Abstract
Objective
Both bipolar spectrum disorders (BPSD) and attention deficit hyperactivity disorder (ADHD) present with emotion-regulation deficits, but require different clinical management. We examined how the neurobiological underpinnings of emotion regulation might differentiate youth with BPSD versus ADHD (and healthy controls, HCs), specifically assessing functional connectivity (FxC) of amygdala-prefrontal circuitry during an implicit emotion processing task.
Methods
We scanned a subset of the Longitudinal Assessment of Manic Symptoms (LAMS) sample, a clinically recruited cohort with elevated behavioral and emotional dysregulation, and age/sex-ratio matched HCs. Our sample consisted of 22 youth with BPSD, 30 youth with ADHD/no BPSD, and 26 HCs. We used generalized psychophysiological interaction (gPPI) to calculate group differences to emerging emotional faces vs. morphing shapes in FxC between bilateral amygdala and ventral prefrontal cortex/anterior cingulate cortex.
Results
FxC between amygdala and left ventrolateral prefrontal cortex (VLPFC) in response to emotions vs. shapes differed by group (p=.05): while BPSD showed positive FxC (emotions>shapes), HC and ADHD showed inverse FxC (emotions<shapes). A group × emotion interaction was found in amygdala-subgenual cingulate FxC (p=.025), explained by differences in FxC in response to negative emotions. While BPSD showed positive FxC, HC showed inverse FxC; ADHD were intermediate. Amygdala-subgenual FxC was also positively associated with depressive symptoms and stimulant medication.
Limitations
Co-morbidity and relatively small sample size.
Conclusions
Youth with BPSD showed abnormally positive FxC between amygdala and regions in the ventral prefrontal cortex during emotion processing. In particular, the amygdala-VLPFC finding was specific to BPSD, and not influenced by other diagnoses or medications.
Keywords: bipolar disorder, neuroimaging, fMRI, implicit emotion processing
INTRODUCTION
Bipolar spectrum disorder (BPSD) and attention deficit hyperactivity disorder (ADHD) often present with overlapping symptomatology (Bowring and Kovacs, 1992; Klein et al., 1998; Leibenluft, 2011). While symptoms are considered to be episodic in BPSD (American Psychiatric Association, 2013; Youngstrom et al., 2008a) and more persistent in ADHD, this distinction is often not so clear-cut. For example, mood lability is quite prominent in the presentation of BPSD (even during euthymia) and is also a hallmark of ADHD, especially when depression is comorbid (Stringaris and Goodman, 2009). Conversely, children with co-morbid ADHD and depression might have episodic periods of low mood and decreased energy, punctuated by periods of elevated mood and hyperactivity (when the depression has remitted and the ADHD symptoms are more obvious) (Youngstrom et al., 2010). Comparing emotion regulation circuitry in BPSD versus ADHD might shed light on the distinct neural underpinnings of superficially similar symptoms, and ultimately inform diagnosis and treatment of these impairing disorders.
Previous neuroimaging work indicates that both BPSD and ADHD are associated with abnormalities in emotion processing networks. A recent critical review of neuroimaging findings in BPSD indicates that the disorder is associated with abnormalities in emotion regulation circuitry (particularly ventrolateral prefrontal cortex; VLPFC), along with abnormal heightened reward-related activity in left orbitofrontal cortex and associated areas (Phillips and Swartz, 2014). Weaker inverse functional connectivity (FxC) between amygdala and prefrontal regions, particularly VLPFC, has been found in adults with BPSD (Townsend et al., 2013). Abnormalities in this circuitry have also been found in youth at risk for BPSD; specifically, at-risk youth (vs. healthy controls) showed weaker inverse/more positive amygdala-VLPFC FxC in response to an implicit emotion processing task (Manelis et al., 2015), but more inverse/weaker positive amygdala-VLPFC FxC during an emotional working memory task (Ladouceur et al., 2013). Similar pathways are thought to be abnormal in ADHD, including reward circuitry, subcortical hyperactivation, and emotion regulation circuitry (Shaw et al., 2014). Task-related FxC between amygdala and VLPFC has also been found to be more positive in ADHD youth vs. healthy controls (HC) (Posner et al., 2011). This overlap in circuitry abnormalities is not surprising, given extensive comorbidity and overlap in symptomatology, particularly difficulties in emotion regulation, across disorder (Shaw et al., 2014). Limited studies comparing blood oxygen level dependent (BOLD) signal in BPSD vs. ADHD have indicated some shared abnormalities (esp. dorsolateral prefrontal cortex; DLPFC) but differences in VLPFC activation in response to an emotional Stroop task (Passarotti et al., 2010).
