Abstract
An Aplysia Trk-like receptor (ApTrkl) was previously shown to be involved in cell wide long-term facilitation (LTF) and activation of ERK when serotonin (5-HT) is applied to the cell soma. The current study investigated the regulation of ApTrkl by overexpressing the receptor and several variants in Aplysia sensory neuron cultures. Kinase activity–dependent constitutive activation of ApTrkl was observed mainly on the plasma membrane. These studies revealed two modes of receptor internalization: (1) kinase activity–dependent internalization and (2) 5-HT-dependent, kinase activity–independent internalization. Both modes of internalization were ligand independent, and the action of 5-HT was mediated through G-protein-coupled receptors (GPCRs). On the other hand, methiothepin, an inverse agonist of 5-HT GPCRs activated endogenous ApTrkl to the same extent as 5-HT, suggesting a transactivation mechanism due to a novel coupling of GPCRs to receptor tyrosine kinase (RTK) activation that is also activated through inverse agonist binding. The neuropeptide sensorin could transiently activate ApTrkl but was not required for 5-HT-induced ApTrkl activation.
Keywords: transactivation, endocytosis, receptor tyrosine kinases
Changes in the strength of the connections between neurons, referred to as synaptic plasticity, underlie how organisms change their behavior through experience. In mammals, there is accumulating evidence for the involvement of receptor tyrosine kinases (RTKs), especially the tropomyosin-related kinase (Trk) family of RTKs (Trk receptors), in long-term synaptic plasticity and behavioral memory. BDNF/TrkB signaling is implicated in long-term potentiation (LTP) in the mammalian hippocampus (Patterson et al., 1992, 1996; Korte et al., 1995, 1998; Kang et al., 1997; Pozzo-Miller et al., 1999), as well as in hippocampus-dependent learning and memory (Mu et al., 1999; Hall et al., 2000; Mizuno et al., 2000; Alonso et al., 2002). In Aplysia, tyrosine kinase activity is involved in long-term memory (LTM) for the sensitization of gill-withdrawal reflex and serotonin (5-HT)-induced long-term facilitation (LTF), the most commonly studied form of synaptic plasticity in Aplysia, through downstream activation of extracellular signal–related kinase (ERK; Purcell et al., 2003). A Trk-like receptor in Aplysia (ApTrkl) is expressed in sensory neurons (Ormond et al., 2004). 5-HT activates ApTrkl, leading to activation of ERK, and ApTrkl is required for cellwide LTF when 5-HT is applied to the cell soma (Ormond et al., 2004). Although ApTrkl is clearly implicated in 5-HT-induced signaling, how 5-HT activates ApTrkl is not clear, especially becuase the extracellular domain of ApTrkl shows no homology with mammalian Trk receptors.
Regulation of Trk receptors through trafficking has been reported. TrkB is internalized by electric stimulation of hippocampal neurons and tyrosine kinase activity is required for the internalization (Du et al., 2003). Nerve growth factor (NGF) induces internalization of TrkA (Ehlers et al., 1995; Grimes et al., 1996). It is suggested that the functional consequence of internalization of growth factor receptors may be to initiate signal transduction (Sorkin and Waters, 1993).
5-HT is known to act through multiple G-protein-coupled receptors (GPCRs) in sensory neurons (Barbas et al., 2003). In mammalian cells, transactivation of RTKs by GPCRs is documented for epidermal growth factor receptor (EGFR), TrkA, and platelet-derived growth factor receptor (PDGFR; Daub et al., 1996; Lee and Chao, 2001; Kotecha et al., 2002). One mechanism of transactivation is for the G-protein-coupled receptor to increase the levels of the ligand for RTK. For example, on GPCR activation, the EGFR is activated by the metalloproteinase-dependent cleavage of proHB-EGF (Prenzel et al., 1999). Interestingly, in Aplysia, the neuropeptide sensorin is released from sensory neurons after repeated application of 5-HT and is involved in the activation of ERK (Hu et al., 2004), similar to the activation by ApTrkl, suggesting that sensorin may be a ligand for ApTrkl. Moreover, K252a, an inhibitor of ApTrkl, blocked many of sensorin’s actions (Hu et al., 2004; Ormond et al., 2004). In other cases, transactivation is ligand independent, and Src tyrosine kinases are involved in the transactivation of EGFR and TrkA (Daub et al., 1997; Luttrell et al., 1997; Maudsley et al., 2000; Lee and Chao 2001). The D4 dopamine receptor transactivates PDGFR and leads to ERK activation, possibly through the recruitment of β-arrestin and Src or through other components of the ERK signaling pathway (Ferguson, 2003). Thus, it is possible that activation of ApTrkl is a result of 5-HT GPCR-mediated transactivation, either by recruitment of a ligand or by activation of intracellular tyrosine kinases such as Src.
