Abstract
Amoeba often use cell movement as a mechanism to find food, such as bacteria, in their environment. The chemotactic movement of the soil amoeba Dictyostelium to folate or other pterin compounds released by bacteria is a well-documented foraging mechanism. Acanthamoeba can also feed on bacteria but relatively little is known about the mechanism(s) by which this amoeba locates bacteria. Acanthamoeba movement in the presence of folate or bacteria was analyzed in above agar assays and compared to that observed for Dictyostelium. The overall mobility of Acanthamoeba was robust like that of Dictyostelium but Acanthamoeba did not display a chemotactic response to folate. In the presence of bacteria, Acanthamoeba only showed a marginal bias in directed movement whereas Dictyostelium displayed a strong chemotactic response. A comparison of genomes revealed that Acanthamoeba and Dictyostelium share some similarities in G protein signaling components but that specific G proteins used in Dictyostelium chemotactic responses were not present in current Acanthamoeba genome sequence data. The results of this study suggest that Acanthamoeba does not use chemotaxis as the primary mechanism to find bacterial food sources and that the chemotactic responses of Dictyostelium to bacteria may have co-evolved with chemotactic responses that facilitate multicellular development.
Keywords: Acanthamoeba, Dictyostelium, Chemotaxis, G proteins
Introduction
Amoeboid organisms exist in various environments where they interact with many other microbial organisms. Dictyostelium and Acanthamoeba are two amoebae of general interest because Dictyostelium has been used as a model for eukaryotic cell movement and differentiation and Acanthamoeba has been documented as a human pathogen (Annesley and Fisher 2009; Artemenko et al. 2014; Janetopoulos and Firtel 2008; Khan 2003; Lam et al. 1997; Nichols et al. 2015; Walochnik et al. 2008; Williams 2010). Both amoebae inhabit soil environments and feed on bacteria and other microbes (Douglas et al. 2013; Khan 2006; Sawyer 1989). Cell movement is a critical feature to allow these cells to find locations where bacteria exist. Mechanisms of cell movement have been extensively studied in Dictyostelium discoideum but relatively little is known about the movement of Acanthamoeba castellanii (Schuster and Levandowsky 1996; Schuster et al. 1993). Since both organisms can inhabit similar environments it is possible that they share similar foraging mechanisms. However, under extreme starvation conditions where food cannot be found the fates of these amoebae are very different. Dictyostelium is a social organism that can form multicellular aggregates when starved and these aggregates undergo an elaborate developmental program to produce a fruiting body with spores that can remain dormant in the absence of nutrients (Loomis 1982). In contrast, starved Acanthamoeba form solitary cysts that allow survival in nutrient poor conditions (Khan 2006; Siddiqui et al. 2012). The formation of dormant spores and cysts are developmental fates for these amoebae if the foraging efforts fail and therefore foraging and dormancy are likely to be tightly regulated with respect to each other.
Dictyostelium chemotactic responses have been regarded as an important model for understanding directed cell movement and the underlying signal transduction pathways (Artemenko et al. 2014). Many Dictyostelium studies have focused on the chemotactic responses to cAMP that occur during the aggregation phase of the developmental life cycle. After several hours of starvation, Dictyostelium increase the expression of cAMP surface receptors and become competent for cAMP chemotaxis (Ginsburg et al. 1995; Insall et al. 1994; Saxe et al. 1991; Sun and Devreotes 1991). The stimulation of cAMP receptors triggers a G protein-mediated signaling pathway that results in chemotactic movement (Kumagai et al. 1989, 1991; Manahan et al. 2004). The response also includes a release of extracellular cAMP so that cells can find each other during the aggregation process. Signaling through these cAMP receptors continues during the multicellular phases of development and contributes to cell sorting within the aggregate and the differentiation of cells into the stalk or spores of the fruiting body (Kim et al. 1998; Louis et al. 1994; Saxe et al. 1993). In contrast, chemotactic movement associated with foraging is present during vegetative growth and enhanced during the first few hours of starvation (De Wit and Bulgakov 1986; Hadwiger et al. 1994; Pan et al. 1972, 1975). This chemotactic response requires receptors for pterin-like compounds such as folate. Foraging cells exhibit substantial meandering during chemotaxis and do not display the elongated morphology typical of aggregating cells. However, both cAMP and folate chemotaxis responses require G proteins that couple to cell surface receptors and many of the downstream cellular responses are quite similar including the transient accumulations of cAMP and cGMP, cytoplasmic influx of calcium, and the activation of regulatory proteins such as mitogen activated protein kinases (MAPKs) (ERK1 and ERK2) (Hadwiger et al. 1994; Kumagai et al. 1991; Maeda et al. 1996; Schwebs and Hadwiger 2015).
The Dictyostelium discoideum genome encodes more than 60 G protein coupled receptors but relatively few of them have been genetically characterized (Prabhu and Eichinger 2006). Four cAMP receptors have been identified and two of these play a role in the cAMP chemotaxis involved with aggregated formation (Ginsburg et al. 1995). Several other receptors have been genetically analyzed, including some close paralogs of the cAMP receptors, and recently a receptor responsible for folate chemotaxis has been identified (Anjard et al. 2009; Pan et al. 2016; Prabhu et al. 2007; Raisley et al. 2004). In regards to G proteins, folate responses require the Gα4 G protein subunit and cAMP responses require the Gα2 subunit (Hadwiger et al. 1994; Hadwiger and Srinivasan 1999; Kumagai et al. 1989). Interestingly, the Gα4 subunit is also required for cellular localization and morphogenesis during multicellular development (Hadwiger and Firtel 1992). Both folate and cAMP chemotaxis responses require the single Gβ subunit encoded by the genome (Lilly et al. 1993). A single Gγ subunit that contributes to the heterotrimeric structure has also been identified (Zhang et al. 2001). These G protein mediated signal transduction pathways for Dictyostelium chemotaxis share many similarities to signaling pathways in chemotactic mammalian cells (e.g., neutrophils) suggesting that many chemotactic signaling components have been evolutionarily conserved in eukaryotes (Artemenko et al. 2014).
Compared to Dictyostelium, very few studies have been conducted on Acanthamoeba cell movement. These studies have assayed Acanthamoeba movement toward a variety of different compounds and to bacteria (Schuster and Levandowsky 1996; Schuster et al. 1993). The results of these studies suggest that Acanthamoeba have variable responses to bacteria and compounds such as cAMP and formylated peptides. These studies did not compare Acanthamoeba cell movement to known chemotactic cells such as Dictyostelium or mammalian neutrophils. These studies were also conducted before the sequencing of any Acanthamoeba genomes and so comparisons of signaling proteins were not considered. The recent sequencing of the Acanthamoeba castellanii genome now provides an opportunity to compare genes that are potentially involved with chemotactic signaling in Acanthamoeba with those genes that have been characterized in chemotactic organisms (Clarke et al. 2013). A recent study has reported similarities in the cAMP-specific phosphodiesterase, RegA, found in both Acanthamoeba and Dictyostelium. RegA regulates the development of spore formation in Dictyostelium and the development of cysts in Acanthamoeba (Du et al. 2014). Like Dictyostelium, the Acanthamoeba genome also encodes many putative G protein coupled receptors and G protein subunits genes that could be potential contributors to their ability to find food sources and undergo cell differentiation (Clarke et al. 2013; de Mendoza et al. 2014).