An important and unanswered question is how the FxC of emotion regulation circuitry differs between youth with primary BPSD vs. ADHD (and no BPSD). The identification of specific abnormalities might shed light on the differing underlying pathophysiology of these disorders, which can sometimes present with superficially similar symptoms. In a previous study using an overlapping sample, we assessed differences in BOLD signal across diagnostic group, and found that youth with BPSD showed less activation in the right VLPFC relative to both clinical controls (primarily with ADHD) and HC (Hafeman et al., 2014). In the current study, we aimed to build on this work by assessing changes in FxC between bilateral amygdala and ventral prefrontal regions in response to an implicit emotion processing task, and how these changes differed across diagnostic group (primary BPSD vs. ADHD/no BPSD vs. HC). Based on findings from a different study of at-risk adolescents that used the same task (Manelis et al., 2015), we hypothesized that those with BPSD (regardless of co-morbidity) would show abnormalities in task-related FxC between amygdala and both VLPFC and ACC, which would be only partially shared by youth with ADHD (and no BPSD).
METHODS
Participants
Participants in the Longitudinal Assessment of Manic Symptoms (LAMS) cohort (Findling et al., 2010; Horwitz et al., 2010) (n=685) were recruited from four sites without regard to diagnostic category, selected based on degree of emotional dysregulation (as measured by the Parent General Behavioral Inventory-10M; PGBI-10M) (Youngstrom et al., 2008b). Follow-up is ongoing for over 72% of participants, with biannual assessments of clinical symptoms, diagnoses and functional impairments. A subset of the LAMS cohort (n=130) was recruited from three sites (Case Western Reserve University, Cincinnati Children’s Hospital, and University of Pittsburgh Medical Center) to participate in the neuroimaging component of the second funding period of the LAMS study. Additionally, 32 age- and gender-matched HCs were scanned for comparison. HCs (8–16 years old) were age- and sex-ratio matched to the LAMS participants, and were recruited from all three sites. Informed consent was obtained from parents or guardians, and youth provided written informed assent. Participants received monetary compensation and a framed structural brain image. Exclusion criteria are described in eMethods.
Forty-seven LAMS youth and four HC were excluded due to incomplete scan, data loss, excessive head movement over the entire task (>4mm), elevated change in BOLD signal from volume to volume (>3 SDs above the mean; reflecting sudden movement) (Power et al., 2012), and/or visible artifacts in scan data. We additionally excluded youth with a task response rate of less than 80% (25 LAMS youth and two HC); given the lack of response, these youth might not have been attending to emotional stimuli, and may have fallen asleep during the scan. LAMS youth were more likely to be excluded than HC (t=13.80, p=.0002). Compared with included LAMS youth, those excluded were younger (p=.008) with lower IQ (p=.002), and had higher scores on the Screen for Childhood Anxiety Related Emotional Disorders (SCARED) (p=.004); they were also more likely to have conduct disorder (p=.04). Proportion of youth excluded also differed significantly across sites (p=.003) (eTable 1).
Assessment
Baseline assessments gathered demographic data including age, sex, and IQ. Diagnoses were determined at baseline and every six months using the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Life Version with the WASH-U mood supplement (K-SADS-PL-W) (Kaufman et al., 1997). Lifetime history of anxiety, mood, and behavioral disorders (up to scanning day) were used in this analysis. Based on previous work that bipolar-disorder-I/II (BD-I/II) and BD-not-otherwise-specified (BD-NOS) youth do not differ significantly on clinical variables or psychosocial functioning (Axelson et al., 2006; Hafeman et al., 2013), we combined these disorders and examined bipolar spectrum disorder (BPSD) as a group. For this analysis, we defined the following groups: (1) primary BPSD (including youth with co-morbidity; n=22); (2) ADHD (n=30); and (3) HC (n=26). Six youth in the clinical sample had never been diagnosed with either ADHD or BPSD, and were excluded from group analyses.
On the scanning day, the youth and a parent/guardian completed multiple clinical rating scales, including the SCARED (measuring anxiety symptoms) (Birmaher et al., 1999), Mood and Feelings Questionnaire (MFQ; measuring depressive symptoms) (Daviss et al., 2006), and the Child Affective Lability Scale (CALS; measuring emotional lability) (Gerson et al., 1996). Additionally, trained clinicians administered the K-SADS Mania Rating Scale (KMRS) (Axelson et al., 2003) and Depression Rating Scale (KDRS) (Kaufman et al., 1997) to assess for hypomanic/manic and depressive symptom severity, respectively. Interviewers determined summary scores based on all available information, including parent and youth report. Four participants did not have all data available on scanning day; data within one month of scanning day was utilized. Within six months of scanning day (at a regular LAMS visit), parents also completed a short questionnaire regarding behavioral and emotional dysregulation in their children (PGBI-10).
Dynamic Faces Paradigm
A block-design emotional dynamic faces task evaluated implicit processing of emotional stimuli (eMethods). During active blocks, participants watched a series of faces that morphed from neutral to full expression of emotion (happy, sad, fearful or angry) in one second. During control blocks, a luminance-equated shape morphed into a larger shape. Participants were instructed to identify the foreground color using a button press; thus the emotional stimuli were not relevant to task. This task robustly activates the amygdala and prefrontal emotion processing circuitry (Fournier et al., 2013; Hafeman et al., 2014; Herringa et al., 2013; Keener et al., 2012).
