Abstract
The paraventricular nucleus of the thalamus (PVT) has been implicated in behavioral responses to reward-associated cues. However, the precise role of the PVT in these behaviors has been difficult to ascertain since Pavlovian-conditioned cues can act as both predictive and incentive stimuli. The “sign-tracker/goal-tracker” animal model has allowed us to further elucidate the role of the PVT in cue-motivated behaviors, identifying this structure as a critical component of the neural circuitry underlying individual variation in the propensity to attribute incentive salience to reward cues. The current study assessed differences in the engagement of specific PVT afferents and efferents in response to presentation of a food-cue that had been attributed with only predictive value or with both predictive and incentive value. The retrograde tracer fluorogold (FG) was injected into the PVT or the nucleus accumbens (NAc), and cue-induced c-Fos in FG-labeled cells was quantified. Presentation of a predictive stimulus that had been attributed with incentive value elicited c-Fos in PVT afferents from the lateral hypothalamus, medial amygdala (MeA), and the prelimbic cortex (PrL), as well as posterior PVT efferents to the NAc. PVT afferents from the PrL also showed elevated c-Fos levels following presentation of a predictive stimulus alone. Thus, presentation of an incentive stimulus results in engagement of subcortical brain regions; supporting a role for the hypothalamic-thalamic-striatal axis, as well as the MeA, in mediating responses to incentive stimuli; whereas activity in the PrL to PVT pathway appears to play a role in processing the predictive qualities of reward-paired stimuli.
Keywords: paraventricular nucleus of the thalamus, incentive salience, motivated behavior, sign-tracking, goal-tracking
Introduction
The paraventricular nucleus of the thalamus (PVT) has recently been implicated in a variety of motivated behaviors (Hsu et al., 2014, Kirouac, 2015, Vertes et al., 2015), including reward-seeking behaviors (Martin-Fardon and Boutrel, 2012, James and Dayas, 2013, Haight and Flagel, 2014, Urstadt and Stanley, 2015). Much of the research surrounding the PVT and reward-seeking behaviors has focused on the role of the PVT in mediating responses to food- or drug-paired cues. For example, paired presentations of a discrete cue-light with a water reward leads to greater c-Fos induction in the PVT, relative to controls who received unpaired presentations of the cue and reward (Igelstrom et al., 2010). Enhanced levels of c-Fos are also found in the PVT in response to presentation of cocaine-paired cues (Matzeu et al., 2015a), as well as following cue-induced reinstatement of ethanol- and cocaine-seeking behavior (Wedzony et al., 2003, Dayas et al., 2008, James et al., 2011). In addition, transient inactivation of the PVT attenuates cue-induced reinstatement of cocaine-seeking behavior (Matzeu et al., 2015b) and the expression of cocaine conditioned place preference (Browning et al., 2014). While these findings demonstrate that the PVT is involved in mediating cue-motivated behaviors, its specific role in these processes is less well known.
Identifying the neural mechanisms underlying cue-motivated behaviors has been complicated by the fact that Pavlovian-conditioned cues can act as both predictive and incentive stimuli (Robinson and Berridge, 1993). A predictive stimulus acquires predictive properties, and thereby the ability to elicit a conditioned response; whereas an incentive stimulus acquires both predictive and incentive motivational properties and thereby the ability to evoke complex emotional and motivational states (Stewart et al., 1984, Childress et al., 1993). Incentive stimuli are defined by three fundamental properties: 1) they can elicit approach behaviors upon presentation, 2) they can act as conditioned reinforcers such that individuals are willing to work for presentation of the stimulus alone, and 3) their presentation can enhance ongoing instrumental actions (Berridge, 2001, Cardinal et al., 2002a). Initially, it was thought that if a cue was predictive of reward delivery (i.e. a predictive stimulus) it was also imbued with incentive properties. Upon further study, however, it was discovered that individuals differ in the extent to which they attribute incentive motivational value or incentive salience to reward-predictive stimuli (Flagel et al., 2009, Robinson and Flagel, 2009). To study this phenomenon, we use a Pavlovian conditioned approach (PCA) procedure that allows us to capture individual variation in the propensity to attribute incentive salience to reward-paired cues, and to thereby explore the underlying neural mechanisms. In this model, where presentation of a discrete lever-cue (conditioned stimulus, CS) is followed by presentation of a food reward (unconditioned stimulus, US), some rats develop a sign-tracking conditioned response (CR). These rats, referred to as “sign-trackers (STs)” (Hearst and Jenkins, 1974), approach and engage the lever-CS upon presentation, and will work for presentation of the lever-CS, even in the absence of a food reward (Robinson and Flagel, 2009). Other rats develop a goal-tracking CR, and these rats, referred to as “goal-trackers (GTs)” (Boakes, 1977), rapidly approach and enter the location of food delivery upon lever-CS presentation, and are less motivated than STs to work for lever presentation in the absence of food reward. The remaining rats develop a mixed CR, vacillating between engagement with the lever-CS and the location of food delivery. Thus, for all individuals, the lever-CS serves as a predictive stimulus, but only for STs does the lever-CS also become an incentive stimulus (Robinson and Flagel, 2009).
The sign-tracker/goal-tracker animal model has been used to show that cortico-thalamic-striatal circuitry is engaged only when a reward cue is attributed with incentive value—that is, to a greater extent in sign-trackers than goal-trackers (Flagel et al., 2011a, Yager et al., 2015). The PVT seems to represent a central node of this differential activity, as there are robust phenotypic differences in food- and drug-cue induced c-Fos in this region, and distinct patterns of correlated neural activity involving the PVT. In sign-trackers, food-cue induced c-Fos mRNA is correlated between the PVT and the shell of the nucleus accumbens (NAc); whereas in goal-trackers, cue-induced c-Fos mRNA is correlated between areas of the prefrontal cortex (PFC), such as the prelimbic cortex (PrL), and the PVT (Flagel et al., 2011b, Haight and Flagel, 2014). Additional evidence supporting a role for the PVT in mediating the propensity to attribute incentive salience to reward cues comes from a lesion study in which we found that PVT lesions attenuate a goal-tracking CR, while concomitantly increasing a sign-tracking CR (Haight et al., 2015). These findings demonstrate a causal link between the PVT and the attribution of incentive salience to a reward cue, suggesting that the PVT may act as a “brake” on incentive salience attribution.
To better elucidate the role of the PVT in mediating the propensity to attribute incentive salience to reward cues, it is crucial to examine the afferent and efferent circuitry of this nucleus. The PVT is situated on the dorsal midline of the thalamus in the rat, directly underneath the 3rd ventricle, and has numerous connections with cortical, limbic and motor areas. Specifically, the PVT receives dense cortical input from much of the anterior-posterior gradient of the PrL, as well as the infralimbic (IL) and cingulate cortices (Vertes, 2004, Li and Kirouac, 2012). Subcortical afferents are widely distributed, and arise from the hypothalamus, ventral subiculum (vSub), and the central and medial amygdala, among other areas (Chen and Su, 1990, Canteras et al., 1995, Van der Werf et al., 2002, Kirouac et al., 2005, 2006, Vogt et al., 2008, Hsu and Price, 2009, Li and Kirouac, 2012, Li et al., 2014, Lee et al., 2015). In addition to its diverse inputs, the PVT sends efferent fibers to a variety of cortical and subcortical structures, including the PrL and IL, NAc core and shell, parts of the bed nucleus of the stria terminalis, and the central and basolateral amygdala, among other areas (Jones et al., 1989, Berendse and Groenewegen, 1990, Su and Bentivoglio, 1990, Moga et al., 1995, Van der Werf et al., 2002, Pinto et al., 2003, Parsons et al., 2006, Parsons et al., 2007, Li and Kirouac, 2008, Vertes and Hoover, 2008). Importantly, several of the sources of afferents, as well as the efferent targets, of the PVT have been implicated in cue- and context-motivated behaviors, including the PrL and IL (Willcocks and McNally, 2013, Moorman and Aston-Jones, 2015), hypothalamus (Petrovich et al., 2012, Cole et al., 2015), amygdala (Parkinson et al., 2000, Mahler and Berridge, 2009), ventral subiculum (Sun and Rebec, 2003, Kufahl et al., 2009), and NAc (Cardinal et al., 2002b, Bossert et al., 2007).
The neuroanatomical location of the PVT allows it to integrate cortical and subcortical inputs and send this information to the NAc to control motivated behavior (Kelley et al., 2005b). We postulate that the PVT acts as a central node to modulate the attribution of incentive salience to reward cues, with STs being more susceptible to subcortical motivational processes and GTs being biased towards greater cortical control of behavior (Haight and Flagel, 2014). Specifically, given that GTs perform better than STs on behavioral tests dependent on cortical processes, including sustained attention (Paolone et al., 2013) and impulsive action (Flagel et al., 2010, Lovic et al., 2011), we hypothesize that these rats will show greater activation of PrL afferents to the PVT, representing greater top-down control of behavior. In contrast, we hypothesize that STs will show greater activation of subcortical inputs to the PVT, including those from the hypothalamus, amygdala, and ventral subiculum. In addition, we expect greater activation of PVT efferents to the NAc in STs, as the sign-tracking, but not the goal-tracking, response has been shown to be dependent on dopamine transmission in the NAc (Flagel et al., 2011b, Saunders and Robinson, 2012), which can be influenced by projections from the PVT (Parsons et al., 2006). These hypotheses were examined by assessing engagement of specific PVT afferent and efferent circuits in response to presentation of a predictive (i.e. for STs and GTs) or incentive (i.e. for STs only) stimulus associated with a food reward.
Experimental Procedures
All experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals: Eighth Edition, revised in 2011. In addition, all procedures were approved by the University of Michigan Institutional Animal Care and Use Committee.
PVT Afferent Labeling
Subjects
Forty male Sprague-Dawley rats were obtained from Envigo (formerly Harlan, Haslett, MI) at approximately 8 weeks of age (~230–300g body weight). Upon arrival, rats were pair-housed in standard acrylic cages (46 × 24 × 22 cm) in a climate-controlled room and allowed to acclimate to the new environment for 10 days prior to any experimental manipulations. Throughout the experiment, rats were maintained on a 12-hour light:dark cycle (lights on at 07:00 hours), and food and water were available ad libitum. Behavioral training was conducted during the light cycle between 12:00 and 17:00 hours.
Surgical Procedures
Following the 10-day acclimation period, all rats underwent stereotaxic surgery in order to infuse the retrograde tracer fluorogold (FG; Fluorochrome, Denver, CO, USA) into the PVT. All surgery was performed under aseptic conditions. First, a surgical plane of anesthesia was induced with inhalation of 5% isoflurane. Once rats were fitted into the stereotaxic frame, anesthesia was maintained throughout the procedure with inhalation of 1–2% isoflurane. The scalp was shaved and sterilized with swabs of 70% alcohol and Betadine solution (Betadine, Stamford, CT, USA). A small incision was made in the scalp to expose bregma and lambda coordinates, and the skull was leveled within +/− 0.1 mm. Small burr holes were then drilled above the PVT, and a 0.5 µl Hamilton Neuros syringe (Hamilton Company, Reno, NV, USA) mounted in a Kopf Model 5000 Microinjection Unit (David Kopf Instruments, Tujunga, CA, USA) was used to make two 50 nl injections of 2% FG solution diluted in 0.9% sterile saline into the PVT, which was the smallest volume that could be reliably injected. Some have argued that FG can be taken up by damaged axons of passage, and possibly undamaged axons, leading to erroneous neuronal labeling (Dado et al., 1990). To minimize this risk, FG injections were performed with a Hamilton Neuros syringe with a small 32 gauge injector tip, minimizing damage to the injection site and limiting uptake by damaged axons. The injections were performed at the following coordinates relative to bregma: AP −2.0, ML −1.0, DV −5.4 and AP −3.0, ML −1.0, DV −5.5 (stereotaxic arm angled at 10° towards the midline). Each injection lasted approximately 2 minutes, and the syringe was left in place for 5 minutes following the injection to minimize diffusion of the FG solution up the injection track. The syringe was then slowly retracted, and the scalp was closed with wound clips. Immediately prior to surgery, and 24 hours after, rats received subcutaneous injections of the nonsteroidal anti-inflammatory drug flunixin (2.5 mg/kg FlunixiJect diluted in 0.9% sterile saline; Butler Schein Animal Health, Dublin, OH, USA) for pain management. Rats were then allowed to recover from surgery for 8–9 days prior to any further experimentation.
