%, n = 12, p < 0.05). This increase was blocked by pre-treatment with rapamycin (for phospho-S6K: 96.5 ± 17.9%, n = 5, p > 0.05; for phospho-S6: 93.3 ± 17.8%, n = 12, p > 0.05). In contrast, forskolin treatment-induced phosphorylation of Akt (Ser473) and ERK (Tyr204) (Fig. 2c and d; for phospho-Akt: 151.3 ± 33.8%, n = 6, p < 0.001; for phospho-ERK: 157.2 ± 20.8%, n = 4, p < 0.001), but these augmentations were not prevented by the addition of rapamycin (for phospho-Akt: 148.6 ± 27.8%, n = 6, p < 0.001; for phospho-ERK: 155.8 ± 16.5%, n = 5, p < 0.001). Both Akt and ERK are upstream of mTOR, thus confirming the specificity of rapamycin. These results demonstrate that forskolin stimulates phosphorylation of S6K and S6 via activation of the mTOR pathway in hippocampal slices.
Fig. 2.
Forskolin (Fsk) increases the activity of several components of the mammalian target of rapamycin (mTOR) pathway in hippocampal slices. (a) Western blot analysis of hippocampal extracts reveals that S6K phosphorylation (Thr389) is significantly increased by the application of 50 μM Fsk (p < 0.01). This increase is completely blocked by pre-treatment of hippocampal slices with 200 nM rapamycin (Fsk + Rap). (b) Fsk also increases the phosphorylation levels of the ribosomal protein S6 (Ser235/236), a TOP-mRNA encoded protein (p < 0.05) which was blocked by Rap. (c) Akt phosphorylation is significantly increased by 50 μM Fsk (p < 0.001) but this augmentation is not prevented by the addition of 200 nM Rap. (d) Similarly, phosphorylation of extracellular signal-regulated kinase (ERK) (Tyr204) is increased by treatment with Fsk (p < 0.001) but this effect was not blocked by pre-treatment with Rap (p < 0.001) indicating that Rap only affected phosphorylation of proteins downstream of mTOR. *p < 0.05; **p < 0.01; ***p < 0.001; NS, non-significant.
Forskolin selectively activates a 5′ TOP reporter via mTOR in HEK cells
While regulation of 5′ TOP mRNAs is usually regulated by mTOR, there are also counterexamples to this paradigm (Tang et al. 2001). Moreover, specific regulation by TOPs has not been investigated in neurons previously, and regulatory pathways in neurons are often specialized. To specifically determine if there is regulation of translation of TOP-containing mRNAs during LTP, we generated two different fluorescent reporters based on previous work examining regulation of 5′ TOP mRNAs (Avni et al. 1994). All 5′ TOP mRNAs have a cap structure followed by a C residue and an uninterrupted stretch of 4–14 pyrimidine residues (Meyuhas 2000; Meyuhas and Hornstein 2000). Mutation of the first C residue to an A residue is sufficient to convert a 5′ TOP transcript into a non-TOP-regulated transcript (Avni et al. 1994). We thus took advantage of this singularity to test the role of the TOP in regulating translation in hippocampal slices. The first construct contains the L32 promoter (required to ensure correct transcriptional initiation at the TOP), the L32 TOP sequence followed by a sequence encoding a destabilized, myristoylated derivative of YFP (5′ TOP reporter: 5′-pL32-myr-dYFP). The second construct is identical, but the first nucleotide of the TOP was converted from a C to an A, removing regulation through the TOP (mutated 5′ TOP reporter: 5′-pL32C-A-myr-dYFP).
We first verified the biological activity of our constructs in HEK293 cells. It has previously been shown that growth arrest or serum deprivation (starving) selectively represses translation of 5′ TOP mRNAs. In contrast, mitogenic stimuli or serum addition preferentially shifts 5′ TOP mRNAs to the polyribosomal fraction and leads to active translation (Meyuhas 2000). Thus, we took advantage of this paradigm to test the activity of our fluorescent reporters.
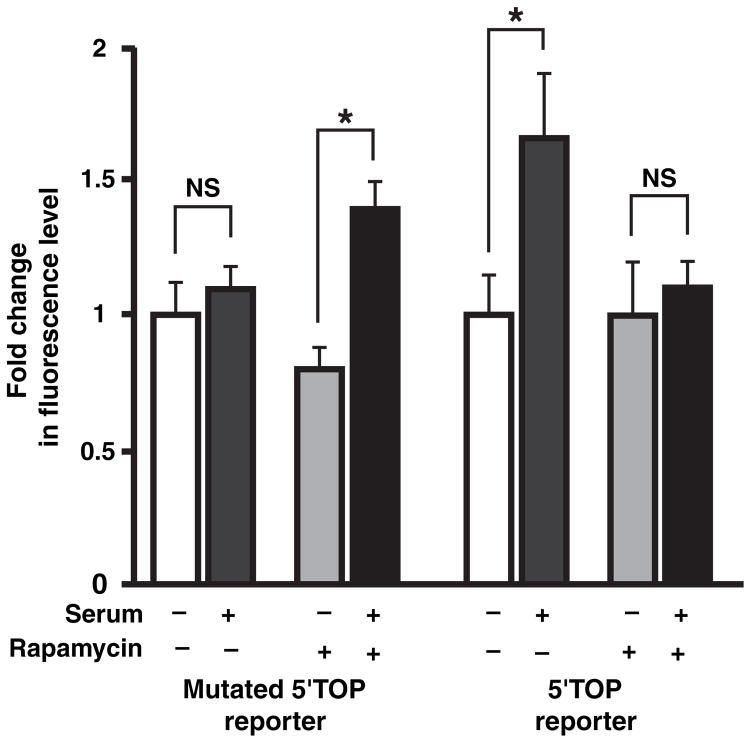
Human embryonic kidney cells were transfected with either the 5′ TOP reporter or the mutated 5′ TOP reporter. After an overnight starving, serum replenishment to cultures increased the fluorescence levels of the 5′ TOP reporter in HEK293 cells, but there was no change in the level of the mutated 5′ TOP reporter (Fig. 3, for the 5′ TOP reporter, n = 33 cells, p < 0.001; for the mutated 5′ TOP reporter, n = 33 cells, p > 0.05). Pre-treatment with rapamycin completely blocked the increase in the level of the 5′ TOP reporter (n = 28 cells, p > 0.05) demonstrating that this was because of mTOR-dependent up-regulation of its translation. Surprisingly, rapamycin pre-treatment revealed an increase in the level of the mutated 5′ TOP reporter by serum replenishment (n = 28 cells, p < 0.01). This may be because of serum activation of other pathways (including possible non-translational pathways), which were suppressed when mTOR was also activated. Nonetheless, the observed rapamycin-sensitive increase in fluorescence levels of the 5′ TOP reporter (TOP-specific) after serum stimulation confirmed that this reporter could effectively monitor TOP-regulated translation.