Neuroimaging Analysis
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM8 http://www.fil.ion.ucl.ac.uk/spm) (eMethods). We used generalized psychophysiological interaction (gPPI) (McLaren et al., 2012) to assess FxC in response to all faces vs. shapes (EMO-SHAPE), positive emotional faces (happy) vs. shapes (POS-SHAPE) and to negative emotional faces (sad, angry, fearful) vs. shapes (NEG-SHAPE). gPPI facilitates the identification of regions that change connectivity to an identified seed region in response to task. It is more efficient than standard PPI methods when there are more than two task conditions (McLaren et al., 2012) and for block designs (Cisler et al., 2014). Details regarding preprocessing and first-level analysis are described in eMethods.
Primary analyses focused on FxC between an anatomically-defined, bilateral amygdala seed and a region of interest (ROI) that included bilateral ventrolateral prefrontal cortex (VLPFC: BA 47) and anterior cingulate cortex (ACC: BA 25/24/32); this ROI was chosen based on previous FxC findings in youth at familial risk for BPSD using this task (Manelis et al., 2015). We first assessed group differences in FxC (between bilateral amygdala and ROI) to all emotions vs. shapes (EMO-SHAPES), using a one-way ANOVA. We then assessed group × emotion interactions, using a 3 (Group: BPSD/ADHD/HC) × 2 (NEG-SHAPES vs. POS-SHAPES) ANOVA, and assessed for clusters within the ROI where amygdala FxC differed according to group × emotion condition. All models were adjusted for age, sex, site, and task accuracy. Clusters showing significant group differences [voxel-wise p<.001, with peak voxel family wise error (FWE)-corrected p<.05 within ROI] were extracted to assess direction of effect and post-hoc individual two-group comparisons. Using multiple regression, we also assessed the effect of dimensional measures of anxiety (parent/child SCARED), depression (parent/child MFQ, KDRS), mood lability (parent/child CALS), and manic symptoms (KMRS) on FxC between amygdala and the VLPFC/ACC ROI.
To address the impact of other potential confounds and dimensions, we used LASSO regression (implemented in SAS 9.4.1) to assess demographic and clinical predictors of mean connectivity within extracted clusters. LASSO is a penalized regression that “shrinks” coefficients below a certain threshold to zero, thus eliminating them from the model, and avoiding highly correlated predictors; this is a more sophisticated alternative to model selection procedures such as stepwise regression, allowing the assessment of a large number of variables (Tibshirani, 1996). Using the LASSO model, we entered in the group variable, performance variables (response rate, accuracy, mean reaction time), IQ, dimensional measures of symptoms, other diagnoses [oppositional defiant disorder (ODD), anxiety, and depression], and psychiatric medication (dichotomized). Next, we entered all selected variables into general linear models (PROC GLM in SAS 9.41) to examine relationships between these variables and mean connectivity within extracted regions.
To further address the effect of heterogeneity within groups, we conducted sensitivity analyses on the extracted clusters to test whether results persisted even after sequentially excluding youth with BD NOS, those with elevated depressive symptoms (KDRS>10) or manic symptoms (KMRS>10), medicated youth and those with both BPSD and ADHD; we also tested whether findings remained within each two-site subgroup. To determine if effects of group on FxC differed depending on levels of anxiety, depression, affective lability, or manic symptoms, we tested interactions between dimensions and group in the extracted clusters.
Finally, we conducted exploratory whole brain analyses. First, a one-group t-test was used to identify regions where amygdala FxC was significantly different to emotional faces versus shapes (EMO-SHAPES). Second, we used a three-group one-way ANOVA and post-hoc two-group comparisons (BPSD vs. HC, BPSD vs. ADHD, ADHD vs. HC) to assess which regions differed according to amygdala FxC (to EMO-SHAPES) between groups. A bilateral amygdala seed was used because we did not have strong a prior hypotheses about laterality; we also conducted analyses using right and left amygdala seeds separately, to explore whether lateralized amygdala FxC differed across group.
Standard measures addressed biases that may arise in multisite neuroimaging studies. As recommended by the Biomedical Informatics Research Network (BIRN; http://www.nbirn.net), a BIRN phantom was utilized monthly at all three sites to ensure longitudinal scanner signal stability, and all analyses were co-varied for scan site.
RESULTS
Sample Characteristics (Table 1)
Table 1.