PVT Efferent Labeling
Subjects
Sixty male Sprague-Dawley rats were obtained from Envigo (formerly Harlan, Haslett, MI) at approximately 8 weeks of age (~225–275g body weight). Upon arrival, rats were pair housed in conditions identical to those described above, and were allowed to acclimate to the new environment for one week prior to any experimental manipulations. Throughout the experiment, rats were maintained on a 12-hour light:dark cycle (lights on at 06:00 hours), and food and water were available ad libitum. All behavioral procedures occurred during the light cycle between 12:00 and 17:00 hours.
Surgical Procedures
Surgical procedures were identical to those described for the PVT afferent experiment, with the exception of the location of FG injection. Bilateral 50-nl injections were made into the border of the NAc core/shell (coordinates from bregma: AP 1.7, ML +/− 1.0, DV −7.2; stereotaxic arm perpendicular to the skull surface).
Behavioral Testing
Pavlovian Conditioned Approach Training
Following recovery from surgery, animals underwent PCA procedures similar to those previously described (Flagel et al., 2011a, Haight et al., 2015, Fraser et al., 2016). Standard behavioral test chambers were used (MED Associates, St. Albans, VT, USA). All chambers were enclosed in sound-attenuating boxes that were equipped with ventilation fans that provided a constant flow of air, as well as background noise. All behavioral data were collected using MED PC software (Med Associates. St. Albans, VT, USA).
Each chamber contained a food cup connected to a pellet dispenser located in the middle of one wall. Activation of the pellet dispenser resulted in the delivery of one 45-milligram banana-flavored grain pellet (Bio-Serve, Flemington, NJ, USA). Each food cup was equipped with an infra-red photo beam, and breaks of the beam were recorded as head entries. Flanking the food cup to the left or right was a retractable, illuminated lever, positioned at equal height with the food cup. All levers were set so that approximately 10 grams of force would cause a deflection of the lever and register as a lever contact. A white house light was placed on the upper middle portion of the wall directly across from the food cup and lever, and was turned on for the duration of each of the Pavlovian conditioning sessions.
For two days prior to behavioral training rats were briefly handled by the experimenters in the housing room, and a small amount of banana-flavored grain pellets (approximately 25 pellets per rat) were placed in the home cage, to familiarize the rats with the experimenters and the novel food. Following these two days, all rats underwent one pretraining session in the test chambers. Each food cup was baited with 3 banana-flavored pellets before the pre-training session in order to direct the rats’ attention to the location of reward delivery. At the beginning of the session, the house light remained off for 5 minutes, to allow the rats to acclimate to the training chamber. Following this acclimation period, the house light was turned on, and 25 food pellets were delivered one at a time into the food cup on a variable interval 30-second schedule (range 0–60s). The lever was retracted for the entire pre-training session, which lasted an average of 12.5 minutes. After pretraining, rats went through 5 sessions of Pavlovian conditioned approach training, one session per day. Each session consisted of 25 trials in which the 8-second insertion of the illuminated lever (CS) into the test chamber was paired with delivery of one banana-flavored pellet (US) into the food cup. CS-US presentation occurred on a variable interval 90-second schedule (range 30–150 seconds). In addition to the rats receiving PCA training, a small subset of rats from each experimental group (Afferent Experiment n=6, Efferent Experiment n=8) were used as an unpaired control group. These rats received the same number of CS and US presentations, but in an unpaired fashion. Each session (PCA and Unpaired Control) lasted approximately 40 minutes. The following data was recorded per trial during each session, in order to quantify Pavlovian conditioned approach behaviors: (1) the number of food cup entries during the 8 second lever-CS period, (2) the latency to first food-cup entry upon lever-CS presentation, (3) the number of lever-CS contacts, (4) latency to first lever-CS contact, and (5) the number of food-cup entries during the inter-trial interval.
Following session 5 of Pavlovian training, rats (in the “paired” group) were classified as STs, GTs, or intermediate responders (INs) based on their average PCA Index scores (Meyer et al., 2012) from sessions 4 and 5. The PCA Index is a composite score that is used to assess the propensity of an individual rat to approach the lever-CS vs. the food cup (location of US delivery). This Index relies on three different metrics: response bias [(total lever contacts – total food cup contacts) ÷ (sum of total contacts)], probability difference score [Prob(lever) – Prob(food cup)], and latency difference score [-(lever contact latency – food cup entry latency) ÷ 8]. These three measures are then averaged together to create the PCA Index score, which ranges from −1.0 to 1.0, with −1.0 representing an individual whose behavior is directed solely towards the food cup, and 1.0 representing an individual whose behavior is directed solely towards the lever-CS.
Context Habituation and Re-Exposure to the CS
Following Pavlovian conditioned approach training, the test chambers were reconfigured such that the food cup and pellet dispenser were removed, the lever was placed in the center of the wall it was previously located on, and new metal grate flooring was inserted. To minimize the influence of contextual cues, rats classified as STs, GTs, and the unpaired control groups (UNs) were placed into the reconfigured test chambers on three consecutive days. During these sessions, following an initial 5-minute acclimation period, the house light was illuminated and the animals remained in the chambers for another 30 minutes, with the lever retracted. On the fourth day (i.e. cue-test session), rats were placed into the chambers, and following the 5-minute acclimation period, the house light was illuminated, and the illuminated lever-CS was inserted into the cage for 2 seconds, once a minute, over a period of ten minutes, for a total of 10 lever-CS presentations. Importantly, these presentations were not paired with pellet delivery, but lever contacts were recorded during the session. Following the 10th lever presentation, rats were placed back into their home cages and transferred to the housing room, where they were left undisturbed for 60 minutes. Following this 60-min period, the rats were deeply anesthetized with an intraperitoneal injection of a cocktail containing ketamine (90 mg/kg) and xylazine (10 mg/kg) and transcardially perfused with approximately 100 mL of room temperature 0.9% saline, followed by approximately 200 mL of room-temperature 4% formaldehyde (pH=7.3–7.4, diluted in 0.1M sodium phosphate buffer; Fisher Scientific, Hampton, NH, USA).
Tissue Processing and Quantification
Tissue Preparation
Following perfusion, brains were extracted and post-fixed overnight in 4% formaldehyde at 4°C. Brains were then cryoprotected over three nights in graduated sucrose solutions (10%, 20%, and 30%, dissolved in 0.1M sodium phosphate buffer, NaPB; pH=7.3–7.4) at 4°C. Following cryoprotection, brains were sectioned at 40 µm on a frozen cryostat (Leica Biosystems Inc, Buffalo Grove, IL, USA). Starting with the anterior PrL and continuing through the thalamus, brain sections were serially collected in 6-well plates. Each well contained a full brain series, with each section approximately 200 µm caudal from the previous section. Towards the hindbrain, where the vSub is located, sections were collected in 48-well plates, one section per well. All sections were stored in 0.1M NaPB at 4°C.
Immunohistochemistry
One series from each brain, as well as the appropriate vSub section, was processed for detection of FG and c-Fos via free-floating immunohistochemistry. All immunohistochemical processing took place at room temperature with gentle agitation. Free-floating sections were washed 3–5 times (5 min each wash) in 0.1M phosphate-buffered saline (PBS) in between incubations. Sections were first incubated in 1% hydrogen peroxide (H2O2; diluted in 0.1M PBS) for 10 minutes. Sections were then incubated in a blocking solution containing 2.5% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and 0.4% Triton X-100 (Acros Organics, Geel, Belgium), diluted in 0.1M PBS, for 1 hour. Following incubation with the blocking solution, sections were incubated overnight in primary antibody solution containing 1:1000 goat anti-c-Fos antibody (lot H2214, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), 1% normal donkey serum, and 0.4% Triton X-100. The next day, sections were incubated in secondary antibody solution containing 1:500 donkey anti-goat antibody (lots 118762 and 119956, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), 1% normal donkey serum, and 0.4% Triton X-100 in 0.1M PBS, for 1 hour. Sections were then incubated for 1 hour in Vectastain Elite ABC solution (1:1000 A and 1:1000 B, diluted in 0.1M PBS, mixed 30 minutes before use; Vector Laboratories, Burlingame, CA, USA). This stain was then visualized by incubating the tissue in a solution containing 0.02% 3,3’-diaminobenzidine tetrahydrochloride, 0.08% nickel sulfate hexahydrate, and 0.012% H2O2, diluted in 0.1M NaPB, for 10 minutes. This caused a dark black precipitate to form at the location of c-Fos detection. Following development for c-Fos staining, the tissue was processed for FG staining. Tissue was again incubated in 1% H2O2 for 10 minutes, and then incubated overnight in rabbit anti-FG primary antibody (1:50,000; this antibody was a generous gift from Dr. Stanley Watson’s Laboratory at the University of Michigan, and is commercially available from Fluorochrome, Denver, CO, USA) with 1% normal donkey serum and 0.4% Triton X-100 in 0.1M PBS. On the third day, sections were incubated in secondary antibody solution containing 1:500 donkey anti-rabbit antibody (lots 119063 and 124459, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), 1% normal donkey serum, and 0.4% Triton X-100 in 0.1M PBS, for 1 hour. Sections were then incubated for 1 hour in Vectastain Elite ABC solution (1:1000 A and 1:1000 B, diluted in 0.1M PBS, mixed 30 minutes before use; Vector Laboratories, Burlingame, CA, USA). This stain was then visualized by incubating the tissue in a solution containing 0.02% 3,3’-diaminobenzidine tetrahydrochloride and 0.012% H2O2, diluted in 0.1M NaPB, for 10 minutes. This caused a brown precipitate to form at the location of FG detection. The sections were then mounted onto SuperFrost Plus microscope slides (Fisher Scientific, Hampton, NH, USA), dehydrated in graduated ethanol solutions followed by xylenes, and coverslipped with Permount medium (Fisher Scientific, Hampton, NH, USA).
