Abstract
The p75 neurotrophin receptor (p75NTR) and its activation of the sphingomyelin signaling cascade are essential for mechanical hypersensitivity resulting from locally injected nerve growth factor (NGF). Here the role of the same effectors, and of the TrkA receptor, are evaluated for thermal hyperalgesia from NGF. Sensitivity of rat hind paw plantar skin to thermal stimulation after local sub-cutaneous injection of NGF (500 ng) was measured by the latency for paw withdrawal (PWL) from a radiant heat source. PWL was reduced from baseline values at 0.5–22h by ~40% from that in naïve or vehicle-injected rats, and recovered to pre-injection levels by 48h. Local pre-injection with a p75NTR blocking antibody did not affect the acute thermal hyperalgesia (0.5–3.5h) but hastened its recovery so that it had reversed to baseline by 22h. In addition, GW4869 (2 mM), an inhibitor of the neutral sphingomyelinase (nSMase) that is an enzyme in the p75NTR pathway, also failed to prevent thermal hyperalgesia. However, C2-ceramide, an analogue of the ceramide produced by sphingomyelinase, did cause thermal hyperalgesia. Injection of an anti-TrkA antibody known to promote dimerization and activation of that receptor, independent of NGF, also caused thermal hyperalgesia, and prevented the further reduction of PWL from subsequently injected NGF. A nonspecific inhibitor of tropomyosin receptor kinases, K252a, prevented thermal hyperalgesia from NGF, but not that from the anti-TrkA antibody. These findings suggest that the TrkA receptor has a predominant role in thermal hypersensitivity induced by NGF, while p75NTR and its pathway intermediates serve a modulatory role.
Keywords: neurotrophin, pain, hypersensitivity, atypical PKC, TRPV1
Graphical abstract
INTRODUCTION
In addition to influencing the development, survival and differentiation of peripheral sensory and sympathetic neurons, Nerve Growth Factor (NGF), induces thermal and mechanical sensitization of these neurons in adult humans (Dyck et al., 1997; Svensson et al., 2003; Rukwied et al., 2010) and decreases both mechanical and thermal nociceptive thresholds in rodent models of pain (Lewin et al., 1993; Woolf et al., 1994; Amann et al., 1995;Woolf 1996; McMahon et al., 1995; Hathway and Fitzgerald, 2006; Mills et al., 2013). The initiation and maintenance of nociceptor hypersensitivity as a part of the inflammatory response after tissue injury manifests as acute and chronic pain (see Pezet and McMahon, 2006). Results of recent clinical trials using NGF-sequestering antibodies attests to the ongoing role of this neurotrophin in chronic pain (Wild et al., 2007; Cattaneo, 2010; Schnitzer et al., 2014; Shelton 2014), although pre-clinical findings show a different contribution of NGF between inflammatory and neuropathic pain (Djouri 2016).
Both the trophic actions and the hyperalgesic effects of NGF have been attributed to tropomyosin receptor kinase A (TrkA) that is expressed on peripheral and central neurons and is distinguished by its high affinity for NGF (Meakin and Shooter, 1992; Barker and Murphy,1992; Fundin et al., 1997). A lower affinity NGF receptor, the p75 neurotrophin receptor (p75NTR), activates a different intracellular pathway than that of TrkA. Traditionally, p75NTR was thought to be exclusively involved in the development and survival aspects of NGF, and hyperalgesia in the developed, adult peripheral nervous system was attributed to TrkA (Watanabe et al., 2008; Mantyh et al., 2011). However, the NGF enhancement of excitability of isolated sensory neurons relies on activation of p75NTR, which triggers the sphingomyelin signaling cascade (for review see Nicol and Vasko, 2007; Zhang et al., 2012). Neutral sphingomyelinase(s) (nSMase), its metabolic product, ceramide, and the atypical PKC (aPKC), PKMζ, are important effector molecules of this intracellular pathway. We recently reported that mechanical sensitivity was rapidly enhanced following NGF injection into the plantar surface of the rat paw and that the p75NTR pathway played a key role, since the hypersensitivity was prevented by antibody blockade of that receptor and by inhibition of either nSMase or an aPKC (Khodorova et al., 2013). In addition, NGF-induced mechanical hypersensitivity was recapitulated by a membrane permeant homologue of ceramide. These findings on mechano-hypersensitivity are all consistent with signaling through the p75NTR-nSMase-aPKC pathway. However, changes in thermal sensitivity caused by NGF, and the involvement of the TrkA pathway were not explored in this preceding work.
Therefore, the present work expands these studies by determining the contributions of the p75NTR- and of the TrkA- coupled pathways to NGF-induced thermal hypersensitivity in (male) rats. The results show that TrkA is essential for this thermal response and that p75NTR plays a modulatory role in shaping the duration, but does not affect the acute phase, of thermal hypersensitivity.
EXPERIMENTAL PROCEDURES
Experiments were conducted on 118 adult male Sprague-Dawley rats (230–300g). Rats were housed 2 per cage under a 12:12 h dark-light cycle and were provided with food and water ad libitum. Animals were experimentally treated and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (Guide, 1996) as reviewed and approved by the Harvard Committee on Animals. For most tests, 8 rats were assigned to each control or treatment group, unless otherwise noted.
Thermal testing
The sensitivity of the plantar paw to noxious radiant heat was determined by the method of Hargreaves et al. (1988) (IITC, Life Science, Inc., Woodland Hills, CA). The animals were habituated and tested on a raised glass platform over 5–7 days before each experiment in order to achieve a consistent paw withdrawal latency (PWL), as free of stress-related effects as possible. A series of 3–4 withdrawal latencies was determined, within each test session, alternately on left and right paws (more than 4 tests were applied in the case of a high variability in the behavioral responses); the first paw tested was assigned randomly. A 20 sec cut-off time was set to avoid overt thermal sensitization from testing per se, and tests of the same paw were separated by 3–4 min intervals. Three to 4 measurements for each intact hind paw, performed on the two days (including the day of the experiment) preceding any injections, were averaged and the mean value taken as the baseline nociceptive PWL. Following any treatment, withdrawal latency measurements were carried out alternately on the NGF (or vehicle)-injected (ipsilateral) paw and the uninjected contralateral, paw.
Injection procedures
All injections were made subcutaneously (s.c.) into the mid-plantar surface of the hind paw, 1 cm distal from the heel using a 30-G needle attached to a 10 µl Hamilton microsyringe (Hamilton Co., Reno, NV, USA). The NGF-injected paw was identified as the Ipsilateral Paw (ILP) and the opposite paw as the Contralateral Paw (CLP). Injections occurred under brief general anesthesia caused by inhalation of the rapidly reversible agent sevoflurane (Abbott Labs, N. Chicago, IL, USA). Recovery of the righting reflex occurred in <30 sec after anesthesia inhalation was discontinued; 5–10 min later “normal” nocifensive responses to paw pinch could be assessed in control animals.
NGF, N-acetyl-D-sphingosine (C2-ceramide), GW4869, K252a or their vehicles, each were injected in 10 µl volumes, and antibodies to p75NTR or the TrkA receptor were injected in 20 µl volumes. The non-selective myristoylated pseudosubstrate inhibitor of atypical PKCs (mPSI- or “ZIP”; Standaert et al., 1997), or its inactive scrambled peptide homologue (scrambled ZIP), both at 40 µg/20 µl, were similarly injected, before NGF.
Drugs
NGF-β (rat, recombinant) (Cat No.N-2513, Sigma-Aldrich, St. Louis MO, USA), or NGF-β/CF (rat) (R&D Systems, Inc., MN, USA) was dissolved in phosphate buffered saline (PBS; In Vitrogen) as a stock solution (1000 ng/10 µL) and stored in small aliquots at −80°C. Prior to the injection, NGF stock aliquots were diluted in PBS (pH 7.4) to the noted final concentration of 50 ng/µl, serving to deliver 500 ng per 10 µL injection. C2-ceramide (Cat No.BML-SL 100-005; Enzo Life Sciences, Inc., NY, USA) was dissolved initially to 250 µg/10 ul in pure DMSO, and stored in aliquots at −20 °C, then diluted to 20 µg/10 µl DMSO before the injection. GW4869 (Cat No. D1692; Sigma-Aldrich) was dissolved in DMSO as a 2 mM stock solution, and aliquots were prepared under a stream of N2 before freezing, to avoid atmospheric oxidation. A wide spectrum inhibitor of tropomyosin receptor kinases, K252a (Cat No.K1639, Sigma-Aldrich), was dissolved in DMSO as a 2 mM stock solution and used either at that concentration or further diluted in PBS to 200 µM or 20 µM before injection. An inhibitor of atypical PKCs, including PKMζ, mPSI (Cat No. ALX-260-155-M001; Enzo Life Sciences, Inc.), was dissolved in PBS as a 50 µg /10 µl stock solution and then diluted in PBS to its final concentration. All aliquots were stored at −80°C. The IgG antibodies to p75NTR and TrkA (Clary et al., 1994), generously given by Professor L. Reichardt, of UCSF and the Simon Foundation, New York, were kept at +4° C for 2–3 days, at most, before injection. The anti-p75NTR antibody blocks the extracellular domain of this receptor and prevents agonist binding and receptor activation (Weskamp and Reichardt, 1991). In contrast, the anti-TrkA antibody blocks the neurotrophin binding site but also activates this receptor, independently of NGF (Clary et al., 1994).
Experimental design
For all experiments, rats were allowed to rest quietly in an isolated behavioral testing room for 30 min before any procedures. Within minutes of the rapid onset of anesthesia by sevoflurane, one paw (ILP) was injected with the modulator of a specific enzyme or pathway and was followed by the “standard dose” of NGF: 500 ng. Rats were then returned to their cages, then later removed briefly for thermal testing beginning at 30 min after NGF delivery; when not on the testing apparatus the rats were resting in their cages. To test the effectiveness of the nSMase inhibitor against NGF-induced hyperalgesia, GW4869 (2 mM, 10 µl) or its control vehicle, was injected into the ILP once, 17 min before NGF. To evaluate the role of either NGF receptor in thermal hyper-sensitivity, the p75NTR blocking antibody or the TrkA blocking antibody was injected in a volume of 20 µl, 4h before NGF, equal to the time for an effect of the anti-p75NTR antibody in our previous study (Khodorova et al, 2013). The IgG fraction from naïve rat serum was used as a control for these antibody experiments. The mean value of PWL measured at 3.5h after the antibody injection was taken as a baseline for comparison with thermal responses after NGF injection. In some experiments, capsaicin was injected after the TrkA antibody to show that thermal hyperalgesia produced by a non-TrkA pathway was still possible.
To further establish the role of TrkA signaling, K252a, a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors (Tapley et al. 1992; Knüsel & Hefti, 1992, but see Kase et al. 1987), was injected in a volume of 10 µl at one of three (as indicated in Methods) concentrations, 0.5 h before NGF. To test the role of atypical PKCs (aPKC), including PKMζ, mPSI was injected intra-plantar into the ILP one day prior to NGF. Responses to thermal stimulation measured on D1, 24 h after the inhibitor injection, were taken as the PWL baseline to which NGF’s effects were compared.
