Abstract
Background
Perfluorinated alkyl substances (PFAS) are suspected endocrine disruptors that are highly persistent and neurotoxic in animals. Human epidemiological studies of exposure-related deviations of children’s behaviors are sparse. We assessed the associations between prenatal, 5- and 7-year PFAS exposures and behavioral problem scores in 7-year Faroese children.
Methods
Concentrations of perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA), perfluorooctane sulfonate (PFOS), and perfluorohexane sulfonic acid (PFHxS) were measured in maternal serum and in serum from children at ages 5 and 7 years (n=539, 508, and 491, respectively). We used multivariable regressions and structural equations models to estimate the covariate-adjusted associations between serum-PFAS concentrations and behavioral difficulties, as assessed by the strengths and difficulties questionnaire (SDQ) at age 7.
Results
Serum-PFOS and PFHxS concentrations declined over time, whereas PFOA, PFNA, and PFDA tended to increase. No associations were observed between prenatal PFAS concentrations and SDQ scores. However, a two-fold increase in 5-year serum-PFOA, PFNA, and PFDA concentrations was associated with increases in total SDQ scores by 1.03 (95% CI: 0.11, 1.95), 0.72 (95% CI: 0.07, 1.38) and 0.78 points (95% CI: 0.01, 1.55), respectively. For SDQ subscales, significant associations were found in regard to hyperactivity, peer relationship, and conduct problems, as well as internalizing and externalizing problems and autism screening composite scores. Cross-sectional analyses at age 7 years showed possible sex-dimorphic associations between PFAS concentrations and SDQ scores, where girls had consistently positive associations with SDQ scores whereas boys exhibited a pattern of negative or null associations.
Conclusions
Higher serum PFAS concentrations at ages 5- and 7-years, but not prenatally, were associated with parent-reported behavioral problems at age 7.
Keywords: behavioral problems, neurotoxicity, neurodevelopment, PFAS, persistent organic pollutants, perfluorinated compounds, SDQ, DOHaD
BACKGROUND
Poly- and perfluoroalkyl substances (PFASs) have been produced since the 1950s for numerous applications, such as surfactants, water and oil repellents, cosmetics, food packaging, furnishings, and clothing (Lau 2012). They are highly persistent and bioaccumulative (Birnbaum and Grandjean 2015). Concerns about the potential toxic impacts of long-chain PFASs have led to the phase out of production of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) by a major global manufacturer in 2002, and the inclusion of PFOS in the Stockholm Convention on Persistent Organic Pollutants (Buck et al. 2011). Nonetheless, nearly all serum samples from the general population contain detectable concentrations of both PFOA and PFOS (Calafat et al. 2007). Dietary exposure is likely a major exposure route, along with contaminated drinking water and consumer products (Wu et al. 2015). Recently, breastfeeding has also been shown to be a significant route of exposure in children (Mogensen et al. 2015).
Neurotoxicity has been suggested as a potential adverse effect (Mariussen 2012) of PFASs. Thus, in experimental studies, PFASs were shown to affect neuronal cell development and cognitive functioning (Slotkin et al. 2008; Viberg et al. 2013). Furthermore, the endocrine disruptive properties of PFAS might alter the regulation of fetal brain development by interfering with the thyroid hormone function (Long et al. 2013; Wang et al. 2014). However, only a limited number of epidemiological studies have focused on neurobehavioral endpoints in relation to PFAS exposure. Cross-sectional studies have reported positive associations between children’s exposures to some PFASs and impulsivity (Gump et al. 2011) and Attention-deficit/hyperactivity disorder (ADHD) (Hoffman et al. 2010; Stein and Savitz 2011). So far, published results from prospective cohort studies have revealed little evidence of PFAS neurotoxicity. Indeed, maternal serum PFAS concentrations were not associated with behavioral problems, including attention deficit/hyperactivity disorder and autism, in children aged 7 to 13 years from the Danish National Birth Cohort (Fei and Olsen 2011; Liew et al. 2015). In addition, studies conducted among children from the C8 Health Project showed no associations between estimated in utero and measured childhood PFOA concentrations and neuropsychological test scores (Stein et al. 2013). Higher in utero PFOA concentrations in the same population were associated with decreases in characteristics of attention deficit/hyperactivity disorder, as measured by the Clinical Confidence Index of Connors' Continuous Performance Test-II, in boys and increases in girls, thus suggesting sex dimorphic effects (Stein et al. 2014). Finally, results from the Taiwan Birth Panel Study showed a negative association between social competence and self-help skills at age 2 and PFOS levels in cord blood (Chen et al. 2013), while another study reported a positive association between prenatal exposure to PFOA and PFOS and behavioral problems including hyperactive behavior (Hoyer et al. 2015). However, no previous study has investigated the possible impact of measured prenatal and postnatal PFAS concentrations in relation to children’s behavioral development, and none of the previous studies examined the potential joint effect of different PFASs on behavioral development.
In this study, we assessed the associations between PFAS exposures prenatally and at ages 5 and 7 years in regard to the children’s behavioral problem scores at age 7.
METHODS
Study population
The birth cohort included 656 children born in the Faroe Islands between 1997 and 2000. Only singleton births were included in the cohort and the cohort can be considered reasonably representative of Faroese births (Grandjean et al. 2012). Obstetric variables, including birth weight, parity and maternal age, were obtained from Obstetrical questionnaires. Information on pre-pregnancy weight and height, socio-economic status, maternal smoking and alcohol use during pregnancy were self-reported. The birth cohort was followed by clinical examinations through age 7. A maternal interview provided information concerning current health and past medical history, lifestyle, duration of breastfeeding, behavior and other characteristics. At the end of the clinical examinations at ages 5 and 7, a non-fasting blood sample was drawn. The study protocol was approved by the ethical review committee serving the Faroe Islands and by the institutional review board at the Harvard T.H. Chan School of Public Health, and written informed consent was obtained from all mothers.
