The heavy light chain (HLC) immunoassay quantifies light chain types of each immunoglobulin (Ig), enabling the calculation of ratios of HLC pairs (Katzmann and Rajkumar 2013). This assay measures the light chain types of each Ig (G/A/K), generating ratios of monoclonal Ig/background polyclonal Ig concentrations as paired ratios. It has shown clinical utility in multiple myeloma (MM), particularly for recognition of small M-proteins that migrate in the beta or gamma fraction among polyclonal immunoglobulins, for monitoring of minimal residual disease and as a predictor of progression of monoclonal gammopathy of undetermined significance to MM (Boyle, et al 2014, Bradwell, et al 2013, Katzmann, et al 2013, Ludwig, et al 2013). Additionally, the HLC assay may be a surrogate marker of normal plasma cell recovery after transplant (Tovar, et al 2012). We report the results of a correlative study testing the additional prognostic role of a normalized HLC and free light chain (FLC) tests over conventional electrophoretic response in predicting outcomes done on archived samples of patients enrolled in Blood Marrow Transplant Clinical Trials Network (BMTCTN) 0102, a randomized phase 3 multicentre trial aimed at determining the role of tandem autologous or allogeneic second transplant after upfront autologous haematopoietic cell transplant (HCT) in MM (Krishnan, et al 2011).
Eligible patients were biologically assigned to receive a tandem allogeneic (alloHCT) or a second autologous (autoHCT) transplant, based on availability of a human leucocyte antigen (HLA)-matched sibling donor. The tandem autoHCT group was re-randomized to receive 1 year of maintenance therapy with thalidomide plus dexamethasone vs. observation. De-identified, archived cryopreserved serum samples collected prior to first transplant, day 56 and 1 year post-second transplant banked at the National Heart Lung and Blood Institute (NHLBI) sample biorepository were used in this study using an Institutional Review Board-approved protocol. Samples were processed at The Binding Site, Birmingham, UK. Testing was performed using a Siemens BNII nephelometer for free kappa and free lambda (FLC Freelite® kits), IgGκ and IgGλ, IgAκ and IgAλ, IgMκ and IgMλ (HLC IgG, IgA and IgM Hevylite® kits). The normal ranges for ratios of the FLC and HLC assays were determined by analysis of serum samples from 146 normal controls and were: FLC κ/λ 0.26–1.65; HLC IgG κ/λ 0.98–2.75; HLC IgA κ/λ 0.8–2.04 and IgM κ/λ 0.96–2.3. Twenty-eight samples could not be analysed due to inadequate sample collection; 1 patient with disease progression at study entry (auto-auto arm) was excluded; 490 samples were analysed.
The International Uniform Response Criteria (Durie, et al 2006) was used to define disease response with the addition of near complete response (CR), which was defined as evidence of disease by immunofixation electrophoresis without morphological evidence of MM in bone marrow.(Krishnan, et al 2011) Normalization of HLC ratios across all 3 measured heavy/light chain pairs or normal clonal isotype with normal ratios of uninvolved pairs was considered HLC-R while normalization of the FLC ratio was called FLC remission (FLC-R). The HLC result of any HLC pair missing for a given time was disregarded, thus decreasing the number of interpretable HLC compared to FLC ratios (Table I).
Table I.
Characteristics of study patients and results of HLC and FLC testing
| AutoHCT N (%) (n=331) |
AlloHCT N (%) (n=166) |
P- value | |
|---|---|---|---|
|
| |||
| Male | 207 (63) | 94 (57) | 0·2 |
|
| |||
| Median age, years (range) | 56 (23–70) | 51 (29–68) | <0·001 |
|
| |||
| Race | 0·03 | ||
| African American | 43 (13) | 9 (5) | |
| Caucasian | 246 (74) | 136 (82) | |
| Other | 42 (13) | 21 (13) | |
|
| |||
| Karnofsky score ≥ 90 | 253 (76) | 129 (78) | 0·6 |
|
| |||
| β-2 Microglobulin, median | 2.1 | 2.0 | 0·9 |
|
| |||
| Durie-Salmon | 0·3 | ||
| Stage I–II | 100 (30) | 40 (24) | |
| Stage III | 231 (70) | 126 (76) | |
|
| |||
| 0102 Risk Status* | 0.01 | ||
| High | 34 (10) | 30 (18) | |
| Standard | 297 (90) | 136 (82) | |
|
| |||
| Disease Status at Transplant | 0·2 | ||
| CR | 33 (10) | 23 (14) | |
| Near CR | 50 (15) | 15 (9) | |
| VGPR | 54 (16) | 36 (22) | |
| PR | 125 (38) | 64 (38) | |
| MR | 24 (7) | 13 (8) | |
| SD | 17 (5) | 7 (4) | |
| Not Evaluable | 22 (7) | 5(3) | |
| Unknown | 6(2) | 3(2) | |
|
| |||
| Treatment arm in auto-auto | – | ||
| Thalidomide/Dexamethasone | 169 (51) | – | |
| Observation | 162 (49) | – | |
|
| |||
| No second transplant | 58 (17) | 31 (19) | 0.8 |
|
| |||
| Median follow-up of survivors from 1st transplant | 87 (7–105) | 81 (15–105) | 0.7 |
|
| |||
| FLC-R pre-1st transplant, total N=490 | 105 (32) | 49 (29) | 0.6 |
|
| |||
| HLC-R pre-1st transplant, total N=4851 | 211(67) | 111 (64) | 0.5 |
-High risk defined as beta2 microglobulin >4 mg/dl and deletion 13 by standard karyotype
HLC results are a composite of all heavy and light chain combinations with normal or low concentration plus any normal heavy chain kappa/lambda ratios if any result is missing it will decrease the total N.