Fig. 3.
Forskolin selectively activates a 5′ terminal oligopyrimidine (5′ TOP) reporter via mammalian target of rapamycin (mTOR) in human embryonic kidney (HEK) cells. HEK cells transfected with fluorescent 5′ TOP and mutated 5′ TOP reporters were tested for serum-induced translation. Summary bar graph showing that after an overnight starving, serum addition selectively increases the fluorescence of a 5′ TOP reporter (p < 0.05) and this is blocked by 20 nM rapamycin (right bars). Mutated 5′ TOP reporter is not stimulated by serum (left bars). Addition of rapamycin reveals the existence of a rapamycin-insensitive increase in the level of the mutated 5′ TOP reporter (p < 0.05). *p < 0.05; NS, not significant.
Forskolin increases somatic 5′ TOP mRNAs translation via mTOR in slice cultures
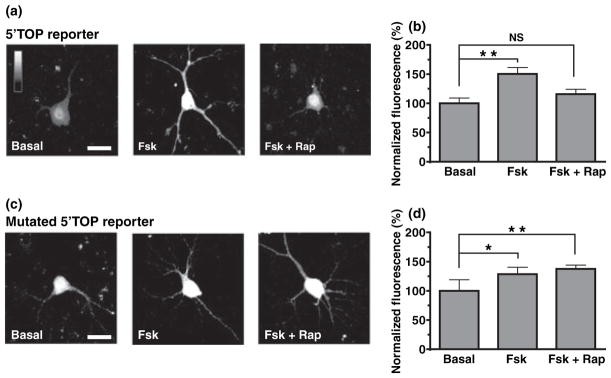
We next investigated the effect of cAMP activation on 5′ TOP mRNAs translation using these two constructs in organotypic hippocampal slices. Neurons in cultured slices were transfected with either the 5′ TOP reporter or the mutated 5′ TOP reporter and fluorescence levels were measured in the cell body of transfected CA1 pyramidal cells. Application of forskolin activated both 5′ TOP and mutated 5′ TOP reporters in slice cultures (Fig. 4a and b 4 h after forskolin application: for 5′ TOP reporter, 150.4 ± 10.9%, n = 14 cells, p < 0.01; for mutated 5′ TOP reporter, 128.5 ± 11.9%, n = 11 cells, p < 0.05). However, pre-treatment with rapamycin selectively prevented increases in 5′ TOP-reporter fluorescence levels (Fig. 4c and d for 5′ TOP reporter, 115.7 ± 8.4%, n = 8 cells, p > 0.05; for mutated 5′ TOP reporter, 137.5 ± 6.5%, n = 6 cells, p < 0.01). This suggests that adenylate cyclase activation can up-regulate somatic 5′ TOP mRNAs translation in an mTOR-dependent manner. Although cAMP stimulation also stimulates general translation in the cell body (i.e., other translational mechanisms that are not governed by the presence of the TOP in the 5′-UTR), these are not mTOR-dependent.
Fig. 4.
Forskolin (Fsk) increases somatic 5′ terminal oligopyrimidine (5′ TOP) mRNAs translation via mammalian target of rapamycin (mTOR) in slice cultures. (a and b) Confocal images (a) and summary bar graph (b) from CA1 pyramidal cells transfected with the 5′ TOP reporter. The fluorescence levels are significantly increased in cells from slices treated with 50 μMFsk, as compared to cells from vehicle-treated slices (p < 0.01). This increase in fluorescence is prevented by pre-treatment with 200 nM rapamycin (Rap). (c and d) Similar experiments showing that the fluorescence levels of a mutated 5′ TOP reporter are significantly increased in cells from slices treated with Fsk (p < 0.05), but, in contrast, this augmentation is not blocked by the pre-treatment with Rap. Scale bar: 20 μm. *p < 0.05; **p < 0.01; NS, not significant.