Assessment of children’s behavioral problems at 7 years
Children’s behavioral development was assessed in 567 children (86 %), using the Parent’s version of the Strengths and Difficulties Questionnaire (SDQ) which has been translated into Faroese (Goodman 1997). The SDQ is a screening test for childhood psychopathology adapted for many different cultures and languages and has demonstrated excellent psychometric properties (http://www.sdqinfo.com/). It is comprised of 25 items scored on a 3-point Likert scale: 0 (“not true”), 1 (“somewhat true”), and 2 (“certainly true”). Five behavioral subscales with a score range of 0 to 10 are calculated from the 25 SDQ items: emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems and prosocial behavior. In addition, a total difficulties score ranging from 0 to 40 was calculated by summing four of the subscales (emotional, conduct, hyperactivity and peer). Higher total, emotional, conduct, hyperactivity and peer SDQ scores indicate higher difficulties, whereas higher prosocial scores indicate lower difficulties. Recently, the authors of the instrument provided theoretical and empirical support for combining the SDQ’s emotional and peer subscales into an internalizing problems subscale and the conduct and hyperactivity subscales into an externalizing problems subscale, both with scores ranging from 0 to 20. Finally, an autism screening score was also proposed, calculated as the peer problems subscale score minus the prosocial subscale (range: −10 to +10) (Goodman et al. 2010).
Exposure assessment
Prenatal PFAS exposures were assessed from analyses of serum obtained from the mothers at the last antenatal examination at week 32 of pregnancy; postnatal exposure was assessed from the serum obtained at ages 5 and 7. PFOA, PFOS, perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) concentrations were measured using online solid-phase extraction and analyzed using high-pressure liquid chromatography with tandem mass spectrometry (Haug et al. 2009). The accuracy and reliability of the data was ensured by including, in each analytical series, quality control serum samples, calibration standards, and reagent and serum blanks. Within-batch and between-batch coefficient of variations were better than 3.0% and 5.2% for all analytes. As potential confounders, we also measured prenatal and postnatal exposures to polychlorinated biphenyls (PCBs) and methylmercury using methods described elsewhere (Heilmann et al. 2010). We used a simplified lipid-based sum of PCBs (ΣPCB) concentrations, calculated as the sum of congeners PCBs 138, 153, and 180 multiplied by 2, to avoid problems with congeners not assessed and concentrations below the detection limit (Grandjean et al. 1995). Methylmercury (MeHg) exposure was assessed from the total mercury concentrations in whole blood and hair collected at childbirth and at the age-5 and 7 examinations. Blood samples were analyzed by cold-vapor atomic absorption spectrometry on a Direct Mercury Analyzer DMA-80 (Milestone, Italy), whereas hair was determined by flow injection cold-vapor technique (FIMS-400 and AS-90; PerkinElmer, Wellesley, MA, USA). In children with valid SDQ scores, measurements of serum-PFAS concentrations were available for 539, 508, and 491 children respectively for prenatal, 5-, and 7-year exposures.
Covariates and potential confounders
Socio-demographic, lifestyle and variables pertaining to medical history and birth outcomes were collected. We used directed acyclic graphs (DAGs) to infer the necessary set of covariates to include in the models from the following potential covariates: child’s exact age (in months), sex (boy; girl), birth weight (grams), maternal age at pregnancy, pre-pregnancy body mass index (BMI, kg/m2), parity (no older siblings; at least one older sibling), duration of exclusive breastfeeding (months), maternal socio-economic status (SES) during pregnancy (based on education, low; intermediate; and high), alcohol consumption during pregnancy (never; ever), and smoking during pregnancy (no; 1–5 cigarettes/day; more than 5 cigarettes/day). Concurrent concentrations of serum-PCBs and hair-Hg were included in all the models a priori. The model for prenatal PFAS exposures additionally included age, sex, maternal age, pre-pregnancy BMI, parity, socio-economic status, and alcohol and smoking during pregnancy. The models for postnatal exposure further included duration of breastfeeding and birth weight.
Statistical analyses
All PFAS concentrations were log-transformed (base 2) to address skewness, and to ensure a normal distribution of residuals. Because scores on the SDQ subscales exhibit an over-dispersed distribution, with the variance exceeding the mean for some subscales, we used negative binomial regressions. Negative binomial regressions intended to model the ratio of the mean SDQ subscale scores among exposed and non-exposed. The results are presented as adjusted mean ratios (aMR) of the SDQ subscales with corresponding 95% confidence intervals (CIs). In all of these models, the exposure estimate was reported for a two-fold increase in PFAS concentrations—for example, an aMR of 1.5 for a two-fold increase in PFOA suggests that the mean SDQ subscale score was 50% higher at a doubling of the PFOA concentrations. For total difficulties scores, internalizing, and externalizing problems scores, as well as the autism screening scores, we used general linear models to infer the adjusted mean differences (aMD) with 95% CI. The aMD is interpretable similar to the β coefficient as the mean difference in the SDQ composite scores for a doubling of PFAS concentrations. Previous studies have reported associations of PFAS exposures with child neurodevelopment that may be modified by child sex (Goudarzi et al. 2016; Stein et al. 2014). Thus, we assessed sex as an effect modifier by introducing a cross-product term (PFAS×sex).