To explore the regulation of ApTrkl, we examined the localization and activation of overexpressed ApTrkl and several variants including extracellular TrkC chimeric ApTrkl constructs with or without intracellular mutations (kinase dead or signaling tyrosine deficient). The results revealed several mechanisms of ApTrkl trafficking that may be related to its regulation. Examination of the endogenous receptor suggested a transactivation mechanism involving signaling downstream of GPCRs, but sensorin was not required for activation of ApTrkl by 5-HT.
MATERIALS AND METHODS
Animals
Aplysia californica specimens (75–125 g) were obtained from Marine Speciments Unlimited (Pacific Palisades, CA) or the Mariculture Facility of the University of Miami (Miami, FL).
Plasmid Construction
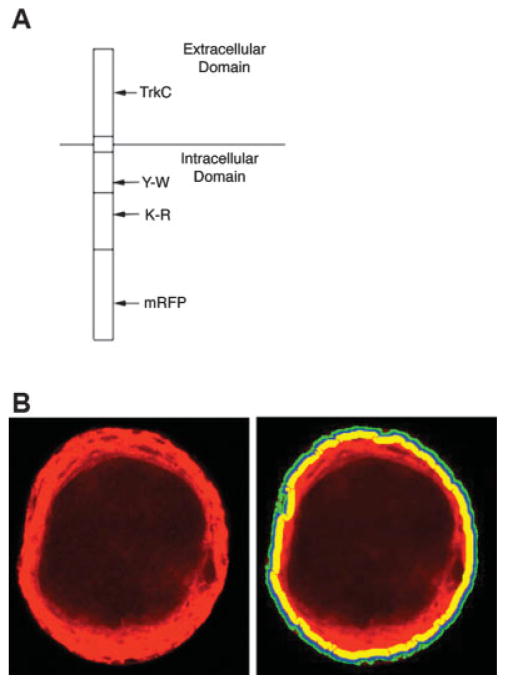
Wild-type (WT), kinase-dead (KR), and phosphor-tyrosine signaling–deficient (YW) ApTrkl-pNEX3 constructs with and without the extracellular domain of mammalian TrkC (TrkC-WT, TrkC-KR, and TrkC-YW, respectively) were generated previously (Ormond et al., 2004). To make TrkC-WT and WT constructs tagged with monocistronic red fluorescent protein (mRFP), the mRFP sequence was amplified by PCR using 3′ primer (GGGTCGCGACTTGTACAGGG CGCCGGT) and 5′ primer (GGGCTCGAGGGATGGCCT CCTCCGAGGACGT) cloned using a TrueBlue vector (Genomics One). The insert was cut out with Nru sites and ligated into the unique Nru site in the sequence encoding the carboxy-terminal insertion in TrkC-WT. The mutants were inserted into the mRFP-tagged constructs by cutting them out of the nontagged constructs at the Kpn and EcoRV restriction sites and ligating into the mRFP constructs at the same sites. In this article, TrkC-WT, TrkC-KR, and TrkC-YW refer to the constructs with mRFP (Fig. 1A).
Fig. 1.
Chimeric ApTrkl constructs and the definition of plasma membrane, juxtamembrane, and cytoplasm. A: Schematic picture of chimeric ApTrkl constructs with extracellular TrkC illustrating the placement of mutations and the site of mRFP insertion. The Y-W (tyrosine to tryptophan) mutation is in the NPxY signaling motif of the juxtamembrane domain, K-R (lysine to arginine) is in the kinase domain, and mRFP is inserted in the middle of the C-terminal extension, leaving a possible PDZ interaction site at the carboxy terminus intact. B: Quantification of ApTrkl localization. Definitions of intracellular boundaries used in this study are demonstrated. The plasma membrane, shown in green, was defined as 2–5 pixels from the outline of each neuron, as identified by IPPlot (Scanalytics, Inc., see the Materials and Methods section). The juxtamembrane, shown in blue, was defined as 7–12 pixels from the outline of each neuron, and the cytoplasm, shown in yellow, was defined as 15–25 pixels from the outline of the neuron. Quantification was done automatically using the IPLab program (Scanalytics, Inc.) in the same way for all the neurons quantified (see the Materials and Methods section for additional details).
Aplysia Sensory Neuron Cultures and Plasmid Injection
Aplysia dissociated sensory neuron cultures were prepared based on a previously described method with some modification (Manseau et al., 2001) and were plated on poly-L-lysine-coated coverslips. In experiments on endogenous ApTrkl, cell cultures were grown for 3–4 days before treatments and fixation. 5-HT (Sigma-Aldrich) and methiothepin (Sigma-Aldrich) were used at concentrations of 20 and 100 μM, respectively. Sensorin peptide (100 ng/mL) and antibody (400 ng/mL) were kind gifts from Dr. Samuel Schacher. In injection experiments, the DNA constructs were microinjected at a concentration ranging from 100 to 400 ng/μL (200 ng/μL in most experiments) together with 0.5% of fast green. Sensory neurons were injected 1–2 days after they were plated on poly-L-lysine-coated coverslips and were treated with 5-HT (Sigma-Aldrich, 20 μM) and fixed 1–2 days after injection.