Forskolin increases dendritic 5′ TOP mRNAs translation via mTOR, ERK, and PI3K in slice cultures
To this point, we have shown that cAMP activation up-regulates 5′ TOP mRNAs in the cell bodies of CA1 pyramidal cells and that this requires mTOR activation. However, recent reports have suggested the role of dendritic translation for the reinforcement of specific synaptic connections (Kelleher et al. 2004b; Sutton and Schuman 2006). Therefore, we next examined whether up-regulation of 5′ TOP mRNAs could also be observed in dendrites. To detect fluorescence in dendrites, we added the MAP2-3′ UTR region to our constructs. It has previously been shown that this sequence contains signals that direct the MAP2 mRNA to dendritic regions (Blichenberg et al. 1999). When cultured slices were transfected with the 5′-TOP-MAP2-3′ UTR reporter, fluorescence levels in CA1 pyramidal cell dendrites were markedly increased by forskolin treatment (Fig. 5a–c). This augmentation was significant throughout the dendritic length examined, from the most proximal region (Fig. 5b and c; at 10 μm from the soma: 209.28 ± 23.87%, n = 13 cells for forskolin-treated slices, as compared to 100.0 ± 9.1%, n = 15 cells for control slices, p < 0.001; normalized to the fluorescence in the first 10 μm in untreated slices) to the most distal part (at 100 μm from the soma: 112.8 ± 21.6%, n = 10 cells for forskolin-treated slices, as compared to 55.5 ± 6.6%, n = 10 cells for control slices, p < 0.05). The forskolin-induced increase in fluorescence was prevented by pre-treatment with rapamycin, but only in the most distal region of the dendrite (for rapamycin-treated slices: at 10 μm from the soma, 163.7 ± 23.5%, n = 14 cells, p < 0.05; at 100 μm from the soma, 62.3 ± 9.2%, n = 13 cells, p > 0.05). In contrast, when neurons were transfected with the mutated 5′-TOP-MAP2-3′ UTR reporter, no significant difference was observed in pyramidal neurons for vehicle-treated, forskolin-treated, or forskolin + rapamycin-treated slices, neither in the proximal dendritic compartment (Fig. 5d–f; at 10 μm from the soma: 100.0 ± 8.0%, n = 26 cells for control slices, 107.3 ± 16.7%, n = 21 cells for forskolin-treated slices, 109.6 ± 21.3%, n = 12 cells, for forskolin + rapamycin treated slices, p > 0.05) nor in the distal dendritic compartment (at 100 μm from the soma: 49.7 ± 5.3%, n = 23 cells for control slices, 41.4 ± 6.0%, n = 18 cells for forskolin-treated slices, 39.9 ± 8.5%, n = 9 cells for forskolin + rapamycin-treated slices, p > 0.05). These data indicate that cAMP stimulation specifically up-regulates 5′ TOP mRNAs translation through mTOR in dendrites of hippocampal pyramidal cells.
Fig. 5.
Forskolin (Fsk) increases dendritic 5′ terminal oligopyrimidine (5′ TOP) mRNAs translation via mammalian target of rapamycin (mTOR), phosphatidylinositol 3-kinase and extracellular signal-regulated kinase in slice cultures. (a and b) Confocal images (a) and summary plot (b) showing that the fluorescence levels of a 5′-TOPMAP2- 3′ UTR reporter are significantly increased in dendrites of pyramidal cells by the application of 50 μM Fsk. This augmentation is prevented by pre-treatment with 200 nM rapamycin. (c) Summary bar graph illustrating that Fsk-induced increase is significant from proximal (at 10 μm, p < 0.001) to distal regions (at 100 μm, p < 0.05). (d and e) Similar experiments indicating that, in contrast, fluorescence levels of a mutated 5′-TOP-MAP2-3′ UTR reporter are not increased by Fsk with or without rapamycin (Rap) pre-treatment. (f) Summary bar graph showing that there is no significant difference in fluorescence throughout the dendritic length in all conditions. (g and h) Confocal images (g) and summary plot (h) from separate experiments showing that the fluorescence levels of a 5′-TOP-MAP2-3′ UTR reporter are significantly increased in dendrites of pyramidal cells by the application of 50 μM Fsk and that this augmentation is prevented by pre-treatment with 50 μM PD98059, 50 μM LY294002, or a high dose of Rap (1 μM). (i) Summary bar graph illustrating that Fsk-induced increase is significant from proximal (at 10 μm, p < 0.001) to distal regions (at 100 μm, p < 0.05). Scale bar: 10 μm. *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant.
Nonetheless, differences in the regulation of 5′ TOP mRNAs seem to exist between proximal and more distal dendrites of cultured hippocampal pyramidal cells. As described above, we observed that fluorescence in proximal dendrites could not be blocked by a low dose of rapamycin, although the same dose completely prevented the increase in fluorescence in more distal dendrites (Fig. 5a–c). There are several possibilities for this discrepancy, including increased mTOR activity and/or presence of additional regulatory pathways in proximal dendrites. To differentiate between these two possibilities, we performed additional confocal imaging experiments with the 5′-TOP-MAP2-3′ UTR reporter using a higher dose of rapamycin as well as mitogen activated protein kinase kinase (MEK)/ERK and PI3K inhibitors (Fig. 5g–i). Under these conditions, fluorescence levels were again markedly increased by forskolin treatment from the most proximal dendritic regions (Fig. 5h and i; at 10 μm from the soma: 149.4 ± 10.4%, n = 14 cells for forskolin-treated slices, as compared to 100.0 ± 3,2%, n = 24 cells for control slices, p < 0.001; normalized to the fluorescence in the first 10 μm in untreated slices) to the most distal dendritic regions (at 100 μm from the soma: 83.2 ± 7.2%, n = 13 cells for forskolin-treated slices, as compared to 60.3 ± 3.2%, n = 23 cells for control slices, p < 0.05). This increase in fluorescence was prevented by the addition of either a high dose of rapamycin (1 μM), an inhibitor of MEK/ERK (PD98059) or an inhibitor of PI3K (LY294002) in both proximal dendrites (at 10 μm from the soma: 107.2 ± 6.8%, n = 19 cells for forskolin + rapamycin-treated slices, 104.5 ± 9,8%, n = 15 cells for forskolin + PD980059-treated slices, 101.1 ± 10.4%, n = 16 cells for forskolin + LY294002-treated slices, p > 0.05) and distal dendrites (at 100 μm from the soma: 62.5 ± 5.2%, n = 19 cells for forskolin + rapamycin-treated slices, 63.1 ± 7.0%, n = 14 cells for forskolin + PD980059-treated slices, 61.4 ± 5.8%, n = 16 cells for forskolin + LY294002-treated slices, p > 0.05). These data contrast with those obtained previously using a lower dose of rapamycin (Fig. 5a–c), in which proximal fluorescence could not be blocked by the addition of the mTOR inhibitor. Moreover, they indicate that throughout the dendritic length, MEK/ERK and PI3K pathways are required for the forskolin-induced increase in 5′ TOP mRNA translation.