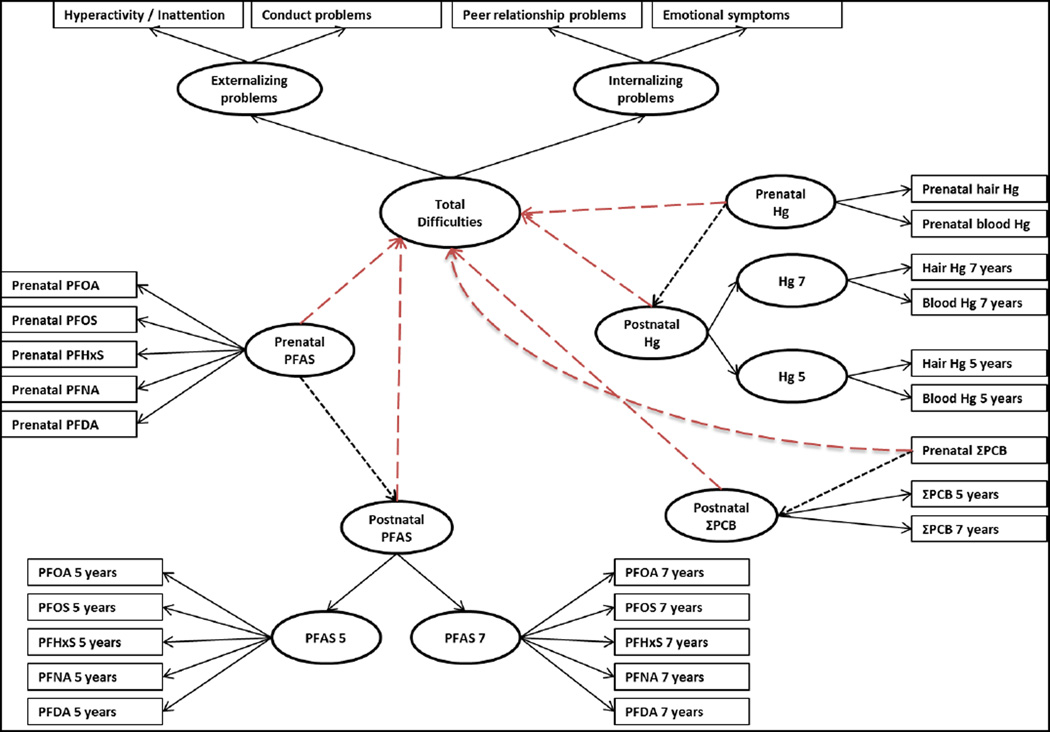
In further analyses, we used structural equations modeling (SEM) with adjustment for the same set of covariates previously described to investigate simultaneously the joint associations between pre- and postnatal exposures, and SDQ total scores, second order (i.e. internalizing and externalizing problems), and first order subscales. In this approach, we also included pre- and postnatal PCB and mercury exposures in all analyses (Figure 1). SEM analyses include a measurement part in which the observed variables are linked to a limited number of latent functions and a structural part describing the relationship between the latent variables (Budtz-Jorgensen et al. 2002). This approach allows us to reduce multiple comparisons testing, and to take into account the two-order factorial structure of the SDQ. In the measurement part, we allowed prenatal, age-5 and 7 PFAS concentrations to be indicators of 3 latent functions representing overall PFAS exposures prenatally and at ages 5 and 7. Additionally, we constructed a second-order latent function reflecting postnatal PFAS exposure indicated by the two 5- and 7-year PFAS latent functions. We also allowed the prenatal PFAS latent function to predict the postnatal PFAS latent function. The same procedure was used for methylmercury exposure using both hair and blood-Hg concentrations to reflect pre- and postnatal latent functions, as well as for PCB exposure (Figure 1). Because the latent functions are hypothetical underlying factors and have no unit, results are presented as the relative change in standard deviation (SD) in terms of the latent behavioral functions associated with a two-fold increase in PFAS concentrations.
Figure 1. Conceptual path diagram for the SEM of the associations between pre and postnatal PFAS concentrations and SDQ scores.
In the measurement part of the SEM, all indicators showed significant correlations to their latent functions (Supplemental Material; Table S1), and the models exhibited an acceptable to very good fit to the data (Comparative fit index between 0.921 and 0.999, and root mean square error of approximation between 0.06 and 0.01). Because there were missing data for some covariates [exclusive breastfeeding duration (n=31) and alcohol consumption during pregnancy (n=1)], we used the Full Information Maximum Likelihood estimation which utilizes all available information and avoids list-wise deletion due to missing data.
In additional analyses, we explored potential nonlinear relations using generalized additive models (GAMs) with penalized smoothing regression splines, and visually inspected plots of the smoothed data. We assessed departure from linearity (at p<0.1) by comparing the model with PFAS concentrations introduced as a spline function with the model with PFAS concentrations introduced as a linear term.
All significance tests were two-sided and the level of significance was set at a P-value<0.05, including for effect modification by sex. DAGs were drawn using DAGitty 2.2 (Textor et al. 2011) and all statistical analyses were conducted using R version 3.1.2 (R Core Team 2014).
RESULTS
The characteristics of the study population are presented in Table 1. Mean age at the age-7 examination was 90 months, and girls were slightly fewer than boys (48% vs 52%). Most of the children had an older sibling (73%), and 32% were exclusively breastfed for 6 months or longer (Table 1).
Table 1.
description of the characteristics of the study population at 7-year examination
| Population characteristic | Mean (SD) or n (%) |
|---|---|
| Age, months | 89.8 (1.2) |
| Birth weight, grams | 3716.6 (498.3) |
| Maternal age at delivery, years | 29.4 (5.1) |
| Maternal pre-pregnancy BMI | 23.9 (3.9) |
| Sex | |
| Boys | 281 (52.1) |
| Girls | 258 (47.9) |
| Parity | |
| No older siblings | 146 (27.1) |
| At least 1 older sibling | 393 (72.9) |
| Maternal SES | |
| Low | 254 (47.1) |
| Medium | 151 (28) |
| High | 134 (24.9) |
| Maternal smoking during pregnancy | |
| No | 395 (73.3) |
| 1–5 cigarettes/day | 64 (11.9) |
| > 5 cigarettes/day | 80 (14.8) |
| Alcohol consumption during pregnancy | |
| Never | 315 (58.5) |
| Ever | 223 (41.5) |
| Missing | 1 |
| Exclusive breastfeeding duration | |
| < 6 months | 344 (67.7) |
| ≥6 months | 164 (32.3) |
| Missing | 31 |
| SDQ scores | |
| Total difficulties | 6.6 (4.9) |
| Emotional symptoms | 1.88 (1.89) |
| Conduct problems | 1.45 (1.41) |
| Hyperactivity/inattention | 2.37 (2.2) |
| Peer relationship problems | 0.9 (1.38) |
| Prosocial behavior | 8.17 (1.45) |
| Internalizing problems | 2.8 (2.7) |
| Externalizing problems | 3.8 (3.2) |
| Autism screening score | −7.3 (2.2) |
The mean scores for SDQ total difficulties was 6.6, and SDQ subscales ranged from 0.9 to 2.4, except for prosocial behavior (mean, 8.2) (Table 1). Mean scores for externalizing and internalizing problems were 3.8 and 2.8, respectively. Finally, the mean for autism screening scores was −7.3. Total difficulties scores were higher among boys, children with no older siblings, from younger mothers, children of mothers with low SES, and of those who reported smoking more than 5 cigarettes/day during pregnancy. For SDQ subscales, scores for the conduct problems, peer relationship problems, and hyperactivity/inattention were higher among children of mothers with low SES, and of those who reported smoking more than 5 cigarettes/day during pregnancy. Further, conduct problems scores were higher among boys, and hyperactivity/inattention scores were also higher among boys, and among children whose mother had lower pre-pregnancy BMI. Scores of peer relationship problems were also higher among children with younger mothers, and among children of mothers that did not report consuming alcohol during pregnancy. Scores of emotional symptoms were higher among children with younger mothers, with no older siblings, and among children of mothers with low SES. Finally, prosocial behavior scores were lower among boys, and among children with birth weight lower than the median: 3750g (Supplemental Material; Table S2).