Immunocytochemistry
After preparation of cell cultures or the injection mentioned above, the samples were treated with reagents and fixed with 4% paraformaldehyde in 30% sucrose and 1× PBS. Cells were then permeabilized by washing with 0.1% Triton X-100 in 30% sucrose and 1× PBS for 10–15 min. Cells were washed with 1× PBS, washed again in NH4Cl to quench free aldehydes for 15–20 min, and blocked for 30 min in a blocking solution of 10% normal goat serum in 0.5% Triton X-100 and 1× PBS. Samples were then incubated in the blocking solution for at least 1 hr with phospho-ApTrkl (P1) antibody, sequence CINKNPT(pY)FSP, at a 1:500 dilution. P1 antibody was preincubated with nonphosphorylated peptide. Following primary antibody incubation, the samples were washed 3–4 times with 1× PBS, and secondary antibody incubation was then carried out for 1 hr in the dark with goat antirabbit CY3 (Jackson Immunochemicals) for methiothepin and sensorin experiments or with goat antirabbit IgG (H + L; Molecular Probes) for injection experiments at dilutions of 1:800 and 1:500, respectively. Cells were then washed 3–4 times with 1× PBS, and coverslips were mounted on slides and fixed using mounting media.
Measurement of Change in Activation and Localization of ApTrkl
All images were taken at near the center of the cell where the nucleus was well defined and obtained from the Zeiss LSM 510 confocal microscope. Samples were imaged using 488- and 543-nm wavelength laser lines, and images were collected at 63× magnification using Zeiss LSM software. The images were then coded, and quantification was done by a blind observer. To quantify the levels of endogenous P1 staining, the NIH image was used. The cytoplasm excluding the nucleus was manually outlined, and mean pixel intensity for P1 staining was measured. In each experiment, both control and experimental cells were normalized to the average of the control cells.
For quantifying the localization of overexpressed constructs, each cell was automatically outlined using the program, IPLab (Scanalytics, Inc.) and concentric circles were then automatically constructed at 1-pixel intervals until the center of the cell was reached. The area 2–5 pixels away from the outermost part of the neuron was defined as the plasma membrane, 7–12 pixels away as the juxtamembrane, and 15–25 pixels away as the cytoplasm (Fig. 1B). Because of varied expression, we always measured the relative intensity of mRFP expression (receptor localization) and/or P1 staining (receptor activation) at the plasma membrane to the juxtamembrane and/or cytoplasm. In some cells, the nucleus was very close to the plasma membrane (e.g., Fig. 2A); in these cells, only the other side of the cell was quantified. Quantification was done blindly, and only experiments with three or more successfully expressed cells in each group were included in the data.
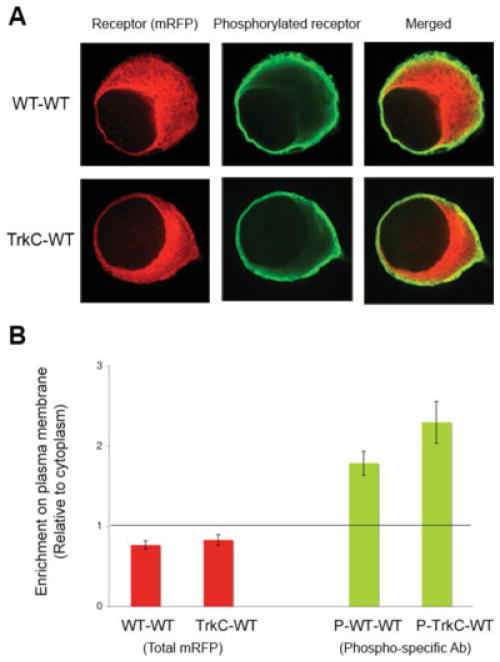
Fig. 2.
Overexpression of wild-type ApTrkl led to a large intracellular pool of the receptor and activation at the plasma membrane. A: WT- and TrkC-WT constructs were injected into sensory neurons. The neurons were fixed (1 or 2 days later) and immunostained with an antibody specific for the phosphorylated ApTrkl Confocal images were taken near the center of the cell. The representative figure shows localization of the receptors (mRFP) distributed throughout the cell and not enriched near the membrane, whereas the phosphorylated receptors (green fluorescence) were more localized at the plasma and juxtamembrane relative to the cytoplasm. B: Quantification of A. Enrichment of the plasma membrane relative to the cytoplasm was measured for the total number of receptors and for the phosphorylated receptors (WT-WT, n = 26; TrkC-WT, n = 13).