Forskolin activates the mTOR pathway in an ERK and PI3K-dependent manner in slice cultures
To date, we have shown that mTOR, MEK/ERK, and PI3K pathways are required for forskolin-induced 5′ TOP mRNAs translation. However, it is still unclear if these pathways regulate 5′ TOP mRNAs in a parallel or linear fashion. To further investigate the mechanism by which cAMP stimulation activates the mTOR pathway, as well as the interactions between MEK/ERK, PI3K and mTOR pathways, we performed additional western blot experiments and examined the phosphorylation levels of S6K at both the mTOR-dependent site (Thr389) and the ERK-dependent site (Thr421/Ser424). Moreover, to match the experiments using the TOP reporter, we did these experiments on slice cultures as opposed to the earlier experiments on acute slices. Application of forskolin to organotypic slices strongly increased the phosphorylation of S6K at both Thr389 and Thr421/Ser424 (Fig. 6a and b; for Thr389: 142.5 ± 12.6%, n = 7, p < 0.05; for Thr421/Ser424: 155.8 ± 14.1%, n = 5, p < 0.05), as well as phosphorylation of S6 (Fig. 6d; 162.5 ± 11.8%, n = 6, p < 0.01). This increase was blocked by addition of PD98059, a specific MEK/ERK inhibitor (for S6K Thr389: 109.4 ± 8,8%, n = 7, p > 0.05; for S6K Thr421/Ser424: 107.5 ± 8.6%, n = 5, p > 0.05; for S6: 114.8 ± 19.1%, n = 6, p > 0.05). In contrast, rapamycin pre-treatment blocked the increase in phosphorylation of S6K at Thr389 and S6 (for S6K Thr389: 90.4 ± 3.5%, n = 7, p > 0.05; for S6: 111.3 ± 9.9%, n = 6, p > 0.05), and had no effect on the increase in phosphorylation of S6K at Thr421/Ser424 (154.5 ± 19.0%, n = 5, p < 0.05). Similarly, addition of LY294002, a specific PI3K inhibitor, prevented the increases in phosphorylation of S6K at Thr389 and S6 (for S6K Thr389: 114.7 ± 13.1%, n = 7, p > 0.05; for S6: 117.1 ± 12.6%, n = 6, p > 0.05) but did not block the augmentation in phosphorylation of S6K at Thr421/Ser424 (160.5 ± 22.6%, n = 5, p < 0.05). Moreover, ERK phosphorylation was increased by cAMP stimulation (Fig. 6c; 150.8 ± 13.6%, n = 6, p < 0.05), which was blocked by PD98059 (102.2 ± 11.7%, n = 6, p > 0.05), but not by rapamycin (162.8 ± 20.2%, n = 6, p < 0.05) or LY294002 incubation (162.9 ± 18.6%, n = 6, p < 0.05). Taken together, these results suggest that forskolin stimulates phosphorylation of S6K and S6 in an ERK-dependent and PI3K-dependent manner. Moreover, they indicate that mTOR activation in organotypic slices requires the conjunction of both MEK/ERK and PI3K activation.
Fig. 6.
Forskolin (Fsk) activates the mammalian target of rapamycin (mTOR) pathway in phosphatidylinositol 3-kinase- and extracellular signal-regulated kinases (ERK)-dependent manner in slice cultures. (a) Western blot analysis of hippocampal extracts reveals that S6K phosphorylation at the mTOR-dependent site (Thr389) is significantly increased by the application of 50 μM Fsk (p < 0.05). This increase is completely blocked by pre-treatment of hippocampal slices with 50 μM PD98059 (Fsk + PD), 50 μM LY294002 (Fsk + LY), and a high dose (1 μM) of rapamycin (Fsk + Rap). (b) Fsk also significantly increases (p < 0.05) S6K phosphorylation at the MEK/ERK-dependent site (Thr421/Ser424). In contrast, this increase is blocked by the addition of PD98059, but not by LY294002 (p < 0.05) or Rap (p < 0.05). (c) Similarly, phosphorylation of ERK (Tyr204) is increased by treatment with Fsk (p < 0.05) and this effect was only blocked by pretreatment with PD98059 and not by LY294002 (p < 0.05) or Rap (p < 0.05). (d) Fsk also significantly augments (p < 0.01) the phosphorylation levels of the ribosomal protein S6 (Ser235/236), an effect that is prevented by the addition of PD98059, LY294002, or Rap. *p < 0.05; **p < 0.01.
