Abstract
Background
Household air pollution (HAP) from indoor biomass stoves contains harmful pollutants, such as polycyclic aromatic hydrocarbons (PAHs), and is a leading risk factor for global disease burden. We used biomonitoring to assess HAP exposure and association with self-reported symptoms in 334 non-smoking Peruvian women to evaluate the efficacy of a stove intervention program.
Methods
We conducted a cross-sectional study within the framework of a community randomized control trial. Using urinary PAH metabolites (OH-PAHs) as the exposure biomarkers, we investigated whether the intervention group (n = 155, with new chimney-equipped stoves) were less exposed to HAP compared to the control group (n = 179, with mostly open-fire stoves). We also estimated associations between the exposure biomarkers, risk factors, and self-reported health symptoms, such as recent eye conditions, respiratory conditions, and headache.
Results
We observed reduced headache and ocular symptoms in the intervention group than the control group. Urinary 2-naphthol, a suggested biomarker for inhalation PAH exposure, was significantly lower in the intervention group (GM with 95% CI: 13.4 [12.3, 14.6] μg/g creatinine) compared to control group (16.5 [15.0, 18.0] μg/g creatinine). Stove type and/or 2-naphthol was associated with a number of self-reported symptoms, such as red eye (adjusted OR with 95% CI: 3.80 [1.32, 10.9]) in the past 48 h.
Conclusions
Even with the improved stoves, the biomarker concentrations in this study far exceeded those of the general populations and were higher than a no-observed-genotoxic-effect-level, indicating high exposure and a potential for increased cancer risk in the population.
Keywords: woodsmoke, household air pollution, stove intervention, polycyclic aromatic hydrocarbon, biomonitoring, 1-hydroxypyrene
1. Introduction
Nearly 40% of the global population uses biomass fuel, such as wood, charcoal, and crop residues, as their primary energy source for cooking and heating (Rehfuess et al., 2014). Household air pollution (HAP) from indoor biomass stoves contains harmful pollutants, such as fine particulate matter (PM2.5), carbon monoxide (CO), and polycyclic aromatic hydrocarbons (PAHs). HAP has been linked to a variety of adverse health outcomes (Naeher et al., 2007; Zhang and Smith, 2007), such as chronic obstructive pulmonary disease (Kurmi et al., 2010), eye diseases (West et al., 2013), adverse birth outcomes (Amegah et al., 2014; Pope et al., 2010), lung cancer (Bruce et al., 2015) and other cancers (Josyula et al., 2015). In the latest Global Burden of Disease, Injury, and Risk Factor Study 2013, HAP was ranked as the 7th leading risk factor globally (Forouzanfar et al., 2015).
Biomass fuel is most commonly used in developing countries, especially in rural areas with limited resources. For example, while 34% of the total population and 13% of the urban population in Peru use solid fuel, over 95% of the rural population rely on solid fuel for cooking and heating (WHO, 2013). Moreover, open-fire pits or inefficient stoves are often used in poorly ventilated conditions, contributing to high levels of harmful incomplete combustion products inside the house and kitchen (Desai et al., 2004; Naeher et al., 2007). Stove improvement programs have been implemented in numerous countries as reviewed elsewhere (Lewis and Pattanayak, 2012; Rehfuess et al., 2014).
As stove improvement programs are being implemented to reduce HAP and associated health burdens globally, there is an urgent need for direct, accurate, and robust exposure assessment tools to evaluate and guide such programs, and provide information to delineate the exposure-response relationship with specific health outcomes (Rylance et al., 2013). However, among studies investigating association between HAP exposure and health outcomes, few had direct exposure measurements and many relied on proxies to characterize exposure, such as stove type and fuel type (Rylance et al., 2013). Moreover, among studies with exposure assessment, it is common to measure smoke components, such as PM2.5 and CO, in kitchen or personal air (Clark et al., 2009; Rosa et al., 2014; Ruiz-Vera et al., 2015). While air pollutant levels can reflect stove emissions, they cannot account for other factors that can significantly impact the effectiveness of the intervention programs, such as personal behavior. Biomonitoring is an effective tool that can assess overall exposure and account for various factors, such as personal behaviors related to stove usage and individual physiological differences.
We conducted a cross-sectional study within the framework of a community randomized control trial (c-RCT) in Peru (Hartinger et al., 2011) to assess HAP exposure through air monitoring and biomonitoring, and, to evaluate the efficacy of a stove intervention program. While the HAP exposure assessment based on air monitoring has been reported previously (Commodore et al., 2013; Hartinger et al., 2013), we report here the biomonitoring results on 10 hydroxylated PAH metabolites (OH-PAHs) in morning urine samples and self-reported health symptoms from 334 non-smoking women. Our objectives are, 1) to investigate whether participants in the intervention group (with new chimney-equipped stoves) were less exposed to HAP than those in the control group (with mostly open-fire stoves) using the urinary OH-PAHs as exposure biomarkers; 2) to study whether the intervention group had less self-reported health symptoms (ocular and respiratory symptoms, headache) than the control group; and 3) to study the associations between the HAP exposure biomarkers, risk factors, and self-reported health symptoms.
2. Material and methods
2.1. Study design
This study was conducted within the framework of a c-RCT involving 51 communities that used wood for cooking and heating in Peru, hereafter referred to as the parent study (Hartinger et al., 2011). The parent study aimed to evaluate reduction of childhood illness through reducing HAP and improving drinking water and kitchen hygiene conditions. The households in the intervention arm received an intervention package that included a new stove, a kitchen sink, and a solar disinfection home-based water treatment. The new stoves were built from red burnt bricks, plastered with a mixture of mud, straw and donkey manure; the stoves consisted of three pot-holes for cooking, a closed combustion chamber, a metal chimney with a regulatory valve, a hood, and metal rods for support (Hartinger et al., 2012). In the control arm, households used their existing stoves, most of which were traditional open-fire stoves. To reduce potential dropout and non-blinding bias in the control arm, households also received a psychomotor stimulation package focusing on early child development, a package that was unrelated to the environmental factors targeted in the study. The new stoves were installed in intervention households between September 2008 and January 2009. No exposure assessment was conducted before the installation of the intervention package in the parent study.
Starting February 2009, 503 households (250 and 253 in the intervention and control arms, respectively) entered the follow-up evaluation phase of the parent study (Hartinger et al., 2011). From June to August 2009, we conducted this cross-sectional study evaluating exposure to HAP (Commodore et al., 2013). Female members of the households (one per household) were eligible for this study if they met the following criteria: 1) were the mother or primary caregiver of the children enrolled in the parent study, 2) used an indoor wood stove, and, 3) agreed to participate in this study and agreed to comply with the project instructions during the 48-h sampling period. Eligible and enrolled participants provided a first-morning urine sample, a 48-h personal CO measurement, and filled out a questionnaire on demographics, smoking status, daily activities, household and community characteristics, and health symptoms, including headache, respiratory and eye-related symptoms. Although field workers visited all households in the parent study, subjects’ availability and willingness for participation, and time and budget constraints limited the total sample size of this study.
After the HAP exposure measurement, we classified post-hoc the intervention group into two sub-groups—“no-repair” sub-group with stoves in good running conditions at the time of the assessment, and, “need-repair” sub-group with stoves that needed repairs, e.g., re-plastering, filling small cracks, and chimney valve replacement. The control group was stratified into three sub-groups based on the type of wood-burning stoves: 1) traditional three-stone open-fire stoves and non-vented stoves (“traditional”), 2) chimney-stoves built by a non-governmental organization (“built-by-NGO”), and, 3) chimney-stoves built by the household members (“self-improved”). A flow diagram for this cross-sectional study is given in Supplemental Material, Fig. S1.