Analysis
Data are graphed for all time points as medians, 25th and 75th percentile values (box plots) and lower and upper 95% Confidence Intervals, shown by the vertical error bars. The mean value is also graphed (filled circle) for comparison with the median value. Data are reported in the Results, although for fewer time points, as “median: lower 95%CI, upper 95% CI”, to allow for better clarity in reading the Results. All statistical parameters were calculated by GraphPad InStat version 3.0 (GraphPad Software, CA, USA).
Since the hind paw withdrawal latencies do not always follow a normal distribution, non-parametric statistical analysis was used, as identified in Results. Friedman test followed by Dunn’s post hoc test was applied to compare repeated measures of PWL with baseline (BSL) values, measured before any injections. Additionally, the two-tailed Mann-Whitney test was used for comparisons of the PWL responses, at one specified time, of a control group, e.g. NGF + vehicle, with the responses at the same time of a treatment group, e.g., NGF + inhibitor (usually pre-treated). In cases where PWL was tested in the same paw before and after a particular treatment, the before and after values were compared using the paired Wilcoxon rank test. The PWL of the contralateral paw (CLP) is reported for the first three experiments (cf. Figs. 1–3); however, since the PWL of the CLP was never significantly affected by NGF, or any of the pre-treatments, it was not analyzed in the later experiments. Precise P values are reported, and P<0.05 is taken as a significant difference.
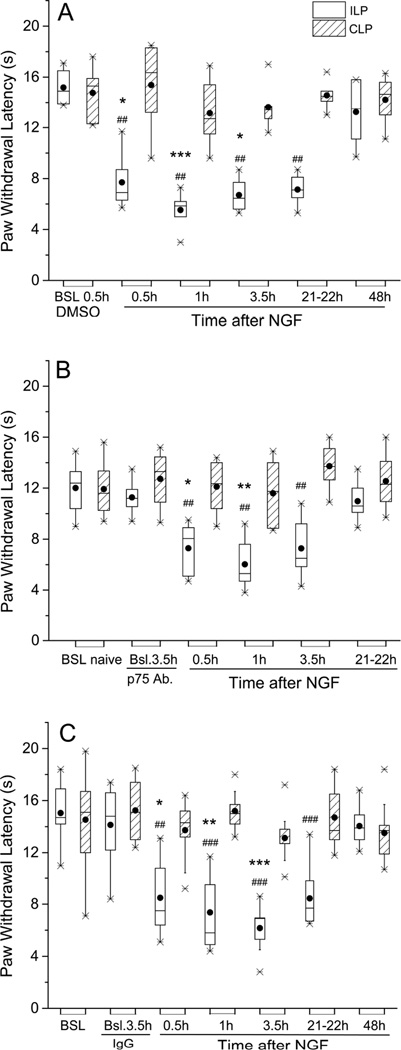
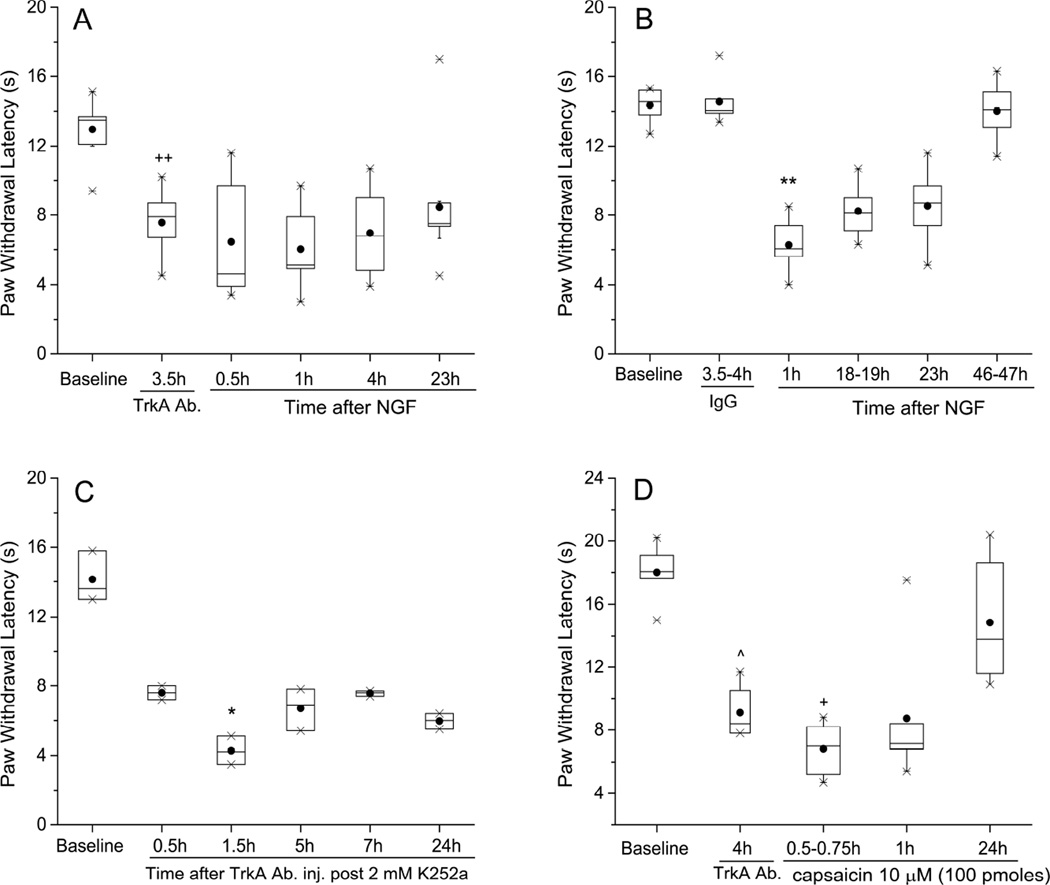
Figure 1.
A polyclonal antibody to p75NTR did not prevent thermal hyperalgesia induced by NGF, but shortened its duration. In this and all other figures, the Paw Withdrawal Latencies are expressed as medians (horizontal lines), with 25th and 75th percentiles (shown by vertical dimension of the box), and the 5th and 95th percentiles indicated by the whiskers. Means values of PWL are shown by the solid circles. (A) The Paw Withdrawal Latency was shortened in controls, where vehicle (PBS, 20 µl) was injected 30 min before NGF, and this index of hyperalgesia lasted from 0.5h through 22h, and resolved back to baseline by 48h after injection of NGF (n=6). *P<0.05, ***P<0.001 vs. baseline ILP (Friedman’s test followed by Dunn’s post hoc test); ## P<0.005 vs. CLP (two-tailed Mann-Whitney test). (B) When pre-injected (20 µl) into the paw 4 h before NGF (500 ng/10 µl), the antibody did not prevent acute thermal hyperalgesia, at 0.5 h – 3.5 h, but accelerated its recovery, which occurred by 21–22 h (n=8). *P<0.05, **P<0.01 vs. baseline ILP (Friedman’s test followed by Dunn’s post hoc test); ## P<0.005 vs. CLP (two-tailed Mann-Whitney test). (C) Thermal hyperalgesic actions of NGF when IgG from naïve rat serum (20 µl) was injected 4 h before NGF (n=9). *P<0.05, **P<0.01, ***P<0.001 vs. baseline after IgG, ILP (Friedman’s test followed by Dunn’s post hoc test). ## P<0.005, ### P<0.0005 vs. CLP (two-tailed Mann-Whitney test).
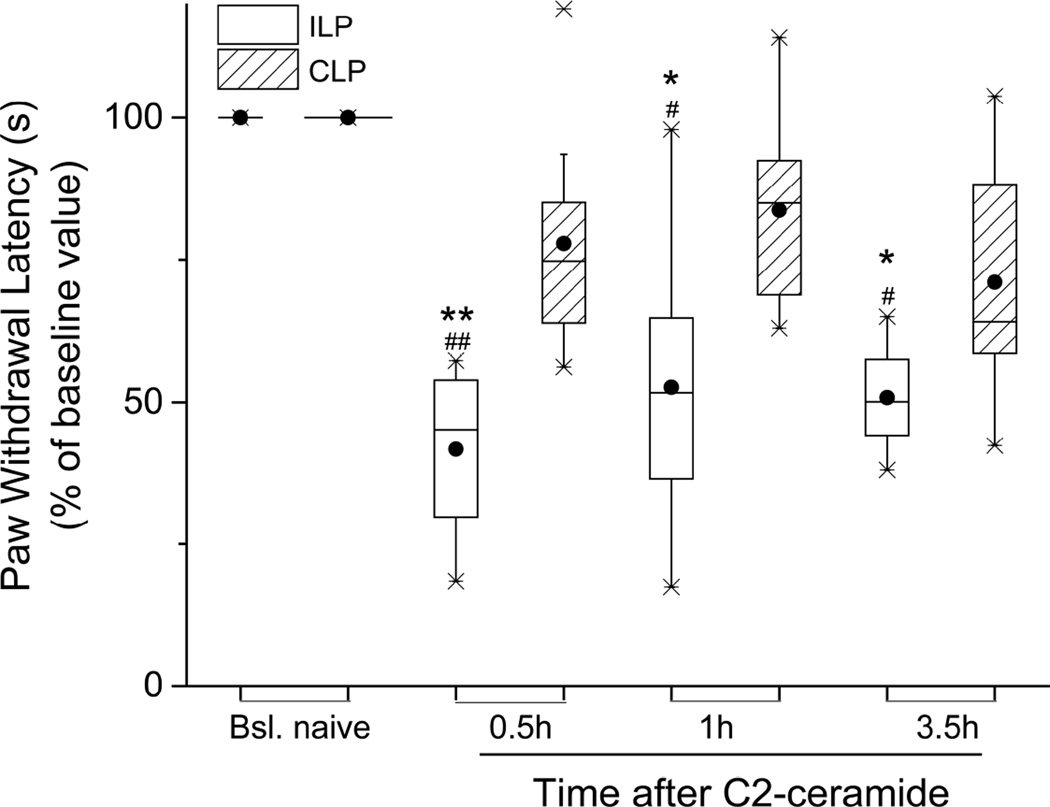
Figure 3.
Thermal hyperalgesia induced by local C2-ceramide. C2-ceramide (20 µg/10 µl) injected s.c. into rat plantar hind paw caused an acute drop in normalized PWL that lasted at least 3.5h (n=8). No change of PWL occurred in the CLP. *P<0.05, **P<0.01 vs. ILP baseline (Friedman’s test followed by Dunn’s post hoc test); #P<0.05, ##P<0.001 vs. the CLP (two-tailed Mann-Whitney test).
