Abstract
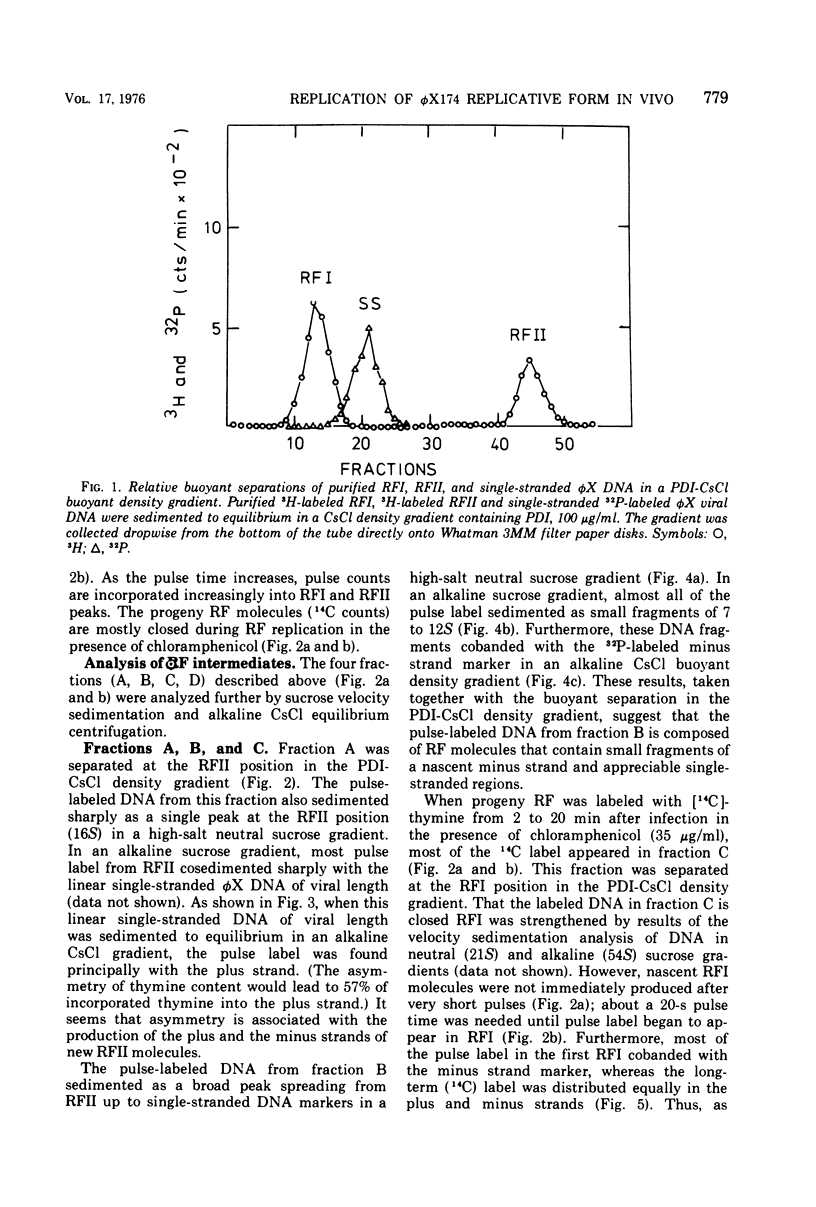
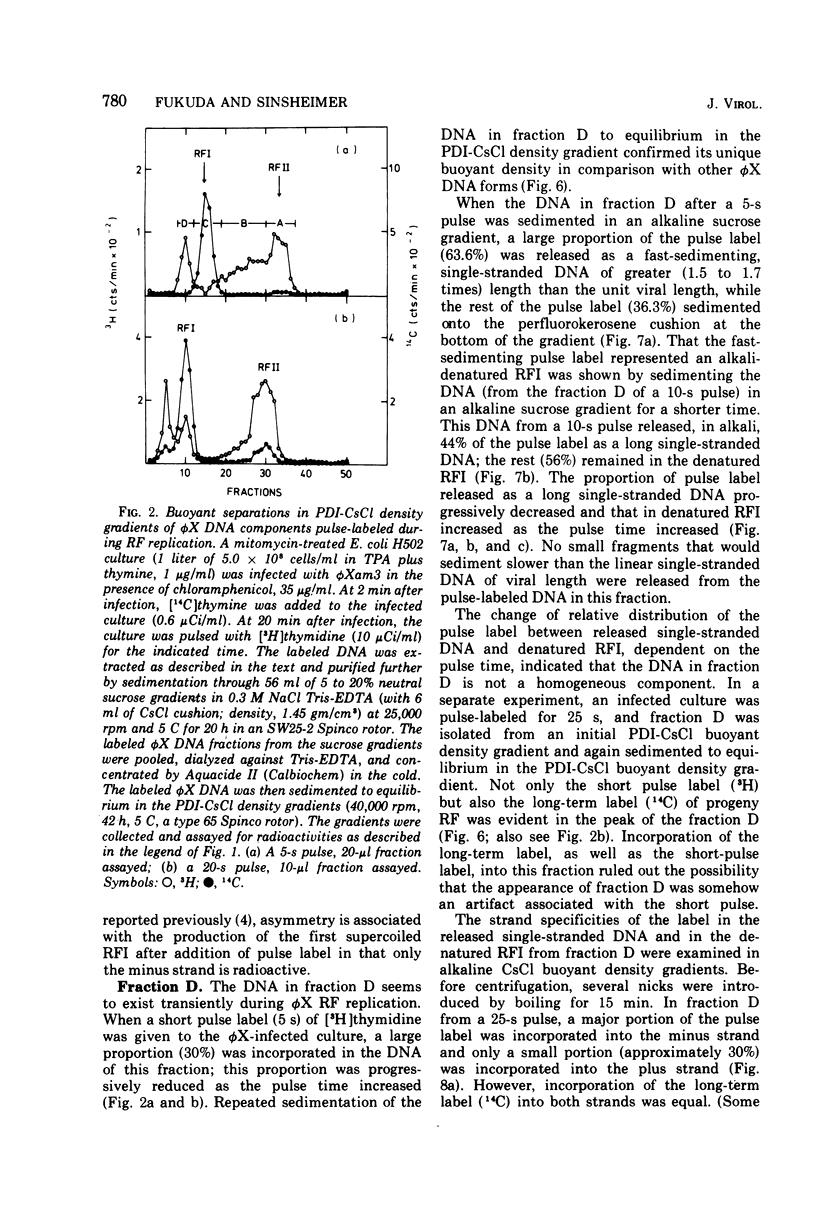
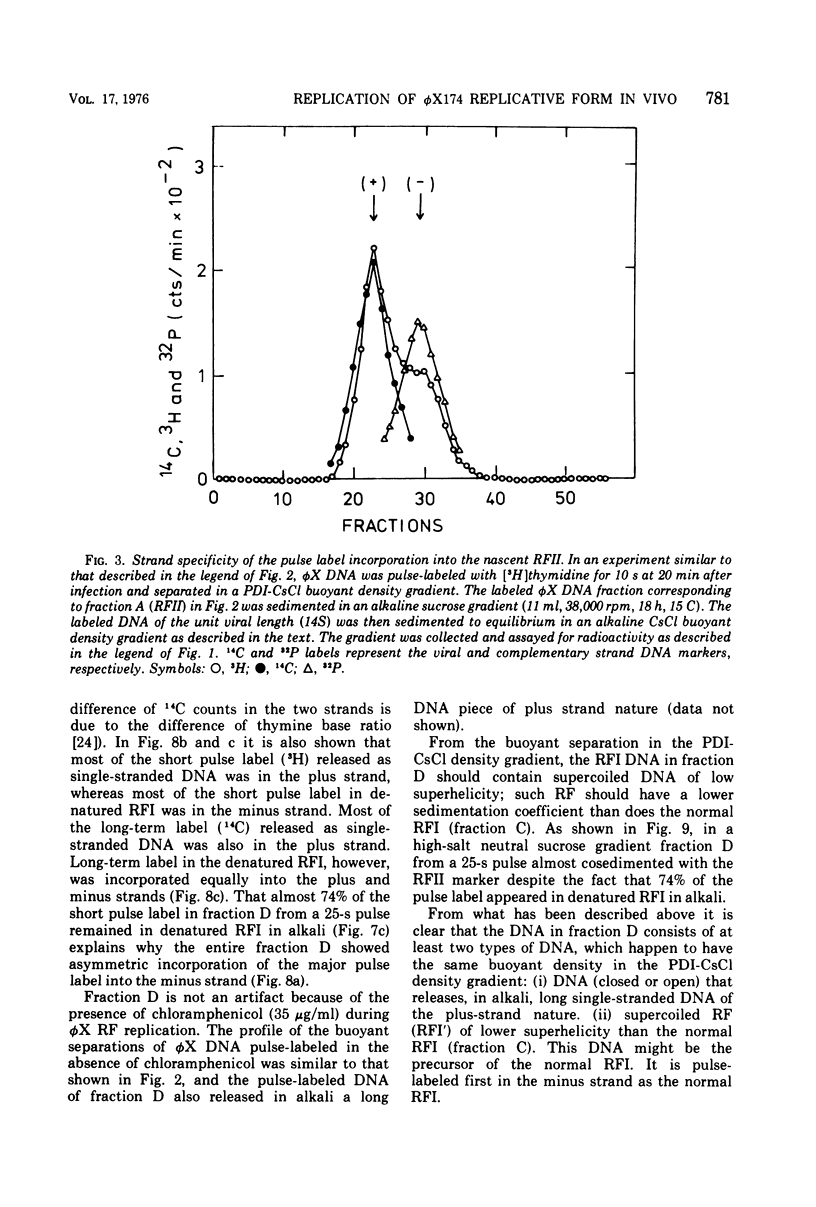
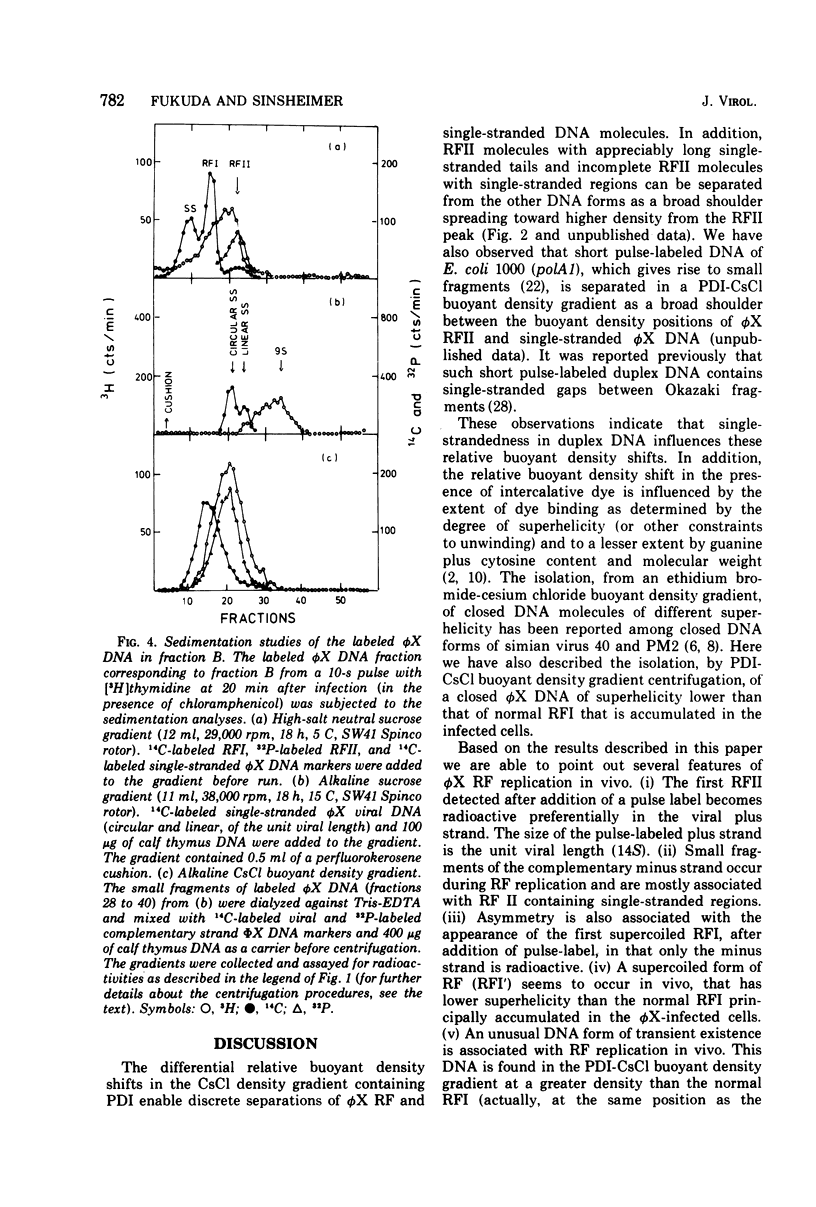
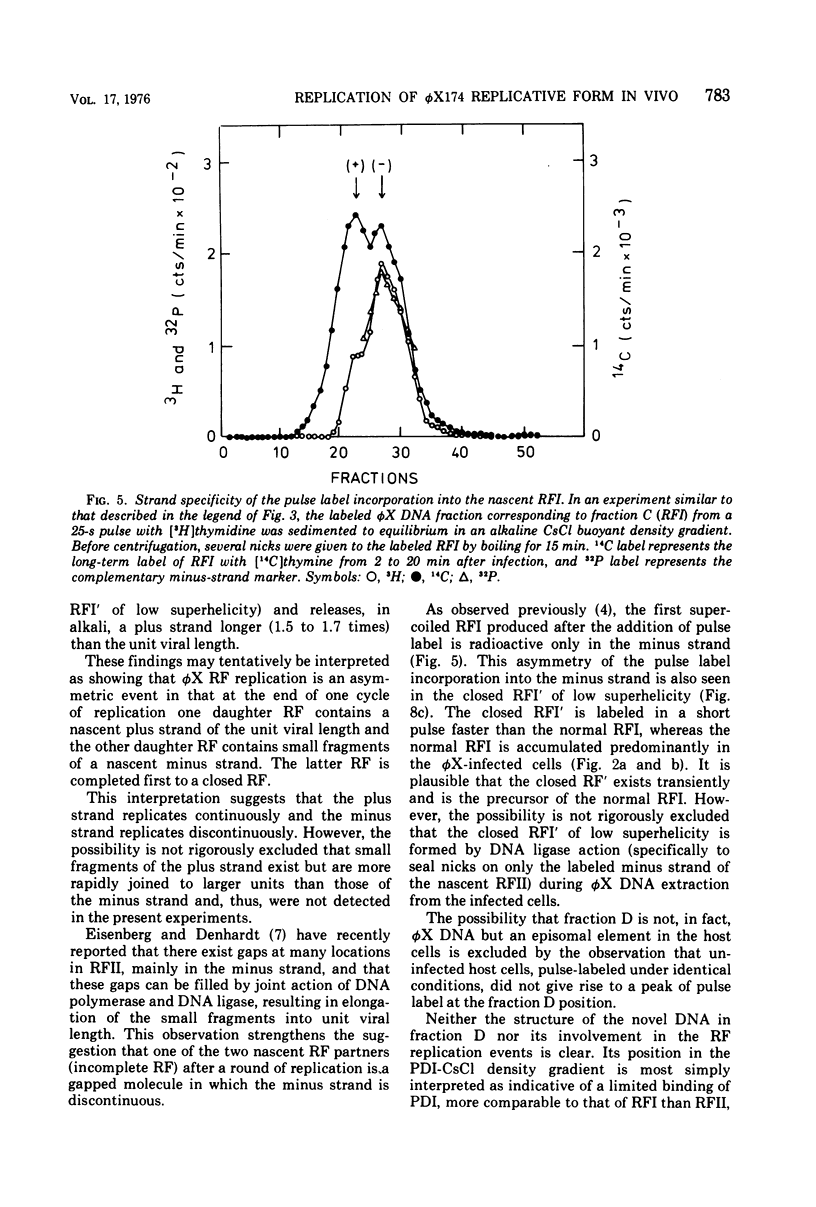
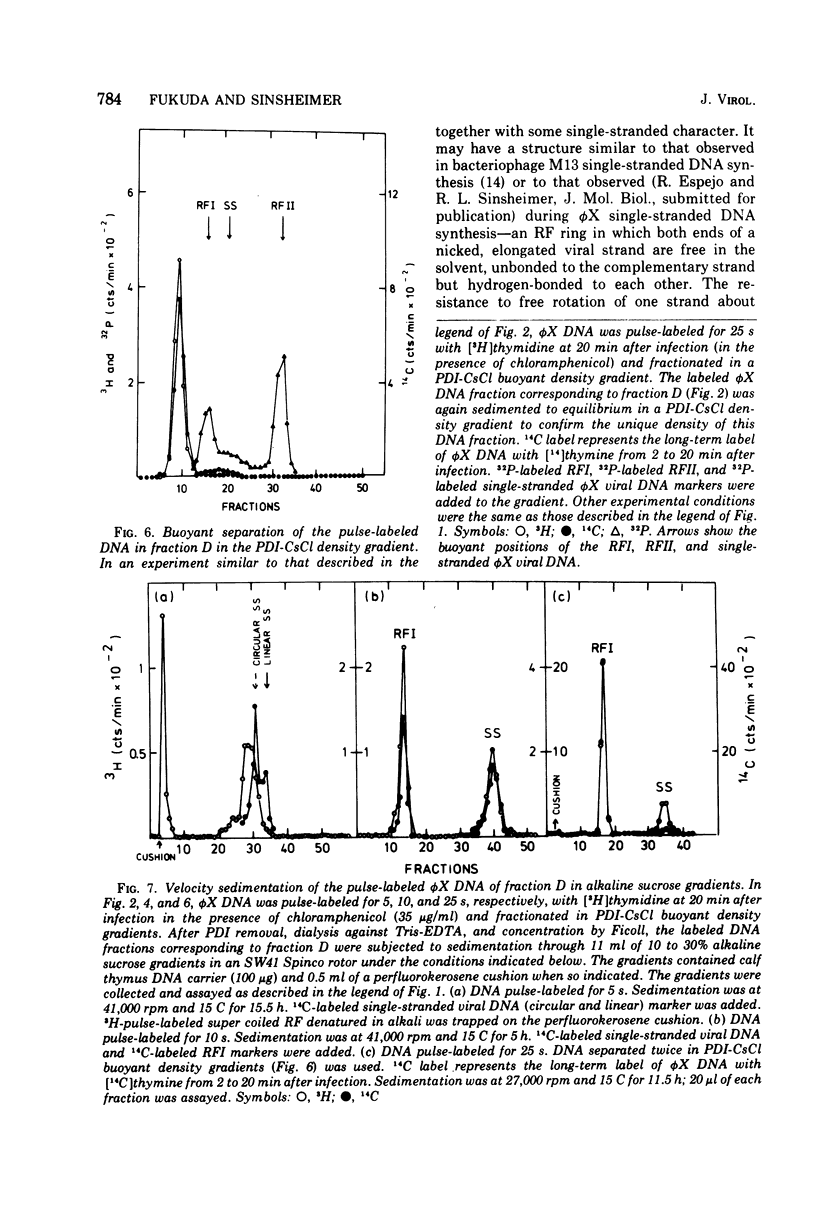
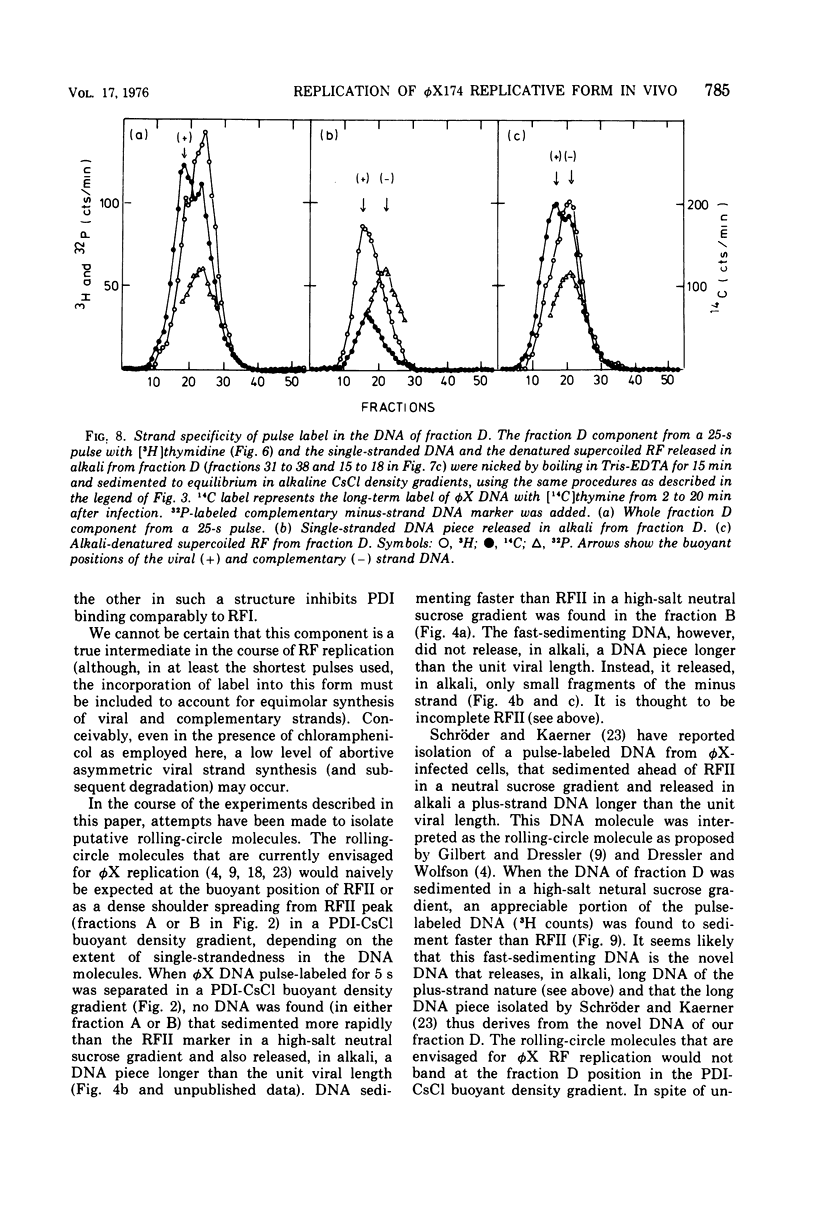
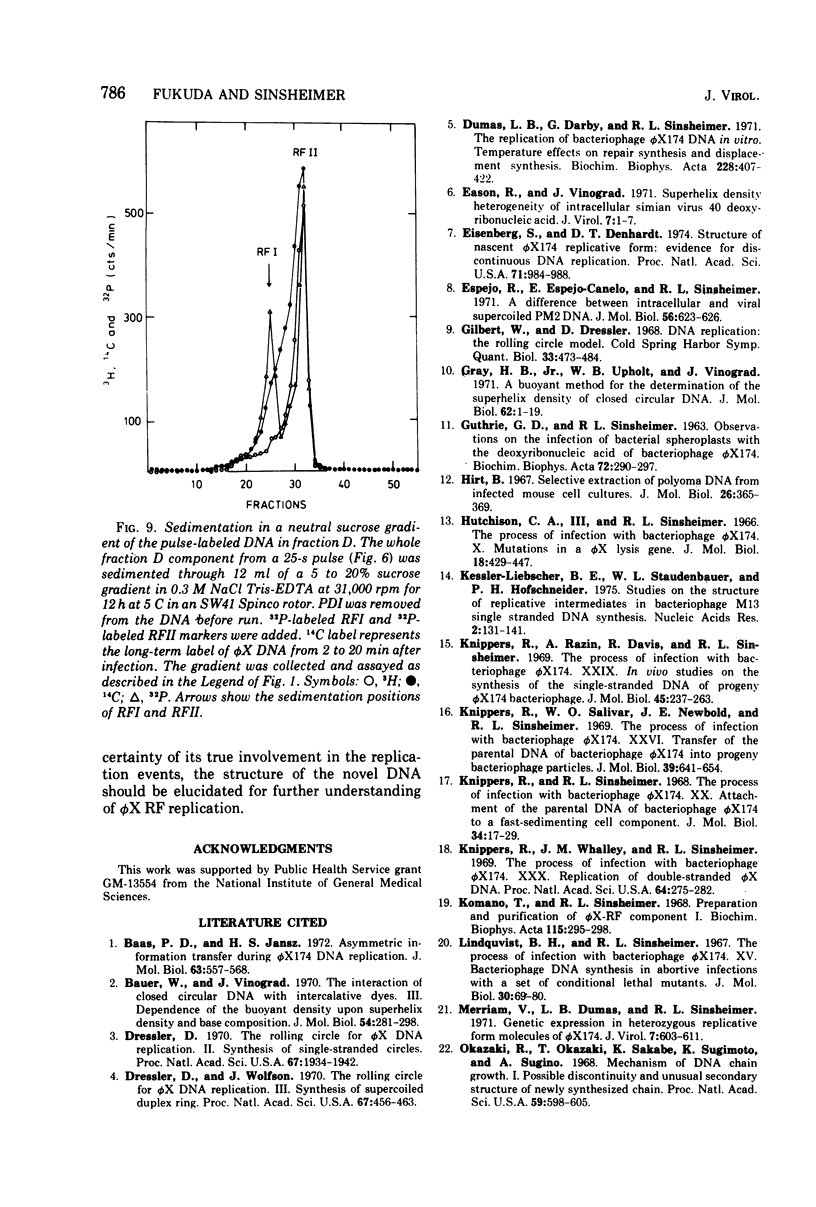
The replication of bacteriophage phi X 174 replicative-form DNA has been studied by structural analysis of pulse-labeled replicative-intermediate molecules. Such intermediates were identified by pulse-labeling with [13H]thymidine and separated into four major fractions (A, B, C, and D) in a propidium diiodide-cesium chloride