2.2. CO measurements, urine sample collection and analytical method
Time-integrated 48-h personal CO measurements were taken from the participants as described elsewhere (Commodore et al., 2013). In brief, the CO measurements were collected using passive CO diffusion tube, i.e., Dräger Diffusion Tube for Carbon Monoxide (Dräger Safety Inc., USA). The sampler uses principles of diffusion and colorimetry where CO passively diffuses into the tube and causes the reduction of sodium palladosulfite to palladium metal, which results in a grayish stain corresponding to a cumulative dose of CO. During the 48-h sampling period, the participants wore the passive CO diffusion samplers in the breathing zone. Field workers recorded the time of tube breakage and capping, which marked the beginning and ending of the CO sampling period, respectively. Upon return to the field station, tubes were read independently by two of the authors (AAC and SMH) and an arithmetic mean was taken. Additional information on the personal CO measurement is given in Supplemental Materials.
At the end of the 48-h personal CO sampling period, the participants collected a morning urine void between 5:00 am and 7:00 am in a pre-labeled sterile polyethylene container and placed the container in an insulated bag with ice packs. Field workers retrieved the urine samples from the participants, recorded the date and volume of the void, and delivered them to the study base, whereupon the samples were transferred into polypropylene tubes and stored at −20 °C until the end of the field work. Samples were then shipped frozen on dry ice to the University of Georgia and the Centers for Disease Control and Prevention (CDC) and stored at −70 °C until analysis. The study protocol was approved by the Human Research Protection Office at the CDC, the Human Subjects Division at University of Georgia, the Ethical Review Board of the Instituto de Investigacion Nutricional and the Universidad Peruana Cayetano Heredia in Lima. Written informed consent was obtained from all participants prior to enrollment in the study.
We analyzed the urine samples for 10 OH-PAHs, i.e. 1-, 2-naphthol, 2-, 3-, 9-hydroxyfluorene, 1-, 2-, 3-, 4-hydroxyphenanthrene and 1-hydroxypyrene. The detailed laboratory method was described elsewhere (Li et al., 2014). Briefly, urine samples (1 mL) were spiked with a 13C-labeled internal standard solution mixed with buffer and enzyme, and hydrolyzed overnight at 37 °C. The OH-PAHs were then extracted by a solvent mixture through semi-automated liquid-liquid extraction. The extracts were evaporated, derivatized, and analyzed by isotope dilution gas chromatography triple quadrupole tandem mass spectrometry. All analyses were subjected to a series of quality control and quality assurance checks as described previously (Li et al., 2014). The limits of detection (LODs) were 0.001–0.019 μg/L, depending on the analyte, and the detection frequency was 100% for all 10 OH-PAHs in this study. The coefficient of variation from 36 quality control samples analyzed with the study sample ranged 1.8–7.3% depending on the analyte. Urinary creatinine was measured according to Roche’s Creatinine Plus Product Application # 03,631,761,003.
2.3. Data and statistical analysis
All urinary OH-PAH concentrations were blank-subtracted. 1-Naph-thol results in two participants in the control group were non-reportable because of chromatographic interference on the 13C-labeled internal standard peak. Creatinine adjustment was made to correct for urine dilution that is known to vary with the hydration status of the individual (Barr et al., 2005). Two urine samples did not have creatinine results and therefore, did not have creatinine-adjusted OH-PAH results.
Statistical analyses were performed using SAS 9.3 software. The Shapiro-Wilk test, histograms and Q-Q plots indicated that OH-PAH concentrations were log-normally distributed. Therefore, creatinine-adjusted OH-PAH concentrations were log-transformed before all statistical analyses. Based on influence statistics and corresponding plots of OH-PAH concentrations, we determined that one participant in the control group was an extreme outlier and excluded the participant in subsequent analyses. Least square geometric means of PAH biomarker by stove type were calculated, controlling for second-hand smoking status, distance between home and road, community traffic, and recent consumption of food cooked directly on open fire (hereafter referred to as “grilled food”). We used t-tests to compare geometric means (GMs) of OH-PAH concentrations by stove type. Both un-adjusted and least square GMs gave similar results. Hence, only un-adjusted GMs are presented here. We calculated Pearson correlation coefficients to assess correlation between urinary OH-PAHs and personal CO levels (log-transformed). Chi-square test and Fisher’s exact test were used to test for differences on the prevalence of health symptoms in the control and intervention groups, and both tests gave the same results regarding statistical significance. Results are considered statistically significant at p < 0.05.
Logistic regression was used to model the association between self-reported health symptoms and OH-PAHs as HAP exposure biomarkers. Health symptoms studied included several eye-related symptoms, several respiratory symptoms, and headache. A stepwise selection procedure was used in which the entry and removal p-values were 0.10. Final model controlled for subject’s age, stove type, CO, wood type, second-hand smoke, distance between home and road, community traffic, pesticide use, fertilizer use, and kitchen type. Urinary OH-PAHs were forced into the model. Sums of fluorene metabolites (summed concentration of 2-, 3- and 9-hydroxyfluorene) and phenanthrene metabolites (summed concentration of 1-, 2-, 3- and 4-hydroxyphenanthrene) were used in place of individual components to reflect exposure to fluorene and phenanthrene, respectively.
3. Results
Among the 503 households in the parent study, 334 women (one participant per household)—155 in the intervention group and 179 in the control group—participated in this study. At the time of this study, the stoves in the intervention group had been in use for 6–8 months (median: 7.4 months). Table 1 gives participants’ demographic information and selected characteristics for their households and communities. Participants from the intervention and control groups had similar demographic, kitchen and community characteristics. Average age at the time of the study was around 30 years and average daily cooking times were approximately 3 h in both groups. Over 70% of the participants in each group had elementary level or less education. All participants were self-reported non-smokers. Over 90% in each group were not exposed to second-hand smoke and had not eaten grilled food during the past 48 h. The communities in this study had limited to no automobile traffic. The only factor with a large difference between the groups was the age of the stove. In the control group, 31% of the stoves had been in use for less than one year, while all stoves in the intervention group were less than one year old by design.
Table 1.
Demographic characteristics of the 334 study participants and their community and household characteristics.
| Control
|
Intervention
|
||||||
|---|---|---|---|---|---|---|---|
| All control | Built-by-NGO | Self-improved | Traditional | All intervention | No-repair | Need-repair | |
| Number of participants | 179 | 23 | 35 | 121 | 155 | 99 | 56 |
| Participants’ demographic characteristics | |||||||
| Age (y) a | 29.4 ± 6.8 | 30.7 ± 7.4 | 28.7 ± 6.9 | 29.4 ± 6.7 | 30.3 ± 8 | 29.5 ± 7.3 | 31.7 ± 8.9 |
| Elementary education or less | 125 (78%) | 14 (70%) | 26 (90%) | 85 (76%) | 96 (72%) | 60 (70%) | 36 (75%) |
| No second-hand smoke | 158 (90%) | 20 (95%) | 31 (91%) | 107 (88%) | 138 (91%) | 89 (91%) | 49 (92%) |
| No grilled food in the last 48 h | 156 (92%) | 20 (100%) | 28 (82%) | 108 (93%) | 142 (93%) | 91 (93%) | 51 (94%) |
| Daily cook time (h) a | 3.0 ± 0.9 | 3.0 ± 0.5 | 3.0 ± 1.0 | 3.0 ± 0.9 | 2.8 ± 0.8 | 2.8 ± 0.8 | 2.8 ± 0.8 |
| Participating household and community characteristics | |||||||
| Enclosed kitchen with 3 or more walls and a roof | 137 (79%) | 15 (71%) | 26 (79%) | 96 (81%) | 114 (76%) | 73 (74%) | 41 (79%) |
| Wood type: Eucalipto or Hualango | 133 (76%) | 13 (62%) | 23 (68%) | 97 (80%) | 103 (68%) | 66 (68%) | 37 (70%) |
| Community traffic: 1–5 cars/day | 107 (65%) | 10 (53%) | 24 (73%) | 73 (65%) | 103 (76%) | 68 (76%) | 35 (78%) |
| Light type: candle | 108 (65%) | 13 (65%) | 23 (74%) | 72 (63%) | 79 (56%) | 53 (60%) | 26 (50%) |
| Length of stove use | |||||||
| <1 y | 46 (31%) | 12 (60%) | 15 (54%) | 19 (19%) | 135 (100%) | 86 (100%) | 49 (100%) |
| 1–4 y | 64 (42%) | 7 (35%) | 10 (35%) | 47 (47%) | 0 | 0 | 0 |
| >5 y | 40 (27%) | 1 (5%) | 3 (11%) | 36 (35%) | 0 | 0 | 0 |
Participants’ age and daily cook time are expressed as mean with standard deviation. All other parameters are expressed as count with percentage of non-missing responses to that question in each group and sub-group.