CR, complete response; VGPR, very good partial response; PR, partial response MR minimal response; SD, stable disease; FLC -R, free light chain remission (i.e., FLC ratio normalization); HLC-R, heavy light chain remission (i.e. HLC ratio normalization)
Sensitivity and specificity of FLC-R and HLC-R were calculated compared to electrophoretic response. Progression-free survival (PFS) was defined from first registration to the first observation of progression. Multivariate models were used to identify baseline variables impacting PFS and overall survival (OS) after transplant and adjusted models were generated to identify the prognostic value of pre-transplant HLC-R and FLC-R for those in electrophoretic CR or very good partial response (VGPR). Model building was performed by Cox regression and survival was estimated by the Kaplan-Meier method. Statistical analyses were performed using SAS v 9 (SAS Institute, Cary, NC).
Table I shows the baseline characteristics and test results. The alloHCT group was younger (median age at diagnosis 51 years versus 56 years, p <0.001), more frequently white (82% versus 74%, p 0.03) and with high-risk disease (18% versus 10%, p 0.01) compared to the autoHCT group, similar to the demographics reported in the parent study.(Krishnan, et al 2011) The median follow-up was similar in both groups.
HLC-R had 100% sensitivity and 100% negative predictive value for identifying electrophoretic CR and non-CR respectively, i.e. a normal HLC result was always concordant with electrophoretic CR while an abnormal HLC result was always concordant with electrophoretic non-CR. Further, HLC-R had high sensitivity (89%) and low specificity (52%) to distinguish ≥VGPR from <VGPR, whereas FLC-R showed high specificity (81%) with low sensitivity (49%) between these groups.
Only 23 patients achieving ≥VGPR had HLC-NR, thus restricting our ability to identify differences in outcomes. While HLC response did not help separate patients with a VGPR or better (Fig 1A), those patients with pre-transplant <VGPR (partial response [PR] or stable disease [SD]) status and HLC-R had better outcomes than those without HLC-R (p 0.03; Fig 1B). It is known that patients with pre-transplant electrophoretic ≥VGPR have good post-transplant outcomes overall (Attal, et al 1996), thus it is not surprising that the HLC had no additional benefit among these patients. Ludwig et al (2013) showed similar results, with improvements in survival among a small group of patients stratified by HLC ratio, with benefit seen particularly in the group with PR rather than ≥VGPR.
Figure 1.
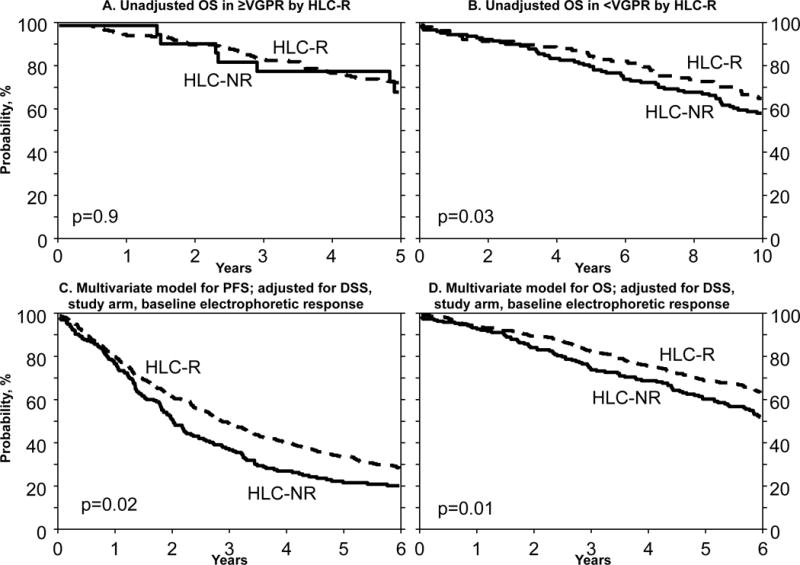
Outcomes stratified by baseline HLC response.
A. Pre-transplant HLC response among patients with electrophoretic VGPR or better (p-value 0.9)
B. Pre-transplant HLC response among patients with electrophoretic PR or worse (P-value 0.03)
C. Multivariate model for progression-free survival (P-value 0.02)
D. Multivariate model for overall survival (P-value 0.01)
HLC, heavy light chain; HLC-R, HLC remission; HLC-NR. HLC non-remission; OS, overall survival; PFS, progression-free survival; VGPR, very good partial response; PR, partial response; DSS, Durie-Salmon Stage.