Sample characteristics according to group
| BPSD (n=22) | ADHD (n=30) | HC (n=26) | Stat | p | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 14.1 (+1.9) | 14.1 (+1.8) | 13.2 (+2.2) | F=1.99 | .14 |
| Sex (% female) | 8 (36.4%) | 10 (33.3%) | 14 (53.8%) | X2=2.70 | .26 |
| IQ | 105 (+15) | 104 (+19) | 105 (+12) | F=0.04 | .97 |
| Performance | |||||
| Response Rate (%) | 97.7 (2.6) | 98.3 (2.4) | 98.5 (2.9) | F=0.62 | .54 |
| Accuracy (%) | 85.4 (11.1) | 91.2 (9.4) | 94.5 (4.6) | F=6.59 | .002 |
| Reaction Time (ms) | 852 (103) | 896 (249) | 898 (261) | F=0.32 | .73 |
| Diagnosis (Lifetime) | |||||
| BD-NOS | 8 (36.4%) | – | – | – | – |
| BD-I | 14 (63.6%) | – | – | – | – |
| Depressive Disorders | – | 16 (53%) | – | – | – |
| ADHD | 13 (59%) | 31 (100%) | – | – | – |
| ODD | 8 (36%) | 16 (53%) | – | X2=0.96 | .33 |
| ADHD and/or ODD | 14 (63.6%) | 31 (100%) | – | – | – |
| Conduct Disorder | 0 (0%) | 1 (3%) | – | – | – |
| Anxiety | 10 (45%) | 7 (23%) | – | X2=2.82 | .09 |
| Medication | |||||
| Medicated (%) | 15 (68%) | 16 (53%) | – | X2=1.16 | .28 |
| Antipsychotic | 11 (50%) | 3 (10%) | – | X2=10.32 | .001 |
| Mood Stabilizer | 5 (23%) | 0 (0%) | – | – | .01* |
| Antidepressant | 4 (18%) | 3 (10%) | – | – | .44* |
| Stimulant | 9 (41%) | 13 (43%) | – | X2=.03 | .86 |
| Current Depressive and Manic Symptoms | |||||
| KDRS | 4.82 (+4.41) | 2.83 (+3.29) | 0.15 (+0.46) | F=13.62 | <.0001 |
| KMRS | 7.77 (+9.06) | 2.40 (+3.81) | 0.08 (+0.39) | F=12.77 | <.0001 |
Fisher’s Exact Test
Groups (BPSD vs. ADHD vs. HC) did not differ according to demographics (age, sex, or IQ). There were group differences in task accuracy (p=.002), with the BPSD group showing lower accuracy relative to both ADHD (p=.05) and HC (p=.001) groups; thus we entered accuracy as a covariate in all neuroimaging analyses. Compared to ADHD, youth with BPSD were more likely to be prescribed antipsychotics (p=.001) and mood stabilizers (p=.01). Other medications and diagnoses were similar across BPSD and ADHD groups. Within the ADHD group, 53% were diagnosed with ODD; 53% were also diagnosed with a depressive disorder. Within the BPSD group, 59% of youth were diagnosed with ADHD and 36% with ODD. Only one participant (within the ADHD group) was diagnosed with conduct disorder.
Group differences in amygdala FxC within the VLPFC/ACC ROI (Table 2)
Table 2.
ROI Analysis: Clusters that show differential group response during emotions vs. shapes or show a group × condition interaction within ROI (VLPFC and ACC). Analyses were conducted at p<.001 threshold. Only clusters with a peak voxel that is FWE-corrected p<.05 within the pre-specified ROI (with small volume correction; SVC) are reported.
| Region | MNI | K | Z | FWE-corrected p (SVC) |
|---|---|---|---|---|
| Effect of group: All emotions vs. shapes | ||||
| Left Inferior Frontal Gyrus (BA 47) | −48 16 0 | 14 | 4.11 | .04 |
| Group × Condition Interaction | ||||
| Subgenual Cingulate (BA 25) | 6 10 −12 | 13 | 4.23 | .03 |
MNI=Montreal Neurological Institute Coordinates; FWE=family-wise error; SVC=small volume correction; BA=Brodmann Area
In response to EMO-SHAPE, a cluster in the left VLPFC showed differential FxC across groups (Figure 1a,b). While the BPSD group showed significant positive FxC in response to EMO-SHAPE (emotions>shapes), HC and ADHD groups showed significant inverse FxC to EMO-SHAPE (emotions<shapes). A cluster within the subgenual anterior cingulate cortex (sgACC) showed a significant group × emotion interaction (Figure 1a,c). Extracted data indicated that this interaction was driven by group differences to NEG-SHAPE, with little effect of POS-SHAPE (Figure 1c). Similar to the VLPFC cluster, the HC group showed significant inverse FxC to NEG-SHAPE, while the BPSD group showed significant positive FxC to NEG-SHAPE; the ADHD group was intermediate.
Figure 1.