RESULTS
Intraplantar NGF injection produced an increase in thermal sensitivity of the ipsilateral hind paw (ILP), as measured by a shortening of paw withdrawal latency (Figure 1A), repeating previously reported findings (Lewin et al., 1993; Woolf et al., 1994; Amann et al., 1995; McMahon et al., 1995). The thermal hypersensitivity was significant within 30 min after the injection of NGF (PWL= 6.9: 5.7,10.0 sec vs BSL,14.9:13.6, 16.7 sec (median:5%, 95% CI); P<0.05), persisted at this increased level through at least 4h (PWL=6.4:5.3, 8.1 sec; P<0.05 vs BSL), and had recovered to baseline, pre-NGF levels by 48h (PWL=13.5:10.6,15.8 sec; P>0.05 vs BSL; all significance calculated from Friedman’s test followed by Dunn’s post-hoc test, n=6). The rats showed no abnormal behavior after NGF injection, did not lick or bite the injected paw, and had normal ambulation and cage behavior for the day after NGF injection. NGF thus appears to cause hyperalgesia but not nociception per se.
To differentiate the receptor dependence of NGF-induced thermal hyperalgesia, between p75NTR and TrkA, a blocking antibody to the p75NTR was injected into the paw 4h before NGF. Acute thermal hyperalgesia followed NGF injection, as in the control animals (0.5h after NGF, PWL=8.0:5.6, 9.0 sec vs BSL,11.2:10.2,12.3 sec; P<0.05, n=8), but the hypersensitivity did not persist as long, instead returning to baseline by 22h after injection (Figure 1B; PWL=10.6:9.7,12.3 sec; P>0.05 vs BSL;). In control animals, prior injection of the IgG fraction from naïve rat serum, 4h beforehand, did not alter the NGF response (0.5h after NGF, PWL=7.5:6.3,10.7 sec vs BSL, 14.8:11.8,16.5 sec; P<0.05, n=9), with hyperalgesia persisting through 22h (PWL=7.7:6.7,10.2, P<0.005 vs BSL; all statistical values from Friedman’s followed by Dunn’s test; Figure 1C), showing the specificity of the anti-p75NTR antibody effect. These results demonstrate that although the return to baseline sensitivity was accelerated by the p75NTR blocking antibody, this treatment did not affect the ability of NGF to significantly lower the PWL. This observation contrasts with the complete prevention of NGF-induced mechanical hypersensitivity by the same dose of anti-p75NTR antibody, as previously reported (Khodorova et al., 2013).
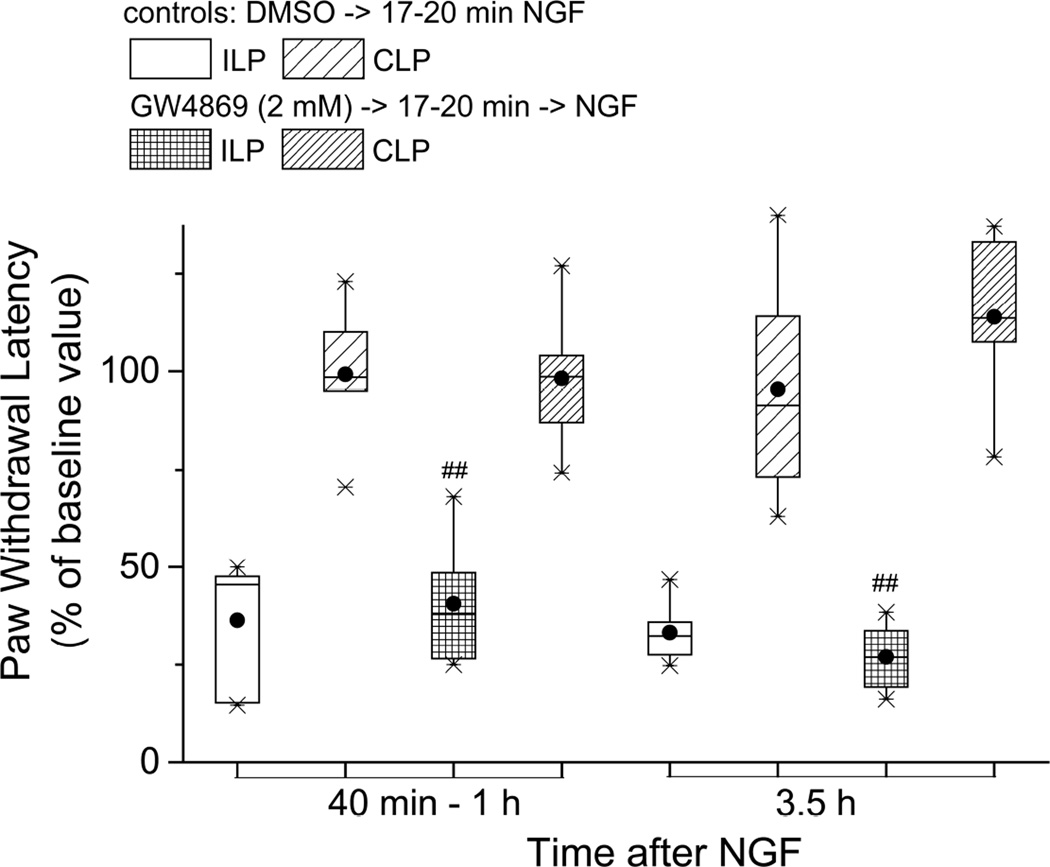
The NGF-activated p75NTR signals intracellularly through neutral sphingomyelinase (nSMase), which generates ceramide (Dobrowsky, et al., 1994), itself eventually converted to sphingosine 1-phosphate (S1P). Therefore, in the next test of p75NTR’s involvement in thermal hyperalgesia, a selective inhibitor of nSMase, GW4869 (Luberto et al., 2002; Marchesini et al., 2003) was injected before NGF. Neither the acute shortening of paw withdrawal latency at 40–60 min after NGF (vehicle-NGF, normalized PWL=45.5:18.9,53.9% of BSL vs GW-NGF,38.0:23.9,57.4% of BSL; P>0.05 Mann-Whitney test) nor that at 3–3.5h after NGF (vehicle-NGF, normalized PWL=32.4:25.0,41.7 % of BSL vs GW-NGF, 26.8:18.1,35.8 % of BSL; P>0.05, Mann-Whitney test) were prevented by GW4869 (n=6, Figure 2), at concentrations which we had previously shown to abolish NGF-induced tactile hypersensitivity (Khodorova et al., 2013). Since GW4869 had no effect at 3.5h, and the inhibition that we previously reported for mechanical hyperalgesia was over by 3.5h (Khodorova et al. (2013), we did not continue the PWL measurements after this time. (In this analysis the different treatment groups had different baseline withdrawal latencies, so for comparison the individual rat data were normalized to the baseline PWL for those animals in their respective groups, and data are reported as the PWL normalized to BSL.) Significant changes from the CLP were also seen in the ILP at both times after NGF following GW4869 injection; at 1h, (GW-NGF ILP, normalized PWL=37.9:23.9,57.4 % BSL vs CLP, 98.6:79.4,117.0 %BSL; P<0.005 Mann-Whitney test) and at 3.5 h (GW-NGF ILP, normalized PWL=26.8:18.1,35.8 %BSL vs CLP, 113.5:91.7, 135.8 %BSL;P<0.005 Mann-Whitney test, Figure 2.) It thus appears that nSMase activity is not required for NGF to induce thermal hyperalgesia.
Figure 2.
A cell-permeant, non-competitive inhibitor of nSmase, GW4869, failed to prevent thermal hyperalgesia induced by NGF; n=6. ## P<0.005 vs the CLP (two-tailed Mann-Whitney test). The PWLs shown here have been normalized to the baseline values of the respective groups because these baseline values differed significantly, so the data are shown as “% baseline” rather than absolute latency times.
We next tested the ability of a membrane-permeant derivative of ceramide, C2-ceramide, to induce thermal hyperalgesia. In addition to being an analogue of the endogenous, longer alkyl chain ceramide that is the precursor of S1P, C2-ceramide is known to directly activate TrkA (MacPhee and Barker, 1999). When injected into the plantar hind paw, this compound shortened PWL acutely as much as NGF did, at 20–40 min after NGF (Figure 3; C2-ceramide, PWL=4.6:2.7,5.4 sec vs BSL, 9.6:8.5,10.7 sec; P<0.01, Friedman’s test followed by Dunn’s test; n=8) and at 3.5 h after NGF (C2-ceramide, PWL=4.6:3.9,5.8 sec vs BSL,9.6:8.5,10.7 sec; P<0.01, Friedman’s test followed by Dunn’s test). This approximate halving of the PWL by C2-ceramide is comparable to NGF’s effect (see Figure 1A). Interestingly, thermal hypersensitivity was still present at 3–3.5h, longer than the elevation of mechanosensitivity caused by this same dose of C2-ceramide (Khodorova et al., 2013), but both modalities of hypersensitivity had resolved by 22–24h (data not shown).