In this study we compared the ability of Acanthamoeba and Dictyostelium to forage for nutrient sources. Chemotactic assays to folate and bacteria were used to determine if these organisms possess similar mechanisms to find nutrients in similar environments. Analyses of these two organisms under identical conditions suggest that these organisms have evolved different mechanisms to find bacteria. The genomes of these organisms were also compared for G proteins that potentially contribute to these responses. The difference in foraging strategies used by these amoebae is supported by the difference in G protein subunits encoded in the Acanthamoeba and Dictyostelium genomes.
Results
Comparison of Amoeboid Chemotaxis to Folate
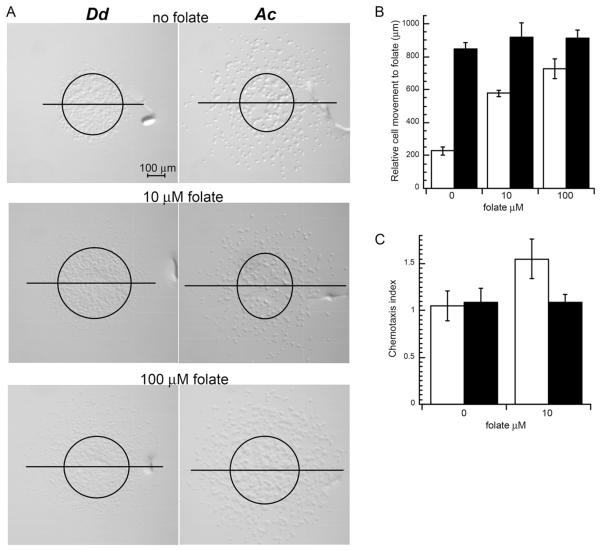
Acanthamoeba and Dictyostelium both feed on bacteria as a food source suggesting that Acanthamoeba might have chemotactic responses to folate as previously described for Dictyostelium (De Wit et al. 1986). To determine if Dictyostelium discoideum and Acanthamoeba castellanii have similar chemotactic responses to folate, both species were examined in an above agar chemotaxis assay. Both amoebae displayed robust cell movement as indicated by the maximum migration distance in the presence of folate (Fig. 1A, B). The migration distance for Dictyostelium was greatly reduced in the absence of folate but Acanthamoeba displayed a similar migration distance in the presence or absence of folate suggesting Acanthamoeba cell movement was not dependent on the folate. This idea is further supported by the Acanthamoeba chemotaxis index of 1.1 compared to Dictyostelium chemotaxis index of 1.6 in the presence of 10 μM folate (Fig. 1C). A chemotaxis index of one is expected for nonchemotactic (i.e., random migration) cells because the number of cells moving toward and away from the chemoattractant source is approximately equal. The greater than one chemotaxis index for Dictyostelium is consistent with previous studies that demonstrate this amoeba is chemotactic to folate (Hadwiger et al. 1994; Nguyen and Hadwiger 2009; Pan et al. 1972). Dictyostelium can inactivate folate as a chemoattractant through a deamination reaction and so as folate diffuses beyond the cell droplet a folate gradient can develop on all sides of the cell droplet (Pan and Wurster 1978). This effect can explain the increased Dictyostelium movement in all directions as the assay proceeds. In comparison, Acanthamoeba moved robustly in all directions regardless of the presence of folate. No significant differences in migration were displayed by Acanthamoeba when exposed to different concentrations of folate, even up to 10 mM (data not shown), but Dictyostelium movement increased in distance and cell density at higher folate concentrations. Acanthamoeba also began moving beyond the original cell droplet perimeter much earlier than Dictyostelium consistent with Acanthamoeba movement not being dependent of the formation of a folate gradient.
Figure 1.
Chemotaxis of Dictyostelium and Acanthamoeba to folate. Chemotaxis assays were set up as described in the methods section. (A) Images of Dictyostelium (Dd) and Acanthamoeba (Ac) 2.5 h after plating with no folate, 10 μM folate, or 100 μM folate. Images are orientated with the folate chemoattractant diffusing from the upper side of the image. Representative images are shown. Each chemotaxis assay typically included the analysis of 6 droplets (minimum of 4) and each assay was repeated at least 2 times. Circles on the image represent the approximate cell droplet perimeter at the time of plating and the horizontal lines bisect the upper and lower halves of the circle. In cases where migrating cells moved beyond a single field of view multiple images were collected to account for all migrating cells. (B) Distance traveled of the leading edge of migrating Dictyostelium (open bars) and Acanthamoeba (black bars) toward the source of folate. Data are the mean distance measured for 6 droplets of cells. For each concentration of folate this chemotaxis assay was repeated 6 times and the data from one representative assay is shown. Unpaired Student’s t-test p values for assays with or without folate were determined (Dictyostelium assays p ⪡ 0.001 and Acanthamoeba assays p > 0.03). (C) Chemotaxis index of Dictyostelium (open bars) and Acanthamoeba (black bars) assayed with folate (10 μM) exposure or without (0 μM). The chemotaxis index was determined by the number of cells outside the original droplet perimeter on the side facing the source of folate divided by the number of cells outside the perimeter facing away from the source of folate. Data is the mean chemotaxis index from 6 droplets of cells. This assay was repeated at least 3 times and data shown represents a typical assay. Error bars represent the standard deviation of the mean. Unpaired Student’s t-test p values for chemotactic index assays with or without folate were determined (Dictyostelium p = 0.0006 and Acanthamoeba p = 1.0).
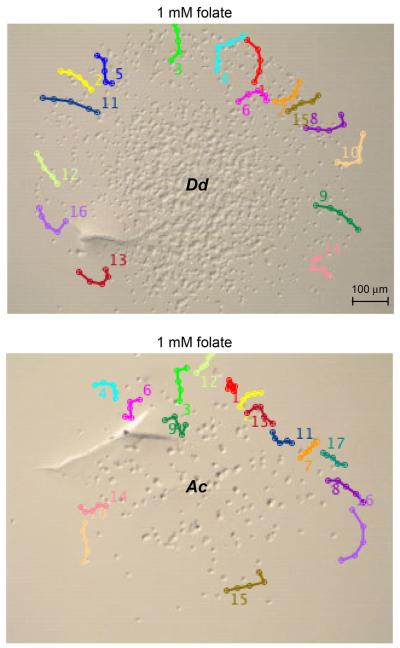
Time-lapse photography of Acanthamoeba and Dictyostelium was also used to monitor cell movement in the presence of folate. Cell tracking software was used to map the movement of individual cells over time (Fig. 2, Supplementary Material Figs S1, S2). Cells located on the edge facing the folate source were chosen because they had little or no contact with other cells. The average distance traveled by Dictyostelium and Acanthamoeba during the 33 min period was comparable (Dd 35.6 ± 5.9 and Ac 29.3 ± 8.9). Many of the Dictyostelium and Acanthamoeba cells displayed movement with noticeable meandering and both populations contained some cells that did not move. This heterogeneity in cell movement is typical of Dictyostelium populations in response to folate (Nguyen and Hadwiger 2009). Cell tracking was also used to assess directionality for Dictyostelium and Acanthamoeba. Migration directionality can be defined as the displacement (direct distance from start point to end point) divided by the total path length of the cell and so cells traveling in a relatively straight line (high persistence) have directionality values near one and cells that meander more have lower values (Petrie et al. 2009). The average directionality values for Acanthamoeba (0.84 ± 0.12) and Dictyostelium (0.83 ± 0.14) were very similar suggesting that both amoebae had similar levels of meandering. However, most Dictyostelium displayed a bias in cell movement toward the source of folate. In contrast, Acanthamoeba movement continued in all directions without being biased by the folate gradient. The Acanthamoeba movement was similar to that previously described for Dictyostelium in the absence of a chemoattractant and this movement has been defined as “random motion” because cells exhibit a stochastic combination of persistence and meandering (Li et al. 2008).