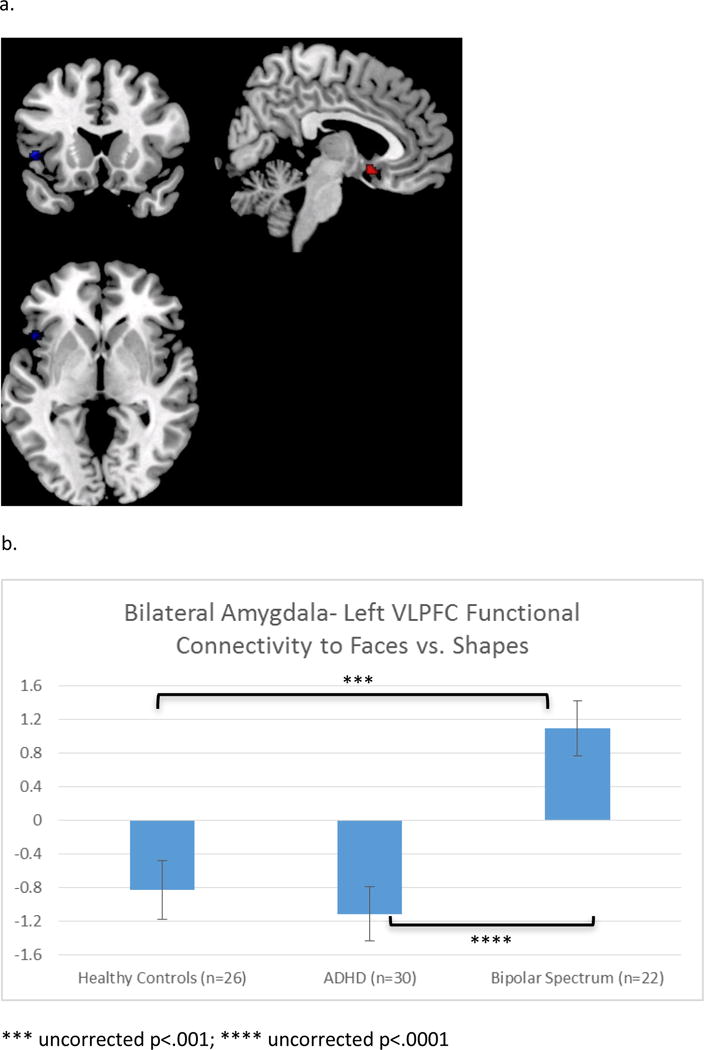
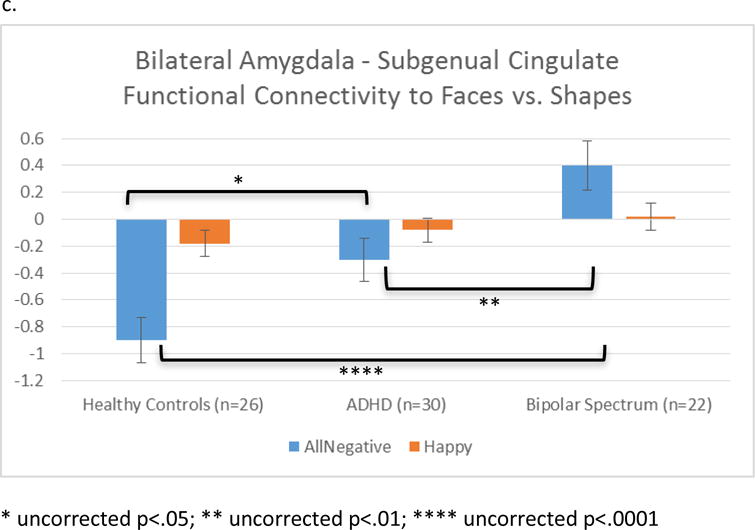
a. Regions within ROI (VLPFC and ACC) that showed differential FxC across groups (voxel-wise p<.001; peak voxel FWE-corrected p<.05 within ROI). b. Overall group effect of emotions vs. shapes. More positive values on the y-axis indicate FxC increases during emotions vs. shapes. c. Condition × group interaction: Differences were significant for negative emotions, but not happy faces.
LASSO regression analyses indicated that the only selected predictor of the VLPFC cluster other than group was task reaction time. In the standard regression model, reaction time was not a significant predictor, and group was still significant after adjusting for this variable (Table 3). For the sgACC cluster, child-rated depressive symptoms (from the MFQ), psychiatric medication, site, and task accuracy were selected as predictors within the LASSO regression model. Amygdala-sgACC FxC was positively associated with both depressive symptoms (t=2.02, p<.05) and medication (t=2.01, p<.05); site and task accuracy were not significant predictors. Further analysis indicated that stimulants were driving the relationship between psychotropic medication and amygdala-sgACC FxC; no other medication subclasses (antipsychotics, antidepressants, or mood stabilizers) were associated with FxC in this cluster (eTable 2). After adjustment for medications and depressive symptoms, the ADHD group no longer differed significantly from the HC group; however, the BPSD group was still different from both HC and ADHD groups (Table 3). Of note, other DSM-IV disorders (depressive disorders, anxiety disorders, and ODD) were not selected as predictors in either model, thus indicating that these disorders did not significantly affect extracted mean connectivity, after adjusting for group. Interactions between group and dimensions were not significant.
Table 3.