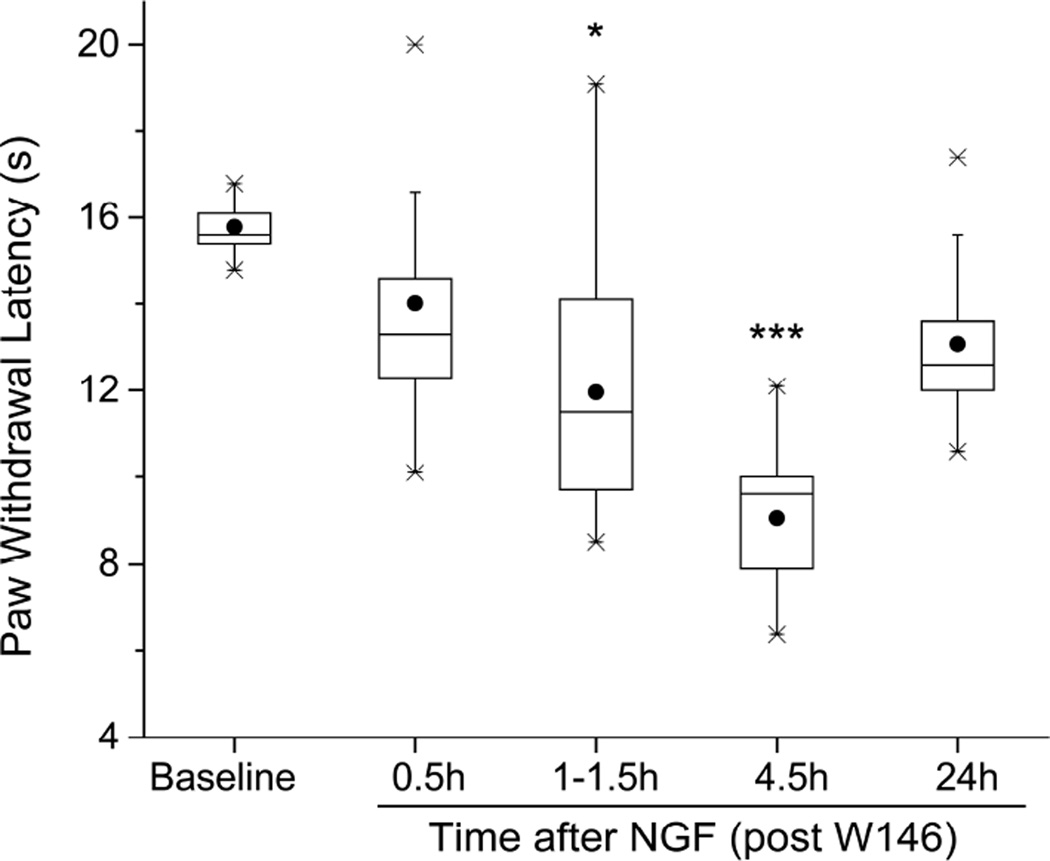
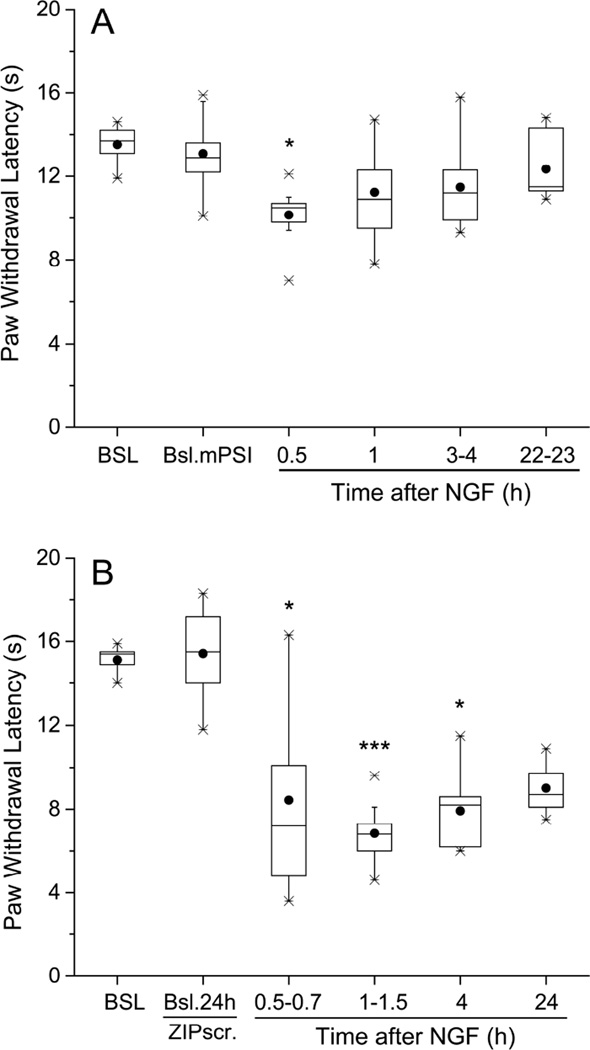
Along the nSMase triggered pathway, ceramide is converted to sphingosine, which is phosphorylated to S1P, an agonist of several different S1P receptors. Therefore, in light of the activity of the ceramide analogue, we next examined the effects of W146, a selective antagonist of the S1P receptor subtype 1 (S1PR1, Sanna et al., 2006), which is effectively activated by S1P and known to be involved in NGF-induced increases in electrical excitability (Zhang et al., 2006 a, b). A single dose of W146, injected into the hind paw 30 min before NGF (Figure 4, n=9), delayed the development of thermal hyperalgesia (no significant change from baseline PWL at 0.5h after W146-NGF, PWL=13.3:11.8,16.2 sec vs BSL 15.6:15.3,16.3 sec; P>0.05 Friedman’s followed by Dunn’s test; compare to significant change from baseline at this time with NGF alone, Figure 1A), and hastened its recovery (at 24h after W146-NGF, PWL=12.6:11.4,14.8 sec vs BSL, 15.6:15.3,16.3 sec; P>0.05, Friedman’s followed by Dunn’s test; compare to significant change from NGF alone that was still present at this time, Figure 1A). Our previous work suggested that p75NTR activation resulted in the downstream involvement of protein kinase M zeta (PKMζ), a member of the atypical PKC (aPKC) family, in both elevated excitability (Zhang et al., 2012) and in mechanical hypersensitivity (Khodorova et al., 2013). Pre-injections of a pseudosubstrate inhibitor of aPKCs, mPSI, known to prevent the development of mechano-hypersensitivity by NGF (Khodorova et al., 2013), also inhibited the thermal hypersensitivity caused by NGF, limiting the duration to only 0.5–1 h and attenuating the peak drop of PWL by 60% from control, n=9 (at 0.5h after NGF, pre-treated with mPSI, PWL=10.5:9.1,11.2 sec vs BSL after mPSI, 13.7:12.8,14.3 sec; P<0.05, Friedman followed by Dunn’s test; Figure 5A; compare to Figure 1A). At none of the other times after NGF injection, when pre-treated by mPSI, did PWL drop significantly from baseline (Figure 5A). By contrast, injection of a scrambled mPSI peptide (ZIPscr), which is an inactive analog of mPSI (Krotova et al., 2006), did not reduce the NGF-induced thermal hyperalgesia, n=9 (Figure 5B); significant deviations from BSL PWL occurred at 0.5–0.7h after NGF (PWL=7.2:5.0,11.9 sec vs BSL after ZIPscr, 15.5:13.8,17.1; P<0.05) and continued through 24h after NGF (PWL=8.7:8.2,9.8 vs BSL after ZIPs, 15.5:13.8,17.1; P<0.005, Friedman’s test followed by Dunn’s test). Thus, thermal hyperalgesia from NGF also appears to involve an atypical PKC.
Figure 4.
A selective antagonist of the S1P receptor 1, W146, injected (4.8 µg/10 µl) 0.5 h before NGF (500 ng/10 µl), delayed the development of thermal hyperalgesia induced by NGF and accelerated the recovery of thermal responsiveness (n=9). *P<0.05, ***P<0.001 vs. BSL (Friedman’s test followed by Dunn’s post hoc test).
Figure 5.
Local pre-treatment with myristoylated pseudosubstrate inhibitor (mPSI) of atypical PKCs decreased the thermal hyperalgesia from NGF. The inhibitor was injected s.c. into the plantar hind paw 24 h before the intraplantar injection of NGF (500 ng/10 µl). (A) mPSI (40 µg/20µl) alone did not change the plantar hind paw thermal responsiveness, when tested at 24h after injection into naïve rats (BSL mPSI), but attenuated NGF-induced peak drop of paw withdrawal latency, measured at 0.5 h, and limited the duration of thermal hyperalgesia to only 1 h (n=9). *P<0.05 vs. baseline after mPSI at 22–23 h, ILP (Friedman’s test followed by Dunn’s post hoc test). (B) Injection into the plantar hind paw of scrambled mPSI (ZIPscr, 40 µg/20 µl), which lacks inhibitory action on aPKCs, 24 h before the intraplantar injection of NGF, neither attenuated nor delayed thermal hyperalgesia from NGF (n=9; compare with Figure 1A). *P<0.05, ***P<0.001 vs. baseline after ZIPscr at 24 h (Friedman’s test followed by Dunn’s post hoc test).
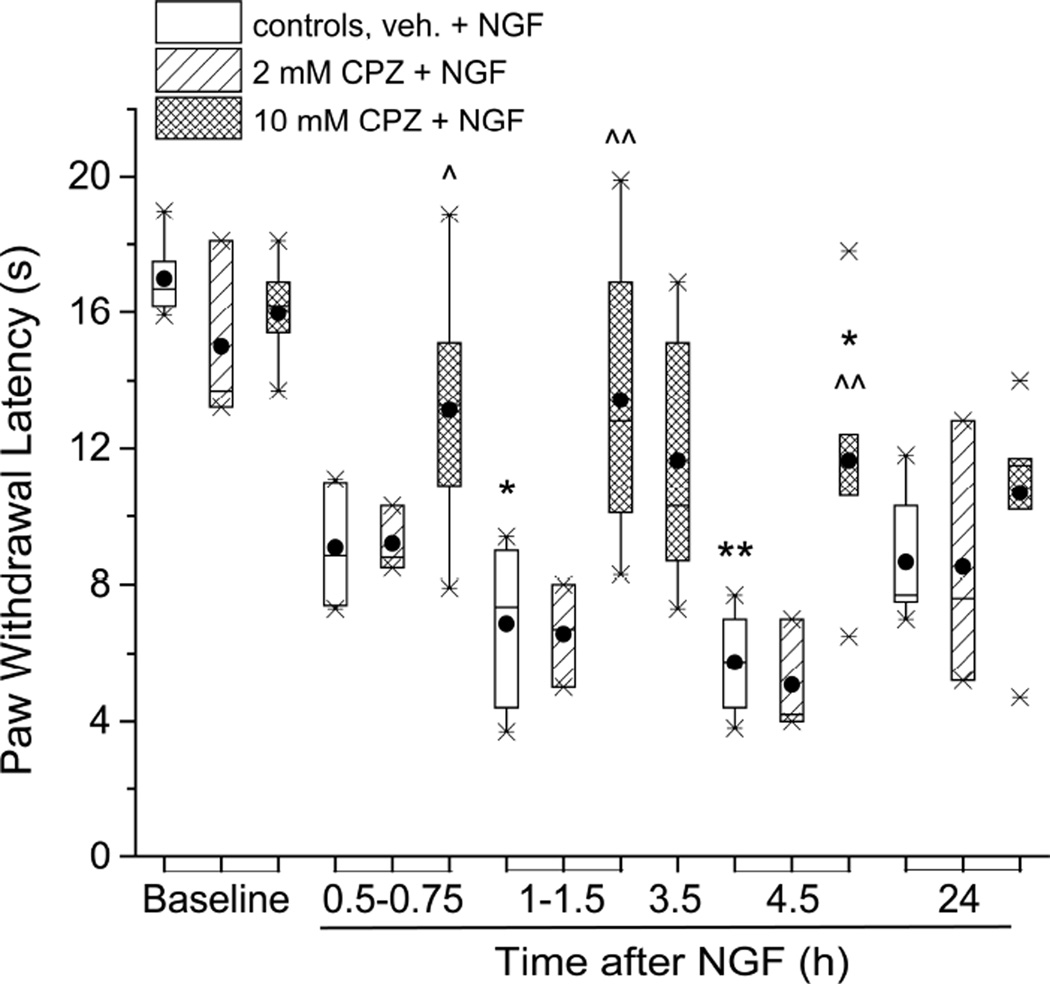
Increased thermal nociceptive sensitivity often involves activation of the TRPV1 channel (Caterina 2007). The same appears to be true for NGF-induced hyperalgesia, since pre-injections of the TRPV1 antagonist capsazepine (CPZ), at 10mM, reduced the NGF-induced shortening of PWL for at least 4.5 h, n=10 (at 4.5h after NGF-CPZ,PWL=11.6:9.3,14.0 sec vs after NGF-VEH, 5.8:4.1,7.3 sec; P<0.05, Mann-Whitney test; Figure 6). At the lower CPZ concentration of 2mM there was no reduction of NGF’s action (at 4.5h, after NGF-CPZ (n=3), PWL=4.2:0.9,9.2 sec vs NGF-VEH (n=6), 5.8:4.1,7.3 sec; P>0.05, Mann-Whitney test). It thus appears that TRPV1 could be one target of PKMζ (or other aPKC) activity, in effecting acute thermal hyperalgesia.
