Abstract
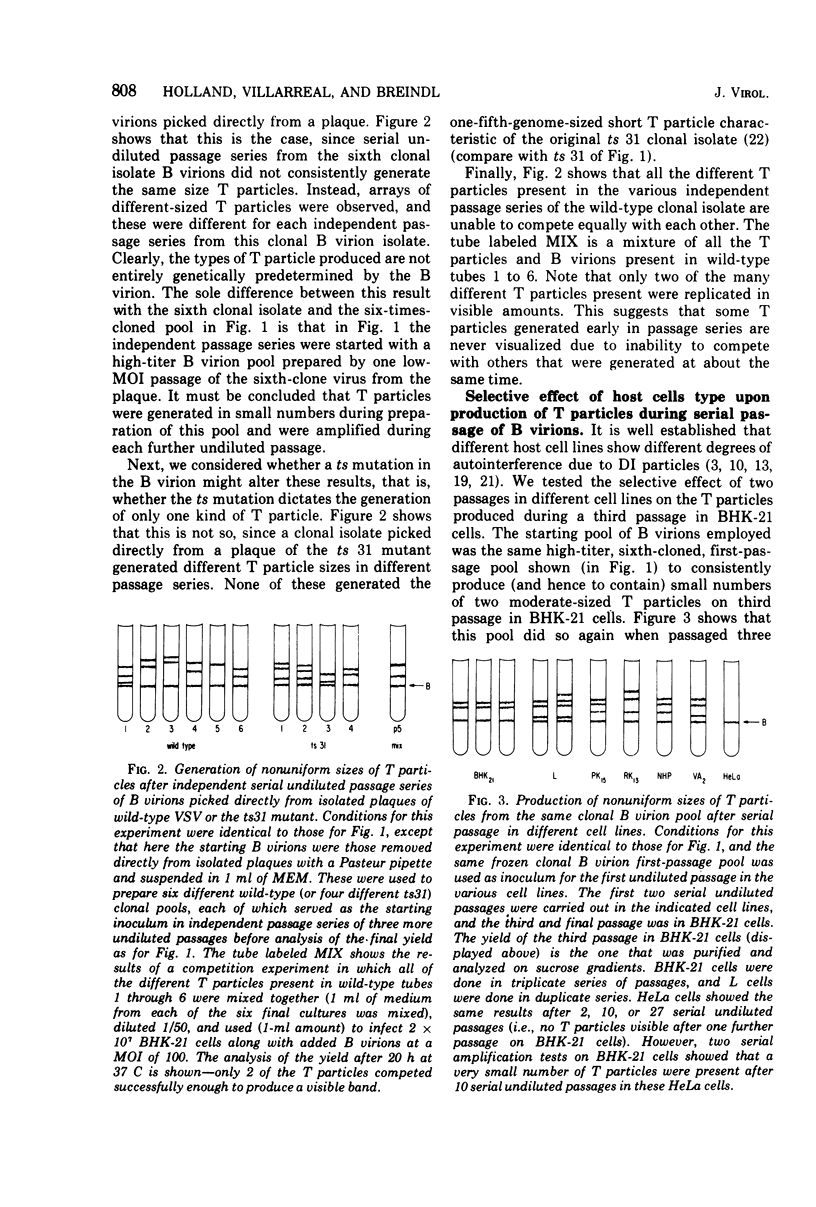
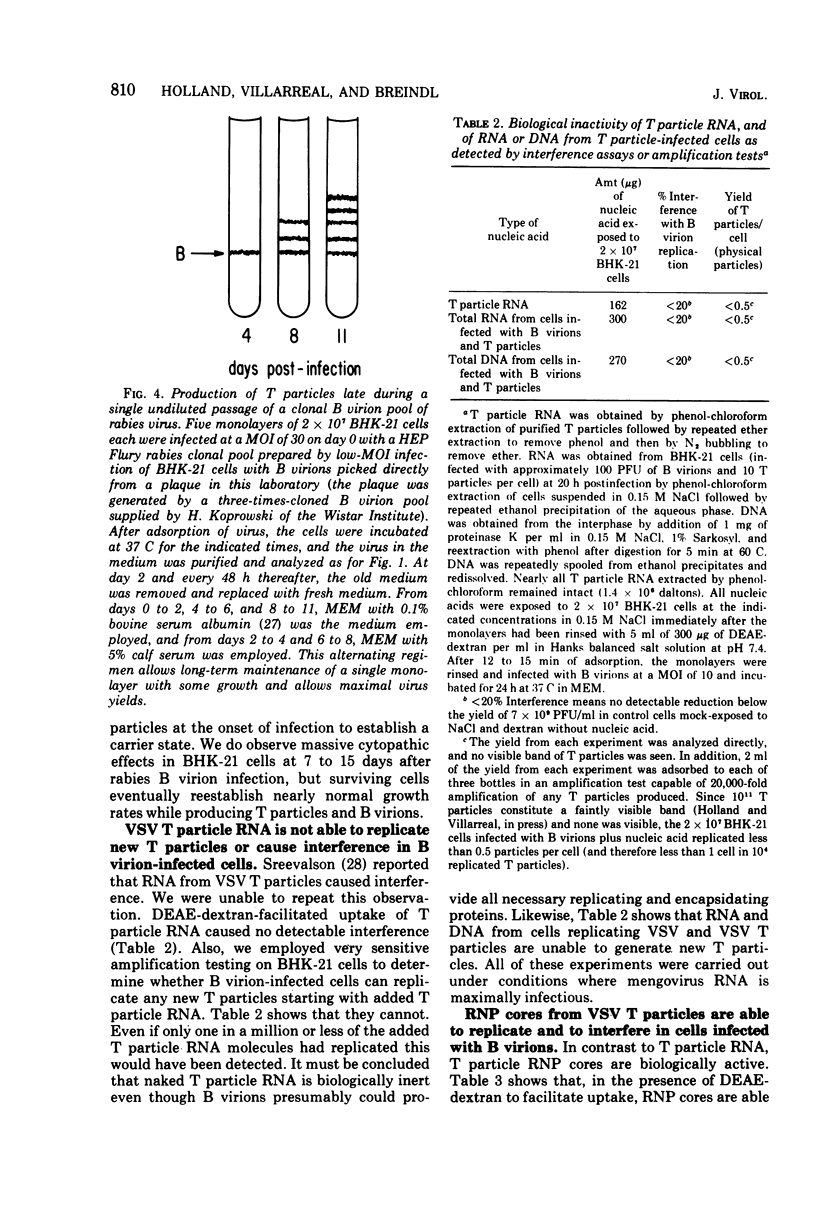
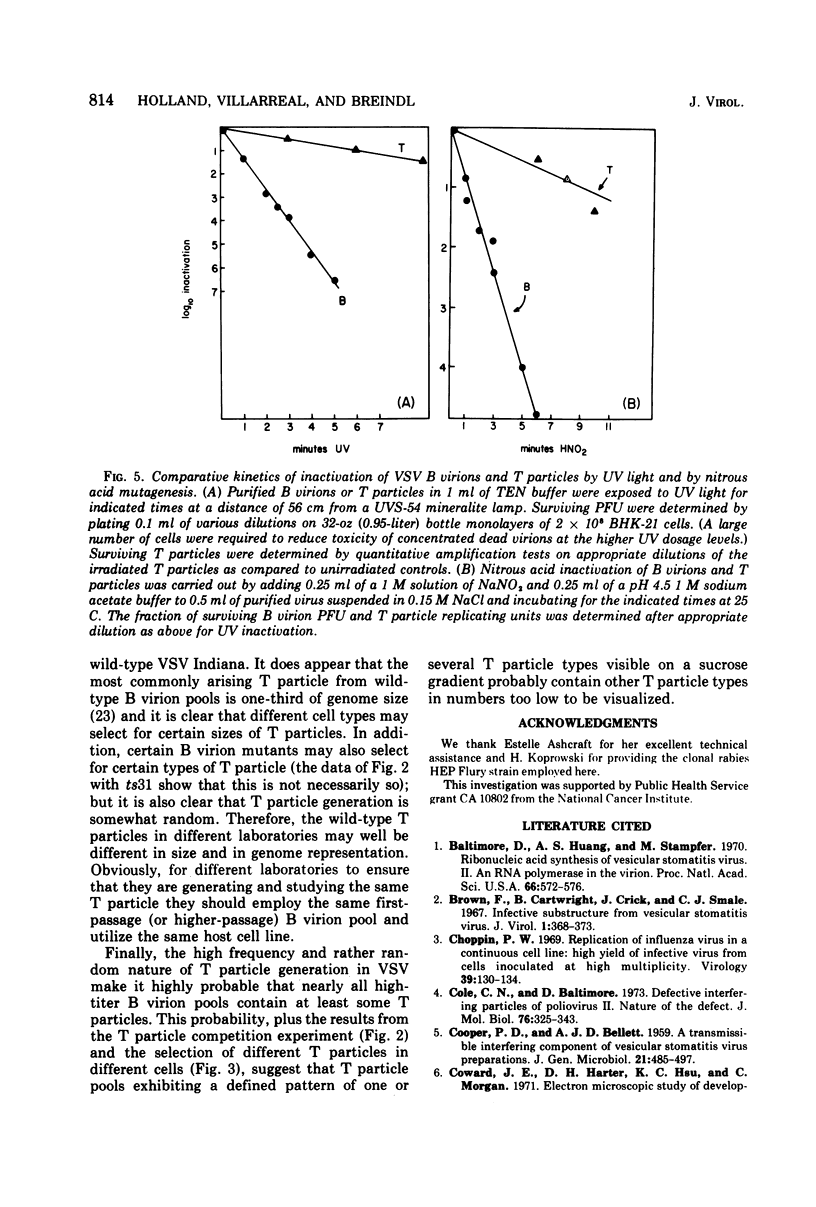
Previous indications that cloned B virions might be genetically predisposed to generate a particular defective T particle are shown to be inaccurate. T particle generation was found to be a much more random process than was previously believed. We show that the previously observed generation of particular sizes of T particles by B virion pools is due to the random generation of T particles during preparation of first-passage pools of cloned B virions, and these breed true during the additional passages needed to produce visible quantities of T particles. It is also shown that different host cell lines selectively amplify different T particles, suggesting a strong role of host cell factors in T particle replication. Surprisingly, our line of HeLa cells did not generate or replicate detectable T particles of vesicular stomatitis virus (VSV) Indiana after either serial undiluted passage or direct addition of T particles, even though the added T particles strongly interfered with B virion replication. In contrast to VSV, rabies virus generates large amounts of T particles during the first passage of cloned B virions, and every rabies-infected baby hamster kidney-21 cell culture evolves into a persistent carrier state. We find that T particle RNA is biologically inactive although T particle nucleocapsid ribonucleoprotein replicates and interferes in cells coinfected with B virions. Efforts to study the mechanism of T particle generation by in vitro attempts to generate T particles or modify their size (using sheared ribonucleoprotein or chemical or UV mutagenesis) were unsuccessful. The kinetics of UV and nitrous acid inactivation