FG/C-Fos Quantification
All immunohistochemical analysis was conducted by an experimenter blind to experimental conditions using the software program Stereo Investigator (MBF Bioscience, Williston, VT, USA) which was connected to a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany) and an Optronics Microfire digital camera (Optronics International, Tulsa, OK, USA). Each section was viewed at 2.5× magnification and a contour was traced around the area of interest using recognizable landmarks from the Rat Brain Atlas (Paxinos and Watson, 2007). Then, at 10× or 20× magnification around the area of interest, Stereo Investigator was used to navigate the sections and mark cells and three measurements were collected by the experimenter: the total number of FG positive cells (distinguishable by a brown cytosolic stain), the total number of c-Fos positive cells (distinguishable by a gray/black nuclear stain), and the total number of FG/c-Fos double-labeled cells (distinguishable by a gray/black nuclear stain that was surrounded by a brown cell body; see Figure 2C for an example image). Target areas were identified for analysis using the patterns of retrograde labeling observed here, as well as published anterograde and retrograde tracing reports by others (Chen and Su, 1990, Van der Werf et al., 2002, Kirouac et al., 2005, Parsons et al., 2006, Li and Kirouac, 2008, Hsu and Price, 2009, Li and Kirouac, 2012, Li et al., 2014). For the Afferent Experiment, areas of quantification included layer 6 of the anterior PrL (approximate bregma level AP +4.2), layer 6 of the posterior PrL and IL (approximate bregma level AP +3.0), the central amygdala and medial amygdaloid complex (approximate bregma level AP −2.6), the hypothalamus (A13 cell group, dorsomedial nucleus, ventromedial nucleus and lateral hypothalamus, which included the perifornical area; approximate bregma level AP −2.8) and ventral subiculum (approximate bregma level AP −5.6). One section was chosen per subject at the appropriate bregma level for each area of interest, and both hemispheres were quantified individually and then summed for one total count for each measure per section. For the Efferent Experiment, three PVT sections were identified and analyzed separately for quantification for each subject; one from the anterior PVT (approximate bregma AP −1.8), one from the mid PVT (approximate bregma AP −2.8), and one from the posterior PVT (approximate bregma AP −3.6)
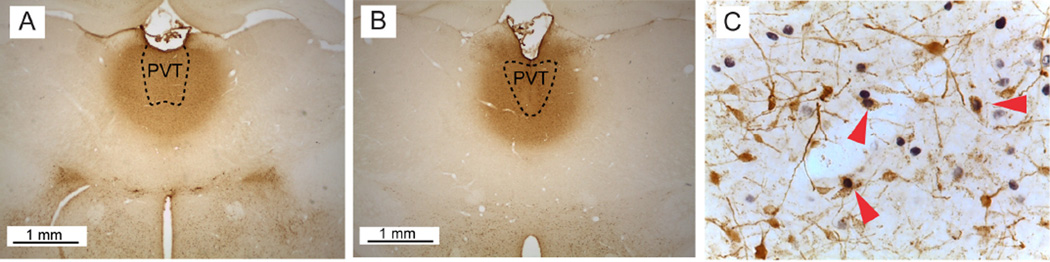
Figure 2. Examples of fluorogold (FG) injections into the PVT and immunohistochemical labeling.
Examples of FG injection shown in A) anterior PVT (approximate bregma AP −2.0) and B) posterior PVT (approximate bregma AP −3.0), shown at 2.5× magnification. Dashed line represents the approximate boundaries of the PVT. C) FG/c-Fos double-labeling following immunohistochemical staining, shown at 40×. Red triangles indicate cells double-labeled for FG and c-Fos. Brightness, color and contrast adjusted for clarity.
Statistical Analyses
To analyze differences on each measure of PCA behavior, linear mixed-effects models were used, with Session as the repeated variable and Group (ST, GT or UN) as the between subjects variable. In each instance, the appropriate covariance structure was determined using Akaike information criteria values. For lever contacts during the cue test session as well as overall levels of c-Fos expression in different brain regions, both the Shapiro-Wilk test and Levene’s Test were used to assess assumptions of normality. When the data sets were considered to be well-modeled by a normal distribution, a univariate ANOVA was used to assess the effects of Group on lever contacts or the total number of c-Fos positive cells. When the assumptions of a normal distribution were violated, these effects were assessed using a Kruskal-Wallis H test.
Activity of specific populations of PVT efferents/afferents (i.e. double-labeled cells) was assessed using the following formula: (the total number of FG and c-Fos double labeled cells) ÷ (the total number of FG positive cells). A binary logistic regression model analysis was used to examine group differences on this measure. For each individual, two values were entered for the dependent variable: the number of double-labeled cells and the total number of FG labeled cells. This allowed the logistic regression to calculate the probability of a cell being double-labeled for each group, which is then incorporated into the analysis. Modeling the dependent variable in this fashion in part controls for the subtle variability within the total population of FG-labeled cells across individuals, as opposed to simply comparing double-labeled cells alone, allowing for a more robust assessment of activity within each population of PVT afferents/efferents. For all analyses, when significant main effects of Group were observed, Bonferroni post-hoc comparisons were performed. For all analyses, significance was set at p ≤ 0.05.
Results
Afferent Experiment
Pavlovian Conditioned Approach Behavior
Similar to previous reports (Flagel et al., 2009, Meyer et al., 2012, Haight et al., 2015), considerable variation was seen in the CRs acquired by individual rats following 5 sessions of PCA training. Some rats directed their behavior towards the lever-CS, and were classified as STs (n = 15; PCA Index range +0.3 to +0.93), while others directed their behavior towards the location of US delivery upon lever-CS presentation, and were classified as GTs (n = 12; PCA Index range −0.34 to −0.91). In addition, some rats showed an intermediate response, and were excluded from the study (n = 7; PCA Index range 0.29 to −0.29; data not shown). Last, control rats (UNs; n = 6) receiving CS-US presentations in an unpaired fashion did not acquire a sign- or goal-tracking CR. Note that additional animals were excluded due to missed FG injections (see below), and their data is not included in the analysis below.
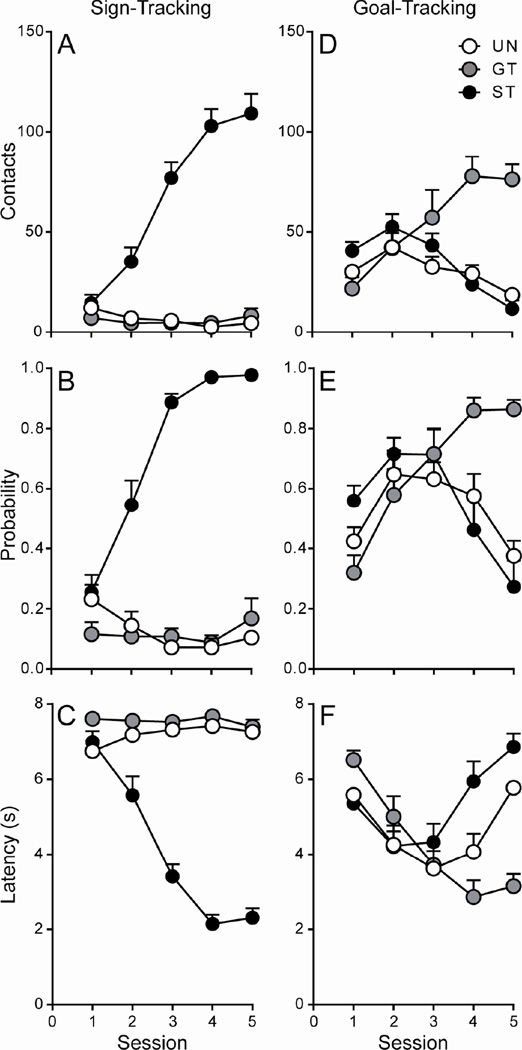
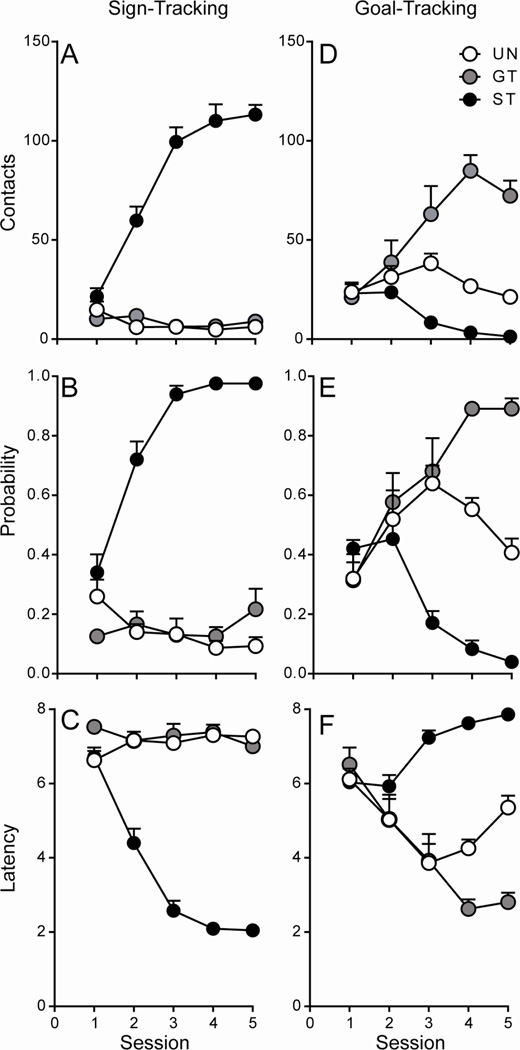
Linear mixed-effects models showed significant overall effects of Session and Group, as well as a significant Session × Group interaction, on all measures of sign-tracking behavior (p < 0.001; Figure 1A–C). For all three measures (contacts, probability, and latency), Bonferroni post-hoc analyses showed a significant within-group effect of Session for STs (p < 0.001), but not for GTs or UNs (p > 0.05). STs began to demonstrate lever-directed behavior as early as session 2, while GTs and rats in the UN group did not develop lever-directed behavior across sessions. For goal-tracking behavior, there was a significant effect of Session, as well as a significant Session × Group interaction, for all three measures (contacts, probability, latency; p ≤ 0.002; Figure 1D–F). In addition, there was a significant effect of Group for food-cup contacts (p = 0.018; Figure 1D) and Bonferroni post-hoc analyses revealed a significant within-group effect of Session for GTs and STs (p < 0.001), but not for rats in the UN group (p > 0.05), on this measure. That is, GTs increased their number of food cup contacts during the 8-second CS period across training sessions, while STs decreased responding directed at the food cup, and the behavior of rats in the UN group did not significantly change across sessions. For probability of food cup contact, as well as latency to food cup contact, Bonferroni post-hoc comparisons showed a significant within-group effect of Session for all three groups (all p ≤ 0.032; Figure 3.1E–F). GTs increased their food-cup-directed behavior across training sessions, while STs and rats in the UN group decreased their food-cup-directed behavior following session 3 and 4, respectively. Taken together, these results indicate that STs developed lever-directed behavior, while GTs developed food-cup-directed behavior, and rats in the UN group developed neither a sign-tracking nor a goal-tracking conditioned response.
Figure 1. Acquisition of the sign- and goal-tracking conditioned response following 5 Pavlovian conditioning sessions (Afferent Experiment).
Mean + SEM for A) lever contacts, B) probability of lever contact, C) latency to lever contact, D) food cup contacts, E) probability of food cup contact, and F) latency to food cup contact. Rats that displayed lever-directed behavior were classified as sign-trackers (STs; n = 11), while those that directed their behavior towards the food cup were classified as goal-trackers (GTs; n = 10). Rats who received unpaired CS-US presentations did not develop a conditioned response (UNs; n = 5).