Figure 6.
The TRPV1 inhibitor, capsazepine reduced NGF-induced acute thermal hyperalgesia. Capsazepine (10 mM, dose 100 nmol/ paw), injected 20 min before NGF (500 ng/10µl) reduced PWL shortening at 0.5 – 4.5 h (n=10). No such effect was found for a lower dose of CPZ (20 nmol/ paw, n=3). In controls, vehicle for CPZ (DMSO, 10 µl/paw) was injected prior to NGF (n=6). *P<0.05, **P<0.01 vs. baseline (Friedman test for the 5 times of the same day, followed by the Dunn’s post hoc test); ^P<0.05, ^^P<0.005 vs. controls at 1.5h and 4.5h (two-tailed Mann-Whitney test).
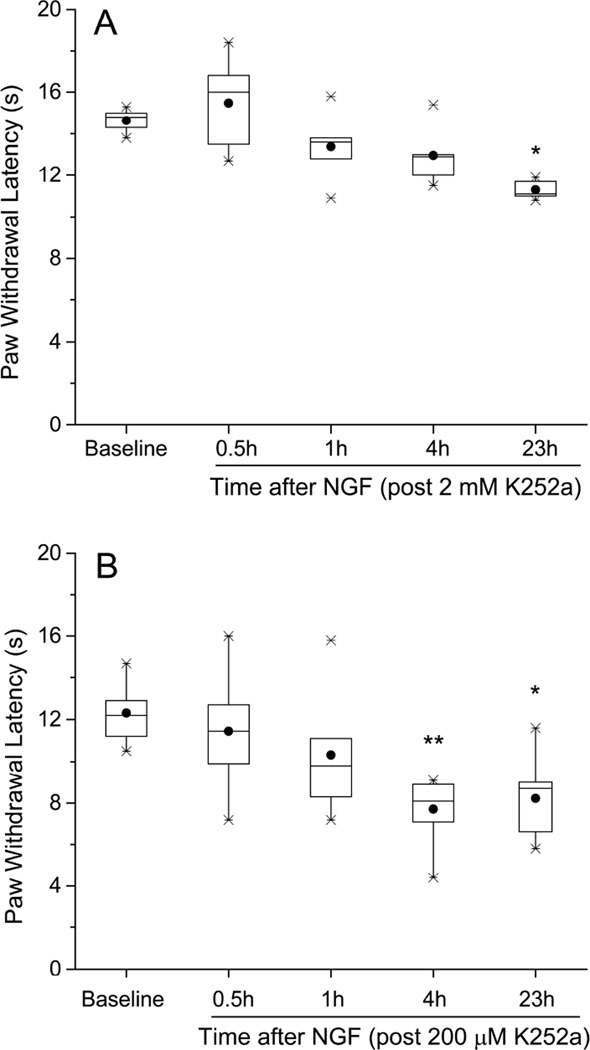
Since blocking the p75NTR with an agonist-blocking antibody did not prevent acute thermal hyperalgesia, yet activating or inhibiting certain p75NTR -coupled steps caused or modified this hyperalgesia, the next test was to manipulate the other NGF receptor, TrkA. As a first step, we injected K252a, a general inhibitor of tropomyosin receptor kinase activity (Tapley et al. 1992; Knüsel & Hefti, 1992) Pre-injection into the hind paw of K252a, at 2 mM, prevented the acute shortening of PWL following NGF (at 0.5h after NGF-K252a, PWL=16:12.6,18.4 sec vs BSL 14.8:13.9,15.4 sec; P>0.05, and at 4h after NGF-K252a, PWL=12.9:11.1,14.8 sec vs BSL 14.8:13.9,15.4 sec; P>0.05, n=5, Friedman’s test followed by Dunn’s test; Figure 7A). At 0.2 mM K252a, the maximum reduction in PWL was delayed until 4h after NGF (compared to the 1h time of maximum effect in controls, see Figure 1A), and the maximum per cent change was less than that from NGF alone, n=7 (NGF-K252a, normalized PWL=33.1:18.9,36.6% of BSL vs NGF-VEH, 45.5:18.9, 53.9 % of BSL (these control data from Figure 2); P<0.05, Mann-Whitney test, Figure 7B). At 0.02 mM, K252a had no effect on the NGF-induced thermal hyperalgesia (data not shown).
Figure 7.
Inhibition of NGF-induced thermal hyperalgesia by K252a, a general inhibitor of tropomyosin receptor kinases. K252a (10 µl) was injected 0.5 h before NGF (500ng/10µl). (A) K252a at 2mM (20 nmol/paw) fully prevented the acute shortening of PWL following NGF (n=5). Dunn’s pair-wise test applied post hoc after Friedman’s test showed significance (*P<0.05) only for 0.5h vs 23h. (B) At a concentration of 0.2 mM (2 nmol/paw), K252a delayed the onset of hyperalgesia by 3–4 h (n=7). *P<0.05, **P<0.001 vs. BSL ILP (Friedman’s test followed by Dunn’s post hoc test).
To further determine the role of TrkA in mediating the thermal hypersensitivity, we injected an antibody known to directly activate this receptor (Clary et al., 1994). Consistent with a TrkA activation mechanism, the antibody by itself caused a shortening of PWL (3.5h after Ab, PWL=7.9:6.0,9.1 sec vs BSL, 13.5:11.7,14.2; P<0.01, paired Wilcoxon test; Figure 8A). Subsequent injection of NGF caused no further reduction of PWL (at 0.5h after NGF, PWL=4.6:4.0,8.9 sec vs 3.5h after Ab, 7.9:6.0,9.1 sec; P>0.05, Friedman’s test followed by Dunn’s test), in agreement with the known ability of this activating antibody to block the neurotrophin binding site (Clary et al., 1994).
Figure 8.
An antibody that activates TrkA causes thermal hyperalgesia. (A) Anti-TrkA antibody (20 µl/paw), when injected into the naïve rat plantar hind paw, led to a shortening of PWL, measured at 3–4 h, but prevented further shortening of PWL following NGF (500 ng/10 µl) (n=9). Significance (P<0.05) was only found for Dunn’s pair-wise comparison of 23h and 0.5h time points, applied after Friedman’s test. (B) Injection of control IgG, from naïve rat serum (20 µl/paw), 4 h before NGF (500 ng/10µl), neither changed PWL, nor affected thermal hyperalgesia from subsequently injected NGF (n=6). **P<0.01 vs. baseline after IgG, ILP (Friedman test followed by Dunn’s post hoc test). (C) Lack of effect of K252a on anti-TrkA antibody-induced thermal hyperalgesia. K252a (20 nmol/paw) was pre-injected 0.5h before the antibody (n=3). ^P<0.05 for baseline vs 24 h after antibody (two-tailed Wilcoxon Paired test). (D) Further reduction of PWL by i.pl. capsaicin, after the anti-TrkA antibody-induced hyperalgesia (n=6). ^P<0.05 vs. baseline ILP, naïve rats (two-tailed Wilcoxon test); +P<0.05 vs. value at 4h after TrkA ab. ILP (two-tailed Wilcoxon test).
This decrease in PWL was not a non-specific effect of the antibody molecule, since the IgG fraction of serum from a naïve rat did not change the PWL (3.5h after IgG, PWL=14.0:13.1,16.0 sec vs BSL 14.6:13.3,15.4 sec; P>0.05, Friedman’s followed by Dunn’s test), and did not affect NGF’s ability to induce thermal hyperalgesia (1h after NGF-IgG, PWL=6.0:4.2, 9.7 sec vs BSL after IgG,14.0: 13.1,16.0 sec; P<0.01, Friedman’s followed by Dunn’s test; Figure 8B).
The anti-TrkA antibody-induced receptor activation does not require trans-phosphorylation of the intracellular receptor domains (Clary et al., 1994) and, in keeping with this action, pre-injection of K252a, that would block this phosphorylation step, had no effect on the TrkA antibody-induced hyperalgesia (see Figure 8C), unlike its profound inhibition of NGF-induced hyperalgesia (Figure 7A).
That the lack of effect of NGF in this circumstance was not due to a functional lower limit in the PWL (after the antibody-induced latency shortening) is proven by the further, brief reduction of PWL caused by capsaicin injection 4 h later into the anti-TrkA antibody-injected paw (at 30–45 min after capsaicin, PWL= 7.0:5.1,8.5 sec vs 4h after Ab, just before capsaicin, PWL=8.4:7.4, 10.8 sec; P<0.05 paired Wilcoxon test; Figure 8D). Interestingly, the PWL recovers to its baseline, pre-Ab level by 24h after capsaicin (PWL=13.8:10.8,18.9 sec vs BSL 18.0:16.2,19.8 sec; P>0.05 Friedman’s test followed by Dunn’s test), whereas in all other conditions tested here (Figs 8A–C), thermal hyperalgesia from the anti-TrkA antibody persisted for at least 24h.
DISCUSSION
These experiments show that the thermal hyperalgesia occurring within the first 24 h after local, subcutaneously injected NGF depends on activation of the TrkA receptor. Activation of this receptor by NGF involves reciprocal cross-phosphorylation of the dimeric sub-unit monomers (Huang and Reichardt, 2003). Inhibition of the intracellular kinase activity of the receptor prevents its activation and, as shown here, the thermal hyperalgesia from NGF. Receptor activation by a highly specific IgG antibody, that does not require receptor homodimer cross-phosphorylation, also results in an acute, local thermal hyperalgesia which is unaffected by the kinase inhibitor and unresponsive to subsequent exposure to NGF, consistent with the blockade of neurotrophin binding by this antibody.
By comparison, blockade of the other receptor for NGF, p75NTR, by a different antibody that is known to fully prevent NGF-dependent electrical hyperexcitability in isolated sensory neurons (Zhang and Nicol 2004) and mechano-hyperalgesia in vivo (Khodorova et al., 2013), has no effect on the magnitude of the acute thermal hypersensitivity after NGF injection. That same antibody, however, does accelerate the recovery of thermal hyperalgesia such that paw withdrawal latency is restored to baseline by ~24h after NGF, not 48h as in naïve animals or with an inactive IgG control. Inhibition of the nSMase enzyme, which is critical for p75NTR -coupled mechano-hypersensitivity in vivo (Khodorova et al., 2013) and for NGF-elevated electrical excitability in isolated sensory neurons (Zhang et al., 2006a), has no effect on thermal hypersensitivity. However, local delivery of C2-ceramide, an analogue of the ceramide that is a product of nSMase activity, by itself causes thermal hyperalgesia (but see below), and inhibition of the S1P receptor S1PR1, an element of the nSMase pathway downstream from ceramide, delays the development of thermal hyperalgesia from NGF.
