Abstract
Macular xanthophylls (MXs) lutein and zeaxanthin are dietary carotenoids that are selectively concentrated in the human eye retina, where they are thought to protect against age-related macular degeneration (AMD) by multiple mechanisms, including filtration of phototoxic blue light and quenching of singlet oxygen and triplet states of photosensitizers. These physical protective mechanisms require that MXs be in their intact structure. Here, we investigated the protection of the intact structure of zeaxanthin incorporated into model membranes subjected to oxidative modification by water- and/or membrane-soluble small nitroxide free radicals. Model membranes were formed from saturated, monounsaturated, and polyunsaturated phosphatidylcholines (PCs). Oxidative modification involved autoxidation, iron-mediated, and singlet oxygen-mediated lipid peroxidation. The extent of chemical destruction (bleaching) of zeaxanthin was evaluated from its absorption spectra and compared with the extent of lipid peroxidation evaluated using the thiobarbituric acid assay. Nitroxide free radicals with different polarity (membrane/water partition coefficients) were used. The extent of zeaxanthin bleaching increased with membrane unsaturation and correlated with the rate of PC oxidation. Protection of the intact structure of zeaxanthin by membrane-soluble nitroxides was much stronger than that by water-soluble nitroxides. The combination of zeaxanthin and lipid-soluble nitroxides exerted strong synergistic protection against singlet oxygen-induced lipid peroxidation. The synergistic effect may be explained in terms of protection of the intact zeaxanthin structure by effective scavenging of free radicals by nitroxides, therefore allowing zeaxanthin to quench the primary oxidant, singlet oxygen, effectively by the physical protective mechanism. The redox state of nitroxides was monitored using electron paramagnetic resonance spectroscopy. Both nitroxide free radicals and their reduced form, hydroxylamines, were equally effective. Obtained data were compared with the protective effects of α-tocopherol, which is the natural antioxidant and protector of MXs within the retina. The new strategies employed here to maintain the intact structure of MXs may enhance their protective potential against AMD.
Keywords: zeaxanthin, carotenoid, oxidative stress, lipid peroxidation, antioxidants, AMD
1. Introduction
Many epidemiological studies suggest that the high consumption of lutein and zeaxanthin is associated with a lower risk of age-related macular degeneration (AMD) [1–3]. Only these two carotenoids are selectively accumulated in the membranes of the retina from blood plasma, where more than 20 other carotenoids are available [4, 5]. Another carotenoid, meso-zeaxanthin (which is a stereoisomer of zeaxanthin), is converted from lutein within the retina [6]. These carotenoids, named macular xanthophylls (MXs), are accumulated primarily in the region of photoreceptor axons [7, 8] but also are detected within photoreceptor outer segments [9, 10] and in the retinal pigment epithelium (RPE) [11]. They are thought to combat light-induced damage mediated by reactive oxygen species by absorbing the most damaging incoming wavelength of light prior to forming reactive oxygen species (a function expected of carotenoids in axons) and by chemically and physically quenching reactive oxygen species once they are formed (a function expected of carotenoids in photoreceptor outer segments and retinal pigment epithelium).
Two main functions explain the selective presence of MXs in the retina. One functional hypothesis states that MXs, due to their appropriate location mostly in the outer plexiform layer [7, 8], form a filter for blue light and block hazardous light before it reaches the potential photosensitizers responsible for photodynamic damage to the retina. Blue-light absorption can be considered an indirect antioxidant action because it prevents the generation of reactive oxygen species that can damage retinal cells. In fact, most ultraviolet light below 300 nm is absorbed by the cornea [12], whereas ultraviolet light in the range of 300 to 400 nm is blocked by the lens. Nevertheless, some fraction of short wavelength blue radiation reaches the retina and may activate endogenous retinal photosensitizers. Another function of MXs is connected with the necessity of protection against oxidative stress, because oxidative stress, in addition to aging, seems to be a major determinant in the pathogenesis of AMD [13]. MXs may act directly as lipid-soluble antioxidants in retina membranes. The antioxidant role of carotenoids involves both quenching of singlet oxygen and scavenging of free radicals. Unfortunately, the same properties that make carotenoids as beneficial as antioxidants also make them highly susceptible to oxidation. In this study, we evaluate how several types of nitroxide free radicals (synthetic antioxidants) may protect macular carotenoids against chemical degradation.
Carotenoids are known to be effective singlet oxygen quenchers, and their activities are much higher than those of another retinal antioxidant α-tocopherol [14, 15]. Singlet oxygen quenching by carotenoids in organic solvents mainly depends on the carotenoid’s triplet state energy level and, thus, on the number of conjugated double bonds. Zea, with 11 conjugated double bonds, is an extremely efficient quencher. At room temperature, the singlet oxygen quenching rate constant for Zea in benzene has been found to be 1.2×1010 M−1 s−1 [16]. The rate constant for lutein, with 10 conjugated double bonds, is two times smaller (~0.66×1010 M−1 s−1). MXs are also capable of quenching excited triplet states of singlet oxygen photosensitizers such as all-trans-retinal [17, 18], retinyl ester [19], all-trans-retinol [20], cytochrome c oxidase [21], porphyrins [22], melanin [23, 24], and lipofuscin [25, 26]. Carotenoids can quench singlet oxygen by two different mechanisms. The first mechanism, involving energy transfer and termed physical quenching, is considered the major pathway of singlet oxygen deactivation. According to this mechanism, carotenoid molecules deactivate singlet oxygen to the less reactive triplet ground state. During that process, carotenoid molecules become excited to the triplet state and can return to the ground state, dissipating the energy excess as heat. The benefit of physical quenching is that carotenoids may act without alteration of their own chemical structure. The second mechanism is called chemical quenching. It involves a chemical reaction between carotenoids and singlet oxygen that results in pigment oxidation. This latter process consumes the carotenoids themselves. Chemical quenching has been reported to contribute less than 0.05% to the overall singlet oxygen quenching by carotenoids [27]. Several carotenoid oxidation products have been found in the retina [11, 28], indicating that this mechanism can take place in vivo and is responsible for destruction of the pigment molecule.
The conjugated double bond system is primarily responsible for the high chemical reactivity of carotenoids with both singlet oxygen [14, 29] and free radicals [30–32]. The selective localization of MXs in domains rich in polyunsaturated phospholipids [33–35], and therefore susceptible to free radical- and singlet oxygen-induced damage, is ideal for their chemical antioxidant action. The scavenging of lipid-derived peroxyl radicals is also an important antioxidant activity of MXs. Carotenoids scavenge lipid peroxyl radicals by forming radical adducts [30], which are less reactive than lipid alkyl peroxyl radicals. Thus, carotenoids are also reported as chain-breaking antioxidants, which delay the oxidation of biomembranes by trapping chain-initiating or chain-propagating peroxyl radicals. This chemical reaction leads to the destruction of carotenoid molecules. The mechanism by which partially consumed carotenoids are replaced or repaired in the human retina is poorly understood.