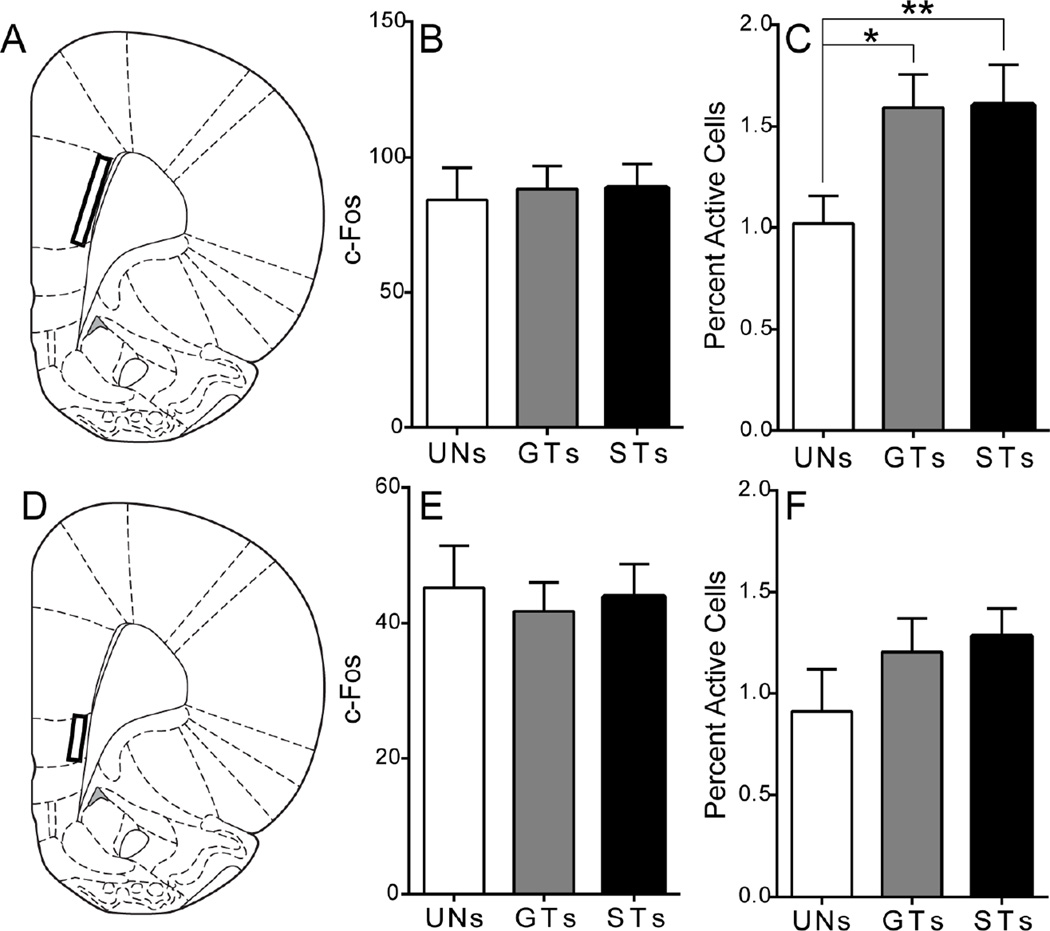
Figure 3. C-Fos expression in the medial prefrontal cortex following cue presentation.
A) Schematic coronal section (AP +3) of the left hemisphere representing the approximate area of quantification for the PrL (AP +4.2 not shown). Mean + SEM for B) overall c-Fos levels in layer 6 of the PrL and C) percent activity specifically in PVT afferents from the prelimbic cortex following stimulus presentation (UNs = 5, GTs = 8, STs = 11). There was an overall effect of Group, and Bonferroni post-hoc tests revealed that both GTs (*p = 0.02) and STs (**p < 0.01) have greater engagement of PVT afferents from the PrL compared to UNs. D) Schematic coronal section (AP +3) of the left hemisphere representing the approximate area of quantification for IL. Mean + SEM for E) overall c-Fos levels in layer 6 of the IL and F) percent activity specifically in PVT afferents from the infralimbic cortex following stimulus presentation (UNs = 5, GTs = 8, STs = 11). Percent activity was calculated as (FG + c-Fos double-labeled cells) / (total FG labeled cells). Atlas images adapted from Paxinos and Watson (2007).
Behavior During the Cue Test Session
During the cue-test session subjects were presented with the lever-CS 10 times, for 2 seconds each time. While many individuals made contact with the lever-CS during its brief presentation [Mean (+/− SEM): UNs = 3.4 (1.0); GTs = 4.7 (1.9); STs = 7.0 (1.6)], a Kruskal-Wallis test showed that there was not a significant effect of Group on this measure during the cue-test session (χ2(2) = 2.01, p = 0.37). Although one might have expected to see differences in lever-directed behavior during the cue-test, it should be reiterated that the lever-CS is presented only very briefly during this session. Furthermore, the number of lever contacts within each group was quite variable, likely due to the presentation of the lever-CS under novel test conditions.
Injection Screening and Retrograde Labeling
Following immunohistochemistry, all brains were screened for FG injection and retrograde labeling accuracy. In general, anterior and posterior injections were centered on the midline and targeted directly at the PVT, completely filling the structure (for example injection sites see Figure 2A–B). Despite the small volume of the injection (50 nl), all subjects had some degree of FG staining in the surrounding nuclei: laterally in portions of the mediodorsal and paratenial nucleus, ventrally in portions of the centromedial nucleus and interanteromedial nuclei (anterior PVT sections) or intermediodorsal and centromedial nuclei (posterior PVT sections), and dorsally in portions of the habenula (Figure 2A–B). This is due to the small size of the PVT, making it extremely difficult to isolate. Two subjects (n = 1 UN, 1 GT) with accurate PVT injections had FG staining that appeared slightly larger around the anterior injection site, spreading ventrally through the interanteromedial nucleus into the borders of the rhomboid/reuniens nucleus on some sections, but the retrograde labeling from these subjects did not appear to differ from the other subjects in their respective groups, so they were included in the study. However, 7 subjects were excluded from the study for seemingly inaccurate FG injections. These subjects either had FG injections that missed the PVT (n = 1 GT, 1 ST); had injections that filled the PVT but were primarily biased towards the left hemisphere (n = 1 UN, 1 GT, 2 ST); or had dense dorsomedial hippocampal staining above the PVT (n = 1 ST). The final number of subjects included in the study was: 5 UNs, 10 GTs, and 11 STs.
Many of the surrounding thalamic nuclei receive similar afferent connections as the PVT. For example, the mediodorsal thalamus also receives input from layer 6 PrL and IL cells (Gabbott et al., 2005). Thus, it is not possible to completely rule out the effect of erroneous labeling in the current study. Importantly, however, our injections entirely filled the PVT, and generally only partially filled the surrounding nuclei, so the majority of retrogradely labeled cells quantified in the current study are likely PVT afferents. In addition, we have previously shown that lesions of the PVT affected the expression of PCA behavior, while damage to the surrounding area in the absence of PVT damage did not appear to affect PCA performance (Haight et al., 2015), supporting the idea that it is indeed PVT afferents, and not afferents to nearby nuclei, that were activated by stimulus presentation in the current study. Furthermore, the areas chosen for quantification showed retrograde labeling similar to previously published findings (Chen and Su, 1990, Van der Werf et al., 2002, Kirouac et al., 2005, 2006, Vogt et al., 2008, Hsu and Price, 2009, Li and Kirouac, 2012, Li et al., 2014).
Quantification Results
In order to evaluate differences in c-Fos protein expression between STs, GTs, and UNs in response to cue presentation, two different assessments were made. The first was an assessment of the overall amount of cue-induced c-Fos in a given region, quantified as the total amount of c-Fos positive nuclei. The second was an assessment of c-Fos specifically in PVT afferents from a given region, which was quantified as double-labeled cells, as indicated above. Of note, some brain areas could not be quantified for certain subjects, for example due to damage that occurred during brain extraction and processing (see figure legend for the n for each region).
Prelimbic and Infralimbic Cortex
Following PVT FG injection, a dense number of retrogradely labeled cells was seen in layer 6 of the PrL. This labeling was observed throughout the anterior-posterior axis of the PrL. Therefore, two different regions of the PrL were quantified: one anterior at approximately AP +4.2, and one posterior at approximately AP +3.0. The pattern of group differences was similar in both the anterior and posterior PrL, so the counts from these sections were averaged for each subject to assess PrL c-Fos expression (AP +3.0 region depicted in Figure 3A). A univariate ANOVA showed no difference between groups in the amount of cue-induced c-Fos in layer 6 of the PrL (effect of Group, F(2,21) = 0.05, p = 0.95; Figure 3B). In order to assess differences in c-Fos counts specifically in PVT afferent cells (i.e. double-labeled cells), a binary logistic regression model analysis was used. Results showed an overall effect of Group (Wald χ2(2,n=24) = 8.34, p = 0.02; Figure 3C). Bonferroni post-hoc comparisons showed that both GTs (p = 0.02) and STs (p < 0.01) have significantly greater engagement of PVT afferents from the PrL compared to rats in the UN group (Figure 3C). These results indicate that presentation of a reward-paired stimulus can evoke activity in PVT afferents from the PrL, regardless of its incentive motivational value.
Layer 6 of the IL, characterized by dense retrograde labeling beneath the posterior PrL, was also quantified (Figure 3D). A univariate ANOVA showed that there was not a significant effect of group for overall c-Fos counts in this region (F(2,21) = 0.10, p = 0.91; Figure 3E). The binary logistic regression model also showed no significant effect of Group (Wald χ2(2,n=24) = 1.87, p = 0.39; Figure 3F) for c-Fos counts specifically in PVT afferents from the IL, demonstrating that cue presentation did not evoke differential activity in the IL between STs, GTs and UNs.
Amygdala
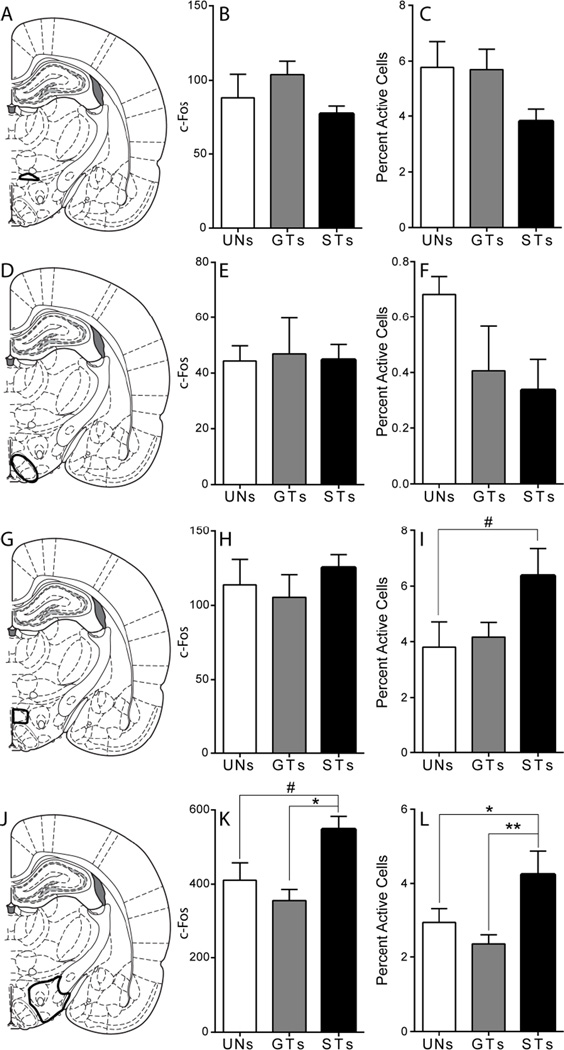
For the CeA (Figure 4A), a univariate ANOVA showed a trend towards a significant effect of Group in overall c-Fos count (F(2,18) = 3.17, p = 0.07; Figure 4B), with a tendency for STs to have more c-fos expression than the other groups. Only a few brains had any observable FG/c-Fos double-labeled cells, and a binary logistic regression model analysis revealed no significant effect of Group, (Wald χ2(2,n=21) = 2.64, p = 0.27; Figure 4C). These data demonstrate that while an incentive stimulus tends to evoke greater c-Fos expression in cells in the CeA, this expression is not in cells projecting to the PVT.
Figure 4. C-Fos expression in the central and medial amygdala in response to cue presentation.