buoyand density gradient. Sedimentation analysis of each of these fractions suggests the following features of phi X replicative-form DNA replication in vivo. (i) At the end of one cycle of replication, one daughter replicative form (RFII) contains a nascent plus (+) strand of the unit viral length, and the other daughter RFII contains small fragments of nascent minus (-) strand. (ii) Asymmetry is also associated with production of the first supercoiled RFI after addition of pulse label in that only the minus strand becomes radioactive. (iii) A supercoiled DNA (RFI') seems to occur in vivo. This DNA is observed at a position of greater density in a propidium diiodide-cesium chloride buoyant density gradient than normal RFI. (iv) A novel DNA component is observed, at a density greater than RFI, which releases, in alkali, a plus strand longer (1.5 to 1.7 times) than the unit viral length. These results are discussed in terms of the possible sequence of events in phi X 174 replicative-form replication in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dressler D., Wolfson J. The rolling circle for phi X DNA replication. 3. Synthesis of supercoiled duplex rings. Proc Natl Acad Sci U S A. 1970 Sep;67(1):456–463. doi: 10.1073/pnas.67.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas L. B., Darby G., Sinsheimer R. L. The replication of bacteriophage phi X174 DNA in vitro. Temperature effects on repair synthesis and displacement synthesis. Biochim Biophys Acta. 1971 Jan 28;228(2):407–422. [PubMed] [Google Scholar]
- Eason R., Vinograd J. Superhelix density heterogeneity of intracellular simian virus 40 deoxyribonucleic acid. J Virol. 1971 Jan;7(1):1–7. doi: 10.1128/jvi.7.1.1-7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Denhardt D. T. Structure of nascent phiX174 replicative form: evidence for discontinuous DNA replication. Proc Natl Acad Sci U S A. 1974 Mar;71(3):984–988. doi: 10.1073/pnas.71.