Discussion
Our results indicate that forskolin induces L-LTP and up-regulation of 5′ TOP mRNAs translation in organotypic hippocampal slices via activation of the mTOR pathway (Fig. 7). First, forskolin, an adenylate cyclase activator, induces a sustained potentiation of fEPSPs that is sensitive to rapamycin treatment. In addition, cAMP activation increases the activity of S6K and S6, two components of the mTOR pathway, in an ERK and PI3K-dependent manner, which underscores the interaction between cAMP, ERK, PI3K, and mTOR pathways. Second, forskolin application increases the fluorescence of a 5′ TOP reporter in both the cell body and dendrites via mTOR, ERK and PI3K, indicating that cAMP activation is also coupled with an increased translation rate of 5′ TOP containing mRNAs.
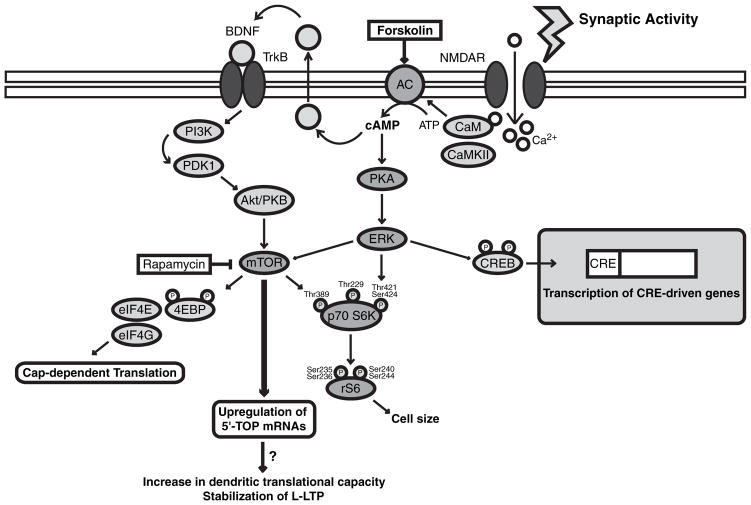
Fig. 7.
A model for the up-regulation of 5′ terminal oligopyrimidine (5′ TOP) mRNAs translation during late-long-term potentiation (LTP). During LTP-inducing stimulation, NMDA receptors are recruited by high-frequency synaptic activity and allow the entry of calcium into the post-synaptic neuron. This in turn activates adenylate cyclase (AC) and leads to the production of cAMP and the activation of cAMPdependent protein kinase (PKA). PKA can then activate the mammalian target of rapamycin (mTOR). The precise mechanism involved remains unclear but PKA could activate mTOR via at least two pathways. First, PKA could activate the extracellular signal-regulated kinase (ERK) pathway. ERK has previously been shown to be required for the activation of downstream targets of mTOR such as 4E-BP and S6 (Kelleher et al. 2004a). Alternatively, cAMP production could cause the release of brain-derived neurotrophic factor (BDNF). Released BDNF can in turn activate TrkB receptors and the phosphatidylinositol 3-kinase (PI3K)/Akt-PKB pathway, which has also been shown to activate mTOR (Patterson et al. 2001; Takei et al. 2004). Finally, activation of mTOR pathway leads to the selective up-regulation of 5′ TOP mRNAs. Our results suggest that a selective increase in 5′ TOP mRNAs translation may be crucial for late-LTP stabilization. CaM, calmodulin; CREB, cAMP responsive element binding protein; CRE, cAMP responsive element.
Induction of L-LTP in hippocampal slice cultures
To date, very few studies have examined late-phase LTP in organotypic hippocampal slices, even though this system is very useful for manipulation of protein expression. Indeed, organotypic slices can be maintained in culture for several days to weeks and therefore allow for the transfection and the long-term expression of fluorescent reporters (in this case, 5′ TOP or mutated 5′ TOP constructs). In previous reports (Muller et al. 1993; Leutgeb et al. 2003; Mellentin et al. 2006), LTP in slice culture has been studied mostly for a short period after LTP induction (approximately 1 h). At such time-point, changes observed at synapses are mostly associated to early-phase LTP rather that to the late-phase of LTP. In our study, we were able to record fEPSP responses for up to 4 h, which corresponds to the maintenance phase of L-LTP, by using chemical induction by forskolin. Our results suggest that this chemical induction protocol in slice culture may be a useful tool for a detailed examination of molecular pathways implicated in L-LTP.
Interactions between cAMP, ERK, PI3K and mTOR pathways during L-LTP
It has previously been shown that cAMP and mTOR signaling pathways are both required for late-phase LTP in the hippocampus (Wong et al. 1999; Tang et al. 2002; Cammalleri et al. 2003; Wang et al. 2004) but it is still unclear whether these two pathways interact with each other. Several studies have shown that forskolin can induce a L-LTP that shares many features of electrically-induced L-LTP (Frey et al. 1993; Otmakhov et al. 2004). On the other hand, various papers demonstrate that rapamycin selectively blocks the late-phase LTP in the hippocampus (Tang et al. 2002; Cammalleri et al. 2003). Nevertheless, it has never been shown that mTOR blockade could also interfere with the late-phase of forskolin-induced LTP. Importantly, our results now demonstrate that forskolin-induced LTP is sensitive to rapamycin pre-treatment, suggesting that adenylate cyclase stimulation is activating the mTOR pathway and that this is a necessary step in forskolin-induced L-LTP. Consistent with cAMP activation acting through mTOR, we found that cAMP activation increased the phosphorylation of S6K and S6, two downstream effectors of mTOR, in a rapamycin-sensitive manner.