Impact of adjustment for variables selected by the LASSO regression on the relationship between group and mean cluster connectivity. P-values are uncorrected for multiple comparisons.
| Model | BPS D vs. HC | ADHD vs. HC | BPSD vs. ADHD | |||
|---|---|---|---|---|---|---|
|
| ||||||
| t | p | t | p | t | p | |
| Left VLPFC | ||||||
| Unadjusted | 3.68 | .0004 | −.59 | .55 | 4.36 | <.0001 |
| Adjusted for Reaction Time | 3.58 | .0006 | −.60 | .55 | 4.27 | <.0001 |
| Subgenual Cingulate | ||||||
| Unadjusted | 5.35 | <.0001 | 2.62 | .01 | 3.02 | .003 |
| Adjusted for site, MFQ, | 2.61 | .01 | .77 | .44 | 2.47 | .02 |
| medication, and accuracy | ||||||
MFQ=Moods and Feelings Questionnaire; VLPFC=Ventrolateral Prefrontal Cortex
Secondary analyses on extracted data indicated that results remained significant even after excluding youth with (1) BD NOS, (2) depressive symptoms (KDRS>10), (3) manic symptoms (KMRS>10), (4) psychotropic medication, and (5) co-morbid BPSD and ADHD (eTable 3). Results also remained significant within each two-site subgroup, indicating that differences were not driven by a single site (eTable 3).
Dimensional Analysis
In response to EMO-SHAPE, there were no significant relationships between dimensional symptoms (anxiety, depression, manic symptoms, and affective lability) and FxC between bilateral amygdala seed and the VLPFC/ACC ROI.
Task-related connectivity patterns in full sample
There were several regions that showed significant inverse FxC in response to EMO-SHAPE, including midline regions (precuneus, medial frontal gyrus), bilateral supramarginal gyrus, and bilateral medial temporal gyrus (all cluster-wise FWE-corrected p<.05; eTable 4a). No regions showed significant positive connectivity with bilateral amygdala to EMO-SHAPE. Similar patterns of FxC were observed to separate right and left amygdala seeds; however, task-related FxC patterns were stronger between left amygdala and cortical regions, particular prefrontal cortex (eTable 4b, c).
Whole Brain Group Differences (eTable 5)
Overall group differences in amygdala FxC to EMO-SHAPE did not survive correction for multiple comparisons; however, post-hoc two-group comparisons yielded three interesting (although exploratory) findings. First, the overall pattern of FxC observed in the ROIs was also observed in two-group whole-brain comparisons at a trend level; specifically, in response to EMO-SHAPE, the BPSD group showed (1) increased amygdala-subgenual FxC relative to the HC group and (2) increased amygdala-left VLPFC FxC relative to the ADHD group (FWE-corrected p<.2). Second, relative to both the BPSD and HC, the ADHD group showed more inverse FxC between the amygdala and the right superior frontal gyrus (FWE-corrected p<.05). Third, there was an overall pattern of BPSD>HC>ADHD regarding FxC to EMO-SHAPE; there were no significant regions where ADHD showed more positive FxC than other groups or where BPSD showed more inverse FxC than other groups.
DISCUSSION
In this sample of youth selected on the basis of elevated behavioral and emotional dysregulation (as well as healthy controls), we assessed functional connectivity (FxC) between amygdala and VLPFC/ACC to an implicit emotion processing task, and examined how this differed according to diagnostic group. FxC between amygdala and both sgACC (to negative emotions vs. shapes) and left VLPFC (to all emotions vs. shapes) differed across groups, differences largely driven by significantly positive FxC in the BPSD youth and significantly inverse FxC in the HC. While the amygdala-left VLPFC FxC abnormality appeared specific to BPSD youth (ADHD was similar to HC), the amygdala-sgACC abnormality was also related to depressive symptoms and current stimulant medication. Exploratory whole brain analyses pointed to decreased FxC between amygdala and right superior frontal gyrus to emotions vs. shapes in the ADHD group, relative to both BPSD and HC. Differences in FxC emerged between the BPSD and ADHD groups, despite the fact that the majority of youth in the BPSD group co-morbid ADHD.
FxC between the amygdala and VLPFC (particularly BA 47) has been repeatedly shown to be abnormal in adults with BPSD, as well as in youth with and at risk for BPSD. In response to emotional stimuli (including an emotion matching task (Vizueta et al., 2012), emotion downregulation (Kanske et al., 2015; Morris et al., 2012), and an emotion labeling task (Foland et al., 2008)), healthy individuals generally show an inverse pattern of connectivity between amygdala and VLPFC, while those with BPSD show an absence of inverse (and even positive) FxC. Similar findings have been shown in youth at familial risk for BPSD (Manelis et al., 2015), specifically between the right amygdala and left VLPFC, although less positive amygdala-VLPFC FxC has also been observed(Ladouceur et al., 2013). Indeed, in our sample, we found that abnormally increased amygdala-VLPFC FxC was specific to BPSD, and not found in ADHD; FxC in this cluster was also not associated with medication. The VLPFC has been implicated in both explicit and implicit emotion regulation. Specifically, VLPFC is part of a network involved in emotion reappraisal (Ochsner et al., 2002) and is also involved in labeling of threatening stimuli, an implicit regulation strategy (Tupak et al., 2014). The VLPFC has been implicated more globally in managing interference during cognitive tasks (Burgess and Braver, 2010), and seems to play a role in the switch between habitual (model-free) and effortful (model-based) strategies (Etkin et al., 2015; Lee et al., 2014). The inverse FxC between bilateral amygdala and left VLPFC observed in the HC and ADHD groups might represent such a regulation of emotional interference, while those with BPSD exhibited deficits in this control.