of T particles indicate a smaller target size relative to B virions, even after correcting for lengths of RNA molecules. The intercalating dye proflavine does not photosensitize VSV B virions or T particles when present during replication, indicating that there is little or no RNA base pairing in the helical nucleocapsids of either.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Cartwright B., Crick J., Smale C. J. Infective virus substructure from vesicular stomatitis virus. J Virol. 1967 Apr;1(2):368–373. doi: 10.1128/jvi.1.2.368-373.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D., BELLETT A. J. A transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:485–497. doi: 10.1099/00221287-21-3-485. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Hsu K. C., Morgan C. Electron microscopic study of development of vesicular stomatitis virus using ferritin-labelled antibodies. J Gen Virol. 1971 Oct;13(1):27–34. doi: 10.1099/0022-1317-13-1-27. [DOI] [PubMed] [Google Scholar]
- Crick J., Brown F. An interfering component of rabies virus which contains RNA. J Gen Virol. 1974 Jan;22(1):147–151. doi: 10.1099/0022-1317-22-1-147. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKETT A. J. A POSSIBLE MORPHOLOGIC BASIS FOR THE AUTOINTERFERENCE PHENOMENON IN VESICULAR STOMATITIS VIRUS. Virology. 1964 Sep;24:51–59. doi: 10.1016/0042-6822(64)90147-3. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDINKO N. Production of noninfectious complement-fixing poliovirus particles in HeLa cells treated with proflavine. Virology. 1958 Oct;6(2):512–524. doi: 10.1016/0042-6822(58)90098-9. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Homologous interference by incomplete Sendai virus particles: changes in virus-specific ribonucleic acid synthesis. J Virol. 