Both the physical and chemical stability of MXs in the retina are significant factors that allow them to perform their protective action effectively and for a prolonged time. Physical stability is manifested by their very slow removal from the retina, observed after discontinuation of xanthophyll supplementation [36]. MXs are also degraded more slowly than other dietary carotenoids such as carotenes and, thus, are chemically more stable [37, 38]. As indicated above, most of the protective actions of MXs in the retina are performed through their physical protective mechanisms, which require that MXs be in their intact structure. Thus, it is timely and important to identify mechanisms whereby the chemical destruction (bleaching) of MXs in retinal membranes can be diminished.
Several studies have postulated that zeaxanthin and α-tocopherol provide synergistic protection of the human retina against chronic oxidative damage caused by photo-induced lipid peroxidation in photoreceptor and RPE cell membranes [39, 40]. The proposed mechanism is the inhibition of xanthophyll consumption by α-tocopherol. One could expect that nitroxide free radicals could act in a similar way by sparing zeaxanthin and allowing it to efficiently quench singlet oxygen.
Lipid soluble nitroxide free radicals can be considered synthetic antioxidants. They can act as chain-breaking antioxidants, inhibiting the formation of lipid alkyl radicals. Being radicals themselves, they react with other radicals, which leads to the termination of radical chain reactions. Both nitroxide free radicals and their reduced forms, EPR-silent hydroxylamines, can react with oxygen-centered and carbon-centered radicals and break the radical chain reaction. Nitroxides, in contrast to other antioxidants such as carotenoids and α-tocopherol, do not act as prooxidants. (See Ref. [41] for a discussion of the prooxidant activities of carotenoids.) They are not depleted during their antioxidant action because their reduction products (hydroxylamines) are also effective antioxidants [42]. It has been reported that carotenoids are less effective in trapping peroxyl radicals then α-tocopherol [43]. On the other hand, lipid-soluble nitroxides provided better protection to polyunsaturated membranes than α-tocopherol [44, 45]. The recycling mechanism of antioxidant action of nitroxides through oxoammonium cations and hydroxylamines seems to be a great advantage as compared with traditional antioxidants: carotenoids and α-tocopherol [46].
Here, we investigated how membrane- and/or water-soluble nitroxide free radicals (spin labels) protect the intact structure of a representative MX, zeaxanthin (Zea), incorporated into model membranes subjected to lipid peroxidation. We compared these data to the protective effect of α-tocopherol, a natural antioxidant within the retina. We also tested whether we could detect a synergistic, inhibitory effect on lipid peroxidation when nitroxides and Zea act together. We theorize that new strategies, like the one employed here, aimed at maintaining the intact structure of MXs during oxidative stress, which is implicated in AMD pathogenesis, should enhance their protective potential.
2. Materials and Methods
2.1. Reagents
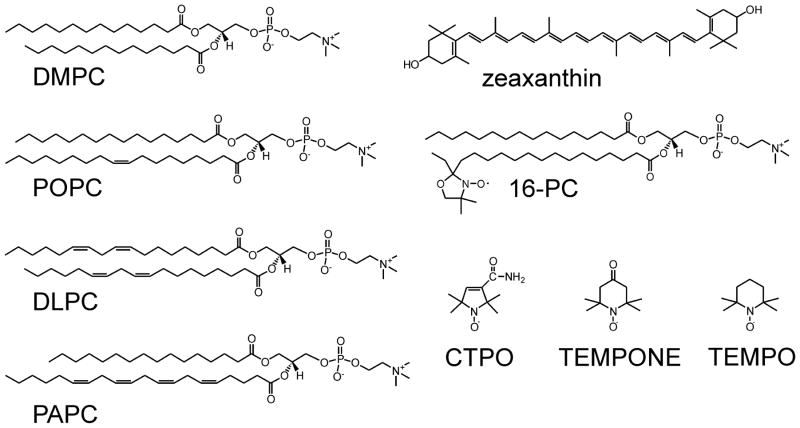
Dilinoleoylphosphatidylcholine (DLPC), dimyristoylphosphatidylcholine (DMPC), 1-palmitoyl-2-arachidonoylphosphochatidyline (PAPC), 1-palmitoyl-2-oleoylphosphatidylcholine (POPC), and 1-palmitoyl-2-(16-doxylstearoyl)phosphatidylcholine (16-PC) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Zea was purchased from CaroteNature (Lupsingen, Switzerland), malondialdehyde (MDA) was obtained from Cayman Chemical (Ann Arbor, MI). CTPO (3-Carbamoyl-2,2,5,5-tetramethyl-3-pyrrolin-1-oxyl), TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl), TEMPONE (2,2,6,6-tetramethylpiperidone-N-oxyl), and other chemicals, of at least reagent grade, were purchased from Sigma-Aldrich Co. (St. Louis, MO).
2.2. Preparation of liposomes
Multilamellar vesicles (liposomes) were prepared by the film deposition method: appropriate amounts of all reagents were dissolved in chloroform and mixed; then, chloroform was evaporated with a stream of nitrogen gas for about 20 minutes to form a lipid film on the bottom of the test tube. The lipid film was thoroughly dried under reduced pressure (about 0.1 mm Hg) for 12 hours. A measured volume of PIPES buffer pH 7 was added to the dried film and vortexed vigorously for about 3 minutes, until a uniform suspension of lipids was obtained. As required for the experiment, liposomes contained appropriate amounts (reported with the results) of Zea, α-tocopherol, and/or various spin labels. Zea, α-tocopherol, and the nonpolar nitroxide (16-PC) were added as chloroform solutions and dried along with the phospholipids. Polar nitroxides (CTPO, TEMPO, and TEMPONE) were dissolved in water and added to PIPES buffer before resuspending the lipid film.
2.3. Oxidation of phospholipids
The oxidation of phospholipids was carried out using several methods: autoxidation–samples were kept in dim light, iron-induced lipid peroxidation–peroxidation was initiated by the addition of iron ions in the form of a hydrophobic complex (ferric-8-hydroxyquinoline [Fe(HQ)3]) at a concentration of 10 μM) prepared according to Korytowski’s method [47], and photosensitizer-induced reaction–peroxidation was initiated with singlet oxygen generated using the photosensitizer rose bengal. For the latter, samples containing 10 μM rose bengal were irradiated with green light using a fluorescent lamp at a power of 0.28 mW/cm2 and a dark yellow green LEE filter (No. 090, LEE Filters, Andover, UK). Since the spontaneous autoxidation depends on small amounts of lipid hydroperoxides and contaminating metal ions in commercial lipids, great care was undertaken to use the same lipid stock solutions for the connected series of experiments. Stock solutions were kept frozen under argon.
At appropriate time intervals, 25 μl aliquots were collected and frozen for further analysis of lipid peroxidation products. From samples containing Zea, 25 μl aliquots were collected at the same intervals for quantification of Zea. All experiments were carried out at room temperature; samples were equilibrated with atmospheric air.
2.4. Measurement of zeaxanthin degradation
Samples containing Zea were extracted with 150 μl of chloroform. UV/VIS spectra of Zea (350 to 600 nm) were collected using a Beckman Coulter DU 800 spectrophotometer (Beckman Coulter, Inc., Brea, CA). Absorbance at 460 nm was used to determine Zea content.