RESULTS
Overexpression of Wild-Type ApTrkl Leads to a Large Intracellular Pool of the Receptor and Activation at the Plasma Membrane
We examined localization and activation of the receptor after overexpressing ApTrkl constructs (Fig. 1A). All experiments were done in sensory neurons of Aplysia. To test for the role of endogenous ligands, we compared a wild-type (WT) ApTrkl construct to one in which we had replaced most of the extracellular domain with the extracellular domain of TrkC (TrkC-ApTrkl; Ormond et al., 2004). We also used a number of constructs with mutations in the intracellular domain to test additional hypotheses. We used constructs either with no changes (-WT), with a K-R mutation that abrogated kinase activity (-KR) or with a Y-W mutation that abrogated the only conserved signaling tyrosine in ApTrkl (-YW) (Ormond et al., 2004). Finally, all constructs contained a red fluorescent protein tag (mRFP) inserted into the nonconserved carboxy-terminal domain to monitor localization of ApTrkl inside the cell. The localization of the receptor could then be monitored in conjunction with the activation state of the receptor using a phospho-specific antibody to the signaling tyrosine of ApTrkl (P1 antibody directed against NPx[pY] site in ApTrkl; Ormond et al., 2004). We defined three areas for localization of the receptors: plasma membrane, juxtamembrane, and cytoplasm (Fig. 1B; see Materials and Methods section). Occasionally, a concentration of staining was observed in a juxtanuclear compartment; as this staining was quite variable and could have been a result of the concentration of the protein in the ER or Golgi after overexpression, this compartment was not included in the quantification.
Overexpression of constructs either containing the WT extracellular domain (WT-WT) or the extracellular domain of TrkC (TrkC-WT) led to constitutive phosphorylation of the receptor, suggesting that overexpression of the receptor leads to dimerization and activation of the receptors (Fig. 2A). This appeared to be independent of any endogenous ligand, as there were no differences in activation when the extracellular domain was switched with the extracellular domain of TrkC (Fig. 2A). This activation occluded any additional actions of possible putative ligands, as phosphorylation of the receptor was not further increased by 5-HT or for the TrKC-WT receptor NT3 (data not shown). The phosphorylated receptor was enriched in the plasma membrane and the juxtamembrane compartment; in contrast, the total receptor was more enriched in an intracellular pool (Fig. 2A,B). At this laser intensity, no staining was observed in noninjected cells; therefore, the signal observed is largely a result of the overexpressed receptor. Thus, from this experiment it can be concluded that when overexpressed, the receptor is much more activated when present in the plasma membrane or the juxtamembrane compartment. However, most of the receptor is localized in an intracellular pool, where it is largely inactive, even when overexpressed.
Kinase Activity, But Not Tyrosine Phosphorylation at the NPxY Site, Is Required for the Presence of a Large Intracellular Pool of ApTrkl
When kinase-dead (KR) constructs (WT-KR, TrkC-KR) were expressed, there was no immunoreactivity with the phospho-specific antibody (Fig. 3A). This result demonstrated that kinase activity is required for the phosphorylation of ApTrkl at the NPxY signaling site. Moreover, it demonstrates that our antibody is phospho-specific even when ApTrkl is overexpressed, as the antibody does not recognize the overexpressed non-phosphorylated receptor. One possible mechanism for the transactivation of RTKs by GPCRs is that GPCRs activate an intracellular kinase (such as Src) that then directly phosphorylates the signaling tyrosines of RTKs, inducing further downstream signaling. If this is true, we would expect 5-HT to increase the staining of the overexpressed kinase-dead ApTrkl with the phospho-specific antibody to the NPxY site, even if ApTrkl has no intrinsic kinase activity. However, no detectable immunoreactivity was observed when 5-HT was added to the cells expressing either WT-KR or TrkC-KR receptor (data not shown).
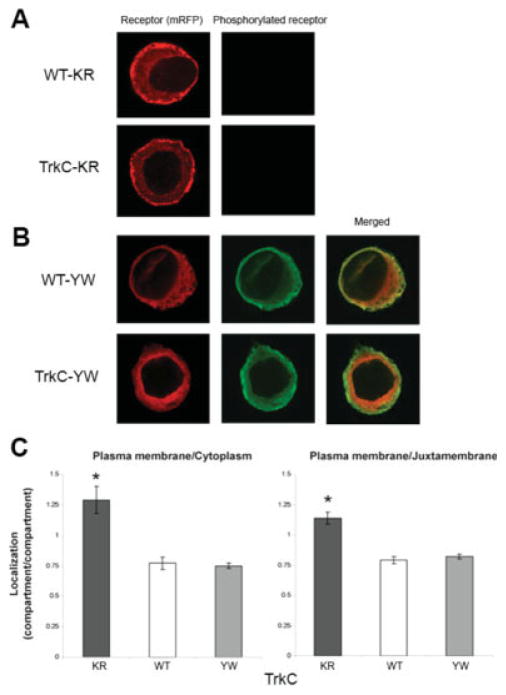
Fig. 3.