Within the intervention group, 64% of the stoves were in good running condition, while 36% were in need of repair at the time of the study. In the control group, 68% households used traditional stoves with no vent in the kitchen, while 13% and 20% used chimney stoves built by an NGO or household members, respectively. As shown in Table 1, most characteristics on the participants, households, and communities were similar among the sub-groups within the intervention or control groups, except for the stove age. A smaller portion of traditional stoves had less than one-year usage, compared to those built by NGO and self-improved stoves.
All 10 OH-PAHs were detected well above the LODs. Table 2 presents the GM with 95% confidence intervals (CI) for creatinine-adjusted concentrations (μg/g creatinine). The unadjusted concentrations and median with interquartile range are given in Supplemental Materials, Tables S1 and S2. Urinary 2-naphthol, a proposed biomarker for inhalational PAH exposure (Kang et al., 2002), was 23% higher in the control group than in the intervention groups (p < 0.001). When comparing the two sub-groups with potentially the most and least exposure (traditional stove sub-group vs. no-repair intervention sub-group), we observed a larger difference (34%). In general, most other OH-PAHs followed similar patterns as those observed for 2-naphthol (i.e. control > intervention), but the differences were smaller and were not statistically significant.
Table 2.
Geometric means and 95% confidence intervals of PAH metabolite concentrations in morning samples from 334 non-smoking women under study (creatinine-adjusted, μg/g creatinine).
| Urinary OH-PAHs | Control
|
Intervention
|
|||||
|---|---|---|---|---|---|---|---|
| All control | Built-by-NGO | Self-improved | Traditional | All intervention | No-repair | Need-repair | |
| N | 179a | 23 | 35 | 121 | 153b | 99 | 54b |
| 1-naphthol | 28.2 [23.8,33.3] | 19.6 [12.9,29.8] | 19.4 [13.7,27.6] | 33.6 [27.3,41.3] | 32.2 [26.1,39.7] | 28.1 [22,35.9] | 41.5 [28.2,61] |
| 2-naphtholc | 16.5c [15,18] | 16.5 [11.9,22.9] | 14.1 [11.7,17] | 17.2 [15.4,19.2] | 13.4 c [12.3,14.6] | 12.8 [11.5,14.2] | 14.5 [12.6,16.8] |
| Sum naphthol | 49.1 [42.8,56.3] | 37.4 [26.1,53.7] | 35.8 [26.9,47.6] | 56.5 [47.6,67.1] | 51.1 [42.9,60.8] | 45.2 [36.8,55.4] | 64.0 [46.2,88.6] |
| 2-OH-fluorene | 2.6 [2.37,2.85] | 2.82 [2.07,3.83] | 2.53 [2.06,3.09] | 2.58 [2.32,2.88] | 2.53 [2.31,2.76] | 2.45 [2.17,2.76] | 2.68 [2.36,3.04] |
| 3-OH-fluorene | 1.1 [1,1.22] | 1.05 [0.75,1.48] | 1.02 [0.83,1.25] | 1.14 [1.01,1.28] | 1.06 [0.96,1.16] | 1.02 [0.9,1.16] | 1.13 [0.97,1.3] |
| 9-OH-fluorene | 2.57 [2.31,2.87] | 2.89 [2.14,3.9] | 2.64 [2.01,3.45] | 2.5 [2.19,2.85] | 2.49 [2.25,2.76] | 2.45 [2.15,2.8] | 2.56 [2.19,2.99] |
| Sum OH-fluorene | 6.49 [5.93,7.12] | 6.95 [5.17,9.35] | 6.44 [5.24,7.93] | 6.42 [5.75,7.18] | 6.28 [5.75,6.85] | 6.12 [5.44,6.88] | 6.58 [5.81,7.45] |
| 1-OH-phenanthene | 1.6 [1.45,1.77] | 1.7 [1.24,2.32] | 1.62 [1.28,2.05] | 1.58 [1.4,1.78] | 1.53 [1.39,1.68] | 1.5 [1.31,1.71] | 1.59 [1.39,1.82] |
| 2-OH-phenanthene | 1.09 [0.99,1.21] | 1.28 [0.91,1.8] | 1.07 [0.85,1.34] | 1.06 [0.94,1.2] | 1.03 [0.93,1.14] | 0.98 [0.86,1.13] | 1.13 [0.97,1.31] |
| 3-OH-phenanthene | 1.18 [1.06,1.31] | 1.26 [0.89,1.77] | 1.12 [0.9,1.39] | 1.18 [1.04,1.34] | 1.18 [1.07,1.3] | 1.13 [1,1.29] | 1.26 [1.09,1.47] |
| 4-OH-phenanthene | 0.27 [0.24,0.3] | 0.27 [0.19,0.4] | 0.24 [0.19,0.3] | 0.28 [0.25,0.32] | 0.26 [0.24,0.29] | 0.26 [0.23,0.3] | 0.27 [0.23,0.32] |
| Sum OH-phenanthene | 4.22 [3.83,4.65] | 4.64 [3.37,6.38] | 4.12 [3.31,5.12] | 4.17 [3.71,4.69] | 4.09 [3.72,4.49] | 3.96 [3.49,4.5] | 4.32 [3.76,4.97] |
| 1-OH-pyrene | 2.52 [2.25,2.83] | 3.02 [2.14,4.27] | 2.46 [1.89,3.2] | 2.46 [2.13,2.83] | 2.66 [2.38,2.96] | 2.59 [2.24,2.99] | 2.79 [2.37,3.29] |
1-Naphthol results in two participants in the control group were non-reportable because of chromatographic interference.
Two urine samples in the need-repair intervention group did not have creatinine results.
2-Naphthol in the Control group was significantly higher than the Intervention group (p < 0.001).
Among the 334 participants in this study, 168 in control and 145 in intervention groups (total n = 313) had valid 48-h personal CO samples that were reported previously (Commodore et al., 2013). All urinary PAH metabolites were significantly associated with personal CO level (p-values ranged <0.01 to 0.049, Supplemental Material, Table S3), another indicator commonly used to characterize HAP exposure. However, the correlations were weak, with Pearson’s correlation coefficients ranging 0.12–0.24 (Supplemental Material, Table S3).
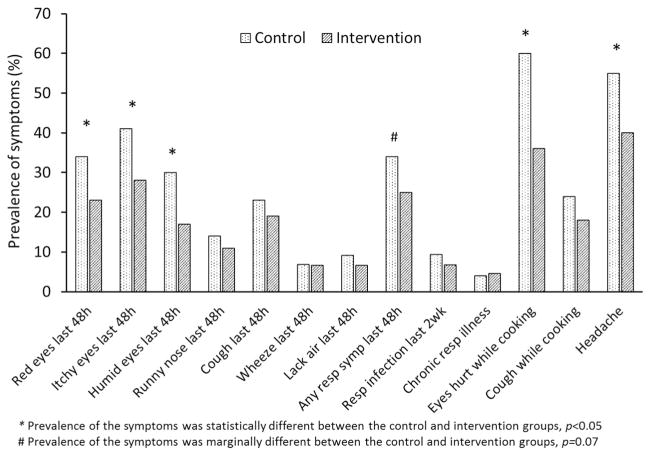
Fig. 1 gives the prevalence of self-reported health symptoms. Participants in the control group had significantly higher prevalence of headache and eye-related symptoms compared to the intervention group. The control group also had marginally higher prevalence (p = 0.07) of recent respiratory symptoms (cough, wheeze, lack of air, or runny nose within the past 48 h).