2.5. Reduction and reoxidation of 16-PC nitroxide; EPR measurements
In order to produce the hydroxylamine form of 16-PC nitroxide, we developed a method in which sodium ascorbate was used as a reducing agent. Using this method, 20 μl of a 1 mM chloroform solution of 16-PC was taken, and chloroform was evaporated under a stream of nitrogen. 16-PC was dissolved in 133 μl of absolute ethanol and mixed with 100 μl of 100 mM sodium ascorbate (or water for the control sample) by vigorous shaking. After 30 minutes, 150 μl of water and 267 μl of chloroform were added, and the mixture was shaken vigorously for 1 minute to extract the 16-PC. The sample was spun down for better separation of the phases, and the organic phase was transferred to a new tube. An aliquot was taken for the electron paramagnetic resonance (EPR) measurement, to confirm the reduction of the characteristic signal of the nitroxide free radical. The amplitude of the EPR signal was reduced to less than 10% of the control. To confirm that the resulting product can undergo reoxidation, we subjected it to a similar procedure with 2 mM potassium ferricyanide used in place of ascorbate. Using this protocol, we confirmed the complete recovery of the characteristic EPR signal.
The EPR spectra were recorded with a Bruker EMX spectrometer. All spectra were collected at room temperature with a modulation amplitude of 1.0 G and an incident microwave power of 5.0 mW.
2.6. Measurement of lipid peroxidation
The accumulation of lipid peroxidation products was measured using a thiobarbituric acid (TBA) assay. Briefly, 25 μl aliquots of liposome suspension that had been frozen were thawed by the addition of 40 μl of 20% TCA containing 0.5 mM BHT (to prevent further oxidation). Samples were centrifuged, 55 μl of the supernatant was transferred to fresh microcentrifuge tubes containing 60 μl of 0.5% TBA, and samples were incubated at 95° C for 30 minutes. After cooling on ice, the absorbance of the samples at 532 nm was measured. The concentration of TBA reactive substances (TBARS) was determined using malondialdehyde as a standard.
2.7. Statistical analysis
In order to estimate the experimental error, standard deviation was calculated from 2 or 3 replicates as indicated in figure legends.
3. Results and Discussion
3.1. The rate of zeaxanthin degradation (bleaching) increased with the degree of PC unsaturation
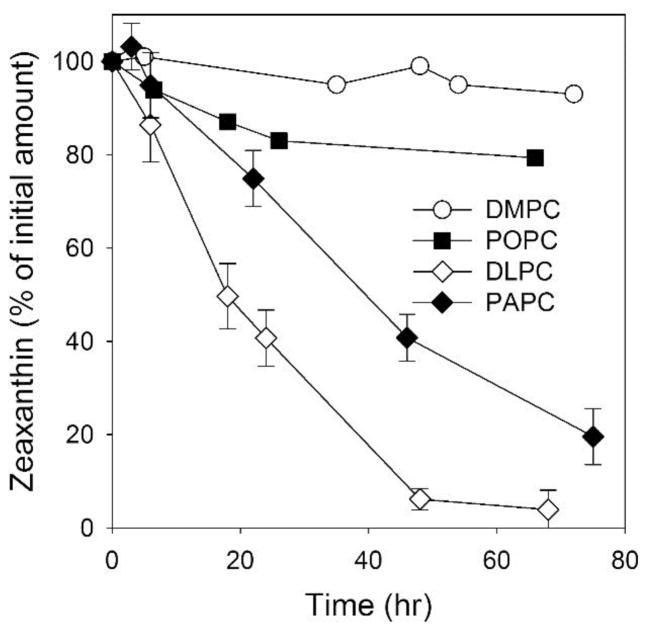
Degradation of the intact structure of Zea is accompanied by the disappearance of its characteristic UV/VIS spectrum, which makes a convenient and sensitive spectrophotometric method of monitoring the bleaching of MXs. We used this approach (see Sect. 2.4 for details) to compare the rates of Zea degradation during lipid peroxidation of membranes made of phospholipids with different degrees of unsaturation. Zea was incorporated into liposomes made of DMPC (saturated PC), POPC (monounsaturated PC), and DLPC and PAPC (polyunsaturated PCs) at a Zea/PC molar ratio of 1/100. For this comparison, we chose to use autoxidation of PCs as a simple model. As shown in Fig. 2, Zea was completely depleted in DLPC membranes after 50 hours and, after the same time frame, by only 60% in PAPC. By contrast, after 50 hours of autoxidation, the amount of Zea decreased only by ~18% in POPC membranes and by less than 3% in DMPC membranes. The fast rate of Zea degradation was observed only in polyunsaturated PC membranes that undergo effective lipid peroxidation.
Fig. 2.
Bleaching of Zea (25 μM) in model membranes made of saturated (DMPC), monounsaturated (POPC), and polyunsaturated (DLPC and PAPC) phospholipids during their autoxidation. The concentration of all phospholipids was 2.5 mM. Error bars, when they are present, represent SD. n=3.
The number of double bonds in one phospholipid molecule of DLPC and PAPC remains the same, namely four. In the DLPC molecule, two conjugated double bonds are in each linoleic acyl chain. In the PAPC molecule, four conjugated double bonds are accumulated in one acyl chain (arachidonic), while the second chain (palmitic) is fully saturated. Lipid peroxidation is initiated by a reactive oxygen species-mediated hydrogen abstraction from a bis-allylic site within a polyunsaturated fatty acid chain. The DLPC molecule has four such hydrogens and PAPC has six; hence, the former should, in theory, undergo slower oxidation than the latter. In our system, however, we observed slower decay of Zea in liposomes made of PAPC. We speculate that, in the PAPC membranes, the separation of polyunsaturated arachidonic acyl chains by saturated palmitic acyl chains decreases the propagation of lipid peroxidation and slows the rate of Zea degradation. Consumption of Zea is most likely a result of its chemical interaction with free radicals generated during the oxidation of DLPC and PAPC. After reacting with a free radical, the Zea molecule becomes a free radical itself. A series of secondary reactions can occur that eventually lead to the degradation of the Zea molecule.