Kinase activity, but not tyrosine phosphorylation at the NPxY site, is required for the presence of a large intracellular pool of ApTrkl. A: WT-KR and TrkC-KR constructs were injected into sensory neurons. The neurons were fixed (1 or 2 days later) and immunostained with an antibody specific for the phosphorylated ApTrkl. At this laser power (similar to that used for the other overexpressed receptors), phosphorylation was not detectable using the phospho-specific antibody to ApTrkl. The representative image shows enrichment of the overexpressed receptor (mRFP) on the plasma membrane. B: Overexpression of WT- and TrkC-YW constructs shows localization and phosphorylation patterns similar to WT constructs (Fig. 2A). That is, more receptors are localized internally, whereas phosphorylated receptors are more localized in the plasma membrane, although the level of phosphorylation is much smaller in the YW constructs. C: Quantification and comparison of mRFP at the plasma membrane relative to the cytoplasm (left) and the juxtamembrane (right) in TrkC-KR (n = 15), -WT (n = 13), and -YW (n = 38) constructs. TrkC-KR is significantly more localized in the plasma membrane than are the other constructs for both the cytoplasmic ratio (one-way ANOVA, F63,2 < .0001; Tukey’s post hoc test, P < .001 for TrkC-KR compared with either TrkC-WT or TrkC-YW) and the juxtamembrane ratio (one-way ANOVA, F63,2 < .0001; Tukey’s post hoc test, P < .001 for KR compared with either YW or WT).
There was a significant difference in the localization of the ApTrkl KR receptor compared with the WT. The TrkC-KR receptor was significantly more enriched in the plasma membrane relative to either the cytoplasm or the juxtamembrane compared with TrkC-WT or -YW (P < .001, Tukey’s post hoc test; Fig. 3C). Thus, the ratio of the receptor at the plasma membrane to that in the intracellular pool was greatly increased in the absence of kinase activity. This suggests that when the kinase is active on the plasma membrane, it induces internalization; in contrast the KR receptor is retained on the membrane because it is always inactive.
Surprisingly, overexpression of the YW receptors showed immunoreactivity with the P1 antibody (Fig. 3B). It should be noted that the level of immunoreactivity with P1 for the YW receptors was much less than with the WT receptor (approximately fivefold based on the anti-P1/mRFP ratio). The P1 antibody is directed at the site that is mutated (Y-W), and we observed no immunoreactivity in previous experiments overexpressing this receptor in a heterologous system (Ormond et al., 2004), suggesting that this immunoreactivity was not derived from the overexpressed receptor. Moreover, the lack of immunoreactivity with the KR mutant rules out any cross-reactivity due to the level of overexpression. Thus, the most likely explanation for this immunoreactivity is heterodimerization with the endogenous receptor and immunoreactivity due to phosphorylation of the endogenous receptor at this site; unlike the KR receptor, the YW receptor is not kinase dead; indeed it was still competent to partially signal ERK activation in heterologous cells (Ormond et al., 2004). Unlike the KR receptor, the YW receptor was not enriched on the plasma membrane (Fig. 3). Thus, although kinase activity is required for the presence of the receptor in the intracellular pool, this is not a result of kinase activity being required for phosphorylation at the NPxY site.
5-HT Induces Kinase Activity- and Ligand-Independent Internalization of ApTrkl
Interestingly, 5-HT treatment led to a decrease in relative plasma membrane enrichment in the KR constructs (P < .01 for WT-KR, P < .05 for TrkC-KR; Fig. 4A,B), suggesting there was 5-HT-induced internalization of the receptor independent of kinase activity. Because this was observed in both WT-KR and TrkC-KR, it is also suggested that 5-HT-induced internalization is ligand independent. However, internalization of the receptor was not observed for the WT constructs with 5-HT treatment (data not shown). It is possible that kinase activity–dependent internalization occluded the 5-HT-induced internalization.
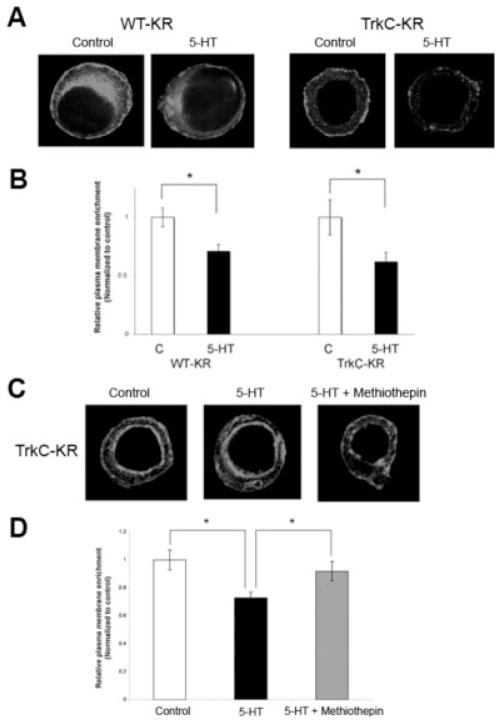
Fig. 4.