PFOS showed the highest serum concentrations on all occasions, followed by PFOA and PFHxS, while PFNA and PFDA showed the lowest concentrations (Table 2). Between-PFAS correlation coefficients for maternal serum or child at ages 5 and 7 ranged from 0.08 to 0.80, and were higher between PFNA and PFDA and lower between PFHxS and other PFASs. Within-PFAS correlation coefficients were higher between concentrations from ages 5 and 7 and lower between maternal and child serum at either age (Supplemental Material, Figure S1). Overall, prenatal PFAS and child 5- and 7-years serum concentrations differed significantly with the child age at the age-7 examination, maternal age at delivery, maternal SES, maternal smoking and alcohol consumption during pregnancy, and exclusive breastfeeding. Maternal PFOA and PFHxS concentrations were higher among nulliparous women, whereas PFNA and PFDA concentrations were higher in women with at least one previous child birth. Further, postnatal PFAS concentrations were generally lower in children of women with higher pre-pregnancy BMI (Supplemental Material, Table S3). PFAS concentrations measured in maternal and child 5- and 7-years serum did not differ significantly according to sex or birth weight (Supplemental Material, Tables S4 and S5).
Table 2.
Descriptive statistics of maternal and child’s PFAS concentrations (µg/L)
| PFASs | GM | Min | P25 | P50 | P75 | P95 | Max | |
|---|---|---|---|---|---|---|---|---|
|
Maternal (n=539) |
PFOS | 27.43 | 9.4 | 23.19 | 27.35 | 33.13 | 43.85 | 66.68 |
| PFOA | 3.19 | 0.82 | 2.56 | 3.34 | 4.01 | 5.62 | 8.43 | |
| PFNA | 0.61 | 0.12 | 0.47 | 0.61 | 0.8 | 1.15 | 1.93 | |
| PFHxS | 4.43 | 0.62 | 2.24 | 4.54 | 8.43 | 15.29 | 26.45 | |
| PFDA | 0.28 | 0.03 | 0.22 | 0.29 | 0.38 | 0.53 | 0.98 | |
|
5 years (n=508) |
PFOS | 16.75 | 6.18 | 13.52 | 16.78 | 21.05 | 27.88 | 48.23 |
| PFOA | 4.09 | 1.33 | 3.33 | 4.06 | 4.98 | 6.93 | 15.44 | |
| PFNA | 1.01 | 0.39 | 0.76 | 0.97 | 1.24 | 2.15 | 6.16 | |
| PFHxS | 0.64 | 0.08 | 0.45 | 0.63 | 0.89 | 1.53 | 19.51 | |
| PFDA | 0.28 | 0.05 | 0.21 | 0.28 | 0.38 | 0.6 | 1.2 | |
|
7 years (n=491) |
PFOS | 15.27 | 5.64 | 12.38 | 15.26 | 18.99 | 25.3 | 35.5 |
| PFOA | 4.51 | 1.72 | 3.53 | 4.37 | 5.66 | 8.4 | 19.16 | |
| PFNA | 1.2 | 0.47 | 0.88 | 1.12 | 1.52 | 2.87 | 9.49 | |
| PFHxS | 0.53 | 0.14 | 0.4 | 0.52 | 0.68 | 1.07 | 8.93 | |
| PFDA | 0.36 | 0.07 | 0.24 | 0.39 | 0.55 | 0.87 | 2.02 | |
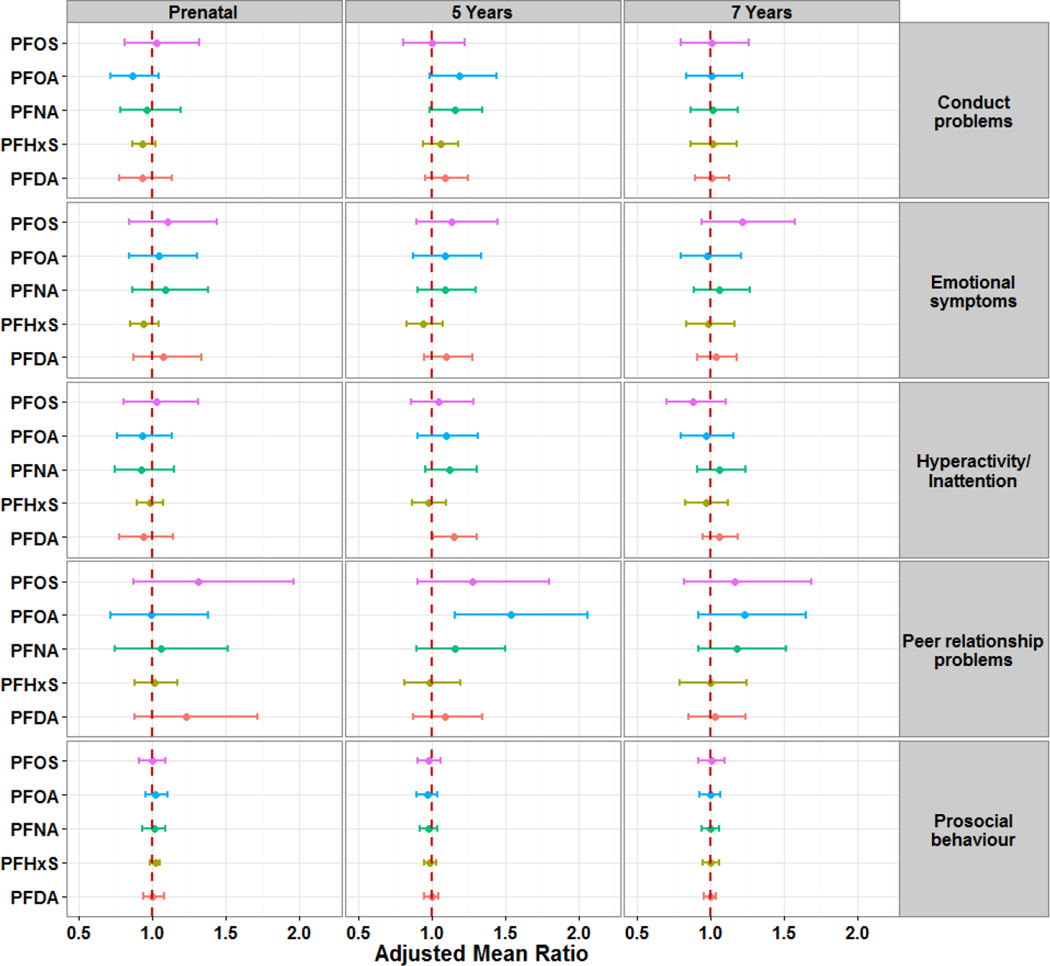
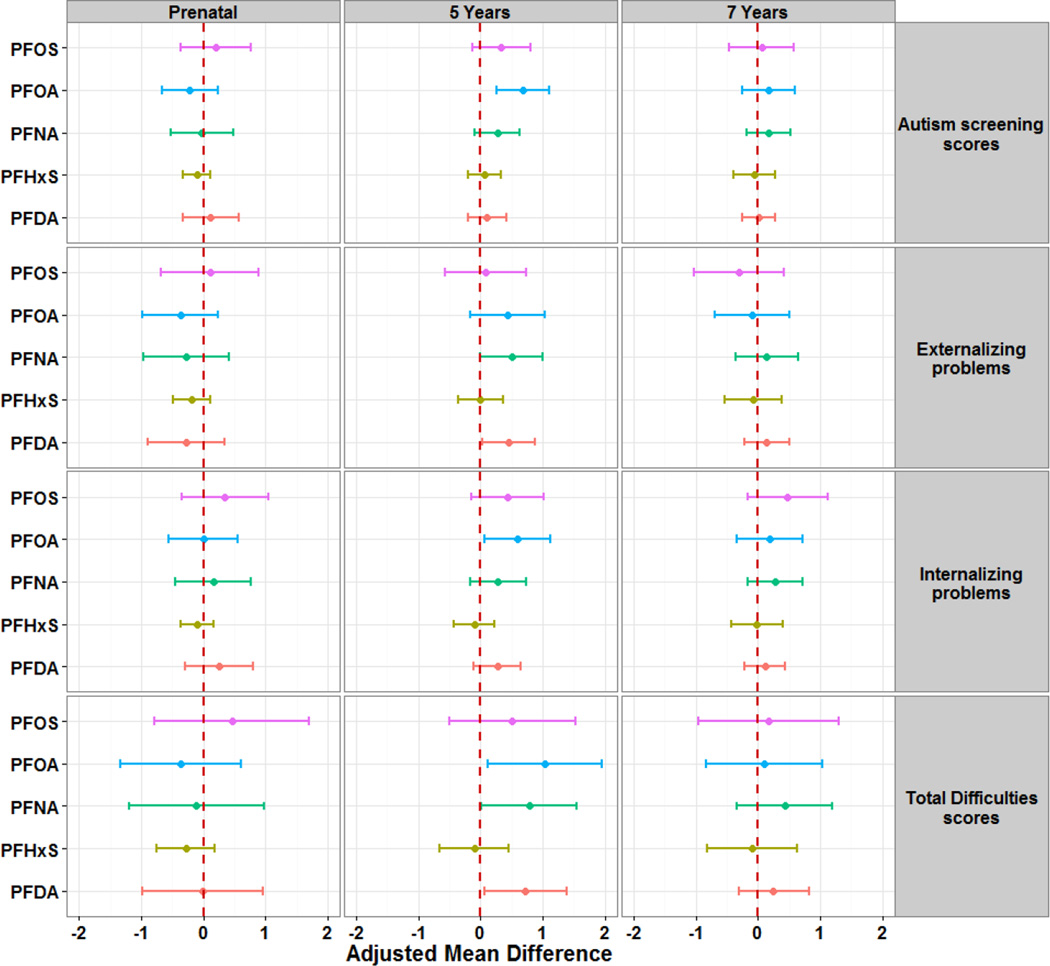
We found no association between maternal or 7-year PFAS concentrations and total SDQ and subscales scores (Figures 2 and 3, with detailed data in supplementary material tables S6 and S7). However, 5-year PFAS concentrations were associated with increased total SDQ scores, as well as subscale scores. For instance, a two-fold increase in serum-PFOA concentrations was associated with increases by 54% in peer relationship problems scores (aMR=1.54; 95% CI: 1.16, 2.06) and 19% in conduct