3.2. Only membrane-located nitroxides effectively protect zeaxanthin from degradation in polyunsaturated PC
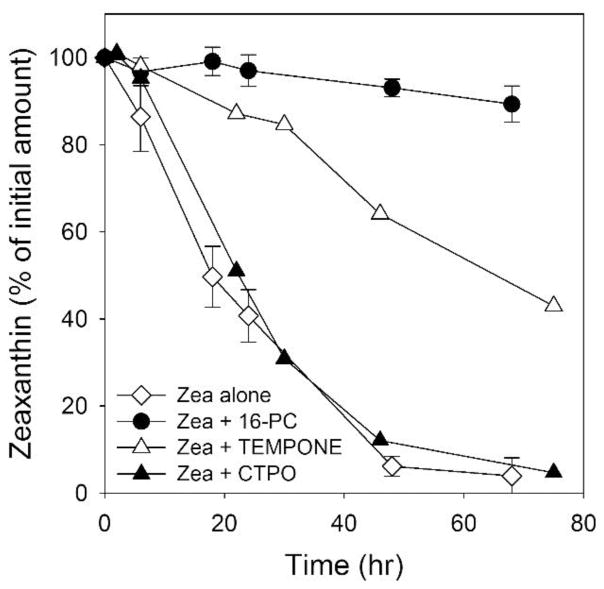
Our previous work using lipid spin labels to study interactions of xanthophylls with model membranes led us to suspect that, during long experiments, the presence of nitroxide free radicals in the membranes may prevent xanthophyll bleaching [33, 34]. Here, we evaluate this possibility, since the resulting information could prove useful in developing strategies to protect the intact MXs structure in situ in the retina. To ensure that the prevention of xanthophyll bleaching occurs exclusively in the lipid bilayer, we tested the effects of nitroxide free radicals with different polarity (i.e., with different partitioning into the lipid bilayer) on the bleaching of Zea during the autoxidation of DLPC (Fig. 3). The most polar spin label CTPO, located mainly in the aqueous phase, had virtually no effect on the rate of bleaching of Zea. TEMPONE, which is soluble in water but which (due to its chemical structure) can also penetrate the nonpolar regions of lipid membranes, can provide partial protection of Zea. The third nitroxide tested in our system was the lipid-soluble spin label 16-PC, which can almost completely protect Zea from degradation. To detect the weak effects of water soluble nitroxides required approximately tenfold higher concentrations than for the membrane-located 16-PC.
Fig. 3.
Bleaching of Zea (25 μM) in DLPC membranes subjected to autoxidation in the absence and presence of CTPO (250 μM), TEMPONE (250 μM), and 16-PC (25 μM). Error bars, when they are present, represent SD. n=3. Data for Zea alone plot are the same as in Fig. 2.
In 16-PC, the nitroxide free radical moiety is attached to the C16 carbon of the stearic chain of the molecule (see Fig. 1), which ensures that it is located inside the lipid bilayer. However, because of the vertical fluctuation and bending of the acyl chain, this moiety is also present at all depths, from the membrane surface to the membrane center [48, 49]. The results presented here indicate that nitroxide free radicals effectively protect Zea, which is transversely located in the lipid bilayer [50], only when they are located also in the lipid bilayer. To standardize our conditions throughout all subsequent experiments, we kept the 16-PC/PC ratio constant, namely at 1/100.
Fig. 1.
Chemical structures of phospholipids and nitroxide free radicals (spin labels) used in these studies.
3.3. Both nitroxide free radicals and their reduced forms, hydroxylamines, were equally effective in protecting against degradation of zeaxanthin
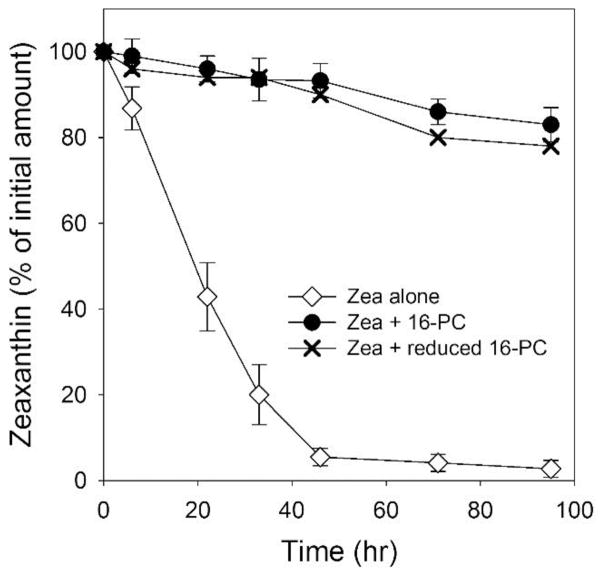
Nitroxide free radicals are very effective scavengers of other free radicals by accepting their unpaired electron. Because of that, they are good chain-breaking compounds during lipid peroxidation [51–53]. After accepting an electron, nitroxides become EPR-silent hydroxylamines. This is a reversible reaction, and a hydroxylamine can give its electron to another free radical in the lipid peroxidation chain. In this way, nitroxides can work in the cycle as one-electron acceptors (being reduced to hydroxylamines), and hydroxylamines as one-electron donors (being oxidized to nitroxides). We tested if this reversible redox process can explain the high effectiveness of nitroxides in the protection of Zea during lipid peroxidation. As shown in Fig. 4, both the nitroxide 16-PC and its reduced form (its hydroxylamine form prepared as described in Sect. 2.5) were equally effective in protecting Zea against degradation during the autoxidation of DLPC. This very promising result indicates that nitroxides are not consumed during that process and can be effective protectors of the intact Zea structure for a prolonged time. When 16-PC protected Zea during DLPC autoxidation, its EPR signal intensity decreased to about 80% of its initial intensity. When the reduced form of 16-PC (its hydroxylamine) protected Zea, the EPR signal (coming from 16-PC) increased to about 80% of the value predicted if all reduced 16-PC returned to the EPR active 16-PC. Both reactions are slower than the bleaching of Zea, and the steady-state level (80% of the EPR signal intensity) is reached after more than 100 hours of autoxidation. These data indicate that 16-PC is involved in the reversible one electron reduction/oxidation process. Here, it should be noted that molecular oxygen, as a two-electron acceptor, is not an effective oxidant for hydroxylamines [54].
Fig. 4.
Degradation of Zea (25 μM) during autoxidation of DLPC membranes in the absence and presence of 16-PC (25 μM) or its reduced form (hydroxylamine, 25 μM). Error bars, when they are present, represent SD. n=3.
3.4. Only for singlet oxygen-mediated lipid peroxidation did membrane-located nitroxides protect zeaxanthin more effectively than α-tocopherol
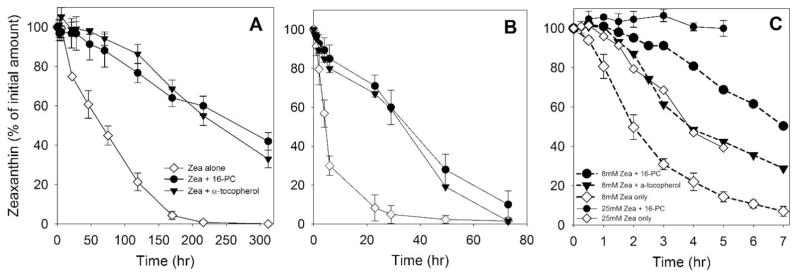
To clarify the mechanism by which membrane-located nitroxides (16-PC) protect the intact structure of Zea, we have to compare the degree of Zea degradation with the degree of lipid peroxidation. To accomplish this, in following experiments we used PAPC instead of DLPC. This PC contains four conjugated double bonds in one acyl chain, which allowed the use of the TBA assay to quantify total phospholipid peroxidation products as TBARS (see Sect. 2.6). In addition to autoxidation, we induced lipid peroxidation in the dark directly in the bilayer by the hydrophobic iron complex [Fe(HQ)3] and by singlet oxygen generated in the photosensitized reactions with rose bengal (see Sect. 2.3). In this section, we focused on the degradation of Zea in PAPC membranes subjected to lipid peroxidation induced by the three methods discussed in Sect. 2.3. We also compared the protection provided by 16-PC with that provided by α-tocopherol.