Various effectors could mediate interactions between cAMP and mTOR pathways. Previous studies have identified Akt/PKB and ERK as upstream regulators of mTOR (Gingras et al. 2001; Hay and Sonenberg 2004; Kelleher et al. 2004a; Lenz and Avruch 2005). Because in our study both phosphorylation of Akt and ERK were increased by forskolin treatment, it is likely that mTOR activation is downstream of these two known activators of mTOR (Fig. 7). While forskolin is not a natural stimulus, activation of adenylate cyclase is critical for ERK activation during both LTP and memory formation (Impey et al. 1998; Sindreu et al. 2007) and ERK is involved in activating the mTOR pathway during LTP (Kelleher et al. 2004a).
Using various biochemical approaches, several recent reports have clearly shown that there is an interaction between ERK and mTOR pathways in hippocampal slices during both LTP and long-term depression (LTD) (Kelleher et al. 2004a; Banko et al. 2006; Tsokas et al. 2007; Antion et al. 2008b). Indeed, phosphorylation of various effectors downstream of mTOR, including S6, S6K, and 4EBP1, are inhibited by treatment with PD98059 or UO126, two MEK/ERK inhibitors (Kelleher et al. 2004a; Banko et al. 2006; Antion et al. 2008b). Similarly, Tsokas et al. 2007 have demonstrated that HFS-induced phosphorylation of mTOR and translational proteins like eEF1A, S6, eEF2, and PABP1 is inhibited by incubation with PD98059 and UO126 (Tsokas et al. 2007). In our system, both phosphorylation of S6K at the mTOR site (Thr389) and S6 were prevented by addition of the specific MEK/ERK inhibitor PD98059, supporting the idea that forskolin activates the mTOR pathway in an ERK-dependent manner. Although our model depicts a direct interaction between ERK and mTOR pathways (Fig. 7), this might not be the case. Indeed, studies have suggested that activation of the mitogen-activated protein kinase (MAPK)/ERK pathway leads to phosphorylation of hamartin and tuberin, two proteins forming the tuberous sclerosis complex (TSC) complex and whose role is to inhibit signaling by mTOR (Roux et al. 2004). Other reports have also shown that the MAPK/ERK pathway might also act directly downstream of mTOR on proteins such as rpS6. Indeed, studies in S6K1−/−/S6K2−/− double knock-out (KO) cells have revealed that, in addition to S6K1 and S6K2, a MAPK-dependent kinase can also phosphorylate rpS6 at Ser235/Ser236 in an mTOR-independent mechanism (Pende et al. 2004). Subsequent work have identified the p90rsks as the kinases mediating the phosphorylation of rpS6 on these residues, and confirmed that Ras/ERK signaling promotes translation initiation (Roux et al. 2007).
Other studies have also suggested that cAMP activation during LTP is accompanied by the extracellular release of brain-derived neurotrophic factor (BDNF) (Patterson et al. 2001). Released BDNF could in turn activate TrkB receptors and the PI3K-Akt/PKB cascade (Patterson et al. 2001), and ultimately lead to the activation of the mTOR pathway (Takei et al. 2004). As phosphorylation of S6K at the mTOR site (Thr389) and S6 were also sensitive to LY294002, a specific PI3K inhibitor, our results not only suggest that S6K and S6 phosphorylation are downstream of ERK, but also of PI3K activation. Therefore, adenylate cyclase stimulation could trigger several cascades that lead to mTOR activation and translational activation (Fig. 7).
Although previous work has also suggested that ERK activation might be downstream of PI3K (Opazo et al. 2003; Tsokas et al. 2007), this might not be the case in our study. Indeed, we found that application of LY294002 did not affect the forskolin-induced increase in ERK phosphorylation. However, it is important to note that previous work used different LTP-inducing paradigms than cAMP stimulation (NMDA application, HFS-stimulation and TBS stimulation) and therefore, different cascades might be recruited. Alternatively, cross-talk between PI3K and ERK pathways may require stronger activation, like four trains of high-frequency stimulation (Tsokas et al. 2007).
L-LTP is associated with the activation of downstream targets of mTOR
Mammalian target of rapamycin effects are mediated by phosphorylation of both 4E-BPs and S6K, and by selective up-regulation of 5′ TOP mRNAs. Previously, it has been shown that LTP- or LTD-inducing stimuli increase the phosphorylation of 4E-BPs, S6K, and S6, and increase the levels of TOP-encoded proteins (Cammalleri et al. 2003; Takei et al. 2004; Banko et al. 2005, 2006; Tsokas et al. 2005, 2007; Antion et al. 2008b); however, the relative role that these pathways play in LTP has not been determined. Further work has shown that genetic deletion of either 4EBP2, S6K1, or S6K2 lead to specific deficits in LTP and memory, thus confirming the importance of these downstream targets of mTOR (Banko et al. 2005; Antion et al. 2008a). Surprisingly, however, removal of S6K1 or S6K2 did not seem to have much impact on L-LTP, suggesting that other downstream targets of mTOR like 4EBP2 might be more crucial for the stabilization of L-LTP (Antion et al. 2008a). Here, we provide evidence that LTP may also specifically involve an increase in 5′ TOP mRNAs through an mTOR-mediated pathway. It would be interesting in the future to determine which downstream pathway of TOR is required for mTOR-mediated synaptic plasticity.
On the other hand, as ERK, PI3K, and mTOR activation are also required for some forms of LTD (Gallagher et al. 2004; Hou and Klann 2004; Banko et al. 2006; Antion et al. 2008b), it would be interesting to determine if different downstream pathways of TOR are important for distinct roles of mTOR-mediated translational regulation in synaptic plasticity. Recently, Sajikumar and Frey (2004) described the phenomenon of ‘cross-tagging’ in which proteins synthesized in response to L-LTP stimuli can be captured by synapses undergoing early-LTD or inversely. This raises the intriguing possibility that the same proteins may be synthesized in response to either L-LTP or late-LTD-inducing stimuli, and captured at synapses that have been appropriately tagged by synaptic activity.