Figure 2.
Migration maps of Dictyostelium and Acanthamoeba in folate chemotaxis assays. Time-lapse photography of Dictyostelium (Dd upper image) and Acanthamoeba (Ac lower image) in response to 1 mM folate (source diffusing from the upper side of each image) after approximately 2 h after plating. Only the final image is shown but images were collected every 20 s for 33 min as described in the methods section. Tracks of individual cells were traced using 5 time points (approximately every 6 min) using MTrackplugin in ImageJ and overlaid on the final image. The number for each track is located near the tail of each track. Cells near the leading edge and with relatively few cell-cell interactions were chosen for track analysis. Movies containing all time-lapse images are included in the Supplementary Material Figures S1, S2.
Comparison of Amoeboid Chemotaxis to Bacteria
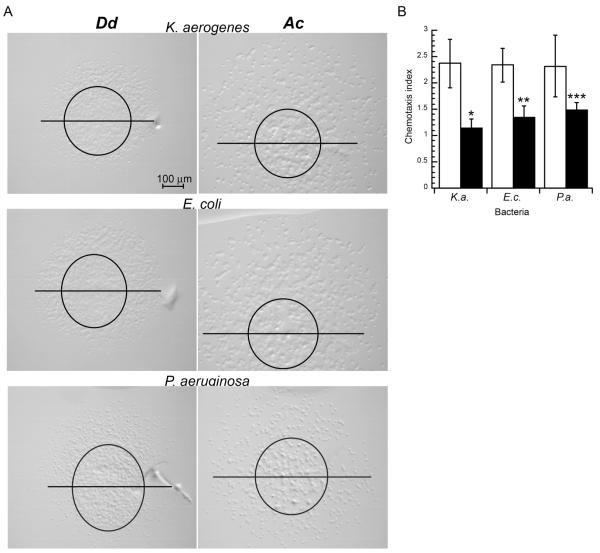
Although Acanthamoeba did not display a chemotactic response to folate it is possible that other molecules released from bacteria might serve as chemoattractants. Therefore chemotaxis assays were conducted with a variety of bacterial species. Chemotaxis to Klebsiella aerogenes and Escherichia coli was analyzed because these species are often used in the laboratory setting as a food source for Dictyostelium. Acanthamoeba was also found to grow efficiently on lawns of these bacteria. Bacteria were harvested and washed with phosphate buffer to remove factors associated with the growth medium. As with the folate chemotaxis assays, Dictyostelium displayed a significant chemotaxis index but Acanthamoeba did not (Fig. 3). However, Acanthamoeba exhibited robust cell movement in all directions allowing some of the cells to reach the bacterial droplet. Both Dictyostelium and Acanthamoeba that reached bacterial droplets were capable of consuming the bacteria (data not shown).
Figure 3.
Chemotaxis of Dictyostelium and Acanthamoeba to bacteria. Chemotaxis assays were set up as described in the methods section. (A) Images of Dictyostelium (Dd) and Acanthamoeba (Ac) 2.5 h after plating with Klebsiella aerogenes, Escherichia coli, and Pseudomonas aeruginosa. Images are orientated with the bacterial source orientated on the upper side of the image. Representative images are shown from an assay that included 6 droplet of either Dictyostelium (Dd) or Acanthamoeba (Ac). Each chemotaxis assay that included a minimum of 4 cell droplets was repeated at least 2 times. Original droplet perimeters and bisector lines were determined as described in Figure 1. In cases where migrating cells moved beyond a single field of view multiple images were collected to account for all migrating cells. (B) Chemotactic index of Dictyostelium (open bars) and Acanthamoeba (black bars) to bacterial droplets. Data is the mean chemotaxis index from 6 droplets of cells. This assay was repeated at least 3 times and data shown represents a typical assay. Error bars represent the standard deviation of the mean. Unpaired Student’s t-test p values for chemotactic index assays with or without bacteria was determined (all Dictyostelium assays p < 0.001 and Acanthamoeba assays *p = 0.6, **p = 0.05, ***p = 0.04).
A previous report has suggested that Acanthamoeba can chemotaxis to Pseudomonas aeruginosa with a chemotactic index of 1.6 in an under agar chemotaxis assay with substantially different parameters than our above agar assay (Schuster et al. 1993). Our above agar assay with Acanthamoeba in the presence of P. aeruginosa produced a comparable chemotaxis index of 1.5 (Fig. 3). This response was significantly less than the chemotactic response of Dictyostelium under the same conditions. Although the chemotaxis response of Acanthamoeba to P. aeruginosa was marginally statistically significant the robust movement of Acanthamoeba in all directions suggests that any chemotactic contributions to cell movement were secondary to the mechanism underlying the process of cell dispersal. Dictyostelium displayed a chemotactic response to P. aeruginosa that was similar to its response to other bacteria suggesting that Dictyostelium might use a similar mechanism to detect all bacteria.
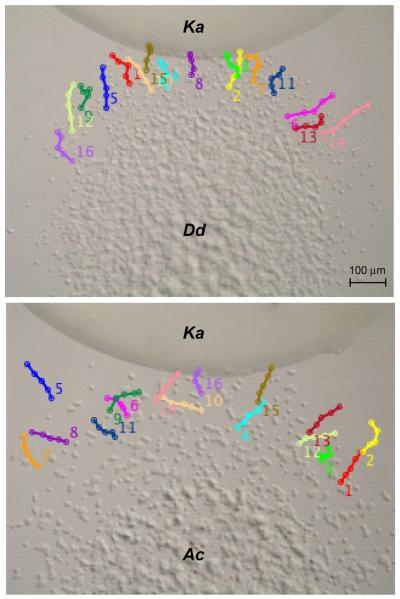
Time-lapse recordings of amoebae migration to bacteria (K. aerogenes) were very comparable to those observed for the amoeboid movement to folate (Fig. 4, Supplementary Material Figs S3, S4). Both amoebae displayed meandering movement and the average migration distances during the 33 min period were similar (Dd 31.1 + 7.1 and Ac 28.8 + 4.2, relative pixel units). The average directionality values for Dictyostelium (0.90 ± 0.10) and Acanthamoeba (0.92 ± 0.12) were also comparable to each other and similar to those observed in response to folate. In many cases, the Dictyostelium and Acanthamoeba movement significantly slowed when the cells reached the bacterial mound but some Acanthamoeba were capable of tunneling further into the bacterial mound (Supplementary Material Fig. S4). This tunneling behavior suggests that some cells might not immediately switch from foraging to feeding when making contact with bacteria. However, Acanthamoeba were never observed leaving the bacterial mound suggesting the foraging movement did not continue indefinitely in while in contact with the bacteria. The larger cell size of Acanthamoeba compared to Dictyostelium might possibly contribute to the ability of individual cells to physically penetrate further into the bacterial mound.
Figure 4.
Migration maps of Dictyostelium and Acanthamoeba in chemotaxis to Klebsiella aerogenes. Time-lapse photography of Dictyostelium (Dd upper image) and Acanthamoeba (Ac lower image) in response to K. aerogenes droplets (positioned on the upper side of each image) after approximately 2 h after plating. Only the final image is shown but images were collected and tracks were traced as described Figure 2. The number for each track is located near the tail of each track. Cells near the leading edge and with relatively few cell-cell interactions were chosen for track analysis. Movies containing all time-lapse images are included in the Supplementary Material Figures S3, S4.