As indicated in Fig. 5A, we observed the slow bleaching of Zea incorporated into PAPC liposomes subjected to autoxidation. Both 16-PC and α-tocopherol slowed this process significantly, and their effects were nearly the same. Autoxidation is a relatively slow process driven, most likely, by traces of redox-active metal ions that initiate lipid peroxidation by reacting with preexisting lipid hydroperoxides [55]. In order to accelerate this process, we supplied our model system with external iron in the form of the hydrophobic [Fe(HQ)3] complex (see Sect. 2.3). Ferric hydroxyquinoline is a convenient initiator of lipid peroxidation as it can easily penetrate into lipid membranes and react with preexisting lipid hydroperoxides; this starts a free radical chain reaction [47]. The addition of 10 μM external iron to the suspension of PAPC liposomes increased the rate of Zea degradation about tenfold (Fig. 5B). Although the rate of oxidative reactions increased markedly as compared with the autoxidative process, the effects of antioxidants 16-PC and α-tocopherol were almost the same as during autoxidation (when comparing the percentage of the protected Zea). Again, 16-PC and α-tocopherol slow the bleaching of Zea very similarly (Fig. 5B).
Fig. 5.
Bleaching of Zea located in membranes made of polyunsaturated phospholipid (PAPC, 2.5 mM) during their peroxidation in the absence and presence of spin label 16-PC (25 μM) or α-tocopherol (25 μM). Lipid peroxidation was induced using three different methods: A – autoxidation, in the presence of 25 μM Zea; B - peroxidation induced by a hydrophobic complex of Fe (10 μM) in the presence of 25 μM Zea, and C - peroxidation induced by rose bengal (10 μM) and green light at two different concentrations of Zea - 25 μM (solid lines) or 8 μM (dashed lines). Error bars, when they are present, represent SD. In A n=3, in B and C n=2.
Fig. 5C shows the results of experiments in which we monitored the degradation of Zea during the singlet oxygen-induced peroxidation of PAPC membranes. In this case, with the rate of bleaching of Zea similar to that for the iron-induced process, the degree of protection by 16-PC was much greater–we could hardly detect any degradation of Zea during the five-hour experiment (Fig. 5C, solid lines). For the set of experiments with rose bengal as a photosensitizer, we introduced a lower (8 μM) concentration of Zea because the synergistic effects of Zea with other antioxidants on singlet oxygen-induced lipid peroxidation were masked by its strong effects when used at a higher, 25 μM, concentration (see Sect. 3.6). At 8 μM, the rate of degradation of Zea increased about twofold. The presence of 16-PC significantly slowed this process. Moreover, 16-PC was about two times more effective in protecting Zea than α-tocopherol (Fig. 5C, dashed lines).
3.5. Lipid-soluble nitroxides effectively protect zeaxanthin against singlet oxygen-induced destruction in saturated PC membranes
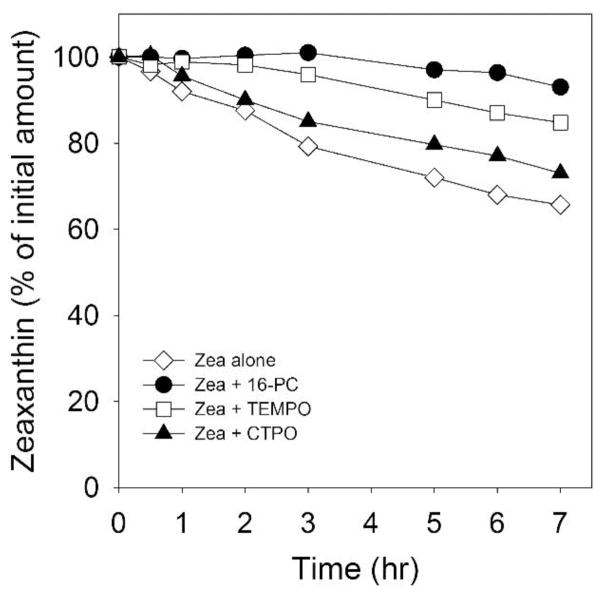
The retina contains certain MXs metabolites; this indicates that chemical quenching of singlet oxygen can take place in this tissue [28, 56]. To better understand this process, we tested the involvement of singlet oxygen itself in the process of Zea destruction. We chose liposomes made of saturated and non-oxidizable phospholipid, DMPC, as the reaction system. The photobleaching of Zea should now result only from its direct interaction with the excited molecules of rose bengal and/or with singlet oxygen. The direct interaction of polar molecules of rose bengal, located in the aqueous phase, and Zea, presumably located in the nonpolar regions of the membrane, is unlikely. Thus, we believe that the 35% decrease in the amount of intact Zea after 7 hours of irradiation can be explained by the singlet oxygen-induced oxidative damage of Zea (Fig. 6).
Fig. 6.
The effect of polarity of nitroxide radicals on bleaching of Zea (25 μM) in DMPC membranes irradiated with green light in the presence of 10 μM rose bengal. CTPO and TEMPO were 250 μM, 16-PC was 25 μM. conclude that singlet oxygen quenching by both spin labels and unsaturated lipids may contribute to protecting Zea from bleaching.
Singlet oxygen, on the other hand, should penetrate into, and diffuse inside, the membranes as easily as triplet (ground) state oxygen, which dissolves better in membranes than in water and diffuses as easily in membranes as in water [57–60]. From the comparison of the destruction rate of Zea in DMPC membranes with that in membranes made of PAPC, we can conclude that singlet oxygen contributes about one-third of the total destruction observed in unsaturated membranes (Figs. 5C and 6). Our interpretation is that the major contribution to this process comes from the degradation of Zea during the “dark” peroxidation of PAPC.
As shown in Fig. 6, nitroxide free radicals can protect Zea from singlet oxygen-induced destruction (bleaching) in DMPC membranes. In a manner similar to what we observed during autoxidation of PC membranes (see Fig. 3), the water soluble CTPO had the smallest protective effect, reducing Zea destruction after 7 hours of irradiation from 35% to 30%. The nitroxide radical TEMPO, which also can easily penetrate into the lipid bilayers [61], effectively inhibits the bleaching of Zea with only 10% Zea bleached after 7 hours of irradiation. The most effective is the membrane-located 16-PC, showing that only 5% of Zea was destroyed during 7 hours of exposure to singlet oxygen. As illustrated in Fig. 3, we used concentrations of both CTPO and TEMPO tenfold higher than of 16-PC, in order to compensate for their solubility in the aqueous phase. Taken together, these outcomes indicate that both Zea destruction and Zea protection occur mainly within the membrane through the singlet oxygen-dependent reactions. Spin labels quench singlet oxygen, although their quenching ability as compared with carotenoids is significantly lower. In the hydrophobic solvents, the quenching rate constant for TEMPO and TEMPO derivatives is ~105–106 M−1s−1 [62–64], while for Zea it is 1.2 ×1010 M−1s−1 [16]. In the aqueous phase, the water-soluble CTPO and the water-located portion of TEMPO can also quench water-located singlet oxygen and further may quench the exited state of rose bengal. We should note here, that the rate constant for the reaction of singlet oxygen with typical unsaturated lipids is in order of 105 M−1s−1 [65–67]. Only in the case of plasmalogens it is as high as ~106 M−1s−1 [67]. Therefore, we
3.6. Combination of zeaxanthin and lipid-soluble nitroxides exerted a strong synergistic protection against singlet oxygen-induced lipid peroxidation
In this paper, we focus mainly on the question of whether selected nitroxide free radicals can protect the intact Zea structure during the oxidation of lipids. In the last section, we investigated whether the protection of Zea by 16-PC can result in the synergistic inhibition of lipid peroxidation, similar (or stronger) to what is known for a combination of Zea and α-tocopherol. To measure the extent of lipid peroxidation, we employed a TBA assay, which is a widely used method for detecting the end products of this process. The TBARS detected in our samples may consist of various lipid peroxidation end products, with relative distribution depending on the position of the hydroperoxide formed, that in turn could potentially be determined by the manner in which peroxidation is induced. Hence, our comparison of data generated with different oxidation methods should be interpreted only in a semiquantitative manner.