problems scores (aMR=1.19; 95% CI: 0.99, 1.44; p=0.06). Further, a two-fold increase in PFOA concentrations at age 5 was associated with higher total SDQ scores (aMD=1.03; 95% CI: 0.11, 1.95), as well as higher internalizing problems (aMD=0.59; 95% CI: 0.06, 1.13) and autism screening scores (aMD=0.68; 95% CI: 0.25, 1.11). PFDA and PFNA concentrations at 5 years also showed significant positive associations with total SDQ scores, externalizing problems, hyperactivity/inattention and conduct problems. No consistent associations were observed for PFHxS and PFOS.
Figure 2. Adjusted mean ratios of SDQ subscales scores at 7 years of age per doubling of PFAS concentrations in maternal, and child 5- and 7-years serum.
Models for prenatal PFAS were adjusted for age, sex, maternal age, pre-pregnancy BMI, parity, socio-economic status, and alcohol and smoking during pregnancy. Models for 5- and 7-year PFAS were further adjusted for exclusive breastfeeding duration and birth weight.
Figure 3. Adjusted mean difference of SDQ composite scores at 7 years of age per doubling of PFAS concentrations in maternal, and child 5- and 7-years serum.
Models for prenatal PFAS were adjusted for age, sex, maternal age, pre-pregnancy BMI, parity, socio-economic status, and alcohol and smoking during pregnancy. Models for 5- and 7-year PFAS were further adjusted for exclusive breastfeeding duration and birth weight.
Analyses including a cross-product term to assess effect modification by child sex showed no significant interactions with maternal and 5-year PFAS concentrations. However, 7-year PFAS concentrations showed some significant sex-specific associations with SDQ scores. Girls had consistently positive associations with SDQ scores whereas boys exhibited a pattern of negative or null associations. Thus, a two-fold increase in 7-year PFOS, PFNA, and PFHxS concentrations was associated with higher total SDQ scores in girls and lower scores in boys (all at p-interaction < 0.05). A similar pattern was observed for externalizing problems and autism screening scores (Supplemental Material; Figure S2), as well as hyperactivity/inattention and conduct problems (Supplemental Material; Figures S3).
Table 3 shows results from the SEM analyses with mutual adjustment for pre- and postnatal PFAS, PCB, and Hg exposures. We observed no significant association between prenatal PFAS exposures and SDQ scores and subscales. In contrast, postnatal exposures, as reflected by PFAS exposures at ages 5 and 7 showed significant positive associations with the latent functions of total difficulties scores (β=0.17 SD; 95% CI: 0.07, 0.27), and externalizing problems (β=0.34 SD; 95% CI: 0.00, 0.67). In addition, we observed a positive association between postnatal PFAS and conduct problems and autism screening scores (β=0.35 SD; 95% CI: −0.01, 0.71 and β=0.56 SD; 95% CI: −0.03, 1.15, respectively). No significant association was observed for prenatal and postnatal PCB or Hg in any of the models.