Fig. 1.
Prevalence of self-reported health symptoms in the control and intervention groups.
Table 3 gives the adjusted odds ratios (ORs) and 95% CI for associations between health symptoms and HAP exposure, controlling for potential risk factors. Only results with p < 0.10 are shown in the table. Stove type (control vs. intervention) was positively associated with itchy eye (2.05 [1.09, 3.88]), humid eye (1.87 [0.90, 3.88]), eyes hurt while cooking (3.37 [1.83, 6.20]), and headache (2.31 [1.29, 4.12]). Urinary 2-naphthol was positively associated with red eye (3.8 [1.32, 10.9]), itchy eye (3.28 [1.15, 9.38]), humid eye (3.93 [1.18, 13.1]), and eyes hurt while cooking (2.32 [0.88, 6.10]). Urinary fluorene metabolite was associated with headache (3.47 [0.93, 12.9]) and recent respiratory symptoms (3.45 [0.83, 14.4]). 1-Hydroxypyrene was negatively associated with cough while cooking (0.34 [0.13, 0.91]).
Table 3.
Results of logistic regression for HAP exposure (using urinary OH-PAHs as exposure biomarkers) and potential risk factors on self-reported health symptoms.
| Self-reported symptoms | N | Variablesa | OR [95%CI] | pc |
|---|---|---|---|---|
| Red eye in the last 48 h | yes: 68 | 2-naphthol | 3.80 [1.32, 10.9] | 0.013 |
| no: 163 | Woman age | 1.04 [1.00, 1.08] | 0.069 | |
| Fertilizer use | 3.65 [1.97, 6.79] | 0.000 | ||
| Itchy eye in the last 48 h | yes: 82 | 2-naphthol | 3.28 [1.15, 9.38] | 0.027 |
| no: 150 | Stove type, Control vs Intervention | 2.05 [1.09, 3.88] | 0.027 | |
| Second-hand smoke | 4.30 [1.64, 11.3] | 0.003 | ||
| Fertilizer use | 3.10 [1.70, 5.66] | 0.000 | ||
| Humid eye in the last 48 h | yes: 58 | 2-naphthol | 3.93 [1.18, 13.1] | 0.026 |
| no: 174 | Woman age | 1.06 [1.01, 1.11] | 0.011 | |
| Stove type, Control vs Intervention | 1.87 [0.90, 3.88] | 0.096 | ||
| Wood type, Eucalipto or Hualango vs Other | 2.11 [0.91, 4.86] | 0.081 | ||
| Second-hand smoke | 6.18 [2.24, 17.0] | 0.000 | ||
| Fertilizer use | 3.80 [1.89, 7.61] | 0.000 | ||
| Runny nose in the last 48 h | yes: 31 | Distance home-to-road, <=20 m vs 20–200 m | 3.03 [1.21, 7.58] | 0.010 |
| no: 201 | Grilled food in the last 48 h | 3.41 [1.00, 11.6] | 0.050 | |
| Cough in the last 48 h | yes: 54 | Fertilizer use | 2.85 [1.48, 5.48] | 0.002 |
| no: 178 | ||||
| Wheeze in the last 48 h | yes: 19 | 1-hydroxypyrene | 0.25 [0.06, 1.05] | 0.059 |
| no: 213 | ||||
| Lack of air/difficult to breathe in the last 48 h | yes: 20 | 1-hydroxypyrene | 0.23 [0.05, 1.05] | 0.057 |
| no: 212 | Second-hand smoke | 3.41 [1.06, 10.9] | 0.039 | |
| Pesticide use | 3.57 [1.31, 9.77] | 0.013 | ||
| Any recent respiratory symptomb | yes: 74 | Sum OH-fluoreneb | 3.45 [0.83, 14.4] | 0.089 |
| no: 158 | 1-hydroxypyrene | 0.45 [0.18, 1.11] | 0.083 | |
| Traffic in community, 24+ cars/day vs <3 per week | 3.77 [1.11, 12.8] | 0.026 | ||
| Fertilizer use | 2.37 [1.31, 4.30] | 0.004 | ||
| Respiratory infection in the last two weeks | yes: 18 | Wood type, Eucalipto or Hualango vs Other | 0.30 [0.10, 0.89] | 0.030 |
| no: 213 | Grilled food in the last 48 h | 9.48 [2.57, 35.0] | 0.001 | |
| Chronic respiratory illness | yes: 7 | Pesticide use | 7.11 [1.32, 38.2] | 0.022 |
| no: 225 | ||||
| Eyes hurt while cooking | yes: 114 | 2-naphthol | 2.32 [0.88, 6.10] | 0.088 |
| no: 117 | Woman age | 1.04 [1.00, 1.09] | 0.040 | |
| Stove type, Control vs Intervention | 3.37 [1.83, 6.20] | 0.000 | ||
| Second-hand smoke | 2.28 [0.87, 5.98] | 0.094 | ||
| Fertilizer use | 2.09 [1.17, 3.75] | 0.013 | ||
| Cough while cooking | yes: 55 | 1-hydroxypyrene | 0.34 [0.13, 0.91] | 0.031 |
| no: 176 | Fertilizer use | 2.75 [1.44, 5.26] | 0.002 | |
| Headache | yes: 111 | Sum OH-fluoreneb | 3.47 [0.93, 12.9] | 0.063 |
| no: 121 | Woman age | 1.04 [1.00, 1.08] | 0.042 | |
| Stove type, Control vs Intervention | 2.31 [1.29, 4.12] | 0.005 |
Variables considered for each model include: woman age, stove type, CO, wood type, second-hand smoke, home-to-road distance, pesticide use, fertilizer use, enclosed kitchen, grilled food, and creatinine-adjusted 1-naphthol, 2-naphthol, 1-hydroxypyrene, summed OH-fluorenes, and summed OH-phenanthrenes.
Any recent respiratory symptoms include cough, wheeze, lack of air, or runny nose within the past 48 h.
Only results either statistically significant (p < 0.05) or marginally significant (0.05 < p < 0.10) are included.
4. Discussion
We evaluated HAP exposure and self-reported symptoms 6–8 months after the installation of intervention stoves and studied the association of HAP exposure with the symptoms. We used urinary PAH metabolites as HAP exposure biomarkers and measured 1-hydroxypyrene and nine other commonly detected OH-PAHs that are present at higher concentrations than 1-hydroxypyrene in non-occupationally exposed populations (CDC, 2015). The communities in this study were all located in rural areas with minimal automobile emissions and industrial activities. The participants were all non-smokers. Over 90% of the participants reported no exposure to second-hand smoke and had not eaten any grilled food (known to contain high levels of PAHs) in the 48 h before sampling. Hence, the PAH biomarkers investigated in this study likely resulted predominantly from HAP exposure.