Initially, it was reported that mTOR activation increased phosphorylation of S6K and S6 and that this was correlated with augmented translation rates of 5′ TOP containing mRNAs (Meyuhas 2000; Meyuhas and Hornstein 2000). However, recent evidence contests this model. Knockout mice lacking both S6K1 and S6K2 showed that up-regulation of 5′ TOP mRNAs in response to mitogens is maintained (Pende et al. 2004) and knock-in mutants of S6, lacking all S6 phosphorylation sites, also have normal mTOR-dependent regulation of 5′ TOP mRNAs (Ruvinsky et al. 2005). Thus, although S6K and S6 phosphorylation levels can still be used as readouts of the activity of the mTOR pathway, these data strongly suggest the existence of another as yet uncharacterized pathway linking mTOR activation and 5′ TOP mRNAs translation.
L-LTP is associated with an increase in 5′ TOP mRNAs translation
Recent reports have suggested that levels of some proteins that are encoded by 5′ TOP containing mRNAs are up-regulated during L-LTP and LTD (Tsokas et al. 2005, 2007; Poon et al. 2006; Liao et al. 2007; Antion et al. 2008b). However, although previous work has correlated LTP-inducing stimulation and increases in the translation of some 5′ TOP mRNAs [for example, eEF1α, see Tsokas et al. (2005, 2007)], no direct causal link has ever been reported. More precisely, it has not been shown that the augmentation observed in these studies were restricted to the 5′ TOP family. Indeed, it might be possible that LTP-inducing protocols cause a general increase in translation of all dendritically targeted mRNAs, rather than a selective up-regulation of 5′ TOP mRNAs. In this study, we thus specifically designed fluorescent reporters to investigate the regulation of 5′ TOP containing mRNAs during L-LTP by comparing two constructs that were identical except for a single residue change that determines TOP regulation (Avni et al. 1994). Interestingly, in the cell body of CA1 pyramidal cells, while levels of both reporters were increased by forskolin, only levels of the proteins encoded by the 5′ TOP-containing mRNAs were sensitive to rapamycin pre-treatment. Moreover, in the dendrites of CA1 pyramidal cells, only levels of the reporter encoded by the 5′ TOP-containing mRNAs were increased, again in an mTOR-dependent manner. Taken together, our results thus clearly suggest that 5′ TOP mRNAs are specifically regulated after LTP induction. A better understanding of the mechanism for TOP regulation downstream of mTOR will allow for selective blockade of this pathway and determining the importance of TOP regulation for LTP.
There are several possible explanations for the differential regulation of 5′ TOP mRNAs in dendrites versus cell bodies. First, different mechanisms for regulation of translation in the cell body and dendrites might be responsible for the more selective up-regulation of 5′ TOP mRNAs observed here. For example, the rate limiting steps for translation could be distinct, and thus in dendrites, the process up-regulated by forskolin in a rapamycin-insensitive manner that acts on the non-TOP mRNAs is not present or not rate limiting. It should also be noted that the construct used for measuring dendritic regulation had the 3′ UTR of MAP2, and this may add additional translational constraints. Thus, the rapamycin-insensitive process up-regulated by forskolin in the cell body may no longer be rate limiting for the construct encoding the 3′ UTR of MAP2 as additional rate-limiting steps are now present. For example, translation of this construct in dendrites may now be regulated by removal of 3′ UTR-mediated repression, and as this process may not be activated by forskolin, it could explain the loss of forskolin-mediated regulation of the non-TOP construct in dendrites. As the only difference between the TOP and non-TOP construct was the C–A conversion at the beginning of the TOP sequence, differences between the activation of these constructs by forskolin and their sensitivity to rapamycin can be directly attributed to regulation of the TOP sequence. Thus, in both cell bodies and dendrites, the TOP sequence provides an additional forskolin-induced rapamycin-sensitive regulation that is likely to be important for the physiological regulation of 5′ TOP containing mRNAs.
We observed a differential sensitivity of the induction by forskolin to rapamycin between proximal and distal dendrites. A larger concentration of rapamycin was required to block the forskolin-induced increase in the 5′ TOP reported construct at proximal dendrites. While it is possible that the higher concentration may affect some other pathways, rapamycin has been reported to be extremely specific for mTOR even at higher concentrations (Bain et al. 2007). It may be because of an increased concentration of mTOR at proximal sites. mTOR has been reported to be linked to gephyrin, a marker of inhibitory synapses that are enriched in proximal dendrites (Sabatini et al. 1999).
Up-regulation of the translational machinery during L-LTP
As described in the literature, the 5′ TOP mRNAs family is mainly composed of ribosomal proteins and elongation factors and includes most components of the translational machinery (Meyuhas 2000; Meyuhas and Hornstein 2000). Ribosome biogenesis is highly energy costing and is therefore finely regulated to meet the changing requirements for the translational machinery. It has been shown that the presence of the TOP motif in the 5′-untranslated region allows for the rapid up-regulation of this class of mRNAs during situations that require high rates of protein synthesis, such as cell proliferation or differentiation (Meyuhas and Hornstein 2000). The shift of 5′ TOP mRNAs from the subpolysomal fraction to the polyribosomal pool can be induced by over-expression of a TOP message, suggesting that 5′ TOP mRNAs translation is regulated by an as yet unidentified repressor (Biberman and Meyuhas 1999). Although modulation of 5′ TOP mRNAs synthesis has been extensively associated with cell growth and related processes, recent work has suggested that this pathway might also be important for the stabilization of LTP in vertebrates (Tsokas et al. 2005, 2007; Poon et al. 2006; Liao et al. 2007) and long term facilitation in Aplysia (Khan et al. 2001; Moccia et al. 2003). Indeed 5′ TOP containing mRNAs are a major class of mRNAs sent out in processes in both vertebrates (Poon et al. 2006) and invertebrates (Moccia et al. 2003; Moroz et al. 2006). Moreover, ribosomal proteins were a major class of proteins translationally up-regulated in synaptoneurosomes after BDNF treatment, and this effect was dependent on activation of mTOR (Liao et al. 2007).
