Abstract
Treatment for substance use disorders (SUD) provides an opportunity to use voucher-based treatment for smoking. Nicotine replacement (NRT) could improve outcomes previously observed with vouchers without NRT.
Methods
A randomized controlled trial compared Contingent Vouchers (CV) for smoking abstinence to Noncontingent Vouchers (NV), when all received counseling and NRT. Smokers who had not sought smoking treatment (n = 340) in residential SUD treatment were provided 14 days of vouchers for complete smoking abstinence per exhaled carbon monoxide (CO) after a 5-day smoking reduction period, or vouchers only for breath samples, plus Brief Advice (four sessions) and 8 weeks of NRT.
Results
Within treatment, 20% had complete abstinence with CV, 5% with NV (p < .001), and participants showed 50% of days abstinent in CV compared to 22% in NV (p < .001). Across 1, 3, 6 and 12 months after randomization, CV resulted in significantly fewer cigarettes per day (p < .01) and fewer days smoking (p < .01), but with small effects. Point-prevalence abstinence differences across follow-up (e.g., 4% CV, 2% in NV at 6 and 12 months) were not significant. No differences in substance use were seen.
Conclusions
Within-treatment effects on abstinence are stronger than in a prior study of the same CV with BA but without NRT, but NRT does not improve abstinence after vouchers end. Implications for voucher-based treatment include investigating effects when combined with stronger smoking medications and using motivational interviewing. Smoking treatment does not harm SUD recovery.
Keywords: contingent vouchers, contingency management, financial incentives, brief advice, nicotine replacement, smoking cessation, substance use disorders, point-prevalence abstinence, nicotine dependence
1. Introduction
Smokers with substance use disorders (SUD) smoke at higher rates than in the general population (e.g., Compton, Thomas, Stimson and Grant, 2007; Moliterno et al, 1994; Roll et al, 1998) and have little success in quitting smoking early in recovery with the common first-line smoking treatments (e.g., Bien and Burge, 1991; Joseph, Willenbring, Nugent, and Nelson, 2005; Kalman et al., 2001; Monti, Rohsenow, Colby, and Abrams, 1995; Prochaska, Delucchi and Hall, 2004). SUD treatment provides an opportunity to provide smoking treatments to smokers with SUD, but stronger approaches may be needed to encourage these smokers to attempt to quit smoking and to sustain abstinence. In this population, motivation to quit smoking is low (Flach and Diener, 2004; Martin, Rohsenow, MacKinnon, Abrams and Monti, 2006; Richter, Gibson, Ahluwalia and Schmelze, 2001; Rohsenow, Martin, Tidey, Monti, and Colby, 2013) and correlates with perceiving more barriers to quitting (Martin, et al., 2006). Smokers in SUD treatment indicated that tobacco abstinence effects are major barriers to attempting smoking cessation (Asher, Martin, Rohsenow, MacKinnon, Traficante, and Monti, 2003; Martin, Cassidy, Murphy, & Rohsenow, in press). Expecting to be unable to tolerate the discomforts of tobacco abstinence predicts less tobacco abstinence for smokers with SUD 3 months later (Rohsenow, Tidey, Kahler, et al., 2015). Therefore, medication to reduce the discomfort of abstinence plus additional incentives to undergo smoking abstinence may be needed for these smokers to even attempt to quit smoking.
Clinical practice guidelines (USDHHS, 2000) suggest providing smokers with behavioral counseling and at least nicotine replacement therapy (NRT). NRT plus counseling results in only an average of 3.5% point-prevalence abstinence at 12 months with currently sober people with a history of alcohol use disorders (AUDs) (per Hughes, 1993; Hurt et al., 1995), and there is little or no data for smokers with mixed SUDs. However, combining NRT and counseling with a stronger method to incentivize initial abstinence is warranted to determine if this would improve initial and sustained smoking abstinence.
Contingent vouchers (CV) to incentivize smoking abstinence on a platform of brief counseling can encourage initial abstinence in unmotivated smokers and provide a foundation for longer term abstinence (Sigmon & Patrick, 2012). Published studies of CV for smoking abstinence among smokers with SUD, mostly with smokers receiving pharmacologic treatment for opiate dependence, showed that CV significantly increased smoking abstinence compared to noncontingent incentives (NV) while the incentives were in place, but not for long after incentives were terminated (Alessi & Petry, 2014; Alessi, Petry & Urso, 2008; Dunn et al., 2008, 2010; Hunt et al., 2010; Robles et al., 2005; Shoptaw et al., 1996, 2002; Wiseman et al., 2005). Most of these studies involved limited or no counseling. One study of smokers in SUD treatment compared CV to NV on a platform of four sessions of Brief Advice (BA; Manley, Epps, Husten, Glynn, & Shopland, 1991) or Motivational Interviewing (MI; Miller & Rollnick, 1991, 2002) adapted to concerns of smokers with SUD but without adjunctive pharmacotherapy (Rohsenow, Tidey, Martin, et al., 2015). CV resulted in significantly more smoking abstinence within-treatment (25% of days in CV, 5% of days in NV), and over 12 months when combined with MI (6.6% of participants abstinent) rather than BA (0% of participants abstinent). However, long-term point-prevalence abstinence rates were still low, suggesting that adding pharmacotherapy such as NRT might improve the outcomes for CV.
In this study, smokers who had not sought smoking treatment in a residential SUD treatment program were randomized to the same CV as in Rohsenow, Tidey, Martin, et al. (2015): 14 days of vouchers for smoking abstinence (based on carbon monoxide (CO) readings twice a day) after a 5-day smoking reduction period, or to the same NV: vouchers not contingent on smoking status. All received BA, a standard of care for smokers not seeking smoking treatment (USDHHS, 2000), and up to 8 weeks of NRT. When this study was started, the results of Rohsenow, Tidey, Martin, et al., 2015, showing that CV was more effective when combined with MI than BA were not available. Therefore, BA adapted for sobriety concerns was chosen since without CV, BA was equivalently effective to MI with smokers in SUD treatment (Rohsenow et al., 2014). Effects on smoking abstinence were investigated both within-treatment and over a year of follow-up. Effects on any substance use was also investigated to ensure no harmful effects, consistent with most smoking treatment studies in this population (reviewed in Rohsenow, 2015).
2. Materials and Methods
2.1 Participants
2.1.1 Site
The clinical sites were two inner-city state-funded residential SUD treatment program (Gateway Healthcare, Inc., and The Providence Center). The abstinence-oriented programs provided SUD education in a group format based on 12-Step models, with outpatient aftercare available. Smoking cessation was not addressed by the programs and patients were able to smoke outdoors. The sites differed primarily in lengths of stay (see Results section). At the outset of the study, we provided in-service training with staff to address the benefits of smoking cessation for people engaged in SUD treatment. Since we had conducted other smoking treatment research there previously, clinical staff posed no barriers.