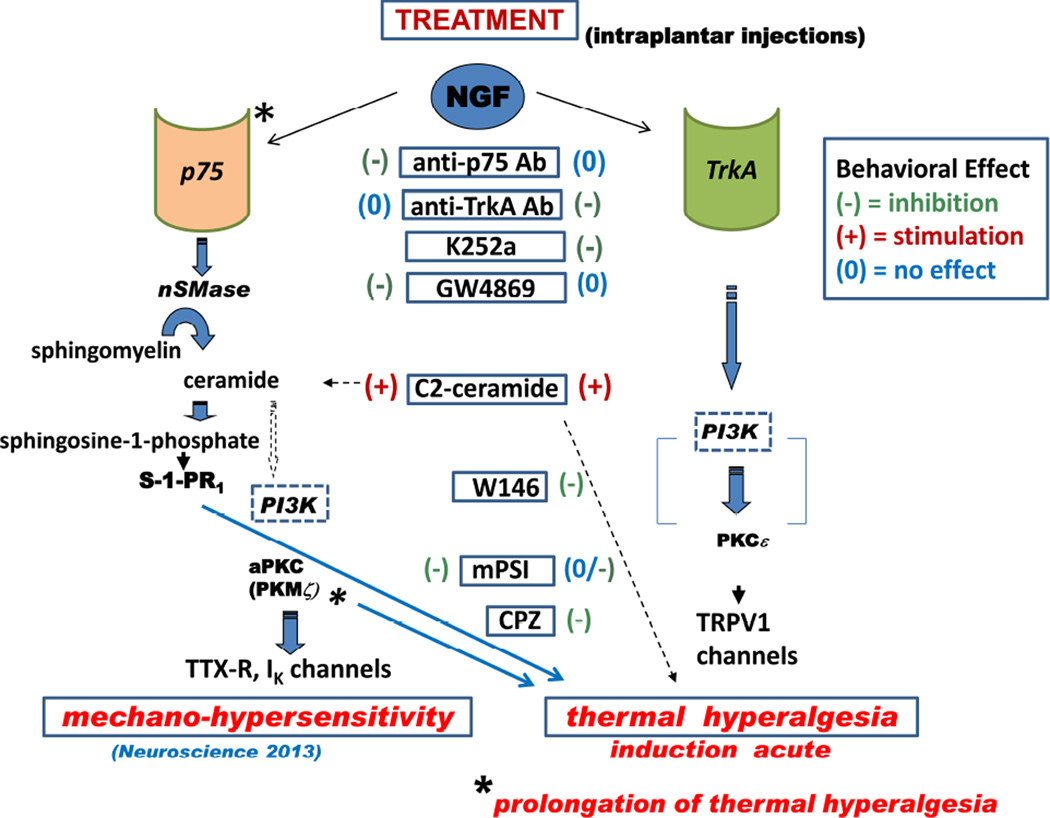
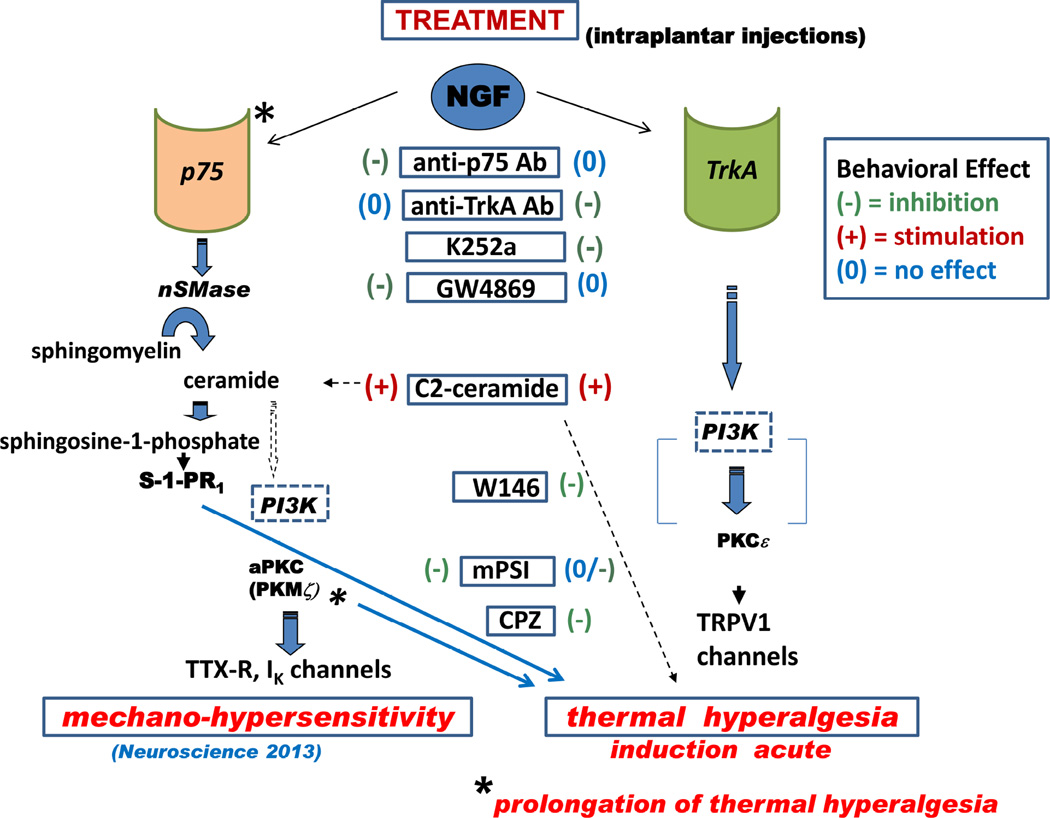
These findings are summarized in the pathway diagram of Figure 9, which shows the predominant intermediates determined experimentally for mediating NGF’s effects on mechanical sensitivity and on thermal sensitivity. The chemical agents used in this and our previous study are shown in boxes aligned vertically in a central column, and identified as having potentiating or inhibiting effects on the behavioral endpoints. Agents are aligned horizontally with their putative target enzymes or receptors, or, in the case of C2-ceramide, with the locus in the NSMase pathway where ceramide would occur. Although we conducted no experiments to test the role of PI3Kinase (PI3K) in the peripheral actions of NGF, its ubiquitous involvement in other cells and systems (see below) suggests that it also will participate in the processes that eventually activate the kinases aPKC (PKMζ ?) and PKCε, that phosphorylate channels and receptors which modulate cellular sensitivities.
Figure 9.
Schematic showing the putative intracellular signaling pathways downstream of neurotrophin receptors, p75NTR and TrkA, which lead to mechanical and thermal hyperalgesia from NGF in rat plantar hind paw, and a summary of the anti-hyperalgesic effects of the treatments used in the study. The steps that were tested experimentally are shown by solid arrows, those that are speculative shown by broken arrows. See text for details.
Altogether, our findings identify the TrkA receptor as the predominant receptor driving NGF-induced acute thermal hyperalgesia. However, there is indirect evidence for an involvement of downstream components generated by the p75NTR pathway in the thermal hyperalgesia measured 24h after NGF, implying that different mechanisms may be involved in the acute and the more prolonged actions of NGF in maintaining thermal hyperalgesia. Whether these different mechanisms are present in the same population of peripheral NGF-activated nociceptors, as in PC12 cells where such receptor interactions have been documented (Negrini et al., 2013), or in different sensory neurons that might be indirectly affected by s.c. NGF (McMahon et al., 1994), or in local peripheral inflammatory cells that are activated by NGF and release neuro-sensitizing substances (Finley et al., 2013; Shutov et al., 2016), or are accounted for by slowly developing changes in the spinal cord and brain after NGF, cannot be determined from these behavioral experiments alone.
Previous reports show that thermal and mechanical hypersensitivities resulting from NGF differ from each other, both in time course - mechanical hypersensitivity lasting much longer (Mills et al., 2013) - and in central MAPKinase involvement (Ostubo et al., 2012) In an earlier paper we detailed the receptor and pathway intermediates involved in mechano-sensitization by NGF, when injected as in the present study (Khodorova et al., 2013). The initial steps in signaling for this sensory modality differ completely from those for thermal hyperalgesia, mechano-sensitization requiring neurotrophin binding to the p75NTR and activation of nSMase, neither of which is involved in the thermal response. However, elements that occur further downstream may be common to these two modalities. Specifically, C2-ceramide, S1P, and an aPKC are implicated in both mechano- and thermal hypersensitivity by behavioral pharmacological experiments. The first of these, C2-ceramide, may mimic the actions of the endogenous ceramide that is cleaved from sphingomyelin by nSMase activity and that produces hyperexcitability in isolated sensory neurons (Zhang et al., 2002, 2006b) and thermal and mechanical hypersensitivity when injected in vivo (Joseph and Levine, 2004; Doyle et al., 2011a). Although the C2 conjugate of ceramide has been less effective than its longer chain, naturally occurring homologues in some systems (Hashizume et al., 1998; Simon and Gear 1998; Takeda et al.; 2006), its actions on sensory neurons in vitro and in causing nociception in vivo are very much in keeping with its simulation of endogenously produced ceramides (Zhang et al., 2002, 2006b; Doyle et al., 2011a; Joseph and Levine, 2004). C2-Ceramide could thusly simulate p75NTR activation. Along these same lines, interestingly, C2-ceramide can activate TrkA via phosphorylation of receptor tyrosines and thus stimulate that receptor’s signaling pathway (MacPhee and Barker 1999). In the present study, however, we did not examine this possible pathway.
Sphingosine 1-phosphate appears to participate in both acute and chronic pain states. Intraplantar injection of S1P itself causes acute hyperalgesia (Doyle et al, 2011b), apparently through activation of the S1PR1 receptor isoform (Mair et al., 2011) and S1P also mediates, via a GPCR (Zhang et al., 2006a), the acute hyperexcitability of isolated sensory neurons caused by NGF (Zhang et al., 2006b). This latter effect involves S1PR1 and S1PR3 (Li et al., 2015), receptors also implicated both in neurite extension caused by NGF exposure in vitro (Toman et al., 2004) and in chronic neuropathic pain from chemotherapeutics (Janes et al., 2014; also see Patti et al., 2012). Initial hydrolysis by sphingomyelinases of sphingomyelin(s), a phospholipid found in many neuronal plasma membranes (Strichartz, 1977), produces ceramide(s) which is subsequently converted to sphingosine, and then to S1P by the enzyme sphingosine kinase (SphK). Whereas p75NTR activation is positively coupled to nSMase, leading to increased ceramide (substrate) production, SphK enzyme activity is enhanced by TrkA activation (Edsall et al., 1997), so that overall S1P production could be elevated by two effects through the two types of NGF receptor. Furthermore, receptors for S1P are translocated to the plasma membrane and there activated by SphK activity per se (Toman et al., 2004), Therefore, our observation of inhibition of NGF-induced thermal hyperalgesia by a stereo-selective antagonist of S1PR cannot be interpreted as evidence for the sole involvement of TrkA in this hypersensitivity.