In Aplysia, rapamycin-sensitive local protein synthesis is important for the stabilization of long-term changes at synapses (Casadio et al. 1999). While complete ribosome biogenesis presumably cannot occur in dendrites, it is possible that during transport, ribosomes lose peripheral proteins, and that local translation of these subunits at stimulated synapses is required to activate dormant ribosomes (Moccia et al. 2003). Increases in the number of translationally competent ribosomes via the selective up-regulation of 5′ TOP mRNAs synthesis would then serve to specifically boost the translational capacity of activated synapses for a prolonged period and thus mark the synapse for persistence of changes. A meta-activation of local translation may be important to promote the translation of newly delivered mRNAs, and thus reconcile the requirement for local translation with the requirement for transcription and transport of newly synthesized mRNAs to synapses.
Taken together, our results demonstrate for the first time that 5′ TOP mRNAs are differentially regulated after LTP-inducing stimulation in an mTOR-dependent manner. Although we cannot rule out the implication of others mTOR targets, our present data clearly suggest that up-regulation of the translational machinery via the selective synthesis of 5′ TOP mRNAs, might play an important role for the stabilization of L-LTP.
Acknowledgments
The authors wish to thank Dr. Oded Meyuhas for the original pL32 and pL32C-A constructs, and Julie Pepin for excellent technical assistance. This work has been supported by CIHR group grant (to JCL, LDG, and WSS), FRSQ (GRSNC) and NSERC (studentship to DG). JCL is recipient of the Canada Research Chair in Cellular and Molecular Neurophysiology.
Abbreviations used
- 5′ TOP
5′ terminal oligopyrimidine tract
- ACSF
artificial cerebrospinal fluid
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal-regulated kinase
- fESPS
field excitatory post-synaptic potentials
- HEK
human embryonic kidney
- LTD
long-term depression
- L-LTP
late-LTP
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- UTR
untranslated region
References
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn Mem. 2008a;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008b;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni D, Shama S, Loreni F, Meyuhas O. Vertebrate mRNAs with a 5′-terminal pyrimidine tract are candidates for translational repression in quiescent cells: characterization of the translational cis-regulatory element. Mol Cell Biol. 1994;14:3822–3833. doi: 10.1128/mcb.14.6.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberman Y, Meyuhas O. TOP mRNAs are translationally inhibited by a titratable repressor in both wheat germ extract and reticulocyte lysate. FEBS Lett. 1999;456:357–360. doi: 10.1016/s0014-5793(99)00983-7. [DOI] [PubMed] [Google Scholar]
- Blichenberg A, Schwanke B, Rehbein M, Garner CC, Richter D, Kindler S. Identification of a cis-acting dendritic targeting element in MAP2 mRNAs. J Neurosci. 1999;19:8818–8829. doi: 10.1523/JNEUROSCI.19-20-08818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci. 2007;27:1942–1953. doi: 10.1523/JNEUROSCI.3208-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann KG, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross-talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, III, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004a;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, III, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004b;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Khan A, Pepio AM, Sossin WS. Serotonin activates S6 kinase in a rapamycin-sensitive manner in Aplysia synaptosomes. J Neurosci. 2001;21:382–391. doi: 10.1523/JNEUROSCI.21-02-00382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Frey JU, Behnisch T. LTP in cultured hippocampal-entorhinal cortex slices from young adult (P25–30) rats. J Neurosci Methods. 2003;130:19–32. doi: 10.1016/s0165-0270(03)00228-0. [DOI] [PubMed] [Google Scholar]
- Liao L, Pilotte J, Xu T, Wong CC, Edelman GM, Vanderklish P, Yates JR., III BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J Proteome Res. 2007;6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- Matthies H, Becker A, Schroeder H, Kraus J, Hollt V, Krug M. Dopamine D1-deficient mutant mice do not express the late phase of hippocampal long-term potentiation. Neuroreport. 1997;8:3533–5. doi: 10.1097/00001756-199711100-00023. [DOI] [PubMed] [Google Scholar]
- Mellentin C, Moller M, Jahnsen H. Properties of long-term synaptic plasticity and metaplasticity in organotypic slice cultures of rat hippocampus. Exp Brain Res. 2006;170:522–531. doi: 10.1007/s00221-005-0236-2. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Meyuhas O, Hornstein E. Translational control of TOP mRNAs. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 2000. pp. 671–694. [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya EEY, Kalachikov S, Spahn CM, Frank J, Kandel ER, Barad M, Martin KC. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci. 2003;23:9409–9417. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, et al. Neuronal transcriptome of aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippocampal organotypic cultures. Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O’Dell TJ. Phos-phatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Khibnik L, Otmakhova N, Carpenter S, Riahi S, Asrican B, Lisman J. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor-dependent. J Neurophysiol. 2004;91:1955–1962. doi: 10.1152/jn.00941.2003. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J, Betz H, Snyder SH. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;21:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol Cell Biol. 2001;21:8671–8683. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol. 2006;575:115–131. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse fore-brain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]