Acanthamoeba Dispersal
The dispersal of Acanthamoeba might possibly be driven through a chemorepulsion mechanism that could potentially mask a response to a chemoattractant at high cell densities. Chemorepulsion has been observed for other amoebae including Dictyostelium and Entamoeba in response to molecules produced during vegetative growth. Although growth medium was washed away prior to the chemotaxis assays, the amoeba might still be capable of releasing chemorepellent molecules. To examine if chemorepulsion contributes to the movement of Acanthamoeba, chemotaxis assays were repeated using different densities of Acanthamoeba because chemorepulsion is expected to correlate with increased amoeba density. High (108 cells/ml) and low (5 × 106 cells/ml) density Acanthamoeba suspensions were used in chemotaxis assays to K. aerogenes (Fig. 5A). Chemotaxis indices were not determined for Acanthamoeba at the high cell density because of the difficulty of counting individual cells but at the lower cell density Acanthamoeba had a chemotaxis index of 1.2 ± 0.4, comparable to the higher cell density assays described in Figure 3.
Figure 5.
Acanthamoeba movement at different cell densities in the presence of bacteria. Chemotaxis assays were set up as described in the methods section. (A) Images of Acanthamoeba (Ac) movement 2 h after plating at different densities (5 × 106 or 1 × 108 cells/ml suspensions) on agar plates near droplets of Klebsiella aerogenes (positioned on the upper side of each image). Representative images are shown from an assay that included 5 droplets of Acanthamoeba (Ac) for each cell density and each assay was repeated 3 times. Original droplet perimeters and bisector lines were determined as described in Figure 1. (B) Time-lapse photography of Acanthamoeba in response to K. aerogenes droplet (positioned on the upper side of each image) after approximately 4.5 h after plating. Only the final image is shown but images were collected and tracks were traced as described Figure 2. The number for each track is located near the head of each track. Cells moving near but not directly toward the bacterial droplet were chosen for track analysis. Movies containing all time-lapse images are included in the Supplementary Material Figure S5.
Chemorepulsion, particularly at high cell densities, might be expected to disperse cells in a uniform radial pattern due the decreasing level of autocrine factors in all directions away from the cell droplet and also the potential repulsion between individual cells. However, Acanthamoeba displayed a disorderly pattern of dispersal (i.e., uneven distribution of migrating cells) at both high and low densities suggesting that dispersal is the result of random motion rather than chemorepulsion. Furthermore, Acanthamoeba at relatively low cell densities near droplets of bacteria were also capable of moving toward or away from the bacteria (Fig. 5B, S). This observation suggests that random motion can occur in close proximity of bacterial cells where potential chemoattractant and chemorepellent concentrations are expected to be relatively high and low, respectively. These results suggest that Acanthamoeba movement is not primarily determined by chemotaxis or chemorepulsion.
Comparison of G Protein Gα Subunits
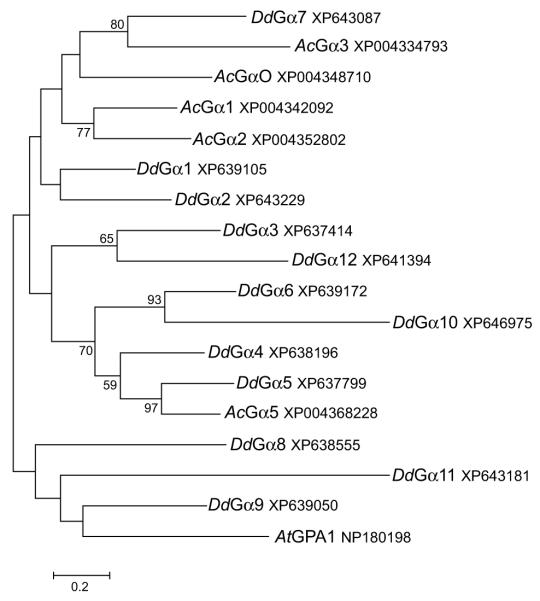
The chemotaxis assays to folate and bacterial sources suggest that the foraging strategy of Acanthamoeba is different than that of Dictyostelium. The basis of this difference could possibly be differences in the signaling components encoded by each organism’s genome. Chemoxtaxis and other chemoresponsive processes (e.g., chemotrophic growth) in eukaryotes are typically associated with G protein-mediated signaling pathways (Artemenko et al. 2014). Given that the Dictyostelium discoideum Gα4 subunit is required for chemotactic responses to folate and bacteria, the sequence of this subunit was used to search for homologous proteins in Acanthamoeba and other amoebae. Dictyostelium discoideum and related dictyostelid species (Dictyostelium purpureum, Polysphondylium pallidum, Dictyostelium fasciculatum, and Actyostelium subglobosum) all possessed a single closely related Gα4 ortholog with sequence identity greater than 90% (Table 1). A previous report has indicated that Dictyostelium purpureum and Polysphondylium pallidum have chemotactic responses to folate and while the other species have not been tested it is likely they also respond to folate given the conservation of the Gα4 subunit (Pan et al. 1972). The most closely related Acanthamoeba Gα subunit to the Dictyostelium Gα4 subunit was a Gα5 ortholog but this subunit has a much lower sequence identity (54%) than the Gα4 orthologs in other amoebae. The Dictyostelium discoideum Gα5 and Gα2 subunits also have a much lower sequence identity (52% and 44%, respectively) with respect to the Gα4 subunit and this is indicative of their functional differences. The Gα2 subunit, like the Gα4 subunit, mediates a chemotactic response but this response is to cAMP. A phylogenetic analysis of the 12 Dictyostelium and 5 Acanthamoeba Gα subunits indicated that other than a similarity between Gα5 orthologs (60% identity), these amoebae do not share closely related Gα subunits (Fig. 6). Out of the other 4 Acanthamoeba Gα subunits, 3 of them shared greatest sequence similarity with each other rather than Dictyostelium Gα subunits. The remaining Acanthamoeba Gα subunit, Gα3, had sequence similarity with the Dictyostelium Gα7 subunit. As previously reported, most Dictyostelium Gα subunits, except for Gα1-Gα2 pair and the Gα4-Gα5 pair share little sequence similarity with each other outside of the highly conserved sequences associated with guanine nucleotide binding. Even the most closely related pairs of Dictyostelium Gα subunits, Gα4-Gα5 and Gα1-Gα2, do not show functional redundancy in chemotactic or developmental phenotypes (Kumagai et al. 1991; Natarajan et al. 2000).
Table 1.
Percent identity of amoeboid Gα subunits to the Dd Gα4 subunit.
| Organism | Gα subunit | % identity |
|---|---|---|
| Dictyostelium purpureum | Gα4 | 99 |
| Polysphondylium pallidum | Gα4 | 94 |
| Dictyostelium fasciculatum | Gα4 | 91 |
| Acytostelium subglobosum | Gα4 | 93 |
| Acanthamoeba castellanii | Gα5 | 54 |
| Polysphondylium pallidum | Gα5 | 53 |
| Dictyostelium discoideum | Gα5 | 52 |
| Dictyostelium discoideum | Gα2 | 44 |
Figure 6.
Phylogenetic tree of Dictyostelium and Acanthamoeba G protein Gα subunits. BLAST searches were used to identify Gα subunit homologs in Dictyostelium discoideum (Dd) and Acanthamoeba castenalli (Ac) genomes. A phylogenetic tree was generated as described in the methods section. The Arabidopsis thaliana Gα subunit (AtGPA1) was used as an out-group. Each sequence has an accession reference number.