A) Schematic coronal section (AP −2.52) of the left hemisphere representing the approximate area of CeA quantification. Mean + SEM for B) overall c-Fos levels in the CeA and C) percent activity specifically in PVT afferents from the CeA following stimulus presentation (UNs = 5, GTs = 7, STs = 9). There was a trend towards a significant effect of Group (F(2,18) = 3.171, #p = 0.07) for overall c-Fos expression in the CeA. D) Schematic coronal section (AP −2.52) of the left hemisphere representing the approximate area of MeA quantification. Mean + SEM for E) overall c-Fos levels in the MeA and F) percent activity specifically in PVT afferents from the MeA following stimulus presentation (UNs = 5, GTs = 6, STs = 8). There was a significant effect of Group, and post-hoc comparisons reveal that STs show greater activity in this circuit compared to rats in the UN group (*p < 0.01). Percent activity was calculated as (FG + c-Fos double-labeled cells) / (total FG labeled cells). Atlas images adapted from Paxinos and Watson (2007).
Retrograde labeling was seen throughout the MeA (Figure 4D), and the pattern of c-Fos expression here was quite different from that in the CeA. A univariate ANOVA showed no significant effect of Group in cue-induced c-Fos induction in the MeA (F(2,16) = 1.85, p = 0.19; Figure 4E), indicating that cue presentation did not lead to differences in overall induction of c-fos this structure. However, when looking specifically at PVT afferents from the MeA, the binary logistic regression model showed a significant overall effect of Group (Wald χ2(2,n=19) = 8.97, p = 0.01; Figure 4F). Bonferroni post-hoc comparisons revealed that STs had greater cue-induced c-Fos in MeA afferents to the PVT compared to rats in the UN group (p < 0.01; Figure 4F), indicating that a reward-paired cue must be attributed with incentive value for it to evoke activity in PVT afferents from the MeA.
Hypothalamus
A large number of retrogradely labeled cells were seen throughout the hypothalamus. These cells were found in several of the hypothalamic subregions including the A13 cell group, the dorsomedial nucleus (DMD), the ventromedial nucleus (VMH), and the lateral hypothalamus (LH), which included the perifornical region (PF). In the A13 cell group (Figure 5A), a univariate ANOVA showed no significant effect of Group on overall c-Fos counts (F(2,18) = 2.39, p = 0.12; Figure 5B). In addition, the binary logistic regression model showed no differences in cue-induced c-Fos in specific PVT afferents from the A13 cell group (overall effect of Group, Wald χ2(2,n=21) = 2.73, p = 0.26; Figure 5C), indicating that presentation of a predictive or incentive stimulus does not lead to increased activity in this nucleus.
Figure 5. C-Fos expression in the hypothalamus in response to cue presentation.
A) Schematic coronal section (AP −2.76) of the left hemisphere representing the approximate area of the A13 cell group quantified. Mean + SEM for B) overall c-Fos levels in the A13 cell group and C) percent activity specifically in PVT afferents from the A13 cell group following stimulus presentation (UN = 5, GTs = 8, STs = 8). D) Schematic coronal section (AP −2.76) of the left hemisphere representing the approximate area of VMH quantified. Mean + SEM for E) overall c-Fos levels in the VMH and F) percent activity specifically in PVT afferents from the VMH following stimulus presentation (UNs = 3, GTs = 7, STs = 5). G) Schematic coronal section (AP −2.76) of the left hemisphere representing the approximate area of the DMD quantified. Mean + SEM for H) overall c-Fos levels in the DMD and I) percent activity specifically in PVT afferents from the DMD following stimulus presentation (UN = 5, GTs = 7, STs = 8). There was a trend towards a significant effect of Group, and post-hoc comparisons show that STs tend to have greater activity in this circuit compared to rats in the UN group (#p = 0.08). J) Schematic coronal section (AP −2.76) of the left hemisphere representing the approximate area of LH quantified. Mean + SEM for K) overall c-Fos levels in the LH and L) percent activity specifically in PVT afferents from the LH following stimulus presentation (UNs = 5, GTs = 7, STs = 6). There was an effect of Group on overall c-Fos levels in the LH (K), and post-hoc comparisons show that STs have higher levels of c-Fos compared to GTs (*p < 0.01), and trend towards a significant increase compared to rats in the UN group (#p = 0.07). For percent activity in PVT afferents from the LH (L), there was an effect of phenotype, and post-hoc comparisons show that STs have greater activity in this circuit compared to GTs (**p < 0.01) and rats in the UN group (*p = 0.01). Percent activity was calculated as (FG + c-Fos double-labeled cells) / (total FG labeled cells). Atlas images adapted from Paxinos and Watson (2007).
Similar to the A13 cell group, cue presentation did not lead to differences in overall c-Fos expression in the VMH (Figure 5D), as there was not a significant effect of Group by Kruskal-Wallis analysis (χ2(2) = 1.43, p = 0.49; Figure 5E). In addition, very few FG/c-Fos double labeled cells were seen in this area (n ≤ 4 per subject), and there was not a significant effect of Group for double-labeled cells (Wald χ2(2,n=15) = 2.04, p = 0.36; Figure 5F). These results indicate that cue presentation has little effect on the activity of PVT afferents from the VMH.
Cue presentation also did not lead to differences in overall c-Fos expression in the DMD (effect of Group, F(2,17) = 0.65, p = 0.54; Figure 5H). However, there was a trend for a significant effect of Group for c-Fos expression in PVT afferents from the DMD (overall effect of Group, Wald χ2(2,n=20) = 5.75, p = 0.06; Figure 5I). Bonferroni post-hoc comparisons showed that there was a tendency for STs to have greater c-Fos expression in this circuit compared to UNs (p = 0.08).
The LH (including the PF, Figure 5J) was amongst the regions with the most robust cue-induced c-fos expression. A univariate ANOVA showed a significant overall effect of Group (F(2,15) = 7.86, p < 0.01; Figure 5K). Bonferroni post-hoc comparisons revealed that STs had significantly greater c-Fos counts in the LH compared to GTs (p < 0.01), and a trend towards greater c-Fos counts compared to rats in the UN group (p = 0.07). A similar effect was seen in PVT afferents from the LH. Binary logistic regression analysis revealed an overall effect of Group (Wald χ2(2,n=18) = 21.63, p < 0.01; Figure 5L), with Bonferroni post-hoc comparisons indicating that cue presentation led to greater c-Fos expression in this circuit in STs compared to both GTs (p < 0.01) and rats in the UN group (p = 0.01).
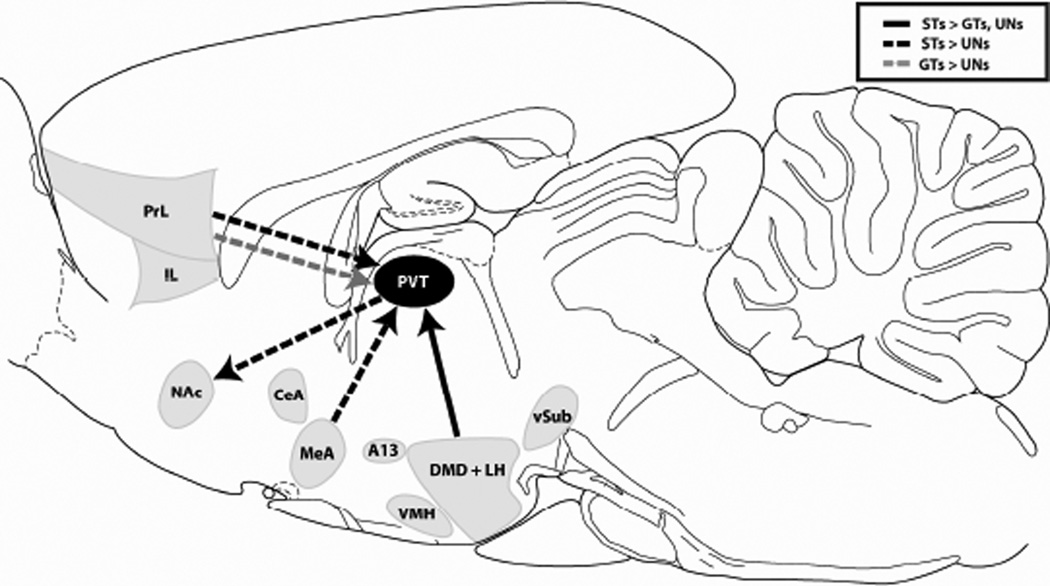
Since the LH and DMD showed similar patterns of c-Fos expression in PVT afferents, with STs tending to have more expression in this circuit, the total counts from these areas were summed in order to get a general picture of activity in dorsomedial/lateral hypothalamic afferents to the PVT. There was a significant overall effect of Group (Wald χ2(2,n=18) = 29.01, p < 0.01) for double-labeled cells in the combined DMD/LH afferents to the PVT (data not shown). Bonferroni post-hoc comparisons indicate that STs had greater c-Fos expression in these hypothalamic afferents compared to GTs and UNs (both p < 0.01). These results confirm that presentation of an incentive, but not a reward-predictive stimulus alone, leads to increased activity in PVT afferents from the dorsomedial/lateral hypothalamus (see Figure 10).
Figure 10. Schematic demonstrating the efferent and afferent circuits of the PVT engaged by cue presentation in STs vs. GTs.
Sagittal schematic representing the efferent and afferent connections of the PVT that had significantly different levels of cue-induced c-Fos between STs and GTs or unpaired controls. Solid black arrow represents the circuit where STs had greater percent activity compared to GTs and rats in the UN group. Dashed black and gray arrows represent connections where STs or GTs had greater percent activity relative to UN controls, respectively. Abbreviations: A13, A13 cell group; CeA, central nucleus of the amygdala; DMD, dorsomedial nucleus of the hypothalamus; IL, infralimbic cortex; LH, lateral hypothalamus; MeA, medial amygdaloid complex; NAc, nucleus accumbens; PrL, prelimbic cortex; PVT, paraventricular nucleus of the thalamus; VMH, ventromedial hypothalamus; vSub, ventral subiculum. Atlas image adapted from Paxinos and Watson (2007).
Ventral Subiculum
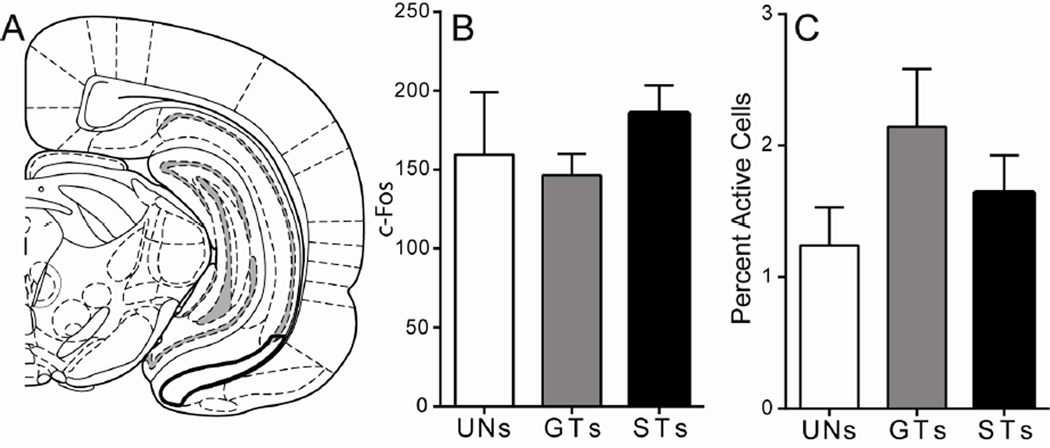
In the VSub (Figure 6A) there was not a significant effect of Group for overall levels of cue-induced c-Fos expression (F(2,16) = 1.09, p = 0.36; Figure 6B), and no significant differences in c-Fos expression in PVT afferents from the VSub following cue presentation (no effect of Group, Wald χ2(2,n=19) = 3.83, p = 0.15; Figure 6C). These results suggest that cells in the VSub that project to the PVT do not encode either the predictive or the incentive motivational value of the reward-paired cue.