2.1.2 Eligibility Criteria
Participants (n = 363) were recruited from patients on site by a member of the research staff, generally in the first week after admission to the SUD treatment program and after any detoxification was completed. Eligibility criteria included meeting current DSM-IV SUD criteria (see 2.4.2) and smoking at least 10 cigarettes per day for the past 6 months. Patients were excluded if they were engaged in smoking treatment; hallucinating or delusional; could not read; or met exclusionary criteria for transdermal NRT (pregnant or nursing; treatment in the last 3 months for unstable angina, severe congestive heart failure, uncontrolled hypertension; lung cancer; supplemental oxygen; history of adverse reactions to NRT; allergies to adhesive; any severe skin disease that requires treatment). Recruits were told the study would provide informational sessions about smoking without requiring cessation, would provide free NRT for 8 weeks, and would offer payments either for reduced smoking followed by abstinence, or just for providing breath samples for 19 days.
2.2 Overview of Procedures
The design was a 2-group (CV vs. NV) randomized controlled clinical trial (RCT). Urn randomization (Stout et al., 1994) on the first day of the voucher period stratified by gender, Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and Smoking Contemplation Ladder (CL; Biener & Abrams, 1991) scores. The median splits for FTND and the CL were based on medians from a previous study with similar participants. Follow ups were at 1, 3, 6 and 12 months. To maximize follow-up rates, we collected detailed contact information, the costs of transportation were covered, reminders were sent, and participants consented to designate a significant other as a locator. All procedures received IRB approval from Brown University and the clinical sites, and we had a federal Certificate of Confidentiality.
2.3 Interventions
2.3.1. NRT
NRT was provided to all for 8 weeks at no charge starting the day before the voucher period started. NRT followed clinical practice guidelines (USDHHS, 2000): 21 mg/day for 4 weeks, 14 mg/day for 2 weeks, 7 mg/day for 2 weeks, with a written instruction sheet provided and reviewed. Because this study was designed to motivate people who had not sought smoking cessation (in addition to assisting with cessation for those who want to quit), we could not require patients to use NRT but we asked everyone to try it (consistent with clinical guidelines for motivating smoking cessation, Fiore et al., 2000).
2.3.2. Brief Advice
Fully manualized BA was provided to all in four sessions: the day before starting the voucher period and 7, 14 and 19 days later. BA used recommended methods (Manley et al., 1991; Hollis et al., 1993), adapted for SUD recovery issues. In the initial session (15 min), therapists assessed interest in quitting, directly advised patients to stop smoking now for their health, assisted by giving advice about useful methods, and asked them to set a quit date within the next week. A handout on common barriers to smoking cessation was provided and corrective information about each was reviewed, especially to correct concerns about effects on sobriety. A handout of cognitive-behavioral coping skills was reviewed together. Patients were given a consumer guide for smoking cessation (Strecher et al., 1989), were encouraged to select from a variety of nationally available published pamphlets on smoking cessation (e.g., effects on pregnancy, children, smoking and food, handling withdrawal, etc.), and were given hard candy on request (chewing gum not allowed on site). In additional sessions (10–15 min each), our counselor checked on progress toward smoking cessation, reviewed their CO record, reminded them of health reasons for quitting, engaged in problem-solving around barriers, noted successes and methods they should continue using, and reminded them of methods available. The last session discussed coping with the transition off of the contingencies.
Interventions were provided by two masters’ level and one bachelor’s level research therapists after 10 hours of training with the treatment manual, including training in being empathic, nonargumentative, and supporting self-efficacy. Treatment session audiotapes (33% of initial sessions, 25% of additional sessions) were reviewed in weekly group supervision with a psychologist trained in the approach, and rated for competence and adherence to the manual (see 2.4.4), with immediate feedback to therapists to prevent drift.
2.3.3. Contingent Voucher procedures
Vouchers were provided during a 5-day reduction phase plus a 14-day abstinent phase (including weekends). Procedures were explained to participants in detail and they were provided a written handout explaining the contingencies. They were encouraged to use the contingencies and NRT to start a lifetime without tobacco. A laptop program tracked all payments earned. To encourage participants to provide a breath CO sample regardless of whether they had been smoking, $1 was provided simply for providing the sample, regardless of results (total possible = $33).
Baseline phase
Breath CO was collected on the afternoon before the voucher period started to assess within-setting baseline CO level. Afternoon was chosen to avoid diurnal variation and as the more valid measure of recent smoking (Benowitz, 1983).
Reduction phase
Breath CO level was collected late each afternoon for 5 days. Participants received a printed voucher with a monetary value of $2 per test for a 25% reduction from baseline CO level, $4 for 50% reduction, and $6 for a 75% or greater reduction.
Abstinence phase
Breath CO level was collected each morning and late afternoon for the next 14 days. An escalating schedule of payments provided increasing levels of payments in vouchers for each successive CO reading ≤ 6 ppm (per Cummings & Richard, 1988; Lamb et al., 2010) starting at $3 for the first sample, and increasing by $0.50 for each consecutive negative test to $16.50 for the 28th consecutive abstinent breath sample, plus $10 bonuses provided every time three consecutive readings showed abstinence. Whenever a breath sample did not meet the criterion for abstinence, the participant earned no voucher and the payment schedule reverted to the initial $3 level, then after three consecutive abstinent samples the schedule returned to the payment level at which the reset occurred, to support efforts to regain abstinence following a lapse.
Total possible payment
Participants who completed all 19 days of samples and missed no more than three of the scheduled breath tests earned a $40 bonus voucher (total possible = $433 + $33 for showing up = $466).
2.3.4 Noncontingent Voucher procedures
In NV, payments were matched to the total average of payments received by those randomized to CV plus NRT in a pilot study plus the added $33 the CV condition now gets, simply for providing breath samples as scheduled. The total of $264 was divided into equal payments ($8 per sample), plus a $40 bonus for providing all 33 samples (total possible = $304). The NV condition controls for the effects of receiving vouchers, providing daily breath samples to be analyzed for CO level, and degree of interaction between patient and research staff. The identical breath sampling schedule, system of records, vouchers and merchandise certificates were used for payments.
2.3.5 Vouchers and payments
Participants immediately received printed vouchers and a “bankbook” with the running totals. Merchandise certificates for the value of the vouchers were deposited in participants’ accounts at the treatment agency to protect them from theft while participants were in residence. Staff maintained a log of earnings in case of loss/theft. Merchandise certificates to popular area stores were given to participants any time the participant requested them (usually when leaving on pass or providing them to family, both only possible on weekends, or on discharge).