5-HT induced kinase activity- and ligand-independent internalization of ApTrkl. A: 5-HT treatment (5 min) induced internalization of the KR mutant receptors at the plasma membrane, which was not through the involvement of ligand because this was observed both for the WT- and TrkC-KR constructs. B: Quantification of A. Relative plasma membrane enrichment (compared to the cytoplasm) with and without 5-HT treatment (control) was calculated and normalized to the control group [for the WT construct, control (C), n = 12); 5-HT, n = 14; P < .01 (two-tailed Student t test); for the TrkC constructs, control, n = 15; 5-HT, n = 12; P < .05 (two-tailed Student t test)]. C: Cells that overexpressed TrkC-KR were treated with either 5-HT or with 5-HT and methiothepin for 5 min. The addition of methiothepin blocks 5-HT-induced internalization of TrkC-KR receptors. D: Quantification of C. Relative plasma membrane staining (to the cytoplasm) showed that 5-HT-induced internalization of TrkC-KR was blocked by the addition of methiothepin [control, n = 18; 5-HT, n = 15; 5-HT+methiothepin, n = 19; one-way ANOVA, F50,2 < .001; Tukey’s post hoc test, 5-HT group was significantly different than control (P < .01) and 5-HT+methiothepin (P < .05)].
We then hypothesized that 5-HT-induced internalization occurs through activation of 5-HT GPCRs, which we examined by using methiothepin, an antagonist for most 5-HT GPCRs in Aplysia (Barbas et al., 2003; Dumitriu et al., 2006). The TrkC-KR chimera was used in order to rule out the involvement of extracellular ligand binding or direct binding of 5-HT to ApTrkl. When methiothepin (100 μM) was applied together with 5-HT (20 μM, 5 min), it blocked the 5-HT-induced internalization of the receptors (P < .01 for control and 5-HT, P < .05 for 5-HT and 5-HT+methiothepin, Tukey’s post hoc test; Fig. 4C,D). Therefore, the results indicate that kinase activity–independent internalization induced by 5-HT occurs through activation of 5-HT GPCRs.
Activation of Endogenous ApTrkl
Because overexpression of the receptor occluded ligand-dependent activation of the receptor, we returned to measurement of the endogenous receptor by different ligands in an attempt to determine the mechanism of receptor activation. To measure endogenous receptor activation required much higher laser power, resulting in higher background signals. Nevertheless, similar to the previous results (Ormond et al., 2004), 5-HT treatment for either 5 min or 1 hr increased activation of the receptor (Fig. 5A,C). However, it should be noted that unlike when the receptor was overexpressed, the activated endogenous receptor was mainly seen in the internal pools, and little if any phospho-specific staining was seen in the plasma membrane (Fig. 5).
Fig. 5.
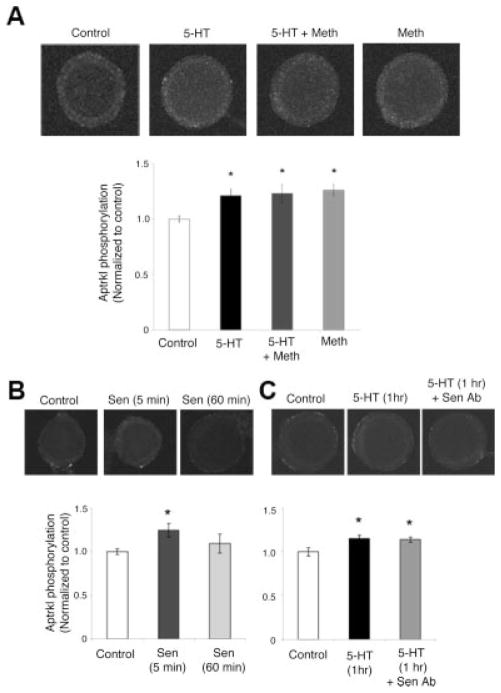
Activation of endogenous ApTrkl. A: Phosphorylation of endogenous ApTrkl was examined for controls (n = 62), treatment with 5-HT (n = 61), treatment with 5-HT and methiothepin (5-HT+Meth; n = 36), or treatment with methiothepin only (n = 45). For the sake of presentation, the pictures shown on top were converted to grayscale as TIF files and brightness and contrast adjusted equally for all groups. Endogenous staining was much less than for the overexpressed receptors. 5-HT treatment increased the level of ApTrkl phosphorylation, which was not blocked by the addition of methiothepin. Indeed, methiothepin itself was enough to phosphorylate ApTrkl to an extent similar to 5-HT. All treatments were done for 5 min. Bottom, quantification with values normalized to controls (one-way ANOVA, F201,3 < .01; Tukey’s post hoc test, the control group was different from all other groups, P < .05; no other groups were differed). B: Middle, treatment with sensorin peptide (Sen; middle; n = 38) for 5 min, which activated ApTrkl significantly more than did the control (left; n = 68); P < .01, two-tailed Student t test. Sen for 1 hr (right; n = 59) was not significantly different from the control. C: Treatment with sensorin antibody (Sen ab). 5-HT-induced activation of endogenous ApTrkl was not blocked by Sen ab (left, control; middle, 5-HT; right, 5-HT+Sen ab); one-way ANOVA, F151,2 < .05; Tukey’s post hoc test, control (n = 35) was different from both 5-HT (1 hr; n = 46) and 5-HT+Sen ab (n = 73), P < .05. 5-HT+Sen ab was not significantly different from 5-HT alone (P > 0.5). The antibody was either applied 5 min before 5-HT application or preincubated for 1 hr before and during 5-HT application. As there was no difference between these conditions, data from both type of experiments were pooled in 5-HT+Sen ab.