Multivariate analysis associated patients with ≥VGPR response prior to first transplant and Durie-Salmon stage (DSS) I/II versus III with superior PFS and OS. After adjusting for electrophoretic response, DSS and study arm (autoHCT versus alloHCT), HLC-R was an independent predictor of superior PFS [hazard ratio (HR) 0.75, 95% confidence interval (CI) 0.59–0.95, p 0.016;Fig 1C], freedom from relapse (HR 0.77, 95% CI 0.6–0.98, p 0.035) and OS (HR 0.69, 95% CI 0.51–0.92, p 0.012; Fig 1D]. No similar prognostic value was seen by incorporating FLC-R into the model, as also recently shown by Martinez-Lopez, et al (2015).
In conclusion, we show that HLC-R is 100% concordant to an electrophoretic CR, but an HLC-R can be achieved without establishment of an electrophoretic CR. The HLC-R status identifies a good prognostic risk group among patients who have not achieved a VGPR or better prior to transplant. For those in an electrophoretic CR, an FLC-R was not additionally prognostic. Our results indicate that HLC-R could be integrated into other response levels to further stratify patients not achieving CR/VGPR.
Acknowledgments
Funding for this study was provided by The Binding Site, in support of BMT CTN Protocol 0201 which was funded by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, the Department of the Navy, Office of Naval Research and the National Marrow Donor Program. Any views, opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not reflect the views or the official policy or position of the above mentioned parties.
Footnotes
This work was presented in part as a poster at the 20th Congress of the European Haematology Association, Vienna, Austria, 2015.
Author Contributions: All authors designed the study, collected and analysed the data, and wrote the manuscript; BL performed the statistical analysis and edited the article. All authors critically revised the manuscript for important intellectual content and approved the final draft.
Disclosure of conflicts of interest: The authors have no conflicts of interest to report.
References
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- Boyle EM, Fouquet G, Guidez S, Bonnet S, Demarquette H, Dulery R, Herbaux C, Noel MP, Manier S, Schraen S, Onraed B, Faucompre JL, Hennache B, Petillon MO, Mathiot C, Avet-Loiseau H, Facon T, Harding SJ, Moreau P, Leleu X. IgA kappa/IgA lambda heavy/light chain assessment in the management of patients with IgA myeloma. Cancer. 2014;120:3952–3957. doi: 10.1002/cncr.28946. [DOI] [PubMed] [Google Scholar]
- Bradwell A, Harding S, Fourrier N, Mathiot C, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Prognostic utility of intact immunoglobulin Ig‘kappa/Ig’lambda ratios in multiple myeloma patients. Leukemia. 2013;27:202–207. doi: 10.1038/leu.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV, for the International Myeloma Working Group International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Katzmann JA, Rajkumar SV. A window into immunoglobulin quantitation and plasma cell disease: antigen epitopes defined by the junction of immunoglobulin heavy and light chains. Leukemia. 2013;27:1–2. doi: 10.1038/leu.2012.201. [DOI] [PubMed] [Google Scholar]
- Katzmann JA, Clark R, Kyle RA, Larson DR, Therneau TM, Melton LJ, 3rd, Benson JT, Colby CL, Dispenzieri A, Landgren O, Kumar S, Bradwell AR, Cerhan JR, Rajkumar SV. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia. 2013;27:208–212. doi: 10.1038/leu.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, Antin JH, Comenzo R, Goodman S, Hari P, Laport G, Qazilbash MH, Rowley S, Sahebi F, Somlo G, Vogl DT, Weisdorf D, Ewell M, Wu J, Geller NL, Horowitz MM, Giralt S, Maloney DG, for the Blood Marrow Transplant Clinical Trials Network Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Milosavljevic D, Zojer N, Faint JM, Bradwell AR, Hubl W, Harding SJ. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia. 2013;27:213–219. doi: 10.1038/leu.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez J, Paiva B, Lopez-Anglada L, Mateos MV, Cedena T, Vidriales MB, Saez-Gomez MA, Contreras T, Oriol A, Rapado I, Teruel AI, Cordon L, Blanchard MJ, Bengoechea E, Palomera L, de Arriba F, Cueto-Felgueroso C, Orfao A, Blade J, San Miguel JF, Lahuerta JJ, Grupo Espanol de Mieloma Multiple/Programa para el Estudio de la Terapeutica en Hemopatias Malignas Cooperative Study, G Critical analysis of the stringent complete response in multiple myeloma: contribution of sFLC and bone marrow clonality. Blood. 2015;126:858–862. doi: 10.1182/blood-2015-04-638742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar N, Fernandez de Larrea C, Elena M, Cibeira MT, Arostegui JI, Rosinol L, Filella X, Yague J, Blade J. Prognostic impact of serum immunoglobulin heavy/light chain ratio in patients with multiple myeloma in complete remission after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1076–1079. doi: 10.1016/j.bbmt.2012.03.004. [DOI] [PubMed] [Google Scholar]