2.4 Assessments
2.4.1 Assessment procedures
Research interviewers blind to treatment conditions conducted assessments pre-treatment and about 1, 3, 6 and 12 months after the initial session. Interviews after discharge were conducted away from the clinical site, after a breath alcohol test (per Sobell and Sobell, 1986) using Alco Sensor IV by Intoximeters ensured breath alcohol ≤ .02 g/dL, and after assurances that clinical staff would not be informed of the information provided. Participants received up to $235 in merchandise certificates for completing all interviews within 14 days of the due date. Family members or close friends received $15 per assessment for four assessments they completed at the four follow-ups (total = $60).
2.4.2 Individual difference measures
The Structured Clinical Interview for DSM-IV-Patient version (First, Spitzer, Gibbon, & Williams, 1995) was used to determine current SUD diagnoses. At pretreatment we also administered a smoking history questionnaire, breath CO, and FTND. Responses to the Barriers to Quitting Smoking in Substance Abuse Treatment (BQS-SAT; Asher et al., 2003), revised to change “alcohol” to “alcohol or drugs” (Martin et al., in press), were provided to therapists for use in the BA.
2.4.3 Within-treatment smoking abstinence
The within-treatment outcome is the number of days during the 14-day period after the 5-day reduction period in which both CO readings met the criterion for abstinence.
2.4.4 Post-treatment outcome measures
The Timeline Followback interview (Brown et al., 1998; Ehrman & Robbins, 1994; Sobell & Sobell, 1980) at baseline (for 6 months preadmission) and follow ups (for the period since the previous interview), was scored for number of cigarettes each day, number of days of drug use, and number of heavy drinking days (at least 6/5 drinks/day for men/women (Flannery et al., 2002). The primary smoking outcome measure during follow-up was 7-day point-prevalence abstinence, confirmed with a CO level ≤ 4 ppm and salivary cotinine ≤ 15 ng/ml (Cropsey et al., 2014; Hughes et al., 2003; Lamb et al., 2010) if not using NRT. At follow-ups, self-reports of drug abstinence were confirmed by urine drug screens (On Trak® test cups for screening confirmed with EMIT, gas chromatography and mass spectrometry). Alcohol abstinence self-reports were used since there is no valid way to confirm these given the unreliability of significant other reports and biochemical methods as confirmation.
2.4.5. Treatment delivery and process measures
Treatment sessions were rated by the treatment supervisors on 1 (not at all) to 5 (extensively) scales for three therapist competence measures (arguing, demonstrating empathy, supporting self-efficacy), and for adherence to delivery of five types of treatment elements (emphasize health benefits for change, explore barriers, problem-solve around barriers/difficulties, discuss methods for changing, discuss NRT). We recorded number of BA sessions attended and number of days using NRT (counting all returned NRT patches while in residence).
At pretreatment and at 1 month, participants completed a Smoking Self-Efficacy Questionnaire (Velicer et al., 1990) and the CL (Section 2.2), a single 10-point fully-anchored scale from 1 (no interest in quitting) to 10 (I have quit smoking and will never smoke again). These were used to determine if CV differentially decreases self-efficacy or motivation.
2.4.6 Adverse effects
Adverse effects were assessed weekly (in-person or by telephone).
2.5 Data Analysis Approach
2.5.1 Preliminary analyses
IBM SPSS Statistics® for PC was used for most analyses, with multiple imputation analyses run using MIANALYZE procedures (SAS/STAT, 2013) and moderation analyses run using PROCESS (Hayes, 2012). Variables were inspected for assumptions of normality, outliers, and other assumptions underlying regression. One extreme outlier for baseline CO was set to 1+ the next highest value (per Tabachnik & Fidell, 2007). Five outliers for length of stay in residential treatment (> 3 SD above the mean) were set to 1+ the next highest value for analyses but the unchanged values are presented in descriptive statistics (Table 1). Log transformation was required for number of drug use days. (Untransformed values are presented for ease of interpretation.) Percentage of heavy drinking days had poor distribution since 70 – 94% of the sample had zeroes at each follow-up, so it was scored dichotomously as relapse to any heavy drinking. All other assumptions underlying the analyses were met. Treatment group differences in baseline characteristics, in treatment session ratings, in length of treatment (days in residence, number of BA sessions attended), and in follow-up rates were examined with between-group (CV vs. NV) t-tests for continuous variables and chi-square tests for dichotomous ones (see Table 2). Site differences were investigated with site by condition analyses of variance (ANOVAs) followed up with simple effects tests. Hypothesis-wide alphas were set at .05.
Table 1.
Participant Pretreatment Characteristics: mean (SD) or percentage
| Full sample Mean (SD) or % |
CV Mean (SD) or % |
NV Mean (SD) or % |
|
|---|---|---|---|
| N | 340 | 172 | 168 |
| Age in years | 37.58 (10.04) | 37.87 (10.14) | 37.28 (9.95) |
| Education (years) | 12.09 (2.15) | 12.15 (2.05) | 12.03 (2.01) |
| Annual Income | |||
| $0–$9,999 | 59% | 61% | 57% |
| $10,000–$29,999 | 26% | 23% | 29% |
| $30,000–$49,999 | 9% | 9% | 9% |
| $50,000 + | 6% | 7% | 5% |
| Days in residential treatment | 120.44 (81.59) | 112.95 (76.26) | 128.07 (86.23) |
| CO level at baseline | 21.58 (11.67) | 21.34 (12.38) | 21.82 (10.03) |
| Cigarettes/day | 19.48 (7.41) | 19.95 (8.03) | 18.99 (6.71) |
| FTND | 5.92 (1.89) | 5.90 (1.81) | 5.94 (1.97) |
| Minutes to first cigarette | 1.07 (.42) | 1.05 (.42) | 1.09 (.43) |
| Years smoked daily | 21.35 (10.33) | 21.34 (10.27) | 21.36 (10.41) |
| Contemplation Ladder | 5.35 (1.60) | 5.31 (1.64) | 5.39 (1.57) |
| % drinking days | 40.19 (38.90) | 42.18 (39.97) | 38.15 (37.79) |
| % drug use days | 54.21(39.83) | 55.62 (40.70) | 52.75 (38.99) |
| Drinks per day | 10.59 (15.71) | 10.89 (16.07) | 10.27 (15.37) |
| Race | |||
| White/Caucasian | 86% | 85% | 87% |
| Black/African American | 10% | 11% | 9% |
| American Indian/Alaskan | 2% | 4% | 0% |
| Asian | < 1% | 0% | 1% |
| Multi-racial | 2% | 0% | 3% |
| Hispanic | 8% | 7% | 9% |
| Male | 67% | 66% | 69.0% |
| Married or living together | 11% | 10% | 12% |
| Unemployed pretreatment | 97% | 99% | 96% |
| Alcohol use disorder | 76% | 74% | 77% |
| Opiate use disorder | 49% | 47% | 51% |
| Cocaine use disorder | 60% | 63% | 57% |
| Marijuana use disorder | 36% | 35% | 37% |
Note: CV= contingent vouchers, NCV= noncontingent vouchers. FTND = Fagerström Test of Nicotine Dependence total score. CO= expired carbon monoxide in parts per million.