Table 3.
Adjusted associations of SDQ scores at 7 years of age per doubling of pre- and postnatal PFAS, PCB, and Hg exposures
| SDQ Scores | Exposure | Prenatal | Prenatal | ||||
|---|---|---|---|---|---|---|---|
| Estimatea | 95% CI | p-value | Estimatea | 95% CI | p-value | ||
| Conduct problems | PFAS | −1.4 | −3.26, 0.45 | 0.14 | 0.35 | −0.01, 0.71 | 0.06 |
| PCB | 0.1 | −0.13, 0.34 | 0.39 | −0.08 | −0.23, 0.07 | 0.29 | |
| Hg | 0.08 | −0.06, 0.21 | 0.27 | 0.2 | −0.15, 0.55 | 0.27 | |
| Emotional symptoms | PFAS | 0.3 | −1.95, 2.55 | 0.79 | 0.29 | −0.21, 0.79 | 0.25 |
| PCB | 0.09 | −0.22, 0.40 | 0.55 | −0.16 | −0.36, 0.04 | 0.11 | |
| Hg | −0.08 | −0.27, 0.10 | 0.37 | 0.21 | −0.30, 0.72 | 0.43 | |
| Hyperactivity/inattention | PFAS | −2.03 | −4.66, 0.60 | 0.13 | 0.44 | −0.12, 1.00 | 0.13 |
| PCB | 0.23 | −0.14, 0.60 | 0.23 | −0.18 | −0.43, 0.08 | 0.17 | |
| Hg | 0.03 | −0.18, 0.23 | 0.81 | 0.51 | −0.09, 1.11 | 0.09 | |
| Peer relationship problems |
PFAS | 0.62 | −0.95, 2.20 | 0.44 | 0.26 | −0.13, 0.65 | 0.19 |
| PCB | −0.07 | −0.31, 0.17 | 0.59 | 0.03 | −0.12, 0.18 | 0.69 | |
| Hg | 0.01 | −0.13, 0.15 | 0.86 | −0.17 | −0.59, 0.24 | 0.4 | |
| Autism screening scores | PFAS | 0.86 | −1.54, 3.25 | 0.48 | 0.56 | −0.03, 1.15 | 0.06 |
| PCB | −0.28 | −0.65, 0.10 | 0.15 | 0.16 | −0.09, 0.41 | 0.2 | |
| Hg | 0.06 | −0.15, 0.28 | 0.56 | −0.35 | −0.97, 0.27 | 0.27 | |
| Externalizing problems | PFAS | −1.46 | −3.19, 0.28 | 0.11 | 0.34 | 0.00, 0.67 | 0.05 |
| PCB | 0.13 | −0.09, 0.35 | 0.25 | −0.1 | −0.25, 0.05 | 0.18 | |
| Hg | 0.06 | −0.06, 0.18 | 0.36 | 0.26 | −0.07, 0.59 | 0.13 | |
| Internalizing problems | PFAS | 0.49 | −0.86, 1.83 | 0.48 | 0.24 | −0.09, 0.57 | 0.16 |
| PCB | −0.03 | −0.23, 0.17 | 0.79 | −0.01 | −0.15, 0.12 | 0.87 | |
| Hg | −0.01 | −0.13, 0.11 | 0.92 | −0.08 | −0.41, 0.25 | 0.64 | |
| Total difficulties | PFAS | −0.58 | −1.55, 0.39 | 0.24 | 0.17 | 0.07, 0.27 | <0.001 |
| PCB | 0.06 | −0.08, 0.20 | 0.38 | −0.06 | −0.15, 0.04 | 0.24 | |
| Hg | 0.03 | −0.05, 0.10 | 0.5 | 0.12 | −0.10, 0.33 | 0.28 | |
All models were adjusted for concurrent exposures in addition to age, sex, maternal age, pre-pregnancy BMI, exclusive breastfeeding duration, birth weight, parity, socio-economic status, and alcohol and smoking during pregnancy.
Estimate for the composite scores is expressed as the SD change in the latent function associated with a twofold increase in exposures.
We explored the shape of the exposure-response relation with GAMs for total difficulties scores and SDQ subscale scores. Although some relationships exhibited a slightly non-linear shape, none of these relations departed significantly from linearity.
DISCUSSION
Overall, in this prospective cohort study from the Faroe Islands, we found no evidence of association between prenatal PFAS exposures and increased scores of behavioral problems. However, we observed a low to moderate, though significant association between postnatal exposure to PFASs and children’s behavioral development, as assessed by the SDQ at 7 years of age. These associations were mainly driven by higher scores on the hyperactivity/Inattention, conduct problems, and peer relationship problems subscales of the SDQ, which were reflected in the constructs of externalizing problems and autism screening scores. Additionally, cross-sectional associations between 7-year PFAS concentrations and SDQ scores showed different patterns by sex with positive associations with SDQ scores in girls (suggesting detrimental effects at higher exposures), but negative associations in boys.
Consistent with the present findings, previous investigations showed no association between prenatal exposure to PFASs and child neurodevelopment (Fei and Olsen 2011; Liew et al. 2015; Ode et al. 2014; Stein et al. 2013; Stein et al. 2014). Previous studies of postnatal PFAS exposures and child neurodevelopment are inconsistent, but most were based on cross-sectional designs (Gump et al. 2011; Hoffman et al. 2010; Stein and Savitz 2011) with methodological limitations, such as unmeasured confounders and the potential for reverse causation.
In regard to postnatal PFAS exposures and child development, childhood PFOA exposure and later neuropsychological problems were investigated in 320 children participating in the C8 health project, with no clear associations with behavioral problems at an average age of 10 years (Stein et al. 2013). The timing of the neuropsychological examination might explain, at least in part, some of the inconsistencies of findings from the two studies, and the children included in the latter study had almost 10-fold higher average serum-PFOA concentrations than the Faroese children. Further, the inter-PFAS correlations in the Faroese population were substantial, while correlation coefficients of PFOA with other PFASs in the U.S. study were weak.
The maternal PFAS concentrations in our cohort were higher than previously reported levels in some other populations (Hoyer et al. 2015), but comparable to those reported from Denmark (Liew et al. 2015). The concentrations in children appeared to decrease over time for PFOS and somewhat for PFHxS, but tended to increase for other PFASs. While child PFAS concentrations were much lower than those reported from the C8 project (Stein et al. 2014), they were comparable to those recorded in U.S. children (Hoffman et al. 2010).