4.1. Assessment of HAP exposure using urinary biomarkers
Among all urinary OH-PAHs, 2-naphthol, a proposed biomarker for inhalational exposure (Kang et al., 2002; Li et al., 2016), had the largest reduction in the intervention group compared to the control group (p < 0.001). Moreover, the extent of reduction increased by 42% when comparing the two sub-groups with presumably the most and least HAP exposure, i.e. traditional stove users within the control group vs. no-repair stove users within the intervention group. The findings are encouraging, especially considering the relatively small sample size, the cross-sectional study design, and considerably large within- and between-person variabilities reported for urinary metabolites (Li et al., 2010b; Siwinska et al., 1998). Urinary naphthols (1- and 2-naphthols) had been reported to give better selectivity and sensitivity for route-specific inhalation PAH exposure than 1-hydroxypyrene (Jansen et al., 1996; Yang et al., 1999). While 1-naphthol can result from naphthalene exposure, it is also a major metabolite of the wide-spectrum carbamate insecticide (Maroni et al., 2000). In contrast, 2-naphthol only results from naphthalene exposure. Additionally, 2-naphthol has shown the expected rise-fall excretion pattern and demonstrated the largest increase compared to other OH-PAHs after a controlled low inhalation exposure (Li et al., 2016). Therefore, 2-naphthol has been suggested as a more suitable biomarker for inhalational PAH exposure than 1-hydroxypyrene and other OH-PAHs (Li et al., 2016; Preuss et al., 2003; Yang et al., 1999). Hence, it is not surprising that 2-naphthol showed the largest difference between control and intervention in this study on HAP exposure. In another stove intervention project in Peru, 2-naphthol also reached a larger reduction than other PAH metabolites three weeks after installing new chimney-equipped stoves (Li et al., 2011).
For the remaining OH-PAHs, there was no significant difference between control and intervention groups, which is consistent with the personal CO results from these participants reported previously (Commodore et al., 2013). Among the 313 participants with both urine and 48-h personal CO measurements, urinary OH-PAHs were significantly associated with the CO. This is consistent with the fact that both PAHs and CO are components in biomass smoke from woodstoves. The weak correlations between urine OH-PAHs and CO can be explained by several factors. The urinary PAH metabolites reflect mostly exposure occurred within a day, due to their short excretion half-lives (4.0–23.5 h) after inhalation exposure (Brzeznicki et al., 1997; Lafontaine et al., 2000; Li et al., 2016; St Helen et al., 2012). In contrast, the CO data in the study were collected over the 48-h period before the urine sampling. In addition, while CO is in the gaseous form entirely, airborne PAHs exist in both gaseous and particle phases. Finally, urinary PAH metabolites reflect PAH exposure from all routes including inhalation, diet and dermal absorption.
The differences in urinary OH-PAHs between the control and intervention in this study are less than those in other stove studies that used PAH metabolites as HAP exposure biomarkers. In an un-related stove intervention study in Peru, the same suite of urinary OH-PAHs were significantly reduced by 19–52% in 57 women three weeks after the installation of new stove to replace the open-fire stoves (Li et al., 2011). In a randomized control trial in Mexico, these 10 biomarkers were reduced by 20–48% post-intervention in 47 women, compared to pre-intervention (Riojas-Rodriguez et al., 2011). A three-stage stove intervention program (removing indoor soot, paving dirt floors, and installing new stoves with chimney) in Mexico found a 29% reduction of 1-hydroxypyrene in 20 residents one month after intervention (Torres-Dosal et al., 2008).
Several factors could lead to the smaller observed reduction in our studies compared to other studies. First and foremost, we did not conduct exposure assessment before the installation of the new stove, and therefore, did not have pre- and post-measurements (self-control) that were available in the above-mentioned studies. Hence, it is not surprising that we observed less differences between intervention and control groups in this cross-sectional study. In addition, our investigation was conducted 6–8 months after the new stoves were installed, compared to few weeks in other studies. The longer lag between intervention and follow-up would likely diminish the observed difference between new and old stoves due to the wear and tear of the new stoves; however, the longer lag can provide valuable information on the long-term effectiveness of the intervention. As shown in this study, durability of the new stoves may be of concern given that over a third of the new stoves were in need of repair at the time of the study.
4.2. Association of HAP exposure with self-reported health symptoms
To the best of our knowledge, this is the first study that investigated the relationship between urinary PAH metabolites (as HAP exposure biomarkers), potential risk factors and self-reported respiratory and eye symptoms in a non-smoking population. We found reduced headache and eye-related symptoms in the intervention group compared to the control group. Stove type (control vs. intervention) and/or 2-naphthol were positively associated with eye symptoms and headache. This is consistent with previous reports that HAP exposure is significantly associated with eye irritations (West et al., 2013). Our finding also demonstrates that the ocular symptoms may be reduced or eliminated through the use of improved stoves.
We did not find statistically significant associations between urinary exposure biomarkers and/or stove type with respiratory symptoms or illnesses. Potential explanations include small number of participants reporting respiratory symptoms or illnesses (Table 3) and possibly longer latency period for respiratory symptoms than eye irritations. As expected, several risk factors were significantly associated with health symptoms. For example, participant’s age was positively associated with red eyes, humid eyes, achy eyes while cooking, and headache. Fertilizer and/or pesticide use were positively associated with a number of respiratory and eye symptoms, potentially due to irritations from fertilizer/pesticide application and usage.
Surprisingly, 1-hydroxypyrene, the most used biomarker for PAH exposure, was negatively associated with cough while cooking. It has been reported that in general populations with no occupational exposure, pyrene is mainly taken up through diet (Jansen et al., 1996; Li et al., 2010a), and urinary 1-hydroxypyrene can act as a route specific biomarker for oral or dermal exposure (Jansen et al., 1996). In this study on inhalational HAP exposure, we did not observe a difference on 1-hydroxypyrene levels between the intervention and control groups, and found opposite effect on association with certain respiratory systems, which suggested that non-inhalational route, e.g., touching surfaces with soot deposit and eating burnt food or food cooked in pots with soot deposit, may be important additional factors affecting 1-hydroxypyrene levels.
Our findings on HAP exposure and sensory irritative symptoms are generally consistent with other stove intervention studies that investigated the impact of improved stoves on women’s respiratory and/or eye health, although those studies used other surrogates to characterize HAP exposure, such as stove type or PM2.5/CO. In a study titled “RESPIRE” in Guatemala, replacing traditional three-stone stoves with chimney-equipped stoves significantly reduced sore eyes and headache 6–18 months after the intervention (Diaz et al., 2007). The study also found reductions of the risk of wheeze and total number of respiratory symptoms, but did not observe significant effects on lung function within a 1.5-year follow-up period (Smith-Sivertsen et al., 2009). In a follow-up study within the RESPIRE cohort, exhaled CO and/or personal CO was significantly associated with several respiratory symptoms, such as wheeze and chronic phlegm (Pope et al., 2015). In a randomized control trial in Mexico, women who used new chimney-equipped stoves most of time had significantly lower risk of respiratory symptoms, eye discomfort, and headache; the use of new stoves was significantly associated with improved lung function comparable to smoking cessation (Romieu et al., 2009). In a cross-sectional survey among 79 Honduran women, users of improved stoves had lower levels of PM2.5 and CO and lower prevalence of self-reported respiratory symptoms compared to open-fire stove users, but no association was found between stove type or air measurements with lung function or C-reactive protein (Clark et al., 2009). Our study findings demonstrate the utility of urinary PAH metabolites as effective exposure biomarkers for evaluation and monitoring purposes in stove intervention projects.