Table 2.
Outcome Variables at Follow up and Process Variables Pre and Post by Voucher Condition: Descriptive Statistics and Univariate Analyses (t-tests or Chi-square Tests)
| Variable | CV N (%) or Mean (SD) |
NV N (%) or Mean (SD) |
Effect Size d or OR |
95% CI |
|---|---|---|---|---|
| Confirmed 7-day abstinence2 | ||||
| 1 Month | 16 (9.3) | 13 (7.7) | 1.22 | 0.57, 2.63 |
| 3 Month | 9 (5.3) | 5 (3.0) | 1.81 | 0.59, 5.52 |
| 6 Month Follow Up | 6 (3.6) | 3 (1.8) | 2.03 | 0.49, 8.23 |
| 12 Month Follow Up | 6 (3.7) | 3 (1.8) | 2.08 | 0.51, 8.45 |
| Average no. cigarettes per day1 | ||||
| 1 Month Follow Up | 5.28 (5.49) | 7.98 (5.49) | −0.49* | −0.71, −0.27 |
| 3 Month Follow Up | 8.84 (7.57) | 9.89 (6.19) | −0.15 | −0.38, 0.08 |
| 6 Month Follow Up | 10.87 (8.05) | 12.18 (6.59) | −0.12 | −.057, 0.33 |
| 12 Month Follow Up | 11.67 (7.70) | 12.78 (7.23) | −0.15 | −0.40, 0.11 |
| Percent smoking days1 | ||||
| 1 Month Follow Up | 77.51 (33.02) | 88.33 (25.48) | −0.37* | −0.59, −0.15 |
| 3 Month Follow Up | 82.39 (30.63) | 88.40 (25.48) | −0.25 | −0.46, 0.02 |
| 6 Month Follow Up | 88.49 (26.45) | 92.30 (21.91) | −0.16 | −0.40, 0.08 |
| 12 Month Follow Up | 87.38 (26.62) | 91.53 (22.86) | −0.17 | −0.42, 0.09 |
| Relapse to any heavy drinking2 | ||||
| 1 Month Follow Up | 12 (7.4) | 8 (5.1) | 1.48 | 0.59, 3.72 |
| 3 Month Follow Up | 23 (15.5) | 24 (16.8) | 0.91 | 0.49, 1.70 |
| 6 Month Follow Up | 38 (27.5) | 35 (26.9) | 1.03 | 0.60, 1.77 |
| 12 Month Follow Up | 38 (30.2) | 36 (31.6) | 0.94 | 0.54, 1.62 |
| Number of drug use days1 | ||||
| 1 Month Follow Up | 1.46 (2.60) | 0.36 (0.51) | −0.01 | −0.23, 0.21 |
| 3 Month Follow Up | 3.35 (10.32) | 3.87 (12.13) | −0.01 | −0.24, 0.22 |
| 6 Month Follow Up | 7.25 (17.21) | 8.69 (20.20) | <0.01 | −0.23, 0.25 |
| 12 Month Follow Up | 21.55 (41.71) | 21.82 (43.24) | 0.02 | −0.23, 0.28 |
| Relapse to any drug use2 | ||||
| 1 Month Follow Up | 13 (8.0) | 16 (10.2) | 0.76 | 0.36, 1.65 |
| 3 Month Follow Up | 38 (49.1) | 32 (50.9) | 1.19 | 0.67, 2.06 |
| 6 Month Follow Up | 50 (36.2) | 42 (32.3) | 1.19 | 0.72, 1.97 |
| 12 Month Follow Up | 54 (42.9) | 50 (43.9) | 0.96 | 0.58, 1.60 |
| Contemplation Ladder1 | ||||
| Pretreatment | 5.40 (1.63) | 5.31 (1.55) | 0.06 | −0.17, 0.29 |
| 1 Month Follow Up | 7.28 (1.73) | 6.89 (1.65) | 0.23 | 0.00, 0.46 |
| Self- Efficacy for Smoking Abstinence1 |
||||
| Pretreatment | 1.78 (0.76) | 1.67 (0.70) | 0.15 | −0.08, 0.38 |
| 1 Month Follow Up | 3.10 (1.03) | 2.83 (1.03) | 0.26 | 0.03, 0.49 |
p < .001
Note: Sample size for continuous variables at 1 month n = 163 in CV and n = 158 in NCV, at 3 months n = 148 in CV and n = 143 in NCV, at 6 months n = 138 in CV and n = 130 in NCV, and at 12 months n = 126 in CV and n = 114 in NCV.
CV = contingent vouchers condition; NV = noncontingent vouchers condition ; CI = confidence interval
Normally distributed variable; effect size is in standardized units of the dependent variable (d).
Dichotomous variable; effect size is expressed as an odds ratio.
2.5.2 Handling missing data
Within the voucher period, participants missing CO data were coded as non-abstinent so data are analyzed on the full n = 340. At follow-up, people who reported smoking or had a CO > 4 ppm or cotinine > 15 ng/ml (if not using NRT), or who were missing CO or cotinine data, were coded as having smoked. People who had a positive, missing or contaminated drug screen were coded as having used drugs for that follow-up interval. Participants who died during follow-up (n = 3 at 3 months, n = 5 at 6 months, and n = 11 at 12 months) were coded as missing for outcomes after death.
Positive imputation (considering missing values to be non-abstinent) is most likely to reflect the true values (Higgins & Green, 2011) but analyses were re-run using multiple imputation methods to provide sensitivity analyses (Rosenbaum, 2005) for those missing verified abstinence, mean cigarettes per day, or number of days of drug use at each follow up. One-hundred-fifty imputed data sets were generated to yield estimates that were better than 95% efficient (Rubin, 1987; Schafer & Graham, 2002). Separate regression analyses were performed using all 150 of the imputed data sets and averaged across the 150 sets of estimates. None of these results differed in significance level from the analyses without multiple imputation, so analyses with multiple imputation are not presented.
2.5.3 Analyses of post-treatment outcomes
Effects of voucher condition on outcomes over time (1, 3, 6 and 12 months) were tested using general estimating equations (GEE; Zeger and Liang, 1986) entering the main effect and the interaction by time. Variables included 7-day point prevalence abstinence, number of days abstinent, cigarettes per day, relapse to any substance use, relapse to any heavy drinking, and number of substance use days, in separate analyses. Analyses of smoking covaried the baseline number of cigarettes per day; substance use outcomes covaried pretreatment number of drug use days or heavy drinking days.