Figure 6. C-Fos expression in the ventral subiculum following cue presentation.
A) Schematic coronal section (AP −5.64) of the left hemisphere representing the approximate area of VSub quantification. Mean + SEM for B) overall c-Fos levels in the VSub and C) percent activity specifically in PVT afferents from the VSub (depicted as [FG + c-Fos double-labeled Atlas image adapted from Paxinos and Watson (2007).
Efferent Experiment
Pavlovian Conditioned Approach Behavior
Similar to the Afferent Experiment, there were differences in the CRs that were acquired following 5 days of PCA training. Rats that directed their behavior towards the lever-CS were classified as STs (n = 23; PCA Index range +0.54 to +0.93). Due to physical experimental limitations, 17 STs with PCA scores ranging from +0.60 to +0.93 were selected from this group to continue on in the study. Rats that exhibited a CR that was directed towards the location of US delivery (food cup) upon lever-CS presentation were classified as GTs (n = 11; PCA Index range −0.58 to −0.93). Rats classified as intermediates vacillated between the lever and the food cup, and they were excluded from the study (n = 18; PCA Index range +0.45 to −0.49; data not shown). In addition, 8 unpaired control rats (UN) received an equivalent number of CS-US presentations, but in an unpaired fashion (n = 8). Note that additional subjects were excluded due to missed FG injections (see below), and their data are not included in the analysis below.
Linear mixed effects models showed significant overall effects of Session and Group, as well as a significant Session × Group interaction, on all measures of sign-tracking behavior (p ≤ 0.001; Figure 7A–C). For all three measures, Bonferroni post-hoc analyses showed a significant within-group effect of Session for STs (p < 0.001), but not for GTs or rats in the UN group (p > 0.05). STs began to demonstrate lever-directed behavior as early as session 2, while GTs and rats in the UN group did not develop this behavior across sessions. For goal-tracking behavior, linear mixed effects models showed significant overall effects of Session and Group, as well as a significant Session × Group interaction, on all three measures (p ≤ 0.001; Figure 7D–F). Bonferroni post-hoc analyses revealed a significant within-group effect of Session for food cup contacts for GTs and STs (p < 0.001), but not for rats in the UN group (p > 0.05; Figure 7D). Thus, GTs increased their food-cup contacts during the 8-second CS period across training sessions, whereas STs decreased their food-cup contacts, and rats in the UN group did not alter their food-cup contacts over the course of training. For the probability of food-cup contact, as well as latency to food-cup contact, Bonferroni post-hoc comparisons showed a significant within-group effect of Session for all three groups (all p ≤ 0.032; Figure 7E–F). Again, GTs increased their food-cup-directed behavior across training sessions, indicating the development of a goal-tracking CR. In contrast, STs began to decrease their food-cup-directed behavior after session 2, and rats in the UN group began to decrease their food-cup-directed behavior following session 3. Taken together, these results indicate that STs developed lever-directed behavior, while GTs developed food-cup-directed behavior, and rats in the UN group developed neither a sign-tracking nor a goal-tracking conditioned response.
Figure 7. Acquisition of the sign- and goal-tracking conditioned response following 5 Pavlovian conditioning sessions (Efferent Experiment).
Mean + SEM for A) lever contacts, B) probability of lever contact, C) latency to lever contact, D) food cup contacts, E) probability of food cup contact, and F) latency to food cup contact. Rats that displayed lever-directed behavior were classified as sign-trackers (STs; n = 15), while those that directed their behavior towards the food cup were classified as goal-trackers (GTs; n = 7). Rats who received unpaired CS-US presentations did not develop a conditioned response (UNs; n = 6).
Behavior During the Cue Test Session
Identical to the Afferent Experiment, subjects were presented with the lever-CS 10 times, for 2 seconds each time, during the cue-test session. However, unlike the results from the cue-test session from the Afferent Experiment, a Kruskal-Wallis test showed a significant effect of Group on lever contacts during the cue-test session (χ2(2) = 15.63, p < 0.01; Figure 9) of the Efferent Experiment. As might be expected, STs (mean = 9.8 +/− 0.7 SEM) showed the greatest number of lever contacts across groups, followed by GTs (mean = 5.1 +/− 1.4 SEM), and then UNs (mean = 1.2 +/− 0.4 SEM).
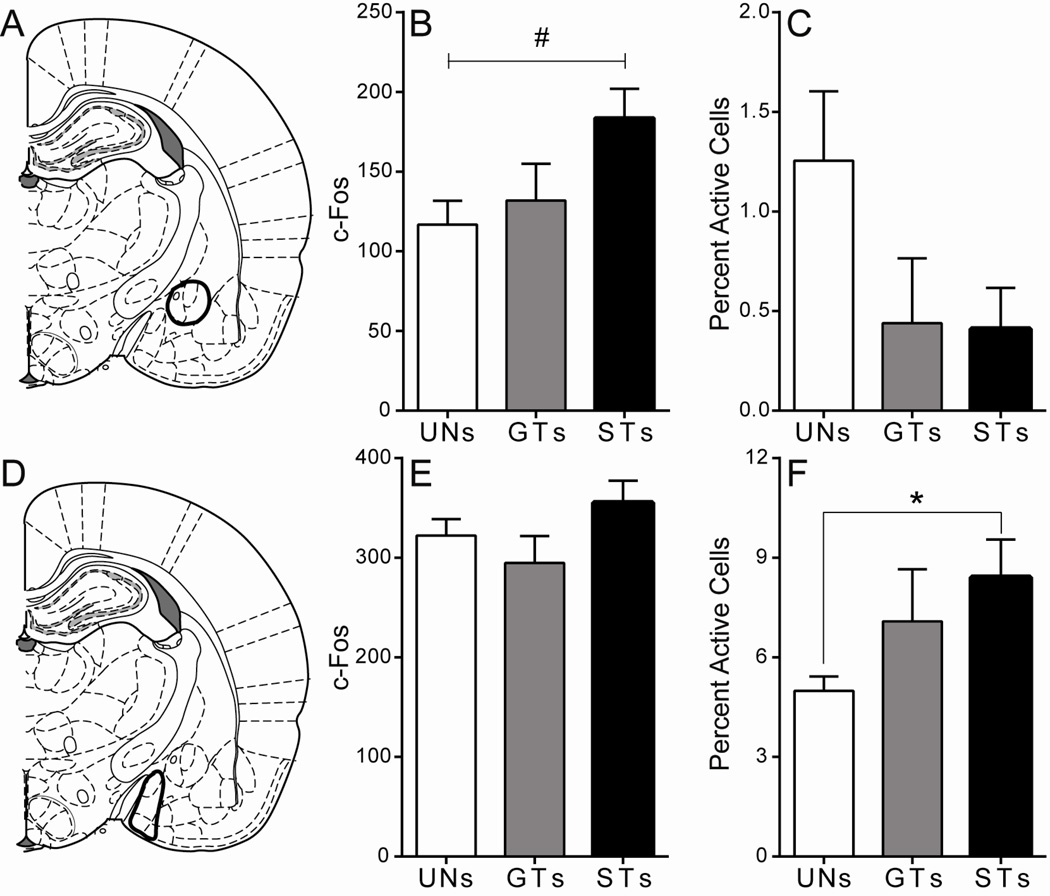
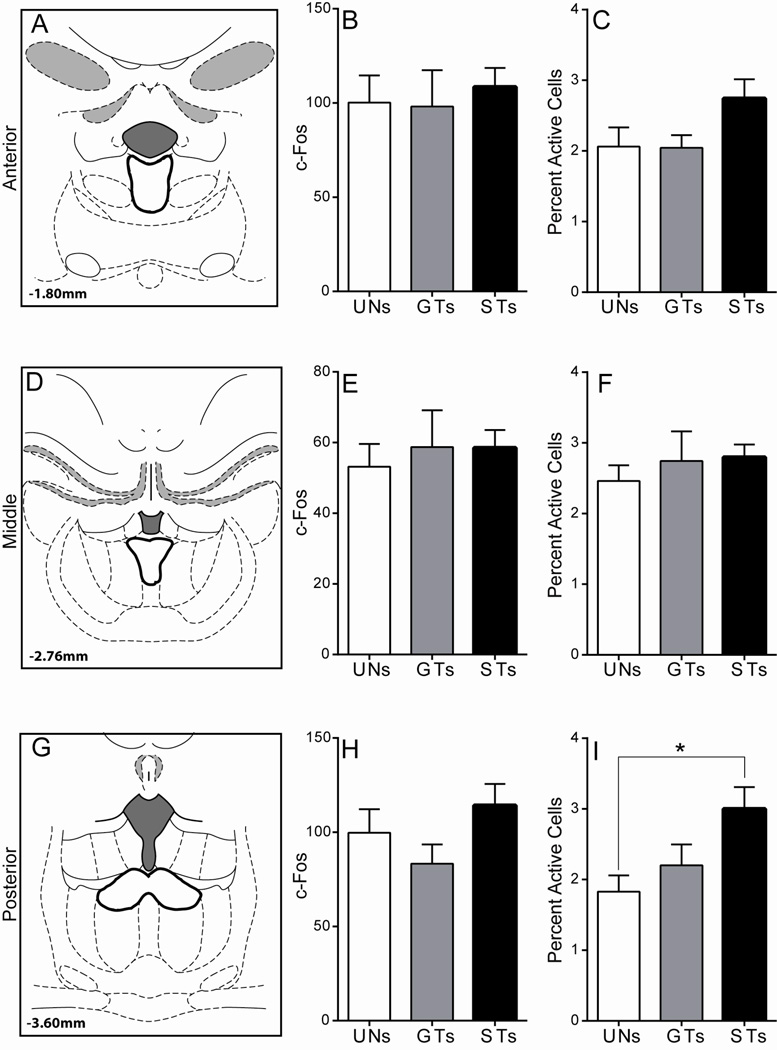
Figure 9. C-Fos expression in the PVT following cue presentation.
A) Schematic coronal section (AP −1.8) of the midline thalamus representing the approximate area of anterior PVT quantification. Mean + SEM for B) overall c-Fos levels in the anterior PVT and C) percent activity specifically in anterior PVT efferents to the NAc following stimulus presentation. D) Schematic coronal section (AP −2.76) of the midline thalamus representing the approximate area of the middle PVT that was quantified. Mean + SEM for E) overall c-Fos levels in the middle PVT and F) percent activity specifically in middle PVT efferents to the NAc following stimulus presentation. G) Schematic coronal section (AP −3.6) of the midline thalamus representing the approximate area of the posterior PVT quantified. Mean + SEM for H) overall c-Fos levels in the posterior PVT and I) percent activity specifically in posterior PVT efferents to the NAc following stimulus presentation. There was a significant effect of Group, and post-hoc comparisons showed that STs have a greater percent activity in posterior PVT cells projecting to the NAc relative to UN controls (*p < .05). Percent activity was calculated as (FG + c-Fos double-labeled cells) / (total FG labeled cells). Atlas images adapted from Paxinos and Watson (2007).
Injection Screening and Retrograde Labeling
Following immunohistochemistry, all brains were screened for accuracy of the FG injection and pattern of retrograde labeling. In general, FG staining at the injection site was primarily contained within the NAc (for an example image see Figure 8A). A small number of rats had unilateral injections, injections that missed the NAc, or injections that were not primarily contained within the borders of the NAc and they were excluded from the study (n= 2 UNs, 3 GTs, and 2 STs). In some rats, there was FG staining that followed up the injection track through the lateral septum/dorsal striatum. While the PVT does send projections to this area, these projections are primarily limited to the anterior PVT (Moga et al., 1995, Li and Kirouac, 2008), and appear slightly less dense than those to the NAc (Li and Kirouac, 2008).