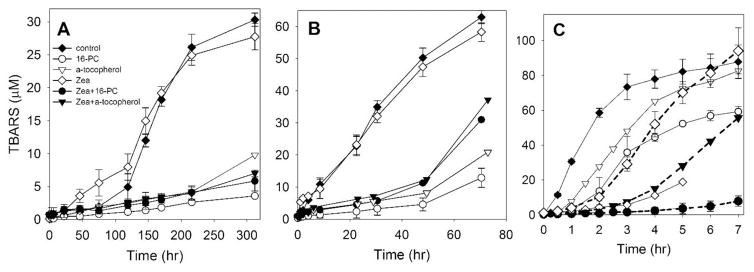
Fig. 7 contains cumulative results showing how Zea itself, lipid-soluble antioxidants (16-PC and α-tocopherol), and a combination of these antioxidants with Zea protect PAPC membranes against peroxidative damage during the autoxidation process (Fig. 7A) and when peroxidation is induced by exogenous iron (Fig. 7B) or singlet oxygen (Fig. 7C). In samples without antioxidants, only in the case of autoxidation was a prolonged lag phase (about 75 hours) observed. In two other cases, peroxidation started immediately after the addition of iron or after turning on the light to start production of singlet oxygen. In all three cases, we followed peroxidation up to the moment when the amount of accumulated TBARS reached (or started to reach) a plateau. It occurred after ~300 hours for autoxidation, after ~60 hours for iron-induced peroxidation, and after ~7 hours during exposure to singlet oxygen. The amount of TBARS produced at those time intervals was 30 μM, 70 μM, and 95 μM, respectively. To compare the intensity of PAPC peroxidation in control experiments (samples without antioxidants), we evaluated the maximal rates of the TBARS production from linear segments of the appropriate TBARS accumulation curves presented in Figs. 7AC. This rate was maximal for singlet-oxygen-induced peroxidation (30 μM/hr), lower for iron-induced peroxidation (1 μM/hr), and very low for autoxidation (0.25 μM/hr). Initiators of lipid peroxidation not only reduced the time of experiments, but also increased the reproducibility of the results, making kinetic analysis more reliable.
Fig. 7.
The effect of 16-PC (25 μM) or α-tocopherol (25 μM), alone or in combination with Zea, on the accumulation of phospholipid peroxidation products (TBARS). Model membranes were made of polyunsaturated phospholipid (PAPC, 2.5 mM). Lipid peroxidation was induced using three different methods: A – autoxidation, in the presence of 25 μM Zea; B - peroxidation induced by a hydrophobic complex of Fe (10 μM) in the presence of 25 μM Zea, and C - peroxidation induced by rose bengal (10 μM) and green light at two different concentrations of Zea - 25 μM (solid lines) or 8 μM (dashed lines). Error bars, when they are present, represent SD. In A and B n=2. In C n=3 except for Zea and Zea + 16-PC, where n=2.
Zea alone differentially affected the peroxidation of PAPC membranes for all three processes. In the case of autoxidation, Zea significantly accelerated the peroxidation process during the lag phase (about the first 100 hours); after that, the time the effect of Zea was negligible (Fig. 7A). Interestingly, during this first 100 hours, Zea was completely bleached and destroyed (Fig. 5A). In the case of iron-induced peroxidation, Zea alone had little effect on peroxidation (Fig. 7B). In that system, Zea was destroyed after 20 hours (Fig. 5B). We can conclude that products of Zea degradation also did not significantly affect the peroxidation process. Only in the case of singlet oxygen-induced peroxidation did Zea significantly inhibit the production of TBARS. In the presence of Zea, we observed a clear lag phase of about 3 hours during which only ~5 μM of TBARS was accumulated; without Zea, accumulation of TBARS reached ~70 μM. In the case of singlet oxygen-induced peroxidation, 60% of Zea was destroyed after 5 hours (Fig. 5C). The concentration of Zea used through our experiments (25 μM) very strongly inhibited singlet oxygen-induced PAPC membrane peroxidation, which masked any possible synergistic effects with other antioxidants. Because of that, we also investigated effects of 8 μM Zea alone and in combination with 16-PC or α-tocopherol (data presented in Fig. 7C, dashed lines). At that concentration, the observed lag phase was about 1.5 hours. A strong antioxidant action of Zea was observed up to 4 to 5 hours after irradiation. After that time, further inhibition of the peroxidation of PAPC membranes was not observed. Instead, signs of the acceleration of the peroxidation after 6 hours of exposure to light and singlet oxygen production were noted (Fig. 7C).
As shown in Figs. 7A and 7B, Zea did not protect PAPC membranes against autoxidation and iron-induced peroxidation. A similar observation was reported by Chen et al. [68]. Both nonpolar and polar carotenoids were easily degraded by free radicals generated from iron, but they were not protective against lipid peroxidation. At the conditions applied here, with a Zea/PAPC ratio of 1/100 and samples equilibrated with air at room temperature, Zea exerts no protective effects and even shows a small pro-oxidant effect. The dual role of carotenoids as both beneficial and harmful agents was connected mainly with their abilities to serve as antioxidants [32, 69, 70], inhibiting free radical production, and as pro-oxidants [71–73], propagating free-radical-induced reactions. Their antioxidant functions and pro-oxidant activities depend on their intrinsic properties (i.e., structure, concentration, location in membranes) as well as on extrinsic factors (i.e., oxygen concentration, interaction with other redox agents, the redox state of the biological environment in which they act) [74]. In membranes, carotenoids demonstrate an inhibitory effect on the formation of lipid oxidative products [32, 69, 70]. There is evidence that they can also enhance the formation of lipid oxidative products, at least under certain conditions (high oxygen concentration, high carotenoid concentration, prolonged oxidative modification) [71, 75]. In our experiments, all of these conditions existed. The goal of this research, however, was to find ways to protect the intact structure of MXs, which should enhance their protective function through physical mechanisms. Therefore, we will refrain from further discussion of the pro-oxidant and antioxidant actions of Zea and direct readers to two chapters on these subjects [74, 76].