2.5.5 Within-treatment, process and adverse effects measures
The primary within-treatment outcome measure was number of days with both CO readings below the criterion for abstinence during the 14-day abstinence induction phase of the voucher period, although number of abstinent readings was also analyzed for reader information. Three process measures were analyzed. Total number of days using NRT was analyzed using t-tests. Pre-post change in self-efficacy and motivation were investigated using two 2 × 2 repeated measures (pretreatment, 1 month) ANOVAs entering Smoking Self-Efficacy Questionnaire and CL scores. No serious adverse effects occurred to be analyzed.
2.5.6 Moderator analyses
Moderation analyses using path analysis-based moderation models (per Hayes, 2012) were conducted by gender, initial CL, and FTND. The outcome variables were confirmed 7-day smoking abstinence at each outcome time, and number of days abstinent during the 14-day voucher abstinence induction phase. Significant interaction effects were followed with simple slopes tests.
Results
3.1 Sample Size and Attrition
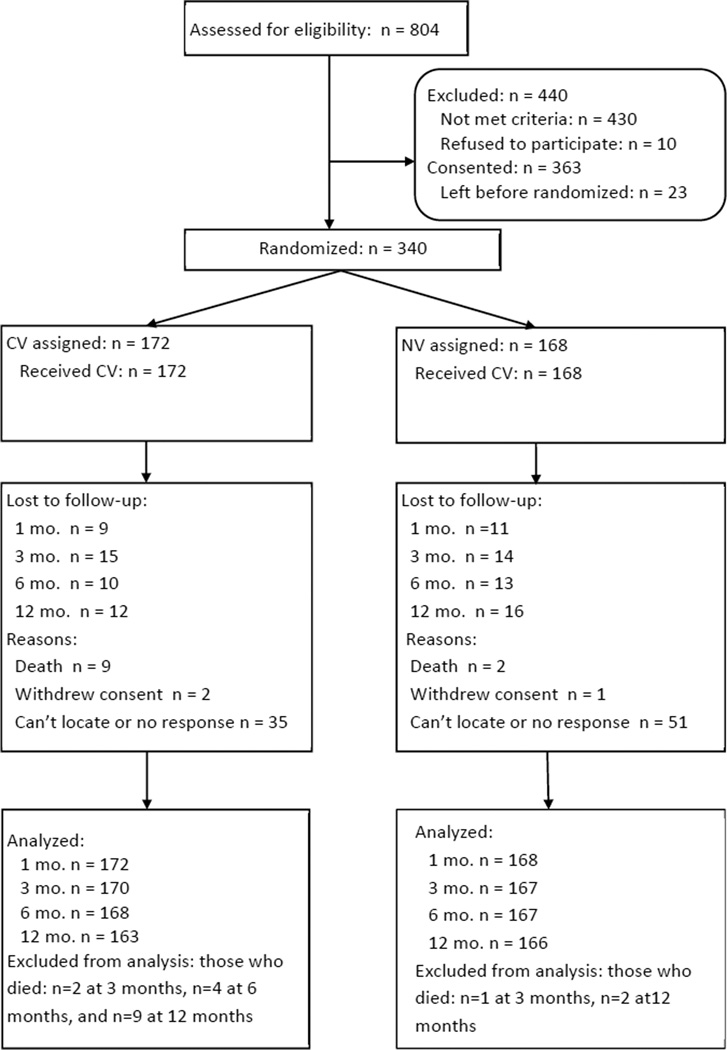
Of 363 participants consented, 340 (94%) were randomized (the intent-to-treat sample). (See Figure 1 for Consort-based flow chart.) Of these, 172 were randomized to CV and 168 to NV (all started their condition). Follow-up completion rates were 94% (n = 320) at 1-month, 86% (n = 291) at 3-months, 80% (n = 268) at 6-months, and 73% (n = 240) at 12-months, with no significant differences by condition. Two-group analyses (t-tests or χ2 analyses) showed no differences between those who did or did not complete the follow up at each time on baseline characteristics (see Table 2).
Figure 1.
Consort-style flow chart of recruitment and retention.
3.2 Participant Characteristics and Site Differences
See Table 1 for baseline characteristics and length of stay. No differences between voucher conditions were significant for any demographic, pretreatment smoking or substance use variable, length of SUD treatment, number of BA sessions completed (M = 3.68), BA treatment delivery ratings, or completion of CO collection within the voucher period or during follow-up (t-tests or χ2 analyses).
Clinical site had no significant main effects or interactions with treatment conditions in ANOVAs on baseline characteristics or clinical variables except for mean number of days in the residential program, treatment condition by clinical site interaction, F (1,335) = 4.84, p < .03. In simple effects tests of the interaction, at one clinical site, voucher conditions did not differ in days in residential treatment (89 days ± 23.56 days [standard deviation]), F (1,335) = 0.00, p = .97, but at the second clinical site participants in the NV condition stayed in residential treatment longer (188 days ± 96.60 days) than those in the CV condition (156 days ± 106.59 days), F (1,335) = 7.73, p < .01. When analyses of treatment effects were rerun including treatment site as a factor, no interactions with voucher condition were significant, so analyses were collapsed across site.
3.3 Confirmation of Abstinence during Follow-up
Of participants reporting 7-day smoking abstinence at any follow up, confirmed abstinence rates were 88% (29/33) at 1 month, 82% (14/17) at 3 months, 75% (9/12) at 6 months, and 90% (9/10) at 12 months. Of participants reporting abstinence from drugs at each follow-up, confirmed abstinence rates per urine screen were 95% (282/291) at 1 month, 98% (217/221) at 3 months, 97% (170/176) at 6 months and 98% (133/136) at 12 months.
3.4 Treatment Outcomes
Table 2 presents descriptive data, unadjusted effect size estimates and 95% confidence intervals (CI) for univariate differences between voucher conditions. Table 3 presents the results for GEE models for smoking outcomes, and Table 4 for substance use outcomes.
Table 3.