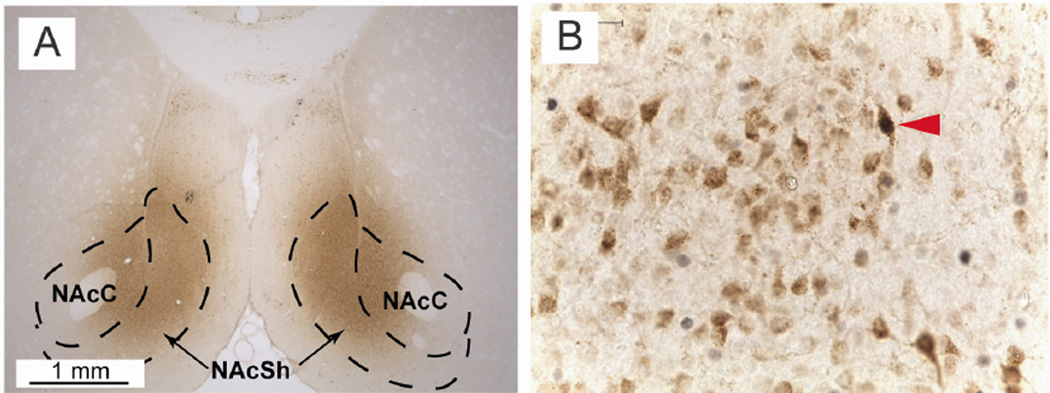
Figure 8. Examples of fluorogold (FG) injections into the NAc and immunohistochemical labeling.
A) Example FG injection in the NAc, shown at 2.5× magnification. Approximate bregma AP 1.7; dashed lines represent the approximate boundaries of the NAc core and shell.
B) FG/c-Fos double-labeling following immunohistochemical staining, shown at 40× magnification. Red triangle indicates a double-labeled PVT cell. Brightness, color and contrast adjusted for clarity.
The pattern of retrograde labeling following the injection was consistent with previous published findings (Li and Kirouac, 2008). Dense FG labeling was seen throughout the rostro-caudal axis of the PVT, and thus one section from the anterior, middle, and posterior PVT was quantified for each brain. Importantly, strong labeling was observed in the PVT in all of the subjects that had a successful surgery, except for one, which was excluded from the study due to minimal FG staining in the PVT (n = 1 GT). The remaining brains (n = 6 UNs, 7 GTs, 15 STs) were further processed and quantified as described above.
Quantification Results
Similar to the Afferent Experiment, two different assessments were made. First, the total amount of c-Fos positive nuclei in a given PVT section was measured. Second, the expression of c-Fos in cells that projected specifically to the NAc was assessed as: (the total number of FG and c-Fos double labeled cells) ÷ (the total number of FG positive cells) within each PVT section (Figure 8B).
Anterior PVT
Within the anterior PVT (Figure 9A), cue presentation did not lead to differences in overall c-Fos expression, as there was not a significant effect of Group using a Kruskal-Wallis test (χ2(2) = 1.07, p = 0.59, Figure 9B). There was also not a significant effect of Group for the expression of c-Fos in anterior PVT neurons that project directly to the NAc (Wald χ2(2,n=28) = 2.92, p = 0.23; Figure 9C). These results suggest that cells in the anterior PVT that project to the NAc do not encode either the predictive or the incentive motivational qualities of reward-paired cues.
Mid PVT
Cue-induced c-Fos expression in the mid PVT was also quantified (Figure 9D). Cue presentation did not have an effect on overall c-Fos activation in the region, as there was not a significant effect of Group using a univariate ANOVA (F(2,25) = 0.16, p = 0.86; Figure 9E). In addition, there was no effect of Group in c-Fos expression specifically in mid-PVT efferents to the NAc (Wald χ2(2,n=28) = 0.54, p = 0.76; Figure 8F). Thus, similar to the anterior PVT, cue presentation did not lead to differential levels of activity between groups in the mid PVT on either measure quantified.
Posterior PVT
In the posterior PVT (Figure 9G), cue presentation did not lead to significant differences between groups in overall c-Fos expression (F(2,25) = 1.60, p = 0.22; Figure 9H). However, c-Fos expression specifically in NAc afferents from the posterior PVT was significantly different between groups (Wald χ2(2,n=28) = 6.16, p < 0.05; Figure 9I). Bonferroni post-hoc comparisons revealed that STs have greater c-Fos counts in cells projecting to the NAc relative to UN controls (p < .05). These data suggest that a reward-paired cue must be attributed with incentive salience for it to elicit activity in posterior PVT efferents to the NAc.
Discussion
The current study measured c-Fos expression in specific PVT afferent and efferent neuronal populations in response to presentation of a predictive and incentive stimulus (in STs), or a predictive-only stimulus (in GTs). This was accomplished using a combination of retrograde tracing and immunohistochemical analyses in a rat model that captures individual variation in Pavlovian conditioned approach behavior. Results indicate that presentation of a reward-predictive stimulus increases activation of PrL cells that project directly to the PVT, evidenced by increased activity in this circuit in both STs and GTs. However, when a reward-predictive stimulus becomes imbued with incentive motivational value, its presentation is able to evoke greater activity in subcortical structures that project to the PVT, mainly the MeA and the dorsomedial/lateral hypothalamus, shown by increased activity in these circuits in STs only. In addition, presentation of an incentive stimulus also leads to greater activity in PVT cells that project to the NAc in STs relative to unpaired controls (summarized in Figure 10).
While changes in the engagement of these circuits were significant, they were subtle in nature, with the average percentage of c-Fos/FG double-labeled cells ranging from approximately 1–9%. These results bring to mind work utilizing various methods surrounding c-Fos expression to examine the role of neuronal ensembles in motivated behavior, and suggest that only a small number of neurons in a given circuit are necessary for the expression of specific learned behaviors, which are often times triggered by presentation of a CS (for review see Cruz et al., 2013, Cruz et al., 2015). Here, we observed small, yet significant, increases in c-fos expression in specific cell populations following 5 days of PCA training, where the association between the lever-CS and the US has been sufficiently learned, evidenced by the development of a conditioned response to CS presentation. Studies utilizing similar retrograde tracing/c-Fos methodology have found equally subtle activation of specific pathways in response to presentation of a CS following fear-conditioning (Orsini et al., 2011, Jin and Maren, 2015). While the current findings suggest that, after 5 days of PCA training, sign- and goal-tracking behaviors are being mediated, at least in part, by small and distinct neuronal ensembles centered around the PVT; it is possible that a different pattern, characterized by a greater number of cue-induced c-fos positive cells, would have emerged had the current experiment been conducted at an earlier time point during the acquisition of these CRs. Follow-up studies at different time points and utilizing more sensitive techniques to better assess the degree of neuronal activation, such as in vivo electrophysiology or fiber photometry, are required to expand the current findings.
These findings extend the theories put forth by Ann Kelley and colleagues (Kelley et al., 2005a, Kelley et al., 2005b), and built upon by others (Martin-Fardon and Boutrel, 2012, James and Dayas, 2013, Urstadt and Stanley, 2015), implicating the involvement of a hypothalamic-thalamic-striatal axis underlying the ability of reward-paired cues to motivate behavior. Specifically, these results highlight the PVT as a potential modulator of incentive salience attribution, via integration of signals from subcortical structures, including the hypothalamus, and sending a coordinated output to the NAc, an area critical for motivated behavior. We previously hypothesized that GTs are more biased towards top-down control of behavior through cortical input to the PVT (Haight and Flagel, 2014, Haight et al., 2015), and this hypothesis was only partially supported by the data in the current study. Previous work from our lab and others has shown that Pavlovian conditioned food cues do not elicit greater c-Fos expression in the PrL in either GTs or STs relative to unpaired controls (Flagel et al., 2011a, Yager et al., 2015), an effect that the current study has replicated. Here we build on these findings, by looking specifically at layer 6 PrL neurons that project directly to the PVT. Contrary to our hypothesis, we found that presentation of a Pavlovian conditioned food cue elicits increased activity in this specific circuit for both GTs and STs, despite a lack of overall changes in total c-Fos expression. This was somewhat surprising, since we have previously shown that cue-induced c-Fos activity in the PrL is correlated with that in the PVT in GTs, but not STs (Haight and Flagel, 2014). This, and the fact that goal-trackers appear to have better top-down cognitive control (Lovic et al., 2011, Paolone et al., 2013) led us to hypothesize that we would find an increase in activity in the PrL to PVT circuit in GTs only. Furthermore, the prelimbic cortex has previously been implicated in other goal-directed behaviors (Balleine and Dickinson, 1998), including cue-motivated behaviors (Sangha et al., 2014, West et al., 2014, Moorman and Aston-Jones, 2015). It is important to note, however, that work in this area has often focused on the PrL connections with different parts of the striatum (Baker and Ragozzino, 2014, Stefanik et al., 2015), so little is known about the role of the PrL to PVT pathway in appetitive cue-motivated behaviors, despite the relatively dense connection (Li and Kirouac, 2012). One possible explanation for the current findings is that the PrL is sending information to the PVT regarding the predictive value of the reward-paired cue for both STs and GTs. Then, only for STs, is this information combined with signals from subcortical structures signaling the incentive value of the cue, overriding communication from the PrL to the PVT. Another possible explanation could depend on the post-synaptic targets of the PrL afferents to the PVT. It has been previously shown that the anterior and posterior regions of the PVT have overlapping, but differential inputs from the anterior and posterior regions of the PrL (Li and Kirouac, 2012). Since we injected FG into both the anterior and posterior portions of the PVT, it is possible that we were not able to detect subtle differences in PrL activity across these connections. Future studies will investigate this further, to determine if the increased PrL activity in STs is targeting a different region of the PVT than that of GTs, and therefore potentially communicating different information about the cue.
The second part of model assumes that STs would show greater activity in subcortical afferents to the PVT, and this hypothesis was supported by the data. The PVT receives a number of subcortical inputs from the hypothalamus, including dopamine, orexin, cocaine-and-amphetamine-regulating-transcript and neuropeptide-Y (Li et al., 2014, Lee et al., 2015, Urstadt and Stanley, 2015). These peptides project from a heterogeneous group of hypothalamic nuclei, including, but not limited to, the A13 cell group, the DMD, the VHM and LH. In the current study, PVT afferents from the DMD and LH showed similar patterns of activity in response to lever-CS presentation, so these regions were combined and assessed as the dorsomedial/lateral hypothalamus. It was found that lever-CS presentation leads to greater activation in dorsomedial/lateral hypothalamic afferents to the PVT in STs, compared to GTs and unpaired controls. While these data indicate that these projections may be specifically involved in processing the incentive value of reward-paired cues, the molecular identity of the cells specifically sending this signal is not known. One hypothalamic input of particular interest is orexin, because of its known role in motivated behavior (Mahler et al., 2012, Sakurai, 2014).
Orexinergic-positive cells that project to the PVT are primarily found distributed throughout lateral hypothalamus, including the perifornical area (Kirouac et al., 2005, Lee et al., 2015), but there appears to be some orexinergic innervation from the dorsomedial hypothalamus as well (Kirouac et al., 2005). These cells have been found to terminate in close proximity to the PVT cells that project to the NAc (Parsons et al., 2006), and administration of orexin into the PVT can elicit dopamine efflux in the NAc (Choi et al., 2012), indicating that this circuit can directly influence one of the main substrates underlying motivated behavior. In addition, presentation of food-paired cues and contexts activate orexin-positive neurons in the hypothalamus (Choi et al., 2010, Petrovich et al., 2012). These studies, combined with the results of the current experiment, highlight the possible involvement of this orexin circuit in incentive salience attribution. We hypothesize that presentation of an incentive stimulus activates orexinergic cells in the lateral/perifornical hypothalamus, and possibly the dorsomedial hypothalamus, that project to the PVT, where they can influence the activity of PVT cells that project to the NAc and affect sign-tracking behavior. Further studies utilizing functional methods, such as pharmacology or optogenetics, are needed to fully examine this hypothesis.