Although the increases measured were small, it should be pointed out that after treating ApTrkl with dsRNA, none of the background signal was removed, but there was complete elimination of the signal that was increased with 5-HT (Ormond et al., 2004). Thus, we assume that in the absence of 5-HT there is little or no activation of the receptor, and the small signal observed was mainly a result of the difficulty in observing the small signal produced by the endogenous receptor in the face of background staining.
To determine if the receptor is activated by 5-HT through transactivation of GPCRs, the effect of methiothepin (100 μM) was examined. Surprisingly, the addition of methiothepin alone was sufficient to activate the receptor to the same level as 5-HT, and there was no additive effect when 5-HT and methiothepin were added together (P < .05 for control and all other groups, Tukey’s post hoc test; Fig. 5A). Thus, for activation of ApTrkl, methiothepin acts as an agonist, similar to 5-HT. This differs from its normal activity and contrasts with the results shown earlier where methiothepin blocked the ability of 5-HT to internalize the KR receptors.
It was shown that repeated 5-HT application to sensory-to-motor neuron culture leads to the release of the neuropeptide sensorin from sensory neurons and that sensorin is involved in LTF and the activation of ERK (Hu et al., 2004). Moreover, K252a, an inhibitor of ApTrkl, blocked the ability of sensorin to activate ERK (Hu et al., 2004; Ormond et al., 2004). To examine the possibility that sensorin is involved in the activation of ApTrkl, either as a ligand or by some other unknown mechanism, sensory neuron cultures were incubated with sensorin peptide (100 ng/mL) for either 5 min or 1 hr. Incubation for 5 min increased the activation of ApTrkl (P < .01), although this activation was transient and not seen after the 1-hr incubation (Fig. 5B). Because sensorin could activate ApTrkl, albeit transiently, we next asked whether 5-HT activation of ApTrkl was mediated through sensorin using an antibody to sensorin that can block most actions of exogenously added sensorin, as well as blocking LTF (Hu et al., 2004). Sen ab (400 ng/mL) did not block the 5-HT-induced activation of ApTrkl (P < .05 for control both from 5-HT and from 5-HT+Sen ab, Tukey’s post hoc test; Fig. 5C). Therefore, sensorin is not required for 5-HT-induced activation of ApTrkl. Finally, because it has been reported that 5-HT can prime the response to sensorin (Hu et al., 2004), we asked if sensorin added for 1 hr after 5 min of 5-HT could activate ApTrkl. This treatment did not activate ApTrkl (0.87 ± 0.08 relative to control, n = 20, P > 0.1, two-tailed Student t test, 5-HT+ sensorin versus control).
DISCUSSION
Regulation of Overexpressed ApTrkl
Overexpression of the wild-type (WT) ApTrkl constructs leads to activation of the receptors, mainly at the plasma membrane, most likely through self-dimerization of overexpressed receptors, whereas more receptors were localized in an intracellular pool (Fig. 2). It is not clear why phosphorylation of the receptor is enhanced in the plasma membrane and juxtamembrane areas compared with the internal pool. Possible explanations are (1) differences in the lipid composition of pH at the membrane, causing conformation changes that increase dimerization; (2) changes in levels of tyrosine phosphatases in the different compartments; or (3) the presence of different binding partners that either activate or inhibit dimerization in the different compartments.
Overexpression of the kinase-dead (KR) ApTrkl constructs showed more localization of the receptors at the plasma membrane, although there was no activation (Fig. 3A,C). This is consistent with constitutive receptor internalization of ApTrkl that is dependent on kinase activity. Consistent with this, tyrosine kinase activity is required for the internalization of TrkB by electric stimulation of hippocampal neurons (Du et al., 2003). Internalization is not a result of phosphorylation of the signaling site (NPXY) because mutations at this site did not affect localization of the receptor.
Interestingly, 5-HT treatment led to internalization of the kinase-dead receptor, as indicated by the decrease in the relative enrichment of the receptor at the plasma membrane (Fig. 4A,B). This suggests that there is a kinase activity–independent mode of internalization, which is not caused by a ligand-binding or direct binding of 5-HT to ApTrkl because the TrkC-KR chimera was capable of internalization. 5-HT is known to activate endocytosis in general, and endocytosis of proteins like Aplysia cell-adhesion molecules (apCAM; Bailey et al., 1992). This effect was mediated by GPCRs because it was blocked by methiothepin. It was surprising that there was no observed internalization of the WT receptor; however, the effect of 5-HT may have been occluded by the kinase-dependent internalization. Alternatively, 5-HT may stimulate insertion of the receptor as well, and although for the WT there was no overall change, the higher expression of the KR receptor at the plasma membrane allowed us to visualize the internalization.