Analyses of Smoking Outcomes across 1, 3, 6 and 12 Months Follow Up by Voucher Condition and Time (GEE)
| Dependent Variable |
Predictor | Model coefficient |
SE | Wald | Effect Size d/OR |
95% CI |
|---|---|---|---|---|---|---|
| 7-day point-prevalence abstinence2 | ||||||
| CV (vs. NV) | 0.25 | 0.38 | 0.44 | 1.29 | 0.62, 2.67 | |
| Pre # cigarettes/day | <−0.01 | 0.02 | 0.15 | 0.99 | 0.94, 1.04 | |
| Time (Centered) | −0.61 | 0.24 | 6.75 | 0.54* | 0.34, 0.86 | |
| Time × CV | 0.24 | 0.29 | 0.67 | 1.27 | 0.72, 2.03 | |
| Number of cigarettes per day1 | ||||||
| CV (vs. NV) | −2.48 | 0.65 | 14.68 | −0.35* | −0.52, −0.18 | |
| Pre # cigarettes/day | 0.19 | 0.40 | 21.22 | 0.03* | 0.02, 0.04 | |
| Time (Centered) | 1.64 | 0.21 | 61.20 | 0.23* | 0.17, 0.29 | |
| Time × CV | 0.52 | 0.31 | 2.90 | 0.07 | −0.01, 0.16 | |
| Percent smoking days1 | ||||||
| CV (vs. NV) | −9.67 | 3.28 | 8.71 | −0.35* | −0.59, −0.12 | |
| Pre # cigarettes/day | 1.36 | 0.15 | 0.88 | −0.01 | −0.24, 0.00 | |
| Time (Centered) | 1.21 | 0.88 | 1.88 | 0.04 | −0.02, 0.11 | |
| Time × CV | 2.25 | 1.41 | 2.96 | 0.09 | −0.13, 0.19 | |
p < .01.
Note: CV = contingent vouchers condition; NV = noncontingent vouchers condition ; CI = confidence interval
Normally distributed variable; effect size is in standardized units of the dependent variable (d).
Dichotomous variable; effect size is expressed as an odds ratio (OR).
Table 4.
Analyses of Substance Use at Follow-up by Voucher Condition and Time (GEE) and Covarying Baseline Value of the Substance Use Variable
| Dependent Variable |
Predictor | Model coefficient |
SE | Wald | d or OR | 95% CI |
|---|---|---|---|---|---|---|
| Relapse to any heavy drinking2 | ||||||
| CV (vs. NV) | 0.09 | 0.33 | 0.35 | 1.09 | 0.57, 2.09 | |
| Pre heavy drinking | <0.01 | <0.01 | 32.24 | 1.01* | 1.00, 1.01 | |
| days | ||||||
| Time (Centered) | 0.64 | 0.33 | 46.60 | 1.89* | 1.58, 2.27 | |
| Time × CV | −0.08 | 0.13 | 0.34 | 0.92 | 0.71, 1.19 | |
| Relapse to any drug use2 | ||||||
| CV (vs. NV) | <−0.01 | 0.29 | <0.01 | 0.99 | 0.56, 1.78 | |
| Pre # drug use | <0.01 | <0.01 | 31.37 | 1.01* | 1.00, 1.01 | |
| days | ||||||
| Time (Centered) | 0.64 | 0.09 | 56.48 | 1.89* | 1.60, 2.24 | |
| Time × CV | 0.04 | 0.12 | 0.11 | 1.04 | 0.83, 1.31 | |
| Number of drug use days1 | ||||||
| CV (vs. NV) | 0.01 | 0.03 | 0.04 | 1.01 | 0.90, 1.14 | |
| Pre # drug use | 0.16 | 0.02 | 62.93 | 1.31* | 1.23, 1.40 | |
| days | ||||||
| Time (Centered) | 0.18 | 0.02 | 64.91 | 1.37* | 1.27, 1.48 | |
| Time × CV | <0.01 | 0.03 | 0.02 | 0.99 | 0.89, 1.10 | |
p < .01.
Note: CV = contingent vouchers condition; NV = noncontingent vouchers condition ; CI = confidence interval
Normally distributed variable; effect size is in standardized units of the dependent variable (d).
Dichotomous variable; effect size is expressed as an odds ratio (OR).
3.4.1 Smoking abstinence during abstinence contingency period by condition
During the 14-day abstinence-induction period after the reduction period, there was a main effect for voucher type on percent of CO readings indicating abstinence, t(338) = 6.35, p < .001, and on number of days when both readings indicated abstinence, t(338) = 6.60, p < .001. In CV, on average 57.4% ± 39.9% of CO readings were abstinent compared to 31.9% ± 34.1% in NV. In CV, on average 7.0 ± 5.7 days (50.0% ± 40.9% of days) showed abstinence (both CO readings abstinent) compared to 3.1 ± 4.6 days (22.0% ± 33.1% of days) in NV. In CV, 19.8% of participants (N = 34) were abstinent for all 28 readings compared to 5.4% in NV (N = 9), χ2 = 15.98, p < .001. Total amount earned within-treatment was $245.61 in CV, $281.68 in NV, t(333) = 2.74, p < .007.
3.4.2 Smoking abstinence at follow ups by voucher condition
Confirmed 7-day point-prevalence smoking abstinence averaged 8.5% (N = 29) at 1 month, 4.2% (N =14) at 3 months, 2.7% (N =9) at 6 months, and 2.7% (N = 9) at 12 months. The analysis was non-significant for voucher type and interaction with time but showed a decrease over time (Tables 2 for means and 3 for statistics).
3.4.3 Cigarettes per day and percent smoking days by condition
For both cigarettes per day and percent days smoking, significant effects were seen for voucher condition and time but not for the interaction (Table 3). Smoking rate was lower in CV than NV and increased over time, but statistical effect sizes were small (Tables 2 and 3).
3.4.4 Moderator analyses
Moderation analyses on smoking abstinence and on cigarettes per day during the post-treatment follow-up period entering gender, pretreatment minutes to first cigarette, or CL score as a factor were nonsignificant.
3.4.5 Substance use outcomes
Analyses of voucher condition effects on number of drug use days and number of participants with any relapse to drug or heavy alcohol use identified no main effect for voucher condition and no interaction with time but did indicate a significant time effect (Tables 2 for means and 4 for statistics). Substance use increased over time (Table 2).
3.5 Process Measures
3.5.1 Contemplation Ladder and Smoking Self-Efficacy
There were no significant effects for voucher condition, time, or interaction with time on CL score. There was a significant effect for voucher condition, F(1, 289) = 5.14, p = .02, and time, F(1, 289) = 370.37, p < .001 for the Smoking Self-Efficacy Questionnaire total score but no interaction with time (see Table 2 for means).
3.5.2 Use of NRT during first 3 months and follow-up
NRT was used on M = 16.9 ± 11.9 days during the voucher period with no significant difference by intervention condition. NRT was used on M = 27.5 days ± 22.7 days during the first 3 months after randomization, with no significant differences by condition. NRT was being used by 10% (n = 32) of participants at 6 months and 9% (n = 31) participants at 12 months (no significant differences by condition).
4.0 Discussion
When added to NRT and counseling, CV resulted in significantly more days abstinent from smoking during the voucher condition than did the NV, with 50% of days abstinent during CV compared to 22% of days without CV. By comparison, when using the same voucher system at one of the same sites without NRT (Rohsenow, Tidey, Martin, et al., 2015), only 25% of days were abstinent during CV and 5% of days with NV, showing that within-treatment abstinence rates with vouchers may be doubled by adding NRT. Similarly, the percent of participants with complete abstinence during the voucher period was 20% for CV and 5% for NV in the present study, compared to 6% and 1%, respectively, when using CV and counseling without NRT (Rohsenow, Tidey, Martin, et al., 2015). The effect of CV + NRT on abstinence in the current study was not due to higher use of NRT by participants in CV condition (i.e., to facilitate meeting abstinence criteria) since the conditions did not differ significantly in NRT use. Thus, adding NRT to CV with counseling results in considerably greater within-treatment abstinence for smokers with SUD than either CV or NRT alone.