Comparison of Other G Protein Subunits
In Dictyostelium, the sole Gβ subunit is required for chemotaxis to both cAMP and folate indicating that both Gα2 and Gα4 complex with the Gβ in these chemotactic responses. The Dictyostelium Gβ subunit sequence was used to search for orthologs in Acanthamoeba but the search did not reveal any closely related proteins as suggested by the low range of sequence identities (20–29%). This contrasts Dictyostelium Gβ searches in other eukaryotes such as humans where Gβ orthologs were identified with much higher sequence identities (60–70%). Several of the Acanthamoeba proteins detected in the sequence similarity searches were identified as Gβ-like proteins because they contain WD-repeats like those found in Gβ subunits. One of these Acanthamoeba WD-repeat proteins has significant similarity to proteins commonly known as Rack homologs (Receptor associated with protein C kinase) that exist in a diverse range of eukaryotes. The Acanthamoeba and Dictyostelium Rack proteins share 59% identity but none of the other Acanthamoeba WD-repeat proteins shared more than 34% sequence identity with the Dictyostelium RACK protein. Phylogenetic analysis of Gβ related proteins from Dictyostelium, Acanthamoeba, and some other eukaryotes indicated that Gβ subunits and Rack proteins form distinct clades suggesting these WD repeat proteins represent two different classes of proteins (Fig. 7A). The Acanthamoeba Rack protein shares comparable relatedness with higher eukaryotes as it does with other amoeboid species.
Figure 7.
Phylogenetic trees of G protein Gβ subunits/Racks and Gγ subunits of some amoebozoan and other select eukaryotes. (A) Phylogenetic tree of Gβ and Rack subunit homologs identified through BLAST searches. Only one Gβ subunit of the multiple Gβ subunits from the human (Hs) genome was selected for comparison purposes. (B) Phylogenetic tree of Gγ subunit homologs identified through BLAST searches. Phylogenetic trees were generated as described in the methods section. Species represented include Dictyostelium discoideum (Dd), Acanthamoeba castenalli (Ac), Homo sapien (Hs), Xenopus laevis (Xl), Arabidopsis thaliana (At), Dictyostelium purpureum (Dp), Polysphondylium pallidum (Pp), Dictyostelium fasciculatum (Df), and Saccharomyces cerevisiae (Sc) genomes. Each sequence has an accession reference number.
G proteins which couple to cell surface receptors are typically heterotrimeric proteins that contain a Gγ subunit. The Gγ subunit binds tightly to Gβ subunit and remains tightly associated with this subunit upon activation of the Gα subunit. A Dictyostelium Gγ subunit has been identified and shown to couple with the Gβ subunit but its requirement in chemotactic movement has not been established (Zhang et al. 2001). The Dictyostelium discoideum Gγ subunit was used to search for related proteins in Acanthamoeba but the search did not identify any closely related proteins. Attempts with human or yeast Gγ to find related proteins in Acanthamoeba were also unsuccessful but the low sequence conservation and the small protein size make the searches for Gγ subunit homologs more challenging than homologs to other G protein subunits in distantly related organisms. However, Gγ subunits from other dictyostelids were detected. A phylogenetic analysis of Gγ sequences indicates that the dictyostelids species have closely related Gγ subunits compared to those found in other unrelated organisms (Fig. 7B). The phylogenetic tree suggests higher sequence similarities between Gγ subunits within a given phylum.
Discussion
The results of this study suggest that Acanthamoeba forage for bacterial food sources using a mechanism distinct from Dictyostelium. Dictyostelium displayed directed movement to both folate and bacterial sources even though the migration paths included substantial meandering. Acanthamoeba displayed a slight bias in the movement toward some bacterial sources but the robust movement in all directions overshadowed any directed movement to the bacterial source suggesting that random motion rather than chemotaxis represents the primary foraging mechanism of Acanthamoeba (Fig. 8). Cell dispersal through random motion is likely to increase the chance that some members of an Acanthamoeba population find new food sources because the population can cover a larger area compared to a population directed by chemotaxis. Cell dispersal through random motion has also been described for Dictyostelium in the absence of a chemotactic signal (Van Haastert and Bosgraaf 2009).
Figure 8.

Model of Acanthamoeba movement. Nutrient deprivation primarily results in random motion allowing cells to disperse in all directions (thick dark gray arrows). Movement toward a chemoattractant (thin light gray arrows) is very weak compared to other amoebae such as Dictyostelium.
The efficient dispersal of Acanthamoeba at even low cell densities suggests that chemorepulsion is not an important contributor for Acanthamoeba dispersal in the conditions tested in this study but such a mechanism could possibly operate in populations of vegetatively growing cells. Under growing conditions Dictyostelium use an autocrine signal, AprA, as a chemorepellent and other amoebae, such as Entamoeba, use ethanol production as a chemorepellent (Phillips and Gomer 2012; Zaki et al. 2006). While the dispersal of cells reduces the competition for food in a localized area it might also have important consequences with respect to cell fate. Acanthamoeba that are unsuccessful in foraging enter into a dormant state (encystation) as solitary cells, allowing survival until local food conditions change. In contrast, Dictyostelium enter into a multicellular state to form dormant cells (spores) if foraging fails and so mechanisms that promote cell dispersal would likely work in opposition to the cell aggregation process. Therefore social amoebae such as Dictyostelium might limit cell dispersal mechanisms to allow for more efficient cell aggregation.
The lack of Acanthamoeba chemotaxis to folate is consistent with the absence of a Gα4 subunit ortholog in the genome given that this subunit is highly conserved among species that can chemotax to folate. While most of the reported folate-responsive amoebae belong to the Dictyostelid classification, a recent study indicates that Vahlkampfia, classified in a different subphylum, can also chemotax to folate (Cavalier-Smith et al. 2015; Maeda et al. 2009). It remains to be determined if the Vahlkampfia genome contains a Gα4 subunit homolog. Many amoebae, including Acanthamoeba, possess a Gα5 subunit homolog that is closely related to Gα4 subunits but phenotypic characterization of the Dictyostelium gα5− mutants indicates that this subunit is not required for folate chemotaxis (Natarajan et al. 2000). Rather, the Gα5 subunit appears to act in opposition to the Gα4 subunit as suggested by the increased folate responsiveness of cells that lack the Gα5 subunit and the decreased folate responsiveness of cells that overexpress the Gα5 subunit (Raisley et al. 2010). The functional relationship of the Gα4 and Gα5 subunits is not fully understood but Gα subunit chimeric studies suggest that the functional differences are not solely due to receptor coupling but rather downstream signaling (Hadwiger 2007).