Lipid-soluble antioxidants α-tocopherol and 16-PC (25 μM) alone effectively inhibited the “dark” peroxidation of model membranes (Figs. 7A and 7B). Their effects, when comparing the ratio of the amount of TBARS formed in the presence and absence of antioxidants, were comparable for both autoxidation and iron-induced peroxidation. The effects also were similar for both antioxidants. In the case of singlet oxygen-induced peroxidation (Fig. 7C), inhibition caused by 16-PC was about two times stronger than that caused by α-tocopherol. Our results are in principal agreement with previously published data showing that, when lipid peroxidation is initiated by free radical reactions, carotenoids are less effective antioxidants then α-tocopherol [43], while lipid-soluble nitroxides provided better lipid protection than α-tocopherol [44, 45]. This can also explain the results presented in Fig. 5C, which show that 16-PC was about two times more effective in protecting Zea than α-tocopherol.
Interestingly, the inhibitory effects of 16-PC and α-tocopherol were significantly weaker than the inhibition caused by both antioxidants during the “dark” processes. This suggests some similarities in peroxidation processes and the mechanisms of their inhibition during autoxidation and iron-induced peroxidation (Figs. 7A and 7B). In both cases, antioxidants delay the oxidation of membrane lipids by trapping chain-initiating and chain-propagating peroxyl radicals. When peroxidation is initiated by a photosensitized generation of singlet oxygen, protection is based on the quenching of this reactive species. This is demonstrated by the inhibitory effect of Zea on the accumulation of peroxidation products (Fig. 7C).
In the case of “dark” peroxidation, the inhibitory effect of combining Zea with 16-PC or α-tocopherol did not differ significantly from the effects of 16-PC and α-tocopherol alone (Figs. 7A and 7B). This is understandable because Zea is not a very efficient chain-breaking antioxidant. In contrast, when light-induced lipid peroxidation was investigated, the combination of Zea and lipid-soluble antioxidants exerted strong synergistic protection against singlet oxygen-induced lipid peroxidation (Fig. 7C). After 7 hours of irradiation, the combination of Zea (8 μM) and α-tocopherol (25 μM) decreased production of TBARS by ~45% and the combination of Zea (8 μM) and 16-PC (25 μM) decreased production of TBARS by ~95%. This is an extremely strong protective effect produced by the combination of these two agents, with the total effect much greater than the sum of the effects of the individual agents. We also observed a similar, very strong synergistic effect, produced by the combination of Zea with another lipid-soluble nitroxide TEMPO, on the protection of PAPC membranes against singlet oxygen-induced peroxidation (data not shown). Together, these results indicate that membrane-located nitroxide free radicals not only protect Zea from destruction, they also enhance the effect of Zea as a lipid-soluble antioxidant during light-induced lipid peroxidation.
4. Final Discussion
Major protection of the retina provided by MXs occurs through blue light filtration, quenching the triplet state of potent photosensitizers, and physical quenching of singlet oxygen. All these physical, photo-related processes require that the MXs remain in their intact structure. However, the conjugated double bond system of carotenoids makes them highly chemically reactive and very susceptible to oxidation. Chemical quenching of singlet oxygen and scavenging of free radicals consume carotenoids themselves and destroy their intact structure. As a consequence, their physical actions, which are major actions in the protection of the retina, are diminished.
The goal of the presented study was to find ways to protect MXs against their destruction in the retina, mainly during peroxidation of retinal membranes. This seems significant, because oxidative stress is one of the major factors that promotes the development of AMD. The protection of the intact structure of MXs should extend and enhance their positive actions in the retina through physical processes (filtration of blue light, quenching triplet states of photosensitizers, physical quenching of singlet oxygen). Membrane-located nitroxide free radicals protected Zea against destruction, and their effect is especially pronounced during the light-induced formation of singlet oxygen.
Two other investigated systems (with autoxidation and iron-induced peroxidation) helped to clarify processes responsible for the bleaching of Zea and identify mechanisms through which Zea was protected against bleaching. All the data presented in Figs. 5A, 5B, 7A, and 7B indicate that Zea was degraded through chemical reactions with the lipid peroxyl radicals. It is also clear that, during lipid peroxidation, lipid-soluble antioxidants (α-tocopherol and 16-PC) protect the intact structure of Zea through the inhibition of these oxidation reactions in the PAPC bilayer. Similarly, the protective effects of 16-PC and α-tocopherol on the bleaching of Zea (Figs. 5A and 5B) correlate with the protection caused by 16-PC and α-tocopherol on the peroxidation of PAPC (Figs. 7A and 7B). Both α-tocopherol and 16-PC may act as chain-breaking antioxidants and protect MXs, which are rather preventive antioxidants and act as the first line of defense against lipid peroxidation. Following are our comments about expected synergistic effect of the action of Zea in combination with other lipid-soluble antioxidants: According to the definition of synergism, the total effect of the combined agents should be greater than the sum of the effects of the individual agents. For these two systems, Zea did not protect PAPC membranes against autoxidation and iron-induced peroxidation. Zea, at conditions applied here (at all times, samples were equilibrated with atmospheric air), exerts no effects and even shows a small pro-oxidant effect (see Sect. 3.6). Thus, the effects of combined antioxidants can be explained only by the antioxidant effect of α-tocopherol or 16-PC. Based on results presented in Figs. 7A and 7B, we observed no synergistic effects for the combination of Zea with other antioxidants (16-PC or α-tocopherol) in these systems.
Only in the case of singlet oxygen-induced lipid peroxidation did Zea show clear protection against peroxidation of PAPC membranes. The protective effect of Zea is especially pronounced during the first stage of peroxidation, when the lag phase of 3 hours or 1.5 hours in the presence of 25 μM or 8 μM Zea, respectively, is clearly observed. During these lag phases ~30% of 25 μM Zea and ~35% of 8 μM Zea were consumed. The addition of 25 μM 16-PC during these lag phases protected 100% of Zea when its concentration was 25 μM, or 97% when its concentration was 8 μM. Thus, 25 μM of 16-PC effectively protects Zea from bleaching during singlet oxygen-induced peroxidation (Fig. 5C). The most impressive observation is a very strong synergistic, inhibitory effect of Zea and 16-PC on lipid peroxidation (Fig. 7C), much stronger than the combination of Zea and α-tocopherol. These results indicate that protection of Zea provided by membrane-located nitroxide free radicals preserves and enhances the photo-related protective actions of Zea. We believe our research indicates a promising new strategy for maintaining an intact MXs structure during oxidative stress.
Highlights.
Nitroxides protect intact structure of zeaxanthin during oxidative stress
Lipid-soluble nitroxides offer better protection than water soluble nitroxides
Effect of nitroxides was comparable or stronger than that of α-tocopherol
Zeaxanthin and lipid-soluble nitroxides exert strong synergistic protection
Acknowledgments
This work was supported by grants EY015526, EY001931, EB002052, and EB001980 from the National Institutes of Health. We would like to thank Dr. Tadeusz Sarna, Dr. Witold Korytowski, Dr. Anna Pawlak and Dr. Jacek Zielonka for helpful discussion during revision of the manuscript.