1971 Oct;8(4):388–394. doi: 10.1128/jvi.8.4.388-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Villarreal L. P., Kohne D., Lesnaw J., Holland J. J. RNA polymerase activity and poly(A) synthesizing activity in defective T particles of vesicular stomatitis virus. Virology. 1974 Mar;58(1):240–249. doi: 10.1016/0042-6822(74)90158-5. [DOI] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Genome homology of vesicular stomatitis virus and defective T particles and evidence for the sequential transcription of the virion ribonucleic acid. J Virol. 1972 Jun;9(6):946–955. doi: 10.1128/jvi.9.6.946-955.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFER F. L. Binding of proflavine by and photoinactivation of poliovirus propagated in the presence of the dye. Virology. 1962 Nov;18:412–425. doi: 10.1016/0042-6822(62)90032-6. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. I. Viral specific RNA and nucleoprotein in infected L cells. Virology. 1970 Nov;42(3):732–743. doi: 10.1016/0042-6822(70)90319-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T. Homologous viral interference: induction by RNA from defective particles of vesicular stomatitis virus. Science. 1970 Sep 4;169(3949):991–993. doi: 10.1126/science.169.3949.991. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Absence of interference during high-multiplicity infection by clonally purified vesicular stomatitis virus. J Virol. 1971 Mar;7(3):409–411. doi: 10.1128/jvi.7.3.409-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON MAGNUS P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Clark H. F. Chronic rabies virus infection of cell cultures. Infect Immun. 1972 Dec;6(6):988–995. doi: 10.1128/iai.6.6.988-995.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