The absence of a Gβ subunit gene in the current Acanthamoeba castellanii genomic data is very surprising given that several putative Gα subunits are present. Biochemical and genetic characterization of Gβ subunits in other organisms suggest the Gβ subunits can have a variety of interactions and roles in downstream signaling and in some cases they provide the primary role in signaling to downstream responses (Neptune et al. 1999; Whiteway et al. 1989). Not detecting a Gβ subunit gene could possibly result from incomplete coverage of the genome by the sequencing analysis. The Acanthamoeba castellanii genome project did not report the depth of sequencing coverage but did indicate 94% coverage of the transcriptome (Clarke et al. 2013). Searches for Gβ subunits were also conducted using recently deposited Acanthamoeba sequences in the AmoebaDB database (AmoebaDB and MicrosporidiaDB:functional genomic resources for Amoebozoa and Microsporidia species). In several Acanthamoeba species, including Acanthamoeba castellanii, these searches revealed partial gene sequences with 50–60% identity to known Gβ subunits suggesting that Gβ homologs are present in the Acanthamoeba genus. It is also possible that other WD-repeat proteins might provide Gβ subunit function. The presence of the Gβ-like Rack protein in Acanthamoeba offers the possibility that this protein could function in G protein signaling pathways. Studies in the yeast S. cerevisiae indicate that the Gpa2 Gα subunit does not couple with the single Gβ or Gγ subunit in this organism (Peeters et al. 2006). Instead the yeast Rack homolog, Asc1, has been reported to serve in place of a Gβ subunit for the Gpa2 signaling pathway (Zeller et al. 2007). The Gpa2/Asc1 G protein pathway mediates the sensing of glucose to control cell growth and division in yeast but thus far this pathway has not been implicated in cell polarity or chemotrophic growth, unlike the Gpa1/Gβ pathway (Busti et al. 2010). While Rack proteins may mediate G protein signaling it is possible that these proteins might not be capable of contributing to chemotactic or chemotrophic responses like Gβ subunits. The Rack ortholog in Dictyostelium cannot compensate for the chemotactic deficiencies that occur in the absence of the Gβ subunit but that does not exclude the possibility that the Rack protein might function in signaling pathways that do not require Gβ proteins (Omosigho et al. 2014).
Conclusion
While Acanthamoeba and Dictyostelium might exist in similar environments and consume common bacteria, these amoebae use very different approaches to finding their food sources based on their cell movement. It is interesting that both amoebae share many families of proteins that participate in G protein-mediated signal transductions but yet very little overlap exists with specific signaling proteins such as specific Gα subunits. Perhaps some of the differences in signaling proteins are the result of social or solitary strategies for surviving starvation. Chemotactic responses of Dictyostelium to folate and bacteria might have co-evolved with chemotactic responses to cAMP because many similarities exist between these responses. These similarities include the activation of MAPKs and other kinases and transient changes in the level of cyclic nucleotides that are important for regulating cell morphology and gene expression (Kumagai et al. 1991; Schwebs and Hadwiger 2015; Veltman and Van Haastert 2006). The use of similar signaling proteins downstream of G protein function for both foraging and cell aggregation could save time and critical energy reserves in the switch between foraging and the cell aggregation process. In addition, the relatively close proximity of cells with each other during chemotactic foraging, a process somewhat analogous to herding, can also help expedite the aggregation process if needed. A potential drawback to “herding” is that it increases the competition between cells for food sources that might be found. However, if food is not found then the close proximity of cells is beneficial because it reduces the distance cells need to migrate to form a multicellular aggregate. In contrast to social amoeba, Acanthamoeba does not require cell aggregation to form cysts and so an investment in chemotactic signaling mechanisms might not be warranted. These differences in developmental fates among social and non-social amoebae might provide some of the basis for the different foraging strategies.
Methods
Strains, growth conditions
The axenic Dictyostelium discoideum strain KAx3 and the Acanthamoeba castellanii strain ATCC 30010 were used in this study. Both amoebae were grown in HL5 medium (Watts and Ashworth 1970). Klebsiella aerogenes was grown on SM+/3 medium and Escherichia coli and Pseudomonas aeruginosa were grown on L broth (Sussman and Sussman 1967). Folate solutions were adjusted to pH 7 using 100 mM NaHCO3.
Chemotaxis assays
Above agar chemotaxis assays were performed as previously described (Nguyen et al. 2010). Cells were grown in fresh HL5 medium 24 hrs prior to harvesting and washing in phosphate buffer (12 mM NaH2PO4 adjusted to pH 6.1 with KOH) and suspended at 1 × 108 cells/ml for Dictyostelium or 2 × 107 cells/ml for Acanthamoeba (Acanthamoeba are approximately 5 times the size of Dictyostelium) unless otherwise noted. Droplets (<1 μl) of cell suspensions were spotted on to non-nutrient agar plates (1.5% agar in phosphate buffer) and then 1 μl of chemoattractant was spotted approximately 2 mm from the cell droplet. Images of the cells were recorded immediately after the plating of the cells and chemoattractant and recorded again 2.5 h later. The agar surface near the cell droplet was scarred with a needle to allow the early and late images to be aligned so that the original cell droplet perimeter could be overlaid on the later image. Cell movement toward the chemoattractant source was determined by measuring the distance from the original cell droplet perimeter to the leading edge of migrating cells. Chemotaxis index (A/B) was defined as the number of cells outside the original cell perimeter that moved toward the chemoattractant (A) divided by the number of cells outside the original cell perimeter that moved away from the chemoattractant (B). Chemotaxis to bacterial cells was performed as that described for folate except 1 μl droplets of bacterial cell suspensions were used as the chemotactic stimulus. Prior to being used in the chemotaxis assays, bacterial cultures were grown overnight in shaking cultures at 22 °C (K. aerogenes) or 37 °C (E. coli and P. aeruginosa). Chemotaxing cells were analyzed using a dissecting microscope (Nikon SMZ2). Videos were created using time-lapse photography with 20 s intervals between images for 33 min. ImageJ with MTrackJ plugin software was used to trace cell migration tracks and determine the migration distance for selected cells. Directionality values were determined using Chemotaxis and Migration Tool Version 1.01 plugin software.
G protein ortholog analysis
G protein sequences were identified using BLASTp searches using default parameters in the non-redundant protein sequences database (NCBI). Amoebae sequences were available primarily due to genome sequencing projects of Dictyostelium discoideum, Acanthamoeba castellanii, Dictyostelium purpureum, Dictyostelium fasciculatum, and Polysphondylium pallidum (Clarke et al. 2013; Eichinger et al. 2005; Heidel et al. 2011; Sucgang et al. 2011). Initial searches were queried with Dictyostelium discoideum protein sequences but queries were also conducted using representative proteins from Acanthamoeba castellanii, mammals and yeast. Molecular phylogenetic analysis was conducted in MEGA7 using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al. 1992; Kumar et al. 2016). The percentage of replicate trees in which the associated proteins clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein 1988). Only branches corresponding to partitions reproduced in more than 50% of the bootstrap replicates are labeled. Each tree is drawn to scale, with branched lengths measured in the substitutions per site. Additional BLAST searches were conducted in the AmoebaDB database (AmoebaDB and MicrosporidiaDB:functional genomic resources for Amoebozoa and Microsporidia species) using the Acanthamoebae data sets (Andrew Jackson, Liverpool, UK).
Supplementary Material
Acknowledgements
We thank D. Schwebs for technical support and S. Khanam and M. Patrauchan for P. aeruginosa cultures. We also thank L. A. Brown for helpful discussions regarding cell dispersal. This work was supported by the grants NIGMS R15 GM097717-01 and OCAST HR13-36 to JAH.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.protis.2016.08.006.