Indeed, there is ample evidence that TrkA and p75NTR exist in the same neurons and interact with one another. Studies show co-localization of TrkA and p75NTR immuno-reactivity in various regions of the brain and in spinal cord (Sobreviela et al., 1994), and in sensory neuron cell bodies in the dorsal root ganglion (Wright and Snider, 1995; Averill et al., 1995; Karchewski et al., 1999). The affinity of NGF for TrkA is increased in the presence of p75NTR (Barker and Shooter, 1994; Hempstead et al., 1991), at least in part due to a faster association rate (Mahadeo et al., 1994), implying that the approach to the neurotrophin binding site is altered when the two receptors interact. The high affinity of TrkA for NGF that occurs when p75NTR is co-expressed does not require that the ligand bind to the latter receptor (Huang and Reichardt, 2003). Although naturally occurring proteolytic activity produces fragments of p75NTR that increase TrkA activity (Matusica et al., 2013), ostensibly through direct, steric interactions (Skeldal et al., 2011), Wehrman et al. (2007) have concluded from a structural analysis that TrkA and p75NTR do not directly interact but rather are coupled by the convergence of downstream signaling cascades. Many such conflicting reports leave unsolved the mechanisms of the interaction between the two NGF receptors.
The nature of effects of p75NTR on the physiological actions of TrkA vary among different systems (Huang and Reichardt, 2003; Reichardt, 2006). Whether this variation follows from interactions at a distance through different signaling pathways, or from differences in their direct interactions due to structural motif differences in different cells, is difficult to know (Mendell, 2002). In an extensive review of the neurotrophin signaling literature, Segal (2003) documented that variations of the specific agonists as well as the rate at which these were presented to the receptor and the receptors’ actual location and stoichiometry (cf. Masoudi et al., 2009) would all determine the signaling pathway for neurotrophin receptors. Thus, the rapid increases of excitability of isolated sensory neurons responding to acutely applied NGF could arise from different pathways than the hypersensitivity due to slower changes in the systemic presence of NGF, e.g., from slow release of endogenous peptide (McMahon and Priestley, 1995) or ligand neutralization by exogenous antibodies or fusion proteins (Koltzenburg et al., 1999; Wild et al., 2007). In the present and our previous study (Khodorova et al., 2013), the acute changes in behavioral responses were documented from 0.5 to 3–4 hours after NGF delivery and had pharmacological sensitivities paralleling those of the responses of isolated sensory neurons (Zhang et al., 2002, 2006a). However, longer term changes in thermal sensitivity, which we, like Mills et al. (2013), detected up to 24 h (and in mechano-sensitivity, which Mills et al. (2013) recorded up to 2 weeks after 3 or 5 µg NGF) , might well recruit different, slower mechanisms, including transcriptional and translational steps controlling protein expression.
In the current paper we also identified two downstream effectors for thermal hyperalgesia, an atypical PKC and the transient receptor TRPV1. Atypical PKCs have been hypothesized to be critical for certain forms of memory encoded in the CNS, and the isoform PKMζ has been specifically involved in hippocampal long-term potentiation (Sacktor et al., 1993; Sacktor 2011). This same enzyme is essential for the excitability increased by NGF in isolated sensory neurons (Zhang et al., 2012), and the mechanical hypersensitivity caused by intraplantar NGF (Khodorova et al, 2013) as well as experimental neuropathic pain (King et al. 2012; Laferrier et al., 2011; Marchand et al., 2011; but cf. Price and Ghosh, 2013). Both NGF-induced sensory neuron excitability changes and heightened mechano-sensitivity are mediated by the p75NTR , so it is noteworthy that an aPKC (possibly PKMζ) is also essential for thermal hyperalgesia from NGF.
Both distal and central terminals of nociceptive neurons might be altered by peripheral NGF, respectively, through the acute hyper-responsiveness at the periphery and the slow transport of a TrkA-NGF complex to the DRG (Grimes et al., 1996), where changes in receptor synthesis and excitability also occur (Marlin and Li, 2015), as well as by regulation of neuropeptides that enhance pain perception and are secreted at both ends of the neuron (Skoff and Adler, 2006). The combined evidence suggests that PKMζ activity in both the periphery and the spinal cord contributes to thermal and mechanical hyperalgesia, although the pathways for activation may differ between these two modalities (Ostubo et al., 2012), and between peripheral and central loci (see Lewin et al., 1994).
The other downstream effector, TRPV1, is also common to mechanical and thermal hypersensitivities (this paper; Mills et al., 2013). Small diameter sensory neurons, that express TRPV1 (Winter et al., 1995; Petruska et al., 2002), respond to capsaicin exposure with an inward current that is acutely enhanced after treatment with NGF (Shu and Mendell, 1999, 2001). Such enhancement may result from biochemical modifications of existing TRPV1 molecules in the plasma membrane (Mohapatra et al., 2003; Bonnington and McNaughton, 2003) as well as from the insertion of new receptors into the membrane from cytoplasmic stores (Ji et al., 2002; Zhang et al., 2005). NGF’s modification of TRPV1 is mediated by the pathway intermediate enzyme PI3Kinase (Bonnington and McNaughton, 2003; Zhuang et al., 2004), which is also involved in the electrical excitability increase (Zhang et al., 2012) and the activation of ERK (Zhuang et al., 2004) in isolated sensory neurons, and which phosphorylates phosphatidylinositols that are known to bind to and modify the gating of TRPV1 (Zhu and Oxford, 2007; Ufret-Vicente et al., 2015). This action may be part of a phospholipid signaling system that is a critical local regulator of thermal nociception and hyperalgesia, and of a global pain systems network (Neely et al., 2012). Modulation of TRPV1 might be one of several endpoint physiological changes, including changes in voltage-gated ion channels (Zhang et al., 2002), that enhance nociceptor excitability, increase peripheral nociceptor activity, and drive the elevation of signaling that underlies the heightened pain responses after disease or injury, at least some of which involve the acute, and possibly sustained, responses to NGF.
CONCLUSIONS
In summary, NGF appears to increase the sensitivity of rat skin to thermal and to mechanical sensitivity by separate and linked pathways, respectively triggered by its binding to TrkA and to p75NTR. The initial downstream pathways of these receptors are separate and non-interacting, but further along there are several possibilities for pathway cross-talk. These interactions are modulatory rather than direct, for example, p75NTR alters the duration of thermal hypersensitivity although blocking that receptor does not prevent thermal hyperalgesia. The ability of exogenous C2-ceramide to effect both modes of hypersensitivity could result from that compound’s promiscuous binding and range of activities, unlike selective actions of longer-chain, naturally occurring ceramides, or to its ability to spread to different biochemical pathway “compartments” which would normally be segregated such that soluble ceramides could not pass between them. Near the effector terminae of the pathways, atypical PKCs are essential for the increased sensitivity to heat and to touch. Data on cellular excitability and from other pain studies in vivo suggest that this enzyme is PKMζ, although from the present experiments other aPKCs cannot be ruled out. At the level of cellular responsiveness, both the thermo-sensitive TRPV1 receptor and voltage-gated Na+ and K+ channels in the neuron’s plasma membrane are probably altered by NGF, but whether both of these changes are mediated by PKMζ or are separately modified by different PKCs ( King et al., 2012; Gallegos and Newton, 2008) remains a question.
HIGHLIGHTS.
NGF sensitizes rat paw skin to thermal and to mechanical stimulation by separate pathways
Thermal sensitization is triggered via TrkA and mechanical by p75NTR
More distal downstream segments of these receptors show evidence of cross-modulation
Atypical PKCs, e.g., PKMζ, are essential for sensitization to both heat and to touch
The thermo-sensitive TRPV1 receptor also contributes to acute thermal hypersensitivity
Acknowledgments
This research was supported by NIH grants NINDS/NIH 078173 (to GN) and NCI/NIH CA080153 (to GS). The authors thank Mr. James Bell, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, for assistance with the graphics.
Abbreviations
- aPKC
atypical protein kinase C
- C2-ceramide
N-acetyl-D-sphingosine
- IgG
immunoglobin
- NGF
nerve growth factor
- nSMase
neutral sphingomyelinase
- p75NTR
p75 neurotrophin receptor
- PKMζ
protein kinase M zeta
- PI3K
phosphatidyl inositol 3 kinase
- PWL
paw withdrawal latency
- S1P
sphingosine 1-phosphate
- S1PR
sphingosine 1-phosphate receptor
- SphK
sphingosine kinase
- TrkA
tropomyosin receptor kinase A
- TRPV1
transient receptor potential vanilloid 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amann R, Schuligoi R, Herzeg G, Donnerer J. Intraplantar injection of nerve growth factor into the rat hind paw: local edema and effects on thermal nociceptive threshold. Pain. 1995;64:323–329. doi: 10.1016/0304-3959(95)00120-4. [DOI] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical Localization of trka receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7(7):1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA, Murphy RA. The nerve growth factor receptor: a multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol Cell Biochem. 1992;110:1–15. doi: 10.1007/BF02385000. [DOI] [PubMed] [Google Scholar]
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75lntr reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, Koltzenburg M, Priestley JV, Shelton DL. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J of Neurosci. 1998;10:1282–1291. doi: 10.1046/j.1460-9568.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- Bergmann I, Priestley JV, McMahon SB, Brocker EB, Toyka KV, Koltzenburg M. Analysis of Cutaneous Sensory Neurons in Transgenic Mice Lacking the Low Affinity Neurotrophin Receptor p75. Eur J Neurosci. 1997;9:18–28. doi: 10.1111/j.1460-9568.1997.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Bergmann I, Reiter R, Toyka KV, Koltzenburg M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci Lett. 1998;255:87–90. doi: 10.1016/s0304-3940(98)00713-7. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003 Sep 1;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. Epub 2003 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. NGF and ProNGF: Regulation of Neuronal and Neoplastic Responses through Receptor Signaling. Adv Biol Regul. 2015;58:16–27. doi: 10.1016/j.jbior.2014.11.003. PMCID: PMC4426037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regulatory Integrative Comp Physiol. 292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr Opin Mol Ther. 2010;12:94–106. [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. P75 and trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L. PG110, A humanized Anti-NGF antibody, reverses established Pain Hypersensitivity in Persistent Inflammatory Pain, but not Peripheral Neuropathic Pain, Rat Models. Pain Med. 2016 Feb 25; doi: 10.1093/pm/pnw007. pii: pnw007. [Epub ahead of print] PMID: 26917622. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the Sphingomyelin Cycle Through the Low-Affinity Neurotrophin Receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Muscoli C, Obeid LM, Salvemini D. Intraplantar-injected ceramide in rats induces hyperalgesia through an NF-kappaB- and p38 kinase-dependent cyclooxygenase 2/prostaglandin E2 pathway. FASEB J. 2011a;25:2782–2791. doi: 10.1096/fj.10-178095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Obeid LM, Salvemini D. Sphingosine-1-phosphate acting via the S1P(1) receptor is a downstream signaling pathway in ceramide-induced hyperalgesia. Neurosci Lett. 2011b;499:4–8. doi: 10.1016/j.neulet.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz T, de Bont DB, de Widt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- Finley A, Chen Z, Esposito E, Cuzzocrea S, Sabbadini R, Salvemini D. Sphingosine 1-Phosphate mediates hyperalgesiua via a neutrophil-dependent mechanism. PLoS One. 2013;8:e55255. doi: 10.1371/journal.pone.0055255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Gallegos LL, Newton AC. Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm. IUBMB Life. 2008;60:782–789. doi: 10.1002/iub.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [PubMed: 8987823] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Washington, DC, U.S.A: National Academy Press; 1996. [Google Scholar]
- Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. PMID: 3340425. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Kageura T, Sato T. Different effects of cell-permeable ceramide analogs on platelet activation. Biochem Mol Biol Int. 1998;44:489–496. doi: 10.1080/15216549800201512. PMID: 9556209. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Fitzgerald M. Time course and dose-dependence of nerve growth factor-induced secondary hyperalgesia in the mouse. J Pain. 2006;7:57–61. doi: 10.1016/j.jpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hempstead BI, Martin ZD, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor (see comments) Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ibanez CF, Ebendal T, Barbany G, Murray-Rust J, Blundell TI, Persson H. Disruption of the low affinity receptor -binding site in NGF allows neuronal survival and differentiation by binding to the trk gene product. Cell. 1992;69:329–341. doi: 10.1016/0092-8674(92)90413-7. [DOI] [PubMed] [Google Scholar]
- Janes K, Little JW, Li C, Bryant L, Chen C, Chen Z, Kamocki K, Doyle T, Snider A, Esposito E, Cuzzocrea S, Bieberich E, Obeidi L, Petrache I, Nicol G, Neumann WL, Salvemini D. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J Biol Chem. 2014;289:21082–21097. doi: 10.1074/jbc.M114.569574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci. 2004;20:2896–2902. doi: 10.1111/j.1460-9568.2004.03750.x. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zance D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Kim FA, Johnston J, McKnight RM, Verge VM. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J Comp Neurol. 1999;413:327–341. doi: 10.1002/(sici)1096-9861(19991018)413:2<327::aid-cne11>3.0.co;2-3. PMID: 10524342. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Nicol GD, Strichartz GR. The p75NTR signaling cascade mediates mechanical hyperalgesia induced by nerve growth factor injected into the rat hind paw. Neuroscience. 2013;254:312–323. doi: 10.1016/j.neuroscience.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Qu C, Okunn A, Melemedjian OK, Mandell EK, Maskaykina IY, Navratilova E, Dussor GO, Ghosh S, Price TJ, Porreca F. Contribution of PKMζ-dependent and independent amplification to components of experimental neuropathic pain. Pain. 2012;153:1263–1273. doi: 10.1016/j.pain.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59(6):1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous ngf prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11(5):1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Krotova K, Hu H, Xia SL, Belayev L, Patel JM, Block ER, Zharikov S. Peptides modified by myristoylation activate eNOS in edothelial cells through Akt phosphorylation. Brit J Pharm. 148:732–740. doi: 10.1038/sj.bjp.0706777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferriere A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. PKMzeta is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain. 2011;7:99. doi: 10.1186/1744-8069-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Nykjaer A. Pro-neurotrophins, sortilin, and nociception. Eur J Neurosci. 2014;39:363–374. doi: 10.1111/ejn.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6(12):1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Li C, Li J, Kays J, Guerrero M, Nicol GD. Sphingosine 1-phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1-phosphate receptors 1 and/or 3. J Neuroinflamm. 2015;12:70. doi: 10.1186/s12974-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubí-Robles M, Quarta S, Biasio W, Kuner R, Gibbins IL, Kress M, Haberberger RV. Genetic evidence for involvement of neuronally expressed S1P1 receptor in nociceptor sensitization and inflammatory pain. PLoS One. 2011 Feb 17;6(2):e17268. doi: 10.1371/journal.pone.0017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee I, Barker PA. Extended ceramide exposure activates the TrkA receptor by increasing receptor homodimer formation. J Neurochem. 1999;72:1423–1430. doi: 10.1046/j.1471-4159.1999.721423.x. [DOI] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao MV, Hempstead BI. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257. doi: 10.1016/bs.ircmb.2014.10.002. 2015 Epub 2014 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of Nerve Growth Factor-TrkA Signaling and the Relief of Pain. Anesthesiol. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, D’Mello R, Yip PK, Calvo M, Muller E, Pezet S, Dickenson AH, McMahon SB. Specific involvement of atypical PKCzeta/PKMzeta in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain. 2011;7:86. doi: 10.1186/1744-8069-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- Masoudi R, Ioannou MS, Coughlin MD, Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D, Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem. 284(27):18424–18433. doi: 10.1074/jbc.M109.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusica D, Skeldal S, Sykes AM, Palstra N, Sharma A, Coulson EJ. An intracellular domain fragment of the p75 neurotrophin receptor (p75 ntr) enhances tropomyosin receptor kinase a (trka) receptor function. J Biol Chem. 2013;288:11144–11154. doi: 10.1074/jbc.M112.436469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Long LH, Phillips HS. Expression and coexpression of trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. PMID: 7514427. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Priestley JV. Peripheral neuropathies and neurotrophic factors: animal models and clinical perspectives. Current Opinion in Neurobiology. 1995;5:616–624. doi: 10.1016/0959-4388(95)80067-0. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Bennett DLH, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trka-igg fusion molecule. Nature Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Does NGF binding to p75 and trkA receptors activate independent signalling pathways to sensitize nociceptors? J Physiol (London) 2002;544:333. doi: 10.1113/jphysiol.2002.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain. 2013;17:469–479. doi: 10.1002/j.1532-2149.2012.00202.x. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Wang SY, Wang GK, Nau C. a tyrosine residue in tm6 of the vanilloid receptor trpv1 involved in desensitization and calcium permeability of capsaicin-activiated currents. Mol Cell Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Neely GG, Rao S, Costigan M, Mair N, Racz I, Milinkeviciute G, Meixner A, Nayanala S, Griffin RS, Belfer I, Dai F, Smith S, Diatchenko L, Marengo S, Haubner BJ, Novatchkova M, Gibson D, Maixner W, Pospislik A, Hirsch E, Wishaw IQ, Zimmer A, Gupta V, Sasaki J, Kanaho Y, Sasaki T, Kress M, Woolf CJ, Penninger JM. Construction of a global pain systems network highlights phospholipid signaling as a regulator of heat nociception. PLOS Genetics. 2012;8(12):e1003071. doi: 10.1371/journal.pgen.1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini S, D’Alessandro R, Meldolesi J. Ngf signaling in pc12 cells: the cooperation of p75ntr with trka is needed for the activation of both mtorc2 and the pi3k signaling cascade. Biol Open. 2013;2:855–866. doi: 10.1242/bio.20135116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Lipid activation of protein kinases. J Lipid Res. 2009;50(Suppl):S266–S271. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7:26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- Ostubo Y, Satoh Y, Kodama M, Araki Y, Satomoto M, Sakamoto E, Pagès G, Pouysségur J, Endo S, Kazama T. Mechanical allodynia but not thermal hyperalgesia is impaired in mice deficient for ERK2 in the central nervous system. Pain. 2012;153:2241–2252. doi: 10.1016/j.pain.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, Shriver LP, Courade JP, Tautenhahn R, Manchester M, Siuzdak G. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nature Chem Biol. 2012;8:232–234. doi: 10.1038/nchembio.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC1, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neurosci. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. 2002. [DOI] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Price TJ, Ghosh S. ZIPping to pain relief: the role (or not) of PKMζ in chronic pain. Mol Pain. 2013;9:6. doi: 10.1186/1744-8069-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Phil Trans R Soc B. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces noninflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U.S.A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006 Aug 2;(8):434–441. doi: 10.1038/nchembio804. Epub 2006 Jul 9 PubMed PMID: 16829954. [DOI] [PubMed] [Google Scholar]
- Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2015;74:1202–1211. doi: 10.1136/annrheumdis-2013-204905. Epub 2014 Mar 13.PMID: 24625625. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: Theme and variations. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shelton D. Development of nerve growth factor (NGF) Inhibition as a strategy for treatment of pain. Journal of the Peripheral Nervous System. 2014;19:S10–S14. doi: 10.1111/jns.12080_3. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, Woodruff TM, Clark JD, Usachev YM. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci. 2016;4(36):5055–5070. doi: 10.1523/JNEUROSCI.3249-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CG, Jr, Gear AR. Membrane-destabilizing properties of C2-ceramide may be responsible for its ability to inhibit platelet aggregation. Biochemistry. 1998 Feb 17;37(7):2059–2069. doi: 10.1021/bi9710636. PMID: 9485333. [DOI] [PubMed] [Google Scholar]
- Skeldal S, Matusica D, Nykjaer A, Coulson EJ. Proteolytic processing of the p75 neurotrophin receptor: a prerequisite for signaling? Bioessays. 2011;33:614–625. doi: 10.1002/bies.201100036. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430–436. doi: 10.1016/j.expneurol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. Trka-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350(4):587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinse C-zeta is a downstream effector of phosphatidyl-3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- Strichartz GR. The Composition and Structure of Excitable Nerve Membrane. In: Jamieson GA, Robinson DM, editors. Mammalian Cell Membranes. Vol. 3. London: Butterworths; 1977. pp. 172–205. [Google Scholar]
- Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Takeda S, Mitsutake S, Tsuji K, Igarashi Y. Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J Biochem. 2006;139:255–262. doi: 10.1093/jb/mvj026. PMID: 16452313. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. PMID: 1312698. [PubMed] [Google Scholar]
- Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166(3):381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty CA, Klein RM, Collins MD, Rosasco MG, Martinez GQ, Gordon SE. Mechanism for phosphoinositide selectivity and activation of TRPV1 ion channels. J Gen Physiol. 2015;145:431–442. doi: 10.1085/jgp.201511354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Ito T, Inoue G, Ohtori S, Kitajo K, Doya H, Takahashi K, Yamashita T. The p75 Receptor Is Associated With Inflammatory Thermal Hypersensitivity. J NeuroSci Res. 2008;86:3566–3574. doi: 10.1002/jnr.21808. [DOI] [PubMed] [Google Scholar]
- Watson JJ, Fahey MS, Van den worm E, Engels F, Nijkamp FP, Stroemer P, McMahon S, Allen SJ, Dawbarn D. Trkad5: a novel therapeutic agent for treatment of inflammatory pain and asthma. J Pharmacol Exp Ther. 2006;316:1122–1129. doi: 10.1124/jpet.105.095844. [DOI] [PubMed] [Google Scholar]
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the trka and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt L. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nersve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral Cell Types Contributing to the Hyperalgesic Action of Nerve Growth Factor in Inflammation. J Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mrna expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol. 2006b;96:1042–1052. doi: 10.1152/jn.00120.2006. PMID: 16723416. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Kays J, Hodgdon KE, Sacktor TC, Nicol GD. Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform, PKMζ. J Neurophysiol. 2012;107:315–335. doi: 10.1152/jn.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–192. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006a;575:101–113. doi: 10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-Kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]