4.3. Comparison of urinary OH-PAHs to other studies
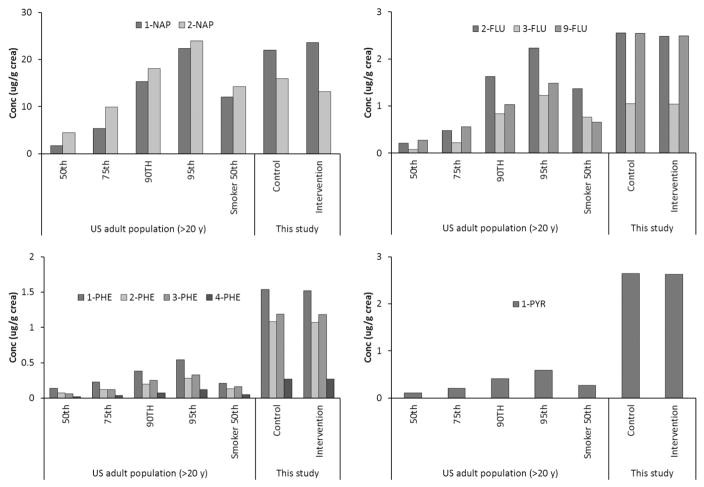
The urinary PAH levels in this study far exceeded the general population levels in national surveys in the U.S. (CDC, 2015), Canada (Health Canada, 2015), and Germany (Becker et al., 2003), c.f. Supplemental Material Table S4. As shown in Fig. 2, median concentrations of the 10 OH-PAHs were equivalent or several times higher than the 95th percentile in the U.S. adults reported in NHANES 2011–2012 (CDC, 2015).
Fig. 2.
Comparison of the median OH-PAH levels in this study to selected percentiles (50th, 75th, 90th, and 95th) and median levels among smokers in the U.S. adult population (CDC, 2015).
Compared to other populations’ exposure to woodsmoke (Table 4), the 1-hydroxypyrene level in our study (median: 2.63 μg/g creatinine) was consistent with those in HAP-exposed populations in Afghanistan (Hemat et al., 2012), Burundi (Viau et al., 2000), Poland (Siwinska et al., 1999), and Mexico (Pruneda-Alvarez et al., 2012), but was several times higher than several Mexican studies on non-smoking women exposed to HAP (Pruneda-Alvarez et al., 2016; Ruiz-Vera et al., 2014, 2015). Compared to occupational exposure to biomass smoke, the 1-hydroxypyrene level in our study was similar to that of workers exposed to rubber woodsmoke in Thailand (Choosong et al., 2014), and was 10 times higher than among charcoal workers in Brazil (Kato et al., 2004).
Table 4.
Median 1-hydroxypyrene concentrations (creatinine-adjusted, μg/g creatinine) in select studies.
| Population | N | Median | Reference |
|---|---|---|---|
| Non-smoking women using woodstoves, Peru | 332 | 2.63 | This study |
| Other studies on populations exposed to wood smoke | |||
| Non-smoking women with old woodstoves without chimney, Peru | 57 | 3.2 | Li et al. (2011) |
| Non-smoking women with chimney-equipped woodstove, Peru | 57 | 2.5 | Li et al. (2011) |
| Non-smoking women with indoor open-fire woodstoves, Afghanistan | 15 | 3.82 | Hemat et al. (2012) |
| Adults using 3-stone woodstoves, Burundi | 18 | 2.89a | Viau et al. (2000) |
| Non-smoking women using indoor open-fire woodstoves, Mexico | 38 | 2.44 | Pruneda-Alvarez et al. (2012) |
| Non-smoking women with biomass as primary energy source, Mexico | 50 | 0.79 | Pruneda-Alvarez et al. (2016) |
| Non-smoking women using wood as the sole energy source, Mexico | 30 | 0.25 | Ruiz-Vera et al. (2014) |
| Non-smoking women using indoor open-fire woodstoves, Mexico | 40 | 0.89c | Ruiz-Vera et al. (2015) |
| Non-smoking workers exposed to rubber wood smoke, Thailand | 41 | 2.04c | Choosong et al. (2014) |
| Charcoal workers exposed to wood smoke, Brazil | 100 | 0.25a | Kato et al. (2004) |
| Children and adults, pre–/post-stove intervention, Mexico | 20 | 13/9.3a | Torres-Dosal et al. (2008) |
| Children in households with biomass as primary energy source, Mexico | 105 | 6.27c | Martinez-Salinas et al. (2010) |
| Children in households with coal stove, Poland | 194 | 1.09b | Siwinska et al. (1999) |
| Reference levels from national surveys | |||
| U.S. adult population (≥20 years) | 2485 | 0.11 | CDC (2015) |
| U.S. adult smokers | 889 | 0.27 | CDC (2015) |
| Canadian population (3–79 years) | 2412 | 0.09 | Health Canada (2015) |
| German adult population (18–69 years) | 573 | 0.10 | Becker et al. (2003) |
| German adult smokers (18–69 years) | 184 | 0.31 | Becker et al. (2003) |
geometric mean;
least square geometric mean;
mean concentration.
Other OH-PAHs were rarely reported in published woodsmoke exposure studies (Supplemental Materials, Table S4). The levels of the 9 other OH-PAHs in this study were similar to another Peruvian stove intervention study (Li et al., 2011). Summed phenanthrene metabolite concentration in this study was consistent with that in a study in Afghanistan (Hemat et al., 2012). However, 2-naphthol in this study (GM: 14.9 μg/g creatinine) was higher than a study on charcoal worker (GM: 9.14 μg/g creatinine) with occupational exposure to woodsmoke (Kato et al., 2004).
These comparisons suggested that people exposed to HAP from indoor woodstoves are among the populations with the highest non-occupational exposure to PAHs (Martinez-Salinas et al., 2010; Pruneda-Alvarez et al., 2016), a group of carcinogenic and mutagenic pollutants formed from incomplete combustions. Furthermore, the median 1-hydroxypyrene in this study was higher than 1.93 μg/g creatinine (1.0 μmol/mol creatinine), a proposed no-observed-genotoxic-effect-level for occupational exposure to PAHs (Jongeneelen, 2014), which indicates an increased risk of cancer among the study subjects.
Several factors may explain the high HAP exposure in this study. First, the chimney-equipped stoves generally do not completely release woodsmoke outside of the house/kitchen, do not improve the combustion efficiency and thus cannot reduce the formation of harmful air pollutants such as PAHs. Second, 36% of the new stoves in this study required some repair at the time of the study (6–8 months after installation), which could affect the effectiveness of exposure reduction. The new stoves were built from materials such as mud, straw and donkey manure. These materials, although readily available, may not be durable for long-term usage. Third, some participants in the intervention group may have used both the new and old stoves at the same time, a practice called “stove-stacking” (Thomas et al., 2013). Although we did not assess the stove usage and adherence in this study, a subsequent study conducted in the same area—including the households in this study—revealed that 32.6% used a traditional stove as the secondary stove (unpublished data). Stove-stacking and adherence issues have also been noted in other stove projects (Romieu et al., 2009; Thomas et al., 2013). Many factors, such as cultural and personal cooking habits, needs and preferences, user training and support, could adversely affect adopting of the new stoves (Lewis and Pattanayak, 2012; Rehfuess et al., 2014) and reduce the benefit of stove-intervention programs. Fourth, although the stoves were replaced, the surfaces in the house may still be contaminated with soot known to contain PAHs, which would lead to continued dermal PAH exposure. Lastly, most study households had permeable roofs and open windows. Therefore, biomass smoke from the chimney and from neighbors could enter or re-enter the kitchens in the intervention households.
4.4. Limitations
There are several limitations in the current study. First, exposure assessment was not conducted before the new stove installation in the intervention households. Pre- and post-intervention assessments would allow for stronger evidence than the cross-sectional data on the effectiveness of the intervention. Second, the current study did not have data to describe stove-stacking and could not assess factors and barriers affecting the adoption of the new stoves. Third, potential recall bias could occur in participants’ self-reporting of health symptoms. Lastly, the study had a small number of participants and an even smaller number of women reporting symptoms. Larger studies with more participants would increase the power of the analyses.