Although not present at age 5 years, sex-specific analyses at age 7 years suggested adverse associations in girls and favorable associations in boys, in accordance with a previous study (Stein et al. 2014). Although sex-dependent differences in neurobehavioral development may lead to differences in vulnerability at different ages, PFASs are highly persistent with mean half-lives in human tissues estimated to range between 3.8 and 8.5 years (Olsen et al. 2007; Zhang et al. 2013), thereby minimizing differences in serum concentrations at different ages. Indeed, the correlation between the latent functions of PFAS exposures at 5 and 7 years was 0.72 (p<0.001), whereas the correlation between the latent functions of prenatal and 5-year PFAS exposures was 0.31 (p<0.001).
The mechanisms behind the association between PFAS exposure and behavioral problems in children are unclear, but some hypotheses have been proposed. Animal studies have shown that exposure to low doses of PFASs induced changes in behavior and habituation through alteration of dopaminergic and cholinergic system (Johansson et al. 2008; Salgado et al. 2015), and alterations in levels of neural proteins that are important for the formation and growth of the synapses (Johansson et al. 2009). PFASs were also shown to alter cell membrane potentials, which in turn affects channel gating properties (Harada et al. 2005). Another potential mechanism by which PFASs might have an adverse effect on neurodevelopment is through interference with thyroid hormone signaling. The thyroid hormone plays a role in synaptogenesis and myelination in the developing brain during the prenatal and postnatal periods, and previous studies in human reported associations between exposure to PFASs and thyroid gland disruption (Melzer et al. 2010; Wang et al. 2014; Wang et al. 2013).
In the present study, the PFASs measured exhibited different associations with children’s reported behavioral problems. Because PFAS concentrations in our study were strongly correlated at different time points, it is difficult to disentangle mixture effects from compound-specific effects. Indeed, the pharmacokinetic properties of PFAS compounds have been shown to be a key determinant of toxicity, and toxic properties may vary with carbon-chain length (Tatum-Gibbs et al. 2011). Of note, analyses of PFASs in human autopsy tissues (Perez et al. 2013) showed elevated brain concentrations of perfluorohexanoic acid, a PFAS that is fairly rapidly cleared from human serum (Russell et al. 2013), and serum-PFAS concentrations may therefore not represent the neurotoxic potential.
The findings from the present study point to a low to moderate association between serum-PFASs and behavioral problems. Even subtle effects of PFASs exposure may shift the distribution of continuous behavioral traits to increase the risk of clinical neurodevelopmental disorders such as autism spectrum disorders and ADHD (Bellinger 2004; Grandjean and Landrigan 2014). We relied on a questionnaire for parent-reported behavioral difficulties with excellent psychometric properties (Croft et al. 2015; Woerner et al. 2004b), and with common usage clinical practice as a screening and/or assessment tool by both school psychologists and clinicians (Borg et al. 2012; Goodman 1999; Yao et al. 2009). The SDQ has also been successfully used to assess children’s behavior across age and culture, and is commonly used in research studies, including longitudinal birth cohorts and national surveys (Chittleborough et al. 2011; Griffiths et al. 2011; Oulhote and Bouchard 2013; Russell et al. 2013). Moreover, recent evidence from exploratory factor analyses in multiple samples across Europe have supported the internal validity of the five subscales of the SDQ (Becker et al. 2006; Woerner et al. 2004a), as well as the newly established alternative factor structure with internalizing and externalizing problems factors, and prosocial behavior (Dickey and Blumberg 2004; Leeuwen et al. 2006). Furthermore, there is little reason to expect that parental report of behavioral problems would be differential by PFAS exposure levels, which is a condition to bias our findings away from the null.
Our study has several strengths. The prospective study design ensures temporality, and the continuous follow-up with high participation rates enables the evaluation of associations between PFAS exposures occurring during critical windows of both pre- and postnatal periods. Additionally, and from a causal inference perspective, simultaneous examination of different time periods of exposure provides a test of the possible presence of confounding bias, thereby greatly increasing the likelihood that an association found is truly causal when the associations are meaningfully differential, which was the case in our study. To our knowledge, this is the first study to investigate associations of both prenatal and postnatal exposure to multiple PFASs and children’s behavioral function. The use of structural equations modeling allowed the mutual adjustment for pre- and postnatal PFAS exposures, as well as PCB and Hg concentrations, thus reducing multiple comparisons testing, while allowing for measurement error in exposures, which is essential for unbiased effect estimation, especially in models that include correlated independent variables (Carroll et al. 2006). Still, insufficient power was available to investigate any potential interactive effects of PFASs, PCB, and Hg exposures. Adjustment for several potentially relevant confounders suggested only marginal impact on the results. The selection of relevant confounders was ascertained a priori using DAGs based on empirical evidence from previous research, thus avoiding over-fitting the statistical models. The potential influence of any unmeasured confounding would appear minimal, especially when taking into regard the homogeneity of the Faroese population in terms of socio-demographic, lifestyle, and genetic characteristics.
CONCLUSIONS
In this prospective study from the Faroe Islands, we found a consistent association between postnatal, but not prenatal exposure to certain PFASs, especially as reflected by the serum concentrations at age 5 years in regard to behavioral problems assessed at age 7. We used structural equation models, which allow for multivariate responses and incorporation of joint exposures. These findings are of potential public health importance, as the PFAS exposure in this population is not elevated above levels commonly reported, and since detectable levels of PFASs occur in almost all members of the general population.
Supplementary Material
HIGHLIGHTS.
-
-
Human epidemiological studies of PFAS exposure-related deviations of children’s behaviors are sparse,
-
-
We assessed the associations between prenatal, 5- and 7-year PFAS exposures and behavioral problem scores in 7-year Faroese children,
-
-
No associations were observed between prenatal PFAS concentrations and SDQ scores
-
-
Higher serum PFAS concentrations at ages 5- and 7-years were associated with behavioral difficulties at age 7.
Acknowledgments
Research reported in this publication was supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number R01ES009797. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no competing interests to declare, financial or otherwise.