This improvement in within-treatment abstinence rates did not result in improved abstinence outcomes at follow ups, however. The 7-day point prevalence abstinence (8.5% at 1 month, 4% at 3 months 3% at 6 and 12 months) did not differ significantly by voucher condition – only 2% more people were abstinent across time after CV than after NV. While CV resulted in significantly fewer cigarettes per day and percent days smoking across the follow-up period, the effects were small, averaging 9 versus 11 cigarettes per day and 84% versus 90% of days smoking for CV versus NV, respectively, effects not likely to be clinically meaningful. This is consistent with other studies of smokers with SUD where improved smoking abstinence within treatment did not result in significant differences in smoking abstinence at 6 or 12 months (reviewed by Thurgood, McNeill, Clark-Carter, & Brose, 2015). The small long-term effects of CV versus NV when combined with BA are consistent with our previous study where CV combined with BA resulted in significantly less point-prevalence abstinence over 12 months than CV combined with MI (Rohsenow, Tidey, Martin, et al., 2015), suggesting that long-term abstinence rates in the current study may have been higher if MI had been used for counseling.
Voucher conditions had no significant effect on degree of motivation to quit smoking or self-efficacy about quitting smoking. This is consistent with other research showing that CV has no harmful effects on intrinsic motivation to quit drugs (Ledgerwood & Petry, 2006) or to quit smoking (Rohsenow, Tidey, Martin, et al., 2015). On the other hand, the combined treatments did not improve self-reported motivation or self-efficacy either. Treatment effects on outcome did not differ by baseline motivation to quit smoking, gender, or degree of nicotine dependence, indicating that the combined treatment is appropriate regardless of these characteristics. Importantly, the CV had no significant effect on drug or alcohol use outcomes after treatment, consistent with a great many studies (reviewed by Rohsenow, 2015) that show no harmful effects on recovery of voluntary smoking treatments for smokers enrolled in SUD treatment.
Future directions should include using MI as the counseling platform and investigating CV combined with pharmacotherapies that may have stronger effects on smoking, such as varenicline or bupropion. For example, varenicline has shown to result in significantly higher rates of abstinence in smokers with SUD 3 and 6 months after starting it (Rohsenow et al., under review), suggesting that CV plus varenicline might be an effective way to increase both initial abstinence and maintenance of abstinence in smokers with SUD, particularly since varenicline reduces relapse rate in smokers attaining initial abstinence (Evins et al, 2014; Tonstad et al, 2006). Since higher levels of withdrawal discomfort predict less smoking abstinence during and after CV for smokers with SUD (Rohsenow, Tidey, Kahler, Martin, Colby, & Sirota, 2015), combining voucher-based smoking treatments with other pharmacotherapies that reduce craving or withdrawal are likely to increase within-treatment abstinence during CV, as the present results suggest.
Limitations
Participants did not need to be motivated for smoking cessation to enroll in this smoking treatment, since the purpose was to use SUD treatment as an opportunity to motivate smoking cessation. Participants’ motivation level was typical of smokers engaged in SUD treatment (Flach & Diener, 2004; Monti et al., 1995), suggesting that the current results will generalize to other smokers in treatment for SUD. However, the combined smoking treatment could be more effective for smokers ready to quit smoking. Results are limited to inner-city smokers with low income in these two treatment centers and might differ in other smokers with SUD.
Conclusions
Within-treatment smoking abstinence was greater with CV than NV, and about double the rates seen in a similar study without NRT (Rohsenow, Tidey, Martin, et al., 2015), suggesting that the NRT improved the effects of CV on short-term smoking abstinence. However, the long-term effects of CV on smoking abstinence were not significant, suggesting that this combination of treatments does not result in lasting effects on tobacco abstinence. Since CV has been shown to have more lasting effects when combined with MI than with BA, adding a stronger pharmacotherapy to the combination of CV and MI would be worth considering.
Highlights.
We provided vouchers contingent on smoking abstinence or noncontingent ones for smokers in drug treatment.
We also provided nicotine patch and brief advice to quit smoking to all the smokers.
More smoking abstinence occurred during treatment with contingent vouchers than without.
Smoking abstinence during treatment was twice as much as in a study of vouchers without patch.
In the year after vouchers, vouchers reduced amount of smoking but did not increase quitting.
Acknowledgments
Supported by 1 R01 DA023995 from the National Institute on Drug Abuse; a Senior Career Research Scientist Award from the Department of Veterans Affairs to the first author; and K05AA019681 from the National Institute on Alcohol Abuse and Alcoholism. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the official views of the National Institutes of Health. Grateful appreciation is expressed to Suzanne Sales for her data analyses and to the clinical and administrative staff of The Providence Center and Gateway Healthcare, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work was performed at: Brown University Center for Alcohol and Addiction Studies, Gateway Healthcare Systems in Woonsocket, RI, and The Providence Center in Providence, RI.
Preliminary results were presented at the annual meetings of the Research Society on Alcoholism, San Francisco, CA, June 2012; of the College on Problems in Drug Dependence, San Diego, CA, June 2013; of the Society for Research on Nicotine and Tobacco - Europe, Santiago de Campostela, Spain, September 2014; and of the College on Problems in Drug Dependence, Phoenix, AZ, June 2015.