In contrast, amygdala-sgACC FxC showed abnormalities in BPSD, but also in ADHD (albeit to a lesser degree); FxC between these regions was also more positive in those medicated for ADHD and in those with depressive symptoms. Thus, in contrast to the amygdala-VLPFC finding, which was specific to BPSD, the amygdala-sgACC finding was influenced by a number of other factors. This is also consistent with previous work that points to weaker inverse (and even positive) amygdala-sgACC FxC in trauma-exposed youth (Thomason et al., 2015), depressed adolescents (Connolly et al., 2013), and emotionally labile youth with ADHD (Hulvershorn et al., 2014). These abnormalities were also found specifically to negative emotional faces (not happy faces), suggesting a particular difficulty with regulation of negative emotions. The sgACC and ventromedial prefrontal cortex are thought to be more involved in “model-free”, implicit emotion regulation (Etkin et al., 2015); such implicit regulation might be deficient in youth who have difficulties with emotion regulation, regardless of diagnosis. Indeed, increased sgACC activation has been associated with major depression (Drevets et al., 2008), and deep brain stimulation of this region has reversed this abnormality, thus treating severe and treatment-resistant depressive episodes (Mayberg et al., 2005). Consistent with this literature, sgACC-amygdala FxC positively correlated with self-reported depressive symptoms in our sample.
Interestingly, we also found that youth on stimulant medication showed weaker inverse amygdala-sgACC FxC to negative emotional faces, which in part explained observed differences between ADHD and HC groups. This is in contrast to previous work, which has generally shown a normalizing impact of stimulants, specifically on activation of VLPFC (Rubia et al., 2014) and amygdala-VLPFC FxC (Posner et al., 2011). Given the nature of our sample, and the fact that only 42% of the ADHD group was on stimulants at the time of scan, it is possible that stimulant medication was a marker for more severe ADHD-related symptoms. Thus the finding that differences between ADHD and HC are more pronounced in those on stimulants might be simply due to severity of disorder, and not the effect of stimulants themselves.
There are several important strengths of this study. First, diagnosis was not based on a single encounter, but, rather, was assessed longitudinally. This is particularly important given that BPSD is often a difficult diagnosis to make from a single assessment point (Youngstrom et al., 2008a), a difficulty which in part motivated this analysis. Second, the sample was large enough (with enough BPSD and ADHD cases) to assess three-group differences with adequate power. Third, extensive longitudinal data were collected regarding dimensional measures, medication, and other potential covariates, thus making it less likely that these variables confound the observed relationships.
These results must also be viewed in light of the following limitations. First, we excluded a large number of participants due to poor task response and data quality, and LAMS youth were more likely to be excluded than the HC group. This led to smaller sample sizes, though all groups still had greater than 20 individuals. In addition, it is possible that FxC patterns of youth who were included (who were, on average, older, less anxious, and had a higher IQ) differed from those who were excluded from analyses. In this case, our results might not generalize to a broader population of youth with BPSD and ADHD. However, including youth with excessive movement or who did not attend to stimuli would likely have compromised the validity of our analyses; thus we have chosen to exclude them. Second, the BPSD and ADHD groups were heterogeneous, and included youth with various co-morbidities and medication. While such confounds complicate analysis, they also mean that the sample is more similar a clinical population (as opposed to youth selected for a single diagnosis). Allowing co-morbidity within the BPSD group also allowed for a more stringent test of our hypothesis, that BPSD would be associated with specific abnormalities not shared by youth with ADHD alone. In addition, findings remained significant in secondary analyses on extracted data that excluded youth with co-morbid BPSD and ADHD. Third, this was a multi-site study, which introduces additional noise into the fMRI assessment. However, we implemented procedures to minimize this effect, including frequent monitoring of signal:noise ratios across all sites and adjustment for site in all analyses; findings remained significant in each two-site subgroup.
In summary, the BPSD group showed significant positive FxC between amygdala and left VLPFC in response to emotional faces, in contrast to both HC and ADHD groups, which showed significant inverse FxC. The BPSD group also showed significantly greater positive FxC between amygdala and sgACC than HC, though this pattern of abnormal FxC was less specific to BPSD, and influenced by depressive symptoms. These findings add to a literature indicating that amygdala-VLPFC FxC might be a specific marker for BPSD, an abnormality also found in those at familial risk for the disorder. Future work will further evaluate the possibility that such altered FxC might represent an endophenotype of disorder, and help to identify those youth with externalizing symptoms who will develop BPSD.
Supplementary Material
Highlights.
Functional connectivity of the amygdala during emotion processing distinguishes youth with bipolar spectrum disorder from both healthy controls and youth with attention-deficit/hyperactivity disorder.