References
- Anjard C, Su Y, Loomis WF. Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium. Development. 2009;136:803–812. doi: 10.1242/dev.032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annesley SJ, Fisher PR. Dictyostelium discoideum–a model for many reasons. Mol Cell Biochem. 2009;329:73–91. doi: 10.1007/s11010-009-0111-8. [DOI] [PubMed] [Google Scholar]
- Artemenko Y, Lampert TJ, Devreotes PN. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cellul Mol Life Sci. 2014;71:3711–3747. doi: 10.1007/s00018-014-1638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti S, Coccetti P, Alberghina L, Vanoni M. Glucose signaling-mediated coordination of cell growth and cell cycle in Saccharomyces cerevisiae. Sensors (Basel) 2010;10:6195–6240. doi: 10.3390/s100606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T, Fiore-Donno AM, Chao E, Kudryavtsev A, Berney C, Snell EA, Lewis R. Multigene phylogeny resolves deep branching of Amoebozoa. Mol Phylogenet Evol. 2015;83:293–304. doi: 10.1016/j.ympev.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Burglin TR, Frech C, Turcotte B, Kopec KO, Synnott JM, Choo C, Paponov I, Finkler A, Heng Tan CS, Hutchins AP, Weinmeier T, Rattei T, Chu JS, Gimenez G, Irimia M, Rigden DJ, Fitzpatrick DA, Lorenzo-Morales J, Bateman A, Chiu CH, Tang P, Hege-mann P, Fromm H, Raoult D, Greub G, Miranda-Saavedra D, Chen N, Nash P, Ginger ML, Horn M, Schaap P, Caler L, Loftus BJ. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013;14:R11. doi: 10.1186/gb-2013-14-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendoza A, Sebe-Pedros A, Ruiz-Trillo I. The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol Evol. 2014;6:606–619. doi: 10.1093/gbe/evu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit RJW, Bulgakov R. Folate chemotactic receptors in Dictyostelium discoideum. II. Guanine nucleotides alter the rates of interconversion and the proportioning of four receptors states. Biochim Biophys Acta. 1986;886:88–95. [Google Scholar]
- De Wit RJ, Bulgakov R, Rinke de Wit TF, Konijn TM. Developmental regulation of the pathways of folate-receptor-mediated stimulation of cAMP and cGMP synthesis in Dictyostelium discoideum. Differentiation. 1986;32:192–199. doi: 10.1111/j.1432-0436.1986.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Douglas TE, Brock DA, Adu-Oppong B, Queller DC, Strassmann JE. Collection and cultivation of dictyostelids from the wild. Methods Mol Biol. 2013;983:113–124. doi: 10.1007/978-1-62703-302-2_6. [DOI] [PubMed] [Google Scholar]
- Du Q, Schilde C, Birgersson E, Chen ZH, McElroy S, Schaap P. The cyclic AMP phosphodiesterase RegA critically regulates encystation in social and pathogenic amoebas. Cell Signal. 2014;26:453–459. doi: 10.1016/j.cellsig.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, van Driessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R, Hauser H, James K, Quiles M, Madan Babu M, Saito T, Buchrieser C, Wardroper A, Felder M, Thangavelu M, Johnson D, Knights A, Loulseged H, Mungall K, Oliver K, Price C, Quail MA, Urushihara H, Hernandez J, Rabbi-nowitsch E, Steffen D, Sanders M, Ma J, Kohara Y, Sharp S, Simmonds M, Spiegler S, Tivey A, Sugano S, White B, Walker D, Woodward J, Winckler T, Tanaka Y, Shaulsky G, Schleicher M, Weinstock G, Rosenthal A, Cox EC, Chisholm RL, Gibbs R, Loomis WF, Platzer M, Kay RR, Williams J, Dear PH, Noegel AA, Barrell B, Kuspa A. The genome of the social amoeba Dictyostelium discoideum. Nature (London) New Biol. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Ginsburg GT, Gollop R, Yu Y, Louis JM, Saxe CL, Kimmel AR. The regulation of Dictyostelium development by transmembrane signalling. J Eukaryot Microbiol. 1995;42:200–205. doi: 10.1111/j.1550-7408.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA. Developmental morphology and chemotactic responses are dependent on G alpha subunit specificity in Dictyostelium. Dev Biol. 2007;312:1–12. doi: 10.1016/j.ydbio.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger JA, Firtel RA. Analysis of Ga4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992;6:38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Srinivasan J. Folic acid stimulation of the Galpha4 G protein-mediated signal transduction pathway inhibits anterior prestalk cell development in Dictyostelium. Differentiation. 1999;64:195–204. doi: 10.1046/j.1432-0436.1999.6440195.x. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Lee S, Firtel RA. The G alpha subunit G alpha 4 couples to pterin receptors and identifies a signaling pathway that is essential for multicellular development in Dictyostelium. Proc Natl Acad Sci USA. 1994;91:10566–10570. doi: 10.1073/pnas.91.22.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel AJ, Lawal HM, Felder M, Schilde C, Helps NR, Tunggal B, Rivero F, John U, Schleicher M, Eichinger L, Platzer M, Noegel AA, Schaap P, Glockner G. Phylogeny-wide analysis of social amoeba genomes highlights ancient origins for complex intercellular communication. Genome Res. 2011;21:1882–1891. doi: 10.1101/gr.121137.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall RH, Soede RD, Schaap P, Devreotes PN. Two cAMP receptors activate common signaling pathways in Dictyostelium. Mol Biol Cell. 1994;5:703–711. doi: 10.1091/mbc.5.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Khan NA. Pathogenesis of Acanthamoeba infections. Microb Pathog. 2003;34:277–285. doi: 10.1016/s0882-4010(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Kim JY, Borleis JA, Devreotes PN. Switching of chemoattractant receptors programs development and morphogenesis in Dictyostelium: receptor subtypes activate common responses at different agonist concentrations. Dev Biol. 1998;197:117–128. doi: 10.1006/dbio.1998.8882. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Hadwiger JA, Pupillo M, Firtel RA. Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J Biol Chem. 1991;266:1220–1228. [PubMed] [Google Scholar]
- Kumagai A, Pupillo M, Gundersen R, Miake LR, Devreotes PN, Firtel RA. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989;57:265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7. 0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Lyon DJ, Fan DS, Houang E. Acanthamoeba keratitis and contact lens wear. Lancet. 1997;350:1481. doi: 10.1016/S0140-6736(05)64251-1. [DOI] [PubMed] [Google Scholar]
- Li L, Norrelykke SF, Cox EC. Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS ONE. 2008;3:e2093. doi: 10.1371/journal.pone.0002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly P, Wu L, Welker DL, Devreotes PN. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 1993;7:986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- Loomis WF, editor. The Development of Dictyostelium discoideum. Academic Press; New York, USA: [Google Scholar]
- Louis JM, Ginsburg GT, Kimmel AR. The cAMP receptor CAR4 regulates axial patterning and cellular differentiation during late development of Dictyostelium. Genes Dev. 1994;8:2086–2096. doi: 10.1101/gad.8.17.2086. [DOI] [PubMed] [Google Scholar]
- Maeda M, Aubry L, Insall R, Gaskins C, Devreotes PN, Firtel RA. Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium – Role of heterotrimeric G proteins. J Biol Chem. 1996;271:3351–3354. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Mayanagi T, Amagai A. Folic acid is a potent chemoattractant of free-living amoebae in a new and amazing species of protist, Vahlkampfia sp. Zool Sci. 2009;26:179–186. doi: 10.2108/zsj.26.179. [DOI] [PubMed] [Google Scholar]