5. Conclusions
To the best of our knowledge, this is the first study that investigated the relationship between urinary PAH metabolites (as HAP exposure biomarkers), potential risk factors and self-reported respiratory and eye symptoms in a non-smoking population exposed to high levels of HAP. We observed reduced headache and ocular symptoms among the new stove users in the intervention group than the control group. Urinary 2-naphthol, a suggested biomarker for inhalation PAH exposure, was significantly lower in the intervention group than the control group. Stove type (control vs. intervention) and/or urinary 2-naphthol were positively associated with self-reported headache and eye symptoms. Even with the improved stoves, the PAH biomarker levels in this study were severely elevated. Further, the median 1-hydroxypyrene level was higher than a proposed no-observed-genotoxic-effect-level for occupational PAH exposure, which indicates an increased risk of cancer among the study subjects. Stove intervention studies should include a variety of tools, such as exposure assessment, to evaluate the effectiveness of the program and help understand the health burden associated with HAP.
Supplementary Material
Acknowledgments
Acknowledgements and Disclaimer
We thank the study participants; Instituto de Investigacion Nutricional staff for logistical help; Hector Verastegui for dataset compilation; field coordinator Selenne Flores, field workers, volunteers, Manual Aguilar Villalobos and Corey Butler for successful fieldwork; Adam Gray for data management; Christopher Fitzgerald for field training; Regina Saavedra, Gayle Lennox, and Anna Adetona for data compilation; Pam Olive for laboratory creatinine measurements.
This work was made possible by NIH Research Grant #5-D43TW005746–04 funded by the Fogarty International Center, National Institutes on Environmental Health Services. This work was also supported by the UBS Optimus Foundation for the parent study (ISRCTN28191222) and grant #2T42OH008436 from the National Institute for Occupational Safety and Health through the University of Alabama, Birmingham. Additional funding was from the University of Georgia Graduate School and Interdisciplinary Toxicology Program. The co-authors of this manuscript do not have any financial conflict of interest with any parties involved in this study. The findings and conclusions in this report are those of the authors and do not necessarily represent the position of the CDC and the Agency for Toxic Substances and Disease Registry.
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- OH-PAH
hydroxylated polycyclic aromatic hydrocarbon metabolite
- CO
carbon monoxide
- PM2.5
fine particulate matter with aerodynamic diameters <2.5 μm
- HAP
household air pollution
- c-RCT
community randomized control trial
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2016.09.011.
References
- Amegah AK, Quansah R, Jaakkola JJ. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PLoS One. 2014;9:e113920. doi: 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzales AJ, Needham LL, Pirkle JL. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Schulz C, Kaus S, Seiwert M, Seifert B. German Environmental Survey 1998 (GerES III): environmental pollutants in the urine of the German population. Int J Hyg Environ Health. 2003;206:15–24. doi: 10.1078/1438-4639-00188. [DOI] [PubMed] [Google Scholar]
- Bruce N, Dherani M, Liu R, Hosgood HD, III, Sapkota A, Smith KR, et al. Does household use of biomass fuel cause lung cancer? A systematic review and evaluation of the evidence for the GBD 2010 study. Thorax. 2015;70:433–441. doi: 10.1136/thoraxjnl-2014-206625. [DOI] [PubMed] [Google Scholar]
- Brzeznicki S, Jakubowski M, Czerski B. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 1997;70:257–260. doi: 10.1007/s004200050216. [DOI] [PubMed] [Google Scholar]
- CDC. National report on human exposure to environmental chemicals, updated tables, February 2015. National Center for Environmental Health, Centers for Disease Control and Prevention; 2015. [Google Scholar]
- Choosong T, Phakthongsuk P, Tekasakul S, Tekasakul P. Urinary 1-hydroxypyrene levels in workers exposed to polycyclic aromatic hydrocarbon from rubber wood burning. Saf Health Work. 2014;5:86–90. doi: 10.1016/j.shaw.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Burch JB, Nelson TL, Robinson MM, Conway S, et al. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. Int J Environ Health Res. 2009;19:357–368. doi: 10.1080/09603120902842705. [DOI] [PubMed] [Google Scholar]
- Commodore AA, Hartinger SM, Lanata CF, Mausezahl D, Gil AI, Hall DB, et al. Carbon monoxide exposures and kitchen concentrations from cookstove-related woodsmoke in San Marcos, Peru. Int J Occup Environ Health. 2013;19:43–54. doi: 10.1179/2049396712Y.0000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MA, Mehta S, Smith KR. Indoor smoke from solid fuels: Assessing the environmental burden of disease at national and local levels. World Health Organization; Geneva: 2004. [Google Scholar]
- Diaz E, Smith-Sivertsen T, Pope D, Lie RT, Diaz A, McCracken J, et al. Eye discomfort, headache and back pain among Mayan Guatemalan women taking part in a randomised stove intervention trial. J Epidemiol Community Health. 2007;61:74–79. doi: 10.1136/jech.2006.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger SM, Lanata CF, Hattendorf J, Gil AI, Verastegui H, Ochoa T, et al. A community randomised controlled trial evaluating a home-based environmental intervention package of improved stoves, solar water disinfection and kitchen sinks in rural Peru: rationale, trial design and baseline findings. Contemp Clin Trials. 2011;32:864–873. doi: 10.1016/j.cct.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Hartinger SM, Lanata CF, Gil AI, Hattendorf J, Verastegui H, Mausezahl D. Combining interventions: improved stove, kitchen sinks and solar disinfection of drinking water and kitchen cloths to improve hygiene in rural Peru. Field Actions Science Reports. 2012 [Online]. Available at: http://factsreports.revues.org/1627.
- Hartinger SM, Commodore AA, Hattendorf J, Lanata CF, Gil AI, Verastegui H, et al. Chimney stoves modestly improved indoor air quality measurements compared with traditional open fire stoves: results from a small-scale intervention study in rural Peru. Indoor Air. 2013;23:342–352. doi: 10.1111/ina.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Third Report on Human Biomonitoring of Environmental Chemicals in Canada - Results of the Canadian Health Measures Survey Cycle. Vol. 3. Ottawa, ON, Canada: 2015. pp. 2012–2013. [Google Scholar]
- Hemat H, Wittsiepe J, Wilhelm M, Muller J, Goen T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J Expo Sci Environ Epidemiol. 2012;22:46–51. doi: 10.1038/jes.2011.33. [DOI] [PubMed] [Google Scholar]
- Jansen EH, Schenk E, Den Engelsman G, Van De Werken G. Route-specific urinary biomarkers in the risk assessment of PAH exposure. Polycycl Aromat Compd. 1996;11:185–192. [Google Scholar]
- Jongeneelen FJ. A guidance value of 1-hydroxypyrene in urine in view of acceptable occupational exposure to polycyclic aromatic hydrocarbons. Toxicol Lett. 2014;231:239–248. doi: 10.1016/j.toxlet.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Josyula S, Lin J, Xue X, Rothman N, Lan Q, Rohan TE, et al. Household air pollution and cancers other than lung: a meta-analysis. Environ Health. 2015;14:24. doi: 10.1186/s12940-015-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JW, Cho SH, Kim H, Lee CH. Correlation of urinary 1-hydroxypyrene and 2-naphthol with total suspended particulates in ambient air in municipal middle-school students in Korea. Arch Environ Health. 2002;57:377–382. doi: 10.1080/00039890209601425. [DOI] [PubMed] [Google Scholar]
- Kato M, Loomis D, Brooks LM, Gattas GFJ, Gomes L, Carvalho AB, et al. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiol Biomark Prev. 2004;13:1005–1012. [PubMed] [Google Scholar]
- Kurmi OP, Semple S, Simkhada P, Smith WC, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65:221–228. doi: 10.1136/thx.2009.124644. [DOI] [PubMed] [Google Scholar]