While both healthy controls and those with attention-deficit/hyperactivity disorder show inverse task-related functional connectivity between amygdala and ventrolateral prefrontal cortex, youth with bipolar spectrum disorder show positive task-related connectivity between these regions.
Amygdala-subgenual functional connectivity in response to negative emotions also distinguishes the three groups, but this relationship is less specific, as it is related to depressive symptoms and stimulant use.
ABBREVIATIONS
- BPSD
bipolar spectrum disorder
- ADHD
externalizing
- HC
healthy controls
- FxC
functional connectivity
- VLPFC
ventrolateral prefrontal cortex
- ADHD
Attention-Deficit Hyperactivity Disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Author; Washington, DC: 2013. [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Bowring MA, Kovacs M. Difficulties in diagnosing manic disorders among children and adolescents. J Am Acad Child Adolesc Psychiatry. 1992;31:611–614. doi: 10.1097/00004583-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Braver TS. Neural Mechanisms of Interference Control in Working Memory: Effects of Interference Expectancy and Fluid Intelligence. PLoS ONE. 2010;5:e12861. doi: 10.1371/journal.pone.0012861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviss WB, Birmaher B, Melhem NA, Axelson DA, Michaels SM, Brent DA. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. J Child Psychol Psychiatry. 2006;47:927–934. doi: 10.1111/j.1469-7610.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry. 2010;71:1664–1672. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, Keener MT, Mullin BC, Hafeman DM, Labarbara EJ, Stiffler RS, Almeida J, Kronhaus DM, Frank E, Phillips ML. Heterogeneity of amygdala response in major depressive disorder: the impact of lifetime subthreshold mania. Psychol Med. 2013:1–10. doi: 10.1017/S0033291712000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. 1996;65:189–198. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- Hafeman D, Axelson D, Demeter C, Findling RL, Fristad MA, Kowatch RA, Youngstrom EA, Horwitz SM, Arnold LE, Frazier TW, Ryan N, Gill MK, Hauser-Harrington JC, Depew J, Rowles BM, Birmaher B. Phenomenology of bipolar disorder not otherwise specified in youth: a comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord. 2013;15:240–252. doi: 10.1111/bdi.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Bebko G, Bertocci MA, Fournier JC, Bonar L, Perlman SB, Travis M, Gill MK, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Frazier TW, Youngstrom EA, Findling RL, Drevets W, Phillips ML. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res. 2014;58:129–136. doi: 10.1016/j.jpsychires.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2013;43:1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. J Clin Psychiatry. 2010;71:1511–1517. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Mennes M, Castellanos FX, Di Martino A, Milham MP, Hummer TA, Roy AK. Abnormal Amygdala Functional Connectivity Associated With Emotional Lability in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:351–361.e351. doi: 10.1016/j.jaac.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Schönfelder S, Forneck J, Wessa M. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Translational psychiatry. 2015;5:e497. doi: 10.1038/tp.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keener MT, Fournier JC, Mullin BC, Kronhaus D, Perlman SB, LaBarbara E, Almeida JC, Phillips ML. Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychol Med. 2012;42:1913–1924. doi: 10.1017/S0033291711002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Pine DS, Klein DF. Resolved: Mania is mistaken for ADHD in prepubertal children. J Am Acad Child Adolesc Psychiatry. 1998;37:1093–1096. doi: 10.1097/00004583-199810000-00020. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, Phillips ML. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci. 2013;5:185–196. doi: 10.1016/j.dcn.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Sang W, Shimojo S, O’Doherty John P. Neural Computations Underlying Arbitration between Model-Based and Model-free Learning. Neuron. 2014;81:687–699. doi: 10.1016/j.neuron.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Hafeman DM, Gill MK, Birmaher B, Phillips ML. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015 doi: 10.1093/brain/awv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational psychiatry. 2012;2:e90. doi: 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. American Journal of Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, Peterson BS. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:828–837.e823. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion Dysregulation in Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2014;171:276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Mood lability and psychopathology in youth. Psychological Medicine. 2009;39:1237–1245. doi: 10.1017/S0033291708004662. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR. Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience. 2015;10:1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society. Series B (Methodological) 1996:267–288. [Google Scholar]
- Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–135. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupak SV, Dresler T, Guhn A, Ehlis AC, Fallgatter AJ, Pauli P, Herrmann MJ. Implicit emotion regulation in the presence of threat: neural and autonomic correlates. Neuroimage. 2014;85(Pt 1):372–379. doi: 10.1016/j.neuroimage.2013.09.066. [DOI] [PubMed] [Google Scholar]
- Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, Altshuler LL. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am J Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: Both artifact and outgrowth of shared mechanisms. Clin Psychol. 2010;17:350–359. doi: 10.1111/j.1468-2850.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: Validity, phenomenology, and recommendations for diagnosis Bipolar Disord. 2008a;10:194–214. doi: 10.1111/j.1399-5618.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J Clin Psychiatry. 2008b;69:831–839. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