- Manahan CL, Iglesias PA, Long Y, Devreotes PN. Chemoattractant signaling in Dictyostelium discoideum. Annu Rev Cell Dev Biol. 2004;20:223–253. doi: 10.1146/annurev.cellbio.20.011303.132633. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Ashley CA, Hadwiger JA. Related Ga subunits play opposing roles during Dictyostelium development. Differentiation. 2000;66:136–146. doi: 10.1046/j.1432-0436.2000.660208.x. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Iiri T, Bourne HR. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274:2824–2828. doi: 10.1074/jbc.274.5.2824. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Hadwiger JA. The Galpha4 G protein subunit interacts with the MAP kinase ERK2 using a D-motif that regulates developmental morphogenesis in Dictyostelium. Dev Biol. 2009;335:385–395. doi: 10.1016/j.ydbio.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Raisley B, Hadwiger JA. MAP kinases have different functions in Dictyostelium G protein-mediated signaling. Cell Signal. 2010;22:836–847. doi: 10.1016/j.cellsig.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JM, Veltman D, Kay RR. Chemotaxis of a model organism: progress with Dictyostelium. Curr Opin Cell Biol. 2015;36:7–12. doi: 10.1016/j.ceb.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Omosigho NN, Swaminathan K, Plomann M, Muller-Taubenberger A, Noegel AA, Riyahi TY. The Dictyostelium discoideum RACK1 orthologue has roles in growth and development. Cell Commun Signal. 2014;12:37. doi: 10.1186/1478-811X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M, Xu X, Chen Y, Jin T. Identification of a chemoattractant G-protein-coupled receptor for folic acid that controls both chemotaxis and phagocytosis. Dev Cell. 2016;36:428–439. doi: 10.1016/j.devcel.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Wurster B. Inactivation of the chemoattractant folic acid by cellular slime molds and identification of the reaction product. J Bacteriol. 1978;136:955–959. doi: 10.1128/jb.136.3.955-959.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Hall EM, Bonner JT. Folic acid as second chemotactic substance in the cellular slime moulds. Nat New Biol. 1972;237:181–182. doi: 10.1038/newbio237181a0. [DOI] [PubMed] [Google Scholar]
- Pan P, Hall EM, Bonner JT. Determination of the active portion of the folic acid molecule in cellular slime mold chemotaxis. J Bacteriol. 1975;122:185–191. doi: 10.1128/jb.122.1.185-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters T, Louwet W, Gelade R, Nauwelaers D, Thevelein JM, Versele M. Kelch-repeat proteins interacting with the Galpha protein Gpa2 bypass adenylate cyclase for direct regulation of protein kinase A in yeast. Proc Natl Acad Sci USA. 2006;103:13034–13039. doi: 10.1073/pnas.0509644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Gomer RH. A secreted protein is an endogenous chemorepellant in Dictyostelium discoideum. Proc Natl Acad Sci USA. 2012;109:0990–10995. doi: 10.1073/pnas.1206350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu Y, Eichinger L. The Dictyostelium repertoire of seven transmembrane domain receptors. Eur J Cell Biol. 2006;85:937–946. doi: 10.1016/j.ejcb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Prabhu Y, Mondal S, Eichinger L, Noegel AA. A GPCR involved in post aggregation events in Dictyostelium discoideum. Dev Biol. 2007;312:29–43. doi: 10.1016/j.ydbio.2007.08.055. [DOI] [PubMed] [Google Scholar]
- Raisley B, Nguyen HN, Hadwiger JA. G{alpha}5 subunit-mediated signalling requires a D-motif and the MAPK ERK1 in Dictyostelium. Microbiology. 2010;156:789–797. doi: 10.1099/mic.0.036541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisley B, Zhang M, Hereld D, Hadwiger JA. A cAMP receptor-like G protein-coupled receptor with roles in growth regulation and development. Dev Biol. 2004;265:433–445. doi: 10.1016/j.ydbio.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Sawyer TK. Free-living pathogenic and nonpathogenic amoebae in Maryland soils. Appl Environ Microbiol. 1989;55:074–1077. doi: 10.1128/aem.55.5.1074-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe CL, 3rd, Johnson RL, Devreotes PN, Kimmel AR. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Dev. 1991;5:1–8. doi: 10.1101/gad.5.1.1. [DOI] [PubMed] [Google Scholar]
- Saxe CL, 3rd, Ginsburg GT, Louis JM, Johnson R, Devreotes PN, Kimmel AR. CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes Dev. 1993;7:262–272. doi: 10.1101/gad.7.2.262. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Levandowsky M. Chemosensory responses of Acanthamoeba castellanii: visual analysis of random movement and responses to chemical signals. J Eukaryot Microbiol. 1996;43:150–158. doi: 10.1111/j.1550-7408.1996.tb04496.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Rahman M, Griffith S. Chemotactic responses of Acanthamoeba castellanii to bacteria, bacterial components, and chemotactic peptides. Trans Am Microsc Soc. 1993;112:43–61. [Google Scholar]
- Schwebs DJ, Hadwiger JA. The Dictyostelium MAPK ERK1 is phosphorylated in a secondary response to early developmental signaling. Cell Signal. 2015;27:147–155. doi: 10.1016/j.cellsig.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Dudley R, Khan NA. Acanthamoeba differentiation: a two-faced drama of Dr. Jekyll and Mr. Hyde. Parasitology. 2012;139:826–834. doi: 10.1017/S0031182012000042. [DOI] [PubMed] [Google Scholar]
- Sucgang R, Kuo A, Tian X, Salerno W, Parikh A, Feasley CL, Dalin E, Tu H, Huang E, Barry K, Lindquist E, Shapiro H, Bruce D, Schmutz J, Salamov A, Fey P, Gaudet P, Anjard C, Babu MM, Basu S, Bushmanova Y, van der Wel H, Katoh-Kurasawa M, Dinh C, Coutinho PM, Saito T, Elias M, Schaap P, Kay RR, Henrissat B, Eichinger L, Rivero F, Putnam NH, West CM, Loomis WF, Chisholm RL, Shaulsky G, Strassmann JE, Queller DC, Kuspa A, Grigoriev IV. Comparative genomics of the social amoebae Dictyostelium discoideum and Dictyostelium purpureum. Genome Biol. 2011;12:R20. doi: 10.1186/gb-2011-12-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TJ, Devreotes PN. Gene targeting of the aggregation stage cAMP receptor cAR1 in Dictyostelium. Genes Dev. 1991;5:572–582. doi: 10.1101/gad.5.4.572. [DOI] [PubMed] [Google Scholar]
- Sussman R, Sussman M. Cultivation of Dictyostelium discoideum in axenic medium. Biochem Biophys Res Commun. 1967;29:53–55. doi: 10.1016/0006-291x(67)90539-6. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Bosgraaf L. Food searching strategy of amoeboid cells by starvation induced run length extension. PLoS ONE. 2009;4:e6814. doi: 10.1371/journal.pone.0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DM, Van Haastert PJ. Guanylyl cyclase protein and cGMP product independently control front and back of chemotaxing Dictyostelium cells. Mol Biol Cell. 2006;17:3921–3929. doi: 10.1091/mbc.E06-05-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walochnik J, Aichelburg A, Assadian O, Steuer A, Visvesvara G, Vetter N, Aspock H. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J Clin Microbiol. 2008;46:338–340. doi: 10.1128/JCM.01177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Ashworth JM. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970;119:71–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, Grant FJ, O’Hara P, MacKay VL. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989;56:467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Williams JG. Dictyostelium finds new roles to model. Genetics. 2010;185:717–726. doi: 10.1534/genetics.110.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M, Andrew N, Insall RH. Entamoeba histolytica cell movement: a central role for self-generated chemokines and chemorepellents. Proc Natl Acad Sci USA. 2006;103:18751–18756. doi: 10.1073/pnas.0605437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller CE, Parnell SC, Dohlman HG. The RACK1 ortholog Asc1 functions as a G-protein beta subunit coupled to glucose responsiveness in yeast. J Biol Chem. 2007;282:25168–25176. doi: 10.1074/jbc.M702569200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Long Y, Devreotes PN. Ggamma in dictyostelium: its role in localization of gbetagamma to the membrane is required for chemotaxis in shallow gradients. Mol Biol Cell. 2001;12:3204–3213. doi: 10.1091/mbc.12.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.