Given the higher level of activation of the overexpressed receptor at the plasma membrane, it is surprising that activation of the endogenous receptor is seen mainly internally, even after a 5-min treatment with 5-HT. This could be a result of a combination of activity-dependent and 5-HT dependent internalization of the activated receptor or because of GPCR-mediated activation of the internal pool of the receptor. However, GPCR-mediated activation must still require kinase activity of ApTrkl; otherwise, it would have been detected by transactivation of the K-R receptor.
Activation of Endogenous ApTrkl
Surprisingly, blocking 5-HT GPCRs with the antagonist methiothepin did not prevent 5-HT-induced activation of endogenous ApTrkl; instead, methiothepin itself activated ApTrkl to the same level as 5-HT (Fig. 5A). Methiothepin acts as an inverse agonist for 5-HT receptors (5-HT1A and 5-HT7 receptors; McLoughlin and Strange, 2000; Krobert et al., 2006), and orthologues of these receptors exist in Aplysia (Angers et al., 1998; Barbas et al., 2002; Cohen et al., 2003). As an inverse agonist, methiothepin binds to the same site of 5-HT and causes a conformational change that favors the inactive state of the receptor. However, it is possible that this conformation may be competent to signal through other pathways. Indeed, methiothepin induces heterologous down-regulation of receptors, and this down-regulation does not depend on the normal signaling pathway of 5-HT receptors (Krobert et al., 2006).
The receptor for sensorin has not been isolated. Although sensorin could transiently activate endogenous ApTrkl, we have not been able to show sensorin activation of heterologously expressed ApTrkl (data not shown), although NT3 could partially activate TrkC-ApTrkl in this heterologous system (Ormond et al., 2004). Moreover, antibodies to sensorin, which are able to block most actions of sensorin (Hu et al., 2004), could not block 5-HT-induced activation of ApTrkl. Thus, we favor a model in which sensorin may activate a G-coupled receptor (like most other neuropeptides) that can also couple to ApTrkl. It is not clear if this is the major action of the sensorin receptor or if this is required for sensorin activation of ERK.
Acknowledgments
Contract grant sponsor: Canadian Institutes of Health Research (CIHR); Contract grant number: MOP 15121 (to W.S.S.).
We thank Dr. Samuel M. Schacher for providing the sensory peptide and antibody. We also thank Xiaotang Fan for generating the mRFP constructs. W.S.S. is a William Dawson Scholar and Fonds de la Recherche en Santé du Québec (FRSQ) Chercheur National.
References
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Angers A, Storozhuk MV, Duchaine T, Castellucci VF, DesGroseillers L. Cloning and functional expression of an Aplysia 5-HT receptor negatively coupled to adenylate cyclase. J Neurosci. 1998;18:5586–5593. doi: 10.1523/JNEUROSCI.18-15-05586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Memory. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Castellucci VF, DesGroseillers L. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. doi: 10.1046/j.0022-3042.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Onyike CU, McElroy VL, Lin AH, Abrams TW. Pharmacological characterization of an adenylyl cyclase-coupled 5-HT receptor in aplysia: comparison with mammalian 5-HT receptors. J Neurophysiol. 2003;89:1440–1455. doi: 10.1152/jn.01004.2002. [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu B, Cohen JE, Wan Q, Negroiu AM, Abrams TW. Serotonin receptor antagonists discriminate between PKA- and PKC-mediated plasticity in aplysia sensory neurons. J Neurophysiol. 2006;95:2713–2720. doi: 10.1152/jn.00642.2005. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Receptor tyrosine kinase transactivation: fine-tuning synaptic transmission. Trends Neurosci. 2003;26:119–122. doi: 10.1016/S0166-2236(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hu JY, Glickman L, Wu F, Schacher S. Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron. 2004;43:373–385. doi: 10.1016/j.neuron.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Andressen KW, Levy FO. Heterologous desensitization is evoked by both agonist and antagonist stimulation of the human 5-HT(7) serotonin receptor. Eur J Pharmacol. 2006;532:1–10. doi: 10.1016/j.ejphar.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- Manseau F, Fan X, Hueftlein T, Sossin W, Castellucci VF. Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed aplysia sensorimotor synapses. J Neurosci. 2001;21:1247–1256. doi: 10.1523/JNEUROSCI.21-04-01247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- McLoughlin DJ, Strange PG. Mechanisms of agonism and inverse agonism at serotonin 5-HT1A receptors. J Neurochem. 2000;74:347–357. doi: 10.1046/j.1471-4159.2000.0740347.x. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Ormond J, Hislop J, Zhao Y, Webb N, Vaillaincourt F, Dyer JR, Ferraro G, Barker P, Martin KC, Sossin WS. ApTrkl, a Trk-like receptor, mediates serotonin- dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron. 2004;44:715–728. doi: 10.1016/j.neuron.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]