Lever-CS presentation also evoked greater c-Fos expression in MeA cells that project to the PVT in STs, compared to UNs. Interestingly, the MeA is a brain area that is far from wholly understood. Early work demonstrated that animals are willing to self-stimulate an electrode that has been planted in the MeA (Kane et al., 1991). More recently, the MeA has been implicated in fear processing (Cousens et al., 2012, Tsuda et al., 2015). Apart from these findings, little else is known about the role of the MeA in motivated behaviors. Here we identify a novel role for the MeA, with MeA afferents to the PVT potentially underlying incentive salience attribution to reward paired cues. Presumably, these afferents are directed towards the anterior pole of the PVT, since retrograde labeling has only been seen in this area following tracer injection into the anterior PVT (Chen and Su, 1990). It is possible, though, that these cells are also targeting the paratenial nucleus of the thalamus. In the previous study (Chen and Su, 1990), as well as the current study, some of the tracer injection did leak into the paratenial nucleus, which is a small thalamic nucleus adjacent to the anterior PVT. On the other hand, an anterograde tracing study showed a dense efferent connection from the MeA to the PVT and medial portions of the mediodorsal nucleus, with only sparse innervation of the paratenial nucleus (Canteras et al., 1995). Nonetheless, the majority of retrograde tracer was injected into the PVT, thus increasing the likelihood that the retrogradely labeled MeA cells are indeed targeting the PVT.
In the last part of the model, it was hypothesized that STs would show greater activity in NAc afferents from the PVT, where they could influence dopamine transmission. The results of the current study lend support to this hypothesis, and indicate that the posterior PVT may play a more prominent role in incentive salience attribution relative than other areas of the PVT. While anterior and posterior aspects of the PVT send similarly dense efferents to the NAc shell, the projection from the posterior PVT to the NAc core is more dense than that from the anterior PVT (Li and Kirouac, 2008). Importantly, sign-tracking behavior is dependent on dopamine transmission in the NAc core, while goal-tracking behavior is not (Flagel et al., 2011b, Saunders and Robinson, 2012). Thus, it is possible that the posterior PVT efferents engaged by presentation of an incentive stimulus are projecting to the NAc core, but follow-up studies using functional techniques will be needed to confirm this hypothesis. In addition, these findings do not preclude a role for the other areas of the PVT, since there could be different levels of activation in efferent PVT populations that were not measured in the current study.
While the results presented here lend further support to the theory that the PVT is important for influencing cue-motivated behavior (Kelley et al., 2005b, Martin-Fardon and Boutrel, 2012, Urstadt and Stanley, 2015), and in particular the propensity to attribute incentive salience to a reward cue (Haight and Flagel, 2014, Haight et al., 2015), they are not in complete agreement with the literature. Previous work has demonstrated that presentation of an incentive stimulus can elicit robust c-Fos expression in the PVT (Flagel et al., 2011a, Yager et al., 2015). To our surprise, in the current study, there were no significant differences between groups in cue-induced c-Fos in the PVT. These discrepant findings may be due to differences in methodology between the current and previous work. In one study, rats received 7 sessions of PCA training (Flagel et al., 2011a), instead of the 5 sessions used in the current study. Thus, it is possible that the two studies were capturing brain states at different stages of acquiring the conditioned response. Also, Flagel et al. (2011a) quantified c-Fos mRNA expression using in situ hybridization, rather than c-Fos protein using immunohistochemistry, which was measured here. Although mRNA and protein levels are most often positively correlated, they measure two different substrates and do not always show the same trends (e.g. Guo et al., 2008). In addition, in situ hybridization quantifies mRNA concentration, and is not necessarily dependent on the number of cells expressing mRNA. In contrast, in the current study using immunohistochemistry, the number of cells expressing c-Fos protein was quantified in a binary fashion. Thus, while our data suggest that the overall number of cells engaged in the PVT did not significantly differ between groups, we were not able to assess whether the concentration of c-Fos signal within specific cells, and/or the number of times a cell has been activated, might have changed to a different degree in STs vs. GTs or UN rats in response to presentation of an incentive stimulus. These differences could explain the discrepant findings that were observed and also highlight an important limitation of utilizing c-Fos immunohistochemistry to study neuronal activation.
In a second published report, c-Fos protein was measured, but there were other important methodological differences with the current study that need to be considered. In the Yager et al (2015) study, the context that was utilized for context habituation and the lever-cue-test day prior to sacrifice included the food cup (Yager et al., 2015); whereas in the current study, the context for habituation and the lever-cue-test day did not contain the food cup. Given that the PVT is known to play a role in both context- and cue-motivated behaviors (Wedzony et al., 2003, Schiltz et al., 2007, Hamlin et al., 2009, Igelstrom et al., 2010, James et al., 2011, Browning et al., 2014), having the food cup present upon the cue re-exposure might have led to differences in c-Fos induction in the PVT, especially since GTs and STs are differentially responsive to contextual cues (Morrow et al., 2011, Saunders et al., 2014).
In conclusion, the current data lend further support to the theory that a hypothalamic-thalamic-striatal axis underlies cue-motivated behavior, by showing that presentation of an incentive stimulus elicits activity specifically in the dorsomedial/lateral hypothalamic-PVT-NAc circuit. In addition, inputs from the MeA likely contribute to the neural circuitry underlying these behaviors. Last, it seems that the PrL to PVT circuit is activated by the predictive, and not the incentive, qualities of a conditioned stimulus. Since the current study was anatomical in nature, follow up studies utilizing functional technologies such as local pharmacology, chemogenetics and optogenetics will be imperative to fully understand how these circuits contribute to cue-motivated behaviors.
Highlights.
Goal-trackers attribute predictive value to reward-paired stimuli
Sign-trackers attribute predictive and incentive value to reward-paired stimuli
Incentive stimuli engage the subcortical hypothalamic-thalamic-striatal circuit
Predictive stimuli engage cortical prelimbic cells projecting to midline thalamus
The paraventricular thalamic nucleus is central to incentive motivational processes
Acknowledgments
The authors would like to acknowledge Dr. Terry Robinson for commenting on a previous version of this manuscript, and Dr. Brady West from the Consulting for Statistics, Computing and Analytics Research team at The University of Michigan for his helpful input on statistical modeling of the data. JLH and SBF designed the experiments. JLH, ZLF and KMF conducted the experiments and collected data. JLH and ZLF analyzed the data. JLH and SBF wrote the paper. This work was supported by the National Institute on Drug Abuse branch of The National Institutes of Health (F31-DA-037680 awarded to JLH and R01-DA-038599 awarded to SBF).
Abbreviations
- CeA
Central amygdala
- CR
conditioned response
- CS
conditioned stimulus
- DMD
dorsomedial hypothalamus
- FG
fluorogold
- GT
goal-tracker
- H2O2
hydrogen peroxide
- IL
infralimbic cortex
- IN
intermediate responder
- LH
lateral hypothalamus
- MeA
medial amygdala
- NAc
nucleus accumbens
- PVT
paraventricular nucleus of the thalamus
- PCA
Pavlovian conditioned approach
- PF
perifornical region
- PBS
phosphate-buffered saline
- PFC
prefrontal cortex
- PrL
prelimbic cortex
- NaPB
sodium phosphate buffer
- ST
sign-tracker
- US
unconditioned stimulus
- UN
unpaired control
- vSub
ventral subiculum
- VMH
ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Memory. 2014;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. In: Medin D, editor. Psychology of Learning and Motivation. Vol. 40. Cambridge, MA: Academic Press; 2001. pp. 223–278. [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Lawrence Erlbaum Associates; 1977. pp. 67–97. [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend. 2014;134:387–390. doi: 10.1016/j.drugalcdep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: A PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002a;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002b;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Chen S, Su H-S. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat — a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens GA, Kearns A, Laterza F, Tundidor J. Excitotoxic lesions of the medial amygdala attenuate olfactory fear-potentiated startle and conditioned freezing behavior. Behav Brain Res. 2012;229:427–432. doi: 10.1016/j.bbr.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Javier Rubio F, Hope BT. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2015;1628(Part A):157–173. doi: 10.1016/j.brainres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado RJ, Burstein R, Cliffer KD, Giesler GJ., Jr Evidence that Fluoro-Gold can be transported avidly through fibers of passage. Brain Res. 1990;533:329–333. doi: 10.1016/0006-8993(90)91358-n. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiat. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KM, Haight JL, Gardner EL, Flagel SB. Examining the role of dopamine D2 and D3 receptors in Pavlovian conditioned approach behaviors. Behav Brain Res. 2016;305:87–99. doi: 10.1016/j.bbr.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fraser KM, Akil H, Flagel SB. Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. Eur J Neurosci. 2015;42:2478–2488. doi: 10.1111/ejn.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Austin, TX: Psychonomic Society; 1974. [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta J-K, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Paraventricular thalamic nucleus: Subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol. 2009;512:825–848. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom KM, Herbison AE, Hyland BI. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience. 2010;168:706–714. doi: 10.1016/j.neuroscience.2010.04.018. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- James MH, Dayas CV. What about me…? The PVT: A role for the paraventricular thalamus (PVT) in drug-seeking behaviour. Front Behav Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S. Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Sci Rep. 2015;5:8388. doi: 10.1038/srep08388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Kilpatrick IC, Phillipson OT. Regulation of dopamine function in the nucleus accumbens of the rat by the thalamic paraventricular nucleus and adjacent midline nuclei. Exp Brain Res. 1989;76:572–580. doi: 10.1007/BF00248914. [DOI] [PubMed] [Google Scholar]
- Kane F, Coulombe D, Miliaressis E. Amygdaloid self-stimulation: A movable electrode mapping study. Behav Neurosci. 1991;105:926–932. doi: 10.1037//0735-7044.105.6.926. [DOI] [PubMed] [Google Scholar]
- Kelley A, Baldo B, Pratt W, Will M. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005a;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005b;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–329. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol. 2006;497:155–165. doi: 10.1002/cne.20971. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Lee EY, Lee HS. Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res. 2015;1598:97–113. doi: 10.1016/j.brainres.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217:257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Li S, Shi Y, Kirouac GJ. The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front Neuroanat. 8. 2014 doi: 10.3389/fnana.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci. 2012:6. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addict Biol Epub ahead of print. 2015a doi: 10.1111/adb.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett. 2015b;608:34–39. doi: 10.1016/j.neulet.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012:7. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci USA. 2015;112:9472–9477. doi: 10.1073/pnas.1507611112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol. 2007;500:1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlingame, MA: Academic Press; 2007. [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology. 2014;39:2405–2413. doi: 10.1038/npp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2015:1–9. doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Su H-S, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda MC, Yeung H-M, Kuo J, Usdin TB. Incubation of fear is regulated by TIP39 peptide signaling in the medial nucleus of the amygdala. J Neurosci. 2015;35:12152–12161. doi: 10.1523/JNEUROSCI.1736-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urstadt KR, Stanley BG. Direct hypothalamic and indirect trans-pallidal, trans-thalamic, or trans-septal control of accumbens signaling and their roles in food intake. Front Syst Neurosci. 2015:9. doi: 10.3389/fnsys.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B, Hof P, Friedman D, Sikes R, Vogt L. Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Struct Funct. 2008;212:465–479. doi: 10.1007/s00429-008-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, Bienkowski P. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology. 2015;40:1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]