References
- Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a RCT of smoking abstinence-contingent incentives in residential substance abuse treatment patients. Nicotine and Tobacco Research. 2014;16:1436–1445. doi: 10.1093/ntr/ntu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. Journal of Applied Behavioral Analysis. 2008;41:617–622. doi: 10.1901/jaba.2008.41-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher MK, Martin RA, Rohsenow DJ, MacKinnon SV, Traficante R, Monti PM. Perceived barriers to quitting smoking among alcohol dependent patients in treatment. Journal of Substance Abuse Treatment. 2003;24:169–174. doi: 10.1016/s0740-5472(02)00354-9. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Cotinine disposition and effects. Clinical and Pharmacology Therapeutics. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- Bien TH, Burge R. Smoking and drinking: A review of the literature. International Journal of Addictions. 1991;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Burling TA, Ramsey TG, Seidner AL, Kondo CS. Issues related to smoking cessation among substance abusers. Journal of Substance Abuse. 1997;9:27–40. doi: 10.1016/s0899-3289(97)90004-3. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence using cotinine as reference. Nicotine & Tobacco Research. 2014;16:1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. American Journal of Public Health. 1988;78:574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavioral Analysis. 2008;41:527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Experimental and Clinical Psychopharmacology. 2010;18:37–50. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, Achtyes ED, Ayer D, Schoenfeld DA. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. Journal of the American Medical Association. 2014;311:145–54. doi: 10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Washington, DC: Public Health Service: USDHHS; 2000. [Google Scholar]
- Flannery BA, Allen JP, Pettinati HM, Rohsenow DJ, Cisler RA, Litten RZ. Using acquired knowledge and new technologies in alcoholism treatment trials. Alcoholism: Clinical and Experimental Research. 2002;26:423–429. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Flach SD, Diener A. Eliciting patients’ preferences for cigarette and alcohol cessation: An application of conjoint analysis. Addictive Behaviors. 2004;29:791–799. doi: 10.1016/j.addbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Retrieved from http://www.afhayes.com/public/process2012.pdf.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org.cochrane-handbook.org. [Google Scholar]
- Hollis JF, Lichenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Annals of Internal Medicine. 1993;118:521–525. doi: 10.7326/0003-4819-118-7-199304010-00006. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Treatment of smoking cessation in smokers with past alcohol/drug problems. Journal of Substance Abuse Treatment. 1993;10:181–187. doi: 10.1016/0740-5472(93)90043-2. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addictive Disorders Treatment. 2010;9:64–74. [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Irving LM, Seidner AL, Burling TA, Thomas RG, Brenner GF. Drug and alcohol inpatients’ attitudes about smoking cessation. Journal of Substance Abuse. 1994;6:267–278. doi: 10.1016/s0899-3289(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking interventions for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2005;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for alcoholics: A pilot study. Journal of Substance Abuse Treatment. 2001;20:233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral R, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology. 2010;78:62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug and Alcohol Dependence. 2006;83:65–72. doi: 10.1016/j.drugalcdep.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Manley M, Epps RP, Husten C, Glynn T, Shopland D. Clinical interventions in tobacco control. A National Cancer Institute training program for physicians. Journal of the American Medical Association. 1991;266:3172–3173. [PubMed] [Google Scholar]
- Martin RA, Rohsenow DJ, MacKinnon SV, Abrams DA, Monti PM. Correlates of motivation to quit smoking among alcohol dependent patients in residential treatment. Drug and Alcohol Dependence. 2006;83:73–78. doi: 10.1016/j.drugalcdep.2005.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RA, Cassidy R, Murphy C, Rohsenow DJ. Barriers to quitting smoking among substance dependent patients predict outcome. Journal of Substance Abuse Treatment. doi: 10.1016/j.jsat.2016.02.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd. New York: Guilford Press; 2002. [Google Scholar]
- Moliterno DJ, Willard JE, Lange RA, Negus BH, Boehrer JD, Glamann DB, Landau C, Rossen JD, Winniford MD, Hillis LD. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. New England Journal of Medicine. 1994;330:454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies and policy. In: Fertig JB, Allen JP, editors. Alcohol and tobacco: From basic science to clinical practice. Research Monograph 30, National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 187–206. [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Crone CC, Whiteside-Mansell L, Conners NA, Bokony PA, Worley LLM, McMillan DE. Voucher-based incentives for cigarette smoking reduction in a women’s residential treatment program. Nicotine & Tobacco Research. 2005;7:111–117. doi: 10.1080/14622200412331328448. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ. The Addictions Newsletter. Spring; 2015. Why we should treat smoking in substance dependence programs; pp. 19–21. [Google Scholar]
- Rohsenow DJ, Martin RA, Tidey JW, Monti PM, Colby SM. Comparison of the Cigarette Dependence Scale with four other measures of nicotine involvement: Correlations with smoking history and smoking treatment outcome in smokers with substance use disorders. Addictive Behaviors. 2013;38:2409–2413. doi: 10.1016/j.addbeh.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Kahler CW, Martin RA, Colby SM, Sirota AD. Intolerance for withdrawal discomfort and motivation predict voucher-based smoking treatment outcomes for smoker with substance use disorders. Addictive Behaviors. 2015;43:18–24. doi: 10.1016/j.addbeh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Monti PM, Colby SM, Day AM, Abrams DB, Sirota AD, Swift RM. Motivational interviewing versus brief advice for cigarette smokers in residential alcohol treatment. Journal of Substance Abuse Treatment. 2014;46:346–355. doi: 10.1016/j.jsat.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, Monti PM. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: Effects on smoking, substance use and depressive symptoms. doi: 10.1111/add.13861. (under review) [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Sirota AD, Swift RM, Monti PM. Contingent vouchers and motivational interviewing for cigarette smokers in residential substance abuse treatment. Journal of Substance Abuse Treatment. 2015;55:29–38. doi: 10.1016/j.jsat.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: A feasibility study. Experimental Clinical Psychopharmacology. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR. Sensitivity analysis in observational studies. Encyclopedia of Statistics in Behavioral Science. 2005;4:1809–1814. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- SAS/STAT User’s Guide: Version 12.3. Cary, NC: SAS Institute; 2013. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. Journal of Substance Abuse Treatment. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Seidner AL, Burling TA, Gaither DE, Thomas RG. Substance-dependent inpatients who accept smoking treatment. Journal of Substance Abuse. 1996;8:33–44. doi: 10.1016/s0899-3289(96)90067-x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Patrick MD. The use of financial incentives in promoting smoking cessation. Preventive Medicine. 2012;55(Suppl):S24–S32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Can we do without alcohol abusers’ self-reports? The Behavior Therapist. 1986;7:141–146. [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: An approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Elmsford, NY: Pergamon Press; 1980. pp. 177–183. [Google Scholar]
- Strecher VJ, Rimer BK, Monaco KD. Development of a new self-help guide - Freedom From Smoking® For You and Your Family. Health Education Quarterly. 1989;16:101–112. doi: 10.1177/109019818901600111. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz P, Carbonari JP, Boca FD. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;(Supplement No. 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th. Boston, MA: Allyn & Bacon; 2007. [Google Scholar]
- Thurgood SL, McNeill A, Clark-Carter D, Brose LS. A systematic review of smoking cessation interventions for adults in substance abuse treatment or recovery. Nicotine and Tobacco Research. 2015 doi: 10.1093/ntr/ntv127. Advance on-line publication July 22, 2015. http://ntr.oxfordjournals.org/content/early/2015/06/10/ntr.ntv127. [DOI] [PMC free article] [PubMed]
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, for the Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:272–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services USDHHS. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Washington, DC: Public Health Service; 2000. [Google Scholar]
- Wiseman EJ, Williams DK, McMillen DE. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine-abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology. 2005;13:102–110. doi: 10.1037/1064-1297.13.2.102. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]