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R., Espejo-Canelo E., Sinsheimer R. L. A difference between intracellular and viral supercoiled PM2 DNA. J Mol Biol. 1971 Mar 28;56(3):623–626. doi: 10.1016/0022-2836(71)90405-0. [DOI] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kessler-Liebscher B. E., Staudenbauer W. L., Hofschneider P. H. Studies on the structure of replicative intermediates in bacteriophage M13 single stranded DNA synthesis. Nucleic Acids Res. 1975 Jan;2(1):131–141. doi: 10.1093/nar/2.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Knippers R., Salivar W. O., Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXVI. Transfer of the parental DNA of bacteriophage phiX174 into progeny bacteriophage particles. J Mol Biol. 1969 Feb 14;39(3):641–654. doi: 10.1016/0022-2836(69)90150-8. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Knippers R., Whalley J. M., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXX. Replication of double-stranded phiX DNA. Proc Natl Acad Sci U S A. 1969 Sep;64(1):275–282. doi: 10.1073/pnas.64.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Sinsheimer R. L. Preparation and purification of phi X-RF component I. Biochim Biophys Acta. 1968 Jan 29;155(1):295–298. doi: 10.1016/0005-2787(68)90360-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Merriam V., Dumas L. B., Sinsheimer R. L. Genetic Expression in Heterozygous Replicative Form Molecules of phiX174. J Virol. 1971 May;7(5):603–611. doi: 10.1128/jvi.7.5.603-611.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schröder C. H., Kaerner H. C. Replication of bacteriophage phichi174 replicative form DNA in vivo. J Mol Biol. 1972 Nov 14;71(2):351–362. doi: 10.1016/0022-2836(72)90356-7. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Yudelevich A., Ginsberg B., Hurwitz J. Discontinuous synthesis of DNA during replication. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1129–1136. doi: 10.1073/pnas.61.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]