- Lafontaine M, Payan JP, Delsaut P, Morele Y. Polycyclic aromatic hydrocarbon exposure in an artificial shooting target factory: assessment of 1-hydroxypyrene urinary excretion as a biological indicator of exposure. Ann Occup Hyg. 2000;44:89–100. [PubMed] [Google Scholar]
- Lewis JJ, Pattanayak SK. Who adopts improved fuels and cookstoves? A systematic review. Environ Health Perspect. 2012;120:637–645. doi: 10.1289/ehp.1104194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD, et al. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. J Environ Monit. 2010a;12:1110–1118. doi: 10.1039/c000689k. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Lewin MD, Pittman EN, Trinidad D, Needham LL, et al. Variability of Urinary Polycyclic Aromatic Hydrocarbon Metabolite Levels in Adults and Comparison of Spot, First-Morning, and 24-Hour Void Sampling. J Expo Sci Environ Epidemiol. 2010b;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sjodin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, et al. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int. 2011;37:1157–1163. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Pittman EN, Hilton D, Hubbard K, et al. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal Bioanal Chem. 2014;406:3119–3129. doi: 10.1007/s00216-014-7676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Trinidad D, Pittman EN, Riley EA, Sjodin A, Dills RL, et al. Urinary poly-cyclic aromatic hydrocarbon metabolites as biomarkers to woodsmoke exposure - results from a controlled exposure study. J Expo Sci Environ Epidemiol. 2016;26:241–248. doi: 10.1038/jes.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni M, Colosio C, Ferioli A, Fait A. Biological monitoring of pesticide exposure: a review. Toxicology. 2000;143:5–118. doi: 10.1016/s0300-483x(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Salinas RI, Elena LM, Batres-Esquivel LE, Dominguez-Cortinas G, Calderon J, Diaz-Barriga F, et al. Exposure of children to polycyclic aromatic hydrocarbons in Mexico: assessment of multiple sources. Int Arch Occup Environ Health. 2010;83:617–623. doi: 10.1007/s00420-009-0482-x. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, et al. Woodsmoke health effects: A review. Inhal Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32:70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Pope D, Diaz E, Smith-Sivertsen T, Lie RT, Bakke P, Balmes JR, et al. Exposure to Household Air Pollution from Wood Combustion and Association with Respiratory Symptoms and Lung Function in Nonsmoking Women: Results from the RESPIRE Trial, Guatemala. Environ Health Perspect. 2015;123:285–292. doi: 10.1289/ehp.1408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss R, Angerer J, Drexler H. Naphthalene - an environmental and occupational toxicant. Int Arch Occup Environ Health. 2003;76:556–576. doi: 10.1007/s00420-003-0458-1. [DOI] [PubMed] [Google Scholar]
- Pruneda-Alvarez LG, Perez-Vazquez FJ, Salgado-Bustamante M, Martinez-Salinas RI, Pelallo-Martinez NA, Perez-Maldonado IN. Exposure to indoor air pollutants (polycyclic aromatic hydrocarbons, toluene, benzene) in Mexican indigenous women. Indoor Air. 2012;22:140–147. doi: 10.1111/j.1600-0668.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- Pruneda-Alvarez LG, Perez-Vazquez FJ, Ruiz-Vera T, Ochoa-Martinez AC, Orta-Garcia ST, Jimenez-Avalos JA, et al. Urinary 1-hydroxypyrene concentration as an exposure biomarker to polycyclic aromatic hydrocarbons (PAHs) in Mexican women from different hot spot scenarios and health risk assessment. Environ Sci Pollut Res Int. 2016;23:6816–6825. doi: 10.1007/s11356-015-5918-0. [DOI] [PubMed] [Google Scholar]
- Rehfuess EA, Puzzolo E, Stanistreet D, Pope D, Bruce NG. Enablers and barriers to large-scale uptake of improved solid fuel stoves: a systematic review. Environ Health Perspect. 2014;122:120–130. doi: 10.1289/ehp.1306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, Masera O, Li Z, Romanoff L, et al. Impact of the improved patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural Mexican women. Environ Health Perspect. 2011;119:1301–1307. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Riojas-Rodriguez H, Marron-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved Biomass Stove Intervention in Rural Mexico Impact on the Respiratory Health of Women. Am J Respir Crit Care Med. 2009;180:649–656. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- Rosa G, Majorin F, Boisson S, Barstow C, Johnson M, Kirby M, et al. Assessing the impact of water filters and improved cook stoves on drinking water quality and household air pollution: a randomised controlled trial in Rwanda. PLoS One. 2014;9:e91011. doi: 10.1371/journal.pone.0091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Vera T, Pruneda-Alvarez LG, Perez-Vazquez FJ, Ochoa-Martinez AC, Orta-Garcia ST, Ilizaliturri-Hernandez CA, et al. Using urinary 1-hydroxypyrene concentrations to evaluate polycyclic aromatic hydrocarbon exposure in women using biomass combustion as main energy source. Drug Chem Toxicol. 2014:1–6. doi: 10.3109/01480545.2014.968932. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vera T, Pruneda-Alvarez LG, Ochoa-Martinez AC, Ramirez-GarciaLuna JL, Pierdant-Perez M, Gordillo-Moscoso AA, et al. Assessment of vascular function in Mexican women exposed to polycyclic aromatic hydrocarbons from wood smoke. Environ Toxicol Pharmacol. 2015;40:423–429. doi: 10.1016/j.etap.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Rylance J, Gordon SB, Naeher LP, Patel A, Balmes JR, Adetona O, et al. Household air pollution: a call for studies into biomarkers of exposure and predictors of respiratory disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L571–L578. doi: 10.1152/ajplung.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwinska E, Mielzynska D, Smolik E, Bubak A, Kwapulinski J. Evaluation of intra- and interindividual variation of urinary 1-hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons. Sci Total Environ. 1998;217:175–183. doi: 10.1016/s0048-9697(98)00186-7. [DOI] [PubMed] [Google Scholar]
- Siwinska E, Mielzynska D, Bubak A, Smolik E. The effect of coal stoves and environmental tobacco smoke on the level of urinary 1-hydroxypyrene. Mutat Res. 1999;445:147–153. doi: 10.1016/s1383-5718(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Smith-Sivertsen T, Diaz E, Pope D, Lie RT, Diaz A, McCracken J, et al. Effect of Reducing Indoor Air Pollution on Women’s Respiratory Symptoms and Lung Function: The RESPIRE Randomized Trial, Guatemala. Am J Epidemiol. 2009;170:211–220. doi: 10.1093/aje/kwp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, III, Benowitz NL. Exposure and Kinetics of Polycyclic Aromatic Hydrocarbons (PAHs) in Cigarette Smokers. Chem Res Toxicol. 2012;25:952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Barstow CK, Rosa G, Majorin F, Clasen T. Use of remotely reporting electronic sensors for assessing use of water filters and cookstoves in Rwanda. Environ Sci Technol. 2013;47:13602–13610. doi: 10.1021/es403412x. [DOI] [PubMed] [Google Scholar]
- Torres-Dosal A, Perez-Maldonado IN, Jasso-Pineda Y, Salinas RIM, Alegria-Torres JA, Diaz-Barriga F. Indoor air pollution in a Mexican indigenous community: Evaluation of risk reduction program using biomarkers, of exposure and effect. Sci Total Environ. 2008;390:362–368. doi: 10.1016/j.scitotenv.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health. 2000;73:331–338. doi: 10.1007/s004209900112. [DOI] [PubMed] [Google Scholar]
- West SK, Bates MN, Lee JS, Schaumberg DA, Lee DJ, Adair-Rohani H, et al. Is household air pollution a risk factor for eye disease? Int J Environ Res Public Health. 2013;10:5378–5398. doi: 10.3390/ijerph10115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Population using solid fuels (estimates), Data by country 2013 [Google Scholar]
- Yang M, Koga M, Katoh T, Kawamoto T. A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol. 1999;36:99–108. doi: 10.1007/s002449900447. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: Measurements, health impacts, and interventions. Environ Health Perspect. 2007;115:848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.