REFERENCES
- Becker A, Steinhausen HC, Baldursson G, Dalsgaard S, Lorenzo MJ, Ralston SJ, et al. Psychopathological screening of children with ADHD: Strengths and Difficulties Questionnaire in a pan-European study. Eur Child Adolesc Psychiatry. 2006;15(Suppl 1):I56–I62. doi: 10.1007/s00787-006-1008-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res. 2004;95:394–405. doi: 10.1016/j.envres.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Grandjean P. Alternatives to PFASs: Perspectives on the Science. Environmental Health Perspectives. 2015;123:A104–A105. doi: 10.1289/ehp.1509944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg AM, Kaukonen P, Salmelin R, Joukamaa M, Tamminen T. Reliability of the strengths and difficulties questionnaire among Finnish 4–9-year-old children. Nord J Psychiatry. 2012;66:403–413. doi: 10.3109/08039488.2012.660706. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1:2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models; A Modern Perspective. Chapman and Hall CRC; 2006. [Google Scholar]
- Chen MH, Ha EH, Liao HF, Jeng SF, Su YN, Wen TW, et al. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology. 2013;24:800–808. doi: 10.1097/EDE.0b013e3182a6dd46. [DOI] [PubMed] [Google Scholar]
- Chittleborough CR, Lawlor DA, Lynch JW. Young maternal age and poor child development: predictive validity from a birth cohort. Pediatrics. 2011;127:e1436–e1444. doi: 10.1542/peds.2010-3222. [DOI] [PubMed] [Google Scholar]
- Croft S, Stride C, Maughan B, Rowe R. Validity of the strengths and difficulties questionnaire in preschool-aged children. Pediatrics. 2015;135:e1210–e1219. doi: 10.1542/peds.2014-2920. [DOI] [PubMed] [Google Scholar]
- Dickey WC, Blumberg SJ. Revisiting the factor structure of the strengths and difficulties questionnaire: United States, 2001. J Am Acad Child Adolesc Psychiatry. 2004;43:1159–1167. doi: 10.1097/01.chi.0000132808.36708.a9. [DOI] [PubMed] [Google Scholar]
- Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A, Lamping DL, Ploubidis GB. When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the Strengths and Difficulties Questionnaire (SDQ): data from British parents, teachers and children. J Abnorm Child Psychol. 2010;38:1179–1191. doi: 10.1007/s10802-010-9434-x. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791–799. [PubMed] [Google Scholar]
- Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, et al. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Sci Total Environ. 2016;541:1002–1010. doi: 10.1016/j.scitotenv.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen E, Budtz-Jørgensen E, et al. SErum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Jr, Sampson EJ, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014 Mar;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths LJ, Dezateux C, Hill A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. Int J Pediatr Obes. 2011;6:e423–e432. doi: 10.3109/17477166.2010.526221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45:8151–8159. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Xu F, Ono K, Iijima T, Koizumi A. Effects of PFOS and PFOA on L-type Ca2+ currents in guinea-pig ventricular myocytes. Biochemical and Biophysical Research Communications. 2005;329:487–494. doi: 10.1016/j.bbrc.2005.01.163. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216:385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum Concentrations of Antibodies Against Vaccine Toxoids in Children Exposed Perinatally to Immunotoxicants. Environmental Health Perspectives. 2010;118:1434–1438. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 2010;118:1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, et al. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years--a prospective study. Environ Health. 2015;14:2. doi: 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, Viberg H. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci. 2009;108:412–418. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Bellinger DC. Invited commentary: persistent organic pollutants and childhood learning and behavioural disorders. Journal of Epidemiology and Community Health. 2007;61:564–565. doi: 10.1136/jech.2006.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. Perfluoroalkyl acids: recent research highlights. Reprod Toxicol. 2012;33:405–409. doi: 10.1016/j.reprotox.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Leeuwen KV, Meerschaert T, Bosmans G, Medts LD, Braet C. The Strengths and Difficulties Questionnaire in a Community Sample of Young Children in Flanders. European Journal of Psychological Assessment. 2006;22:189–197. [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. Attention Deficit/Hyperactivity Disorder and Childhood Autism in Association with Prenatal Exposure to Perfluoroalkyl Substances: A Nested Case–Control Study in the Danish National Birth Cohort. Environmental Health Perspectives. 2015;123:367–373. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Ghisari M, Bonefeld-Jorgensen EC. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int. 2013;20:8045–8056. doi: 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol. 2012;86:1349–1367. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between Serum Perfluorooctanoic Acid (PFOA) and Thyroid Disease in the U.S. National Health and Nutrition Examination Survey. Environmental Health Perspectives. 2010;118:686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol. 2015;49:10466–10473. doi: 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BAG, Olofsson P, et al. Fetal Exposure to Perfluorinated Compounds and Attention Deficit Hyperactivity Disorder in Childhood. PLoS ONE. 2014;9:e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environmental Health Perspectives. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Bouchard MF. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect. 2013;121:1378–1384. doi: 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, Farré M. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2014 [Google Scholar]
- Russell G, Rodgers LR, Ford T. The Strengths and Difficulties Questionnaire as a Predictor of Parent-Reported Diagnosis of Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder. PLoS ONE. 2013;8:e80247. doi: 10.1371/journal.pone.0080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere. 2013;93(10):2419–2425. doi: 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Salgado R, Lopez-Doval S, Pereiro N, Lafuente A. Perfluorooctane sulfonate (PFOS) exposure could modify the dopaminergic system in several limbic brain regions. Toxicol Lett. 2015 doi: 10.1016/j.toxlet.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116(6):716–722. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect. 2011;119:1466–1471. doi: 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children. Paediatr Perinat Epidemiol. 2014;28:146–156. doi: 10.1111/ppe.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB, et al. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology. 2011;281:48–55. doi: 10.1016/j.tox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013;304:185–191. doi: 10.1016/j.tox.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122:529–534. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, et al. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ Health. 2013;12:76. doi: 10.1186/1476-069X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerner W, Becker A, Rothenberger A. Normative data and scale properties of the German parent SDQ. Eur Child Adolesc Psychiatry. 2004a;13(Suppl 2):Ii3–Ii10. doi: 10.1007/s00787-004-2002-6. [DOI] [PubMed] [Google Scholar]
- Woerner W, Fleitlich-Bilyk B, Martinussen R, Fletcher J, Cucchiaro G, Dalgalarrondo P, et al. The Strengths and Difficulties Questionnaire overseas: evaluations and applications of the SDQ beyond Europe. Eur Child Adolesc Psychiatry. 2004b;13(Suppl 2):Ii47–Ii54. doi: 10.1007/s00787-004-2008-0. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, et al. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res. 2015;136:264–273. doi: 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Zhang C, Zhu X, Jing X, McWhinnie CM, Abela JR. Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J Adolesc Health. 2009;45:55–62. doi: 10.1016/j.jadohealth.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.