Abbreviations
- AMD
age-related macular degeneration
- MXs
macular xanthophylls
- Zea
zeaxanthin
- RPE
retinal pigment epithelium
- TCA
tri-chloroacetic acid
- BHT
butylated hydroxytoluene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mares JA. Potential value of antioxidant-rich foods in slowing age-related macular degeneration. Arch Ophthalmol. 2006;124(9):1339–1340. doi: 10.1001/archopht.124.9.1339. [DOI] [PubMed] [Google Scholar]
- 2.Richer SP, Stiles W, KGraham-Hoffman K, Levin M, Ruskin D, Wrobel J, Park DW, Thomas C. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry. 2011;82(11):667–680. doi: 10.1016/j.optm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 4.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res. 2000;71(3):239–245. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 5.Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69(10):1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- 6.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- 7.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25(11):1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 8.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25(6):674–685. [PubMed] [Google Scholar]
- 9.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41(5):1200–1209. [PubMed] [Google Scholar]
- 10.Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999;19(6):491–495. doi: 10.1076/ceyr.19.6.491.5276. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72(3):215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 12.Cullen AP. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int J Toxicol. 2002;21(6):455–464. doi: 10.1080/10915810290169882. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DP, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 15.DP, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53(1 Suppl):194S–200S. [PubMed] [Google Scholar]
- 16.Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants--a review. J Photochem Photobiol B. 1997;41(3):189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 17.Delmelle M. An investigation of retinal as a source of singlet oxygen. Photochem Photobiol. 1978;27(6):731–734. [Google Scholar]
- 18.Pawlak A, Wrona M, Rozanowska M, Zareba M, Lamb LE, Roberts JE, Simon JD, Sarna T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem Photobiol. 2003;77(3):253–258. doi: 10.1562/0031-8655(2003)077<0253:cotapo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Lamb LE, Zareba M, Plakoudas SN, Sarna T, Simon JD. Retinyl palmitate and the blue-light-induced phototoxicity of human ocular lipofuscin. Arch Biochem Biophys. 2001;393(2):316–320. doi: 10.1006/abbi.2001.2492. [DOI] [PubMed] [Google Scholar]
- 20.Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971;172(3978):76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- 21.Pautler EL, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem Photobiol. 1990;51(5):599–605. doi: 10.1111/j.1751-1097.1990.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 22.Gottsch JD, Pou S, Bynoe LA, Rosen GM. Hematogenous photosensitization. A mechanism for the development of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1990;31(9):1674–1682. [PubMed] [Google Scholar]
- 23.Gurrey RK, Ham WT, Mueller HA. Light toxicity in the posterior segment. In: Duane TD, editor. Clinical Ophthalmology. Harper & Row; Philadelphia, PA, USA: 1985. pp. 1–17. [Google Scholar]
- 24.Rozanowska M, Korytowski W, Rozanowski B, Skumatz C, Boulton ME, Burke JM, Sarna T. Photoreactivity of aged human RPE melanosomes: a comparison with lipofuscin. Invest Ophthalmol Vis Sci. 2002;43(7):2088–2096. [PubMed] [Google Scholar]
- 25.Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61(5):448–453. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 26.Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270(32):18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 27.WStahl W, HSies H. Antioxidant effects of carotenoids: Implication in photoprotection in humans. In: Packer L, editor. Implication in Photoprotection in Humans: Handbook of Antioxidants. Marcel Dekker; Basel, Switzerland: 2002. pp. 223–234. [Google Scholar]
- 28.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38(9):1802–1811. [PubMed] [Google Scholar]
- 29.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991;11(1):41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- 30.Burton GW, Ingold KU. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 31.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 32.Palozza P, IKrinsky N. Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 1992;213:403–420. doi: 10.1016/0076-6879(92)13142-k. [DOI] [PubMed] [Google Scholar]
- 33.Wisniewska A, Subczynski WK. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic Biol Med. 2006;41(8):1257–1265. doi: 10.1016/j.freeradbiomed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Wisniewska A, Subczynski WK. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic Biol Med. 2006;40(10):1820–1826. doi: 10.1016/j.freeradbiomed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Subczynski WK, Wisniewska A, Widomska J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem Biophys. 2010;504(1):61–6. doi: 10.1016/j.abb.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65(1):57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 37.Blanquet-Diot S, Soufi M, Rambeau M, Rock E, Alric M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J Nutr. 2009;139(5):876–883. doi: 10.3945/jn.108.103655. [DOI] [PubMed] [Google Scholar]
- 38.Siems WG, Sommerburg O, van Kuijk FJ. Lycopene and beta-carotene decompose more rapidly than lutein and zeaxanthin upon exposure to various pro-oxidants in vitro. Biofactors. 1999;10(2–3):105–113. doi: 10.1002/biof.5520100204. [DOI] [PubMed] [Google Scholar]
- 39.Olchawa MM, Herrnreiter AM, Pilat AK, Skumatz CM, Niziolek-Kierecka M, Burke JM, Sarna TJ. Zeaxanthin and alpha-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells. Free Radic Biol Med. 2015;89:873–82. doi: 10.1016/j.freeradbiomed.2015.10.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrona M, Rozanowska M, Sarna T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic Biol Med. 2004;36(9):1094–101. doi: 10.1016/j.freeradbiomed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385(1):20–7. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 42.Samuni AM, Barenholz Y. Stable nitroxide radicals protect lipid acyl chains from radiation damage. Free Radic Biol Med. 1997;22(7):1165–74. doi: 10.1016/s0891-5849(96)00509-6. [DOI] [PubMed] [Google Scholar]
- 43.Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1126(2):178–84. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- 44.Cimato AN, Piehl LL, Facorro GB, Torti HB, Hager AA. Antioxidant effects of water- and lipid-soluble nitroxide radicals in liposomes. Free Radic Biol Med. 2004;37(12):2042–51. doi: 10.1016/j.freeradbiomed.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Samuni AM, Lipman A, Barenholz Y. Damage to liposomal lipids: protection by antioxidants and cholesterol-mediated dehydration. Chem Phys Lipids. 2000;105(2):121–34. doi: 10.1016/s0009-3084(99)00136-x. [DOI] [PubMed] [Google Scholar]
- 46.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42(11):1632–50. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korytowski W, Wrona M, Girotti AW. Radiolabeled cholesterol as a reporter for assessing one-electron turnover of lipid hydroperoxides. Anal Biochem. 1999;270(1):123–132. doi: 10.1006/abio.1999.4070. [DOI] [PubMed] [Google Scholar]
- 48.Merkle H, Subczynski WK, Kusumi A. Dynamic fluorescence quenching studies on lipid mobilities in phosphatidylcholine-cholesterol membranes. Biochim Biophys Acta. 1987;897(2):238–248. doi: 10.1016/0005-2736(87)90420-2. [DOI] [PubMed] [Google Scholar]
- 49.Yin JJ, Subczynski WK. Effects of lutein and cholesterol on alkyl chain bending in lipid bilayers: a pulse electron spin resonance spin labeling study. Biophys J. 1996;71(2):832–839. doi: 10.1016/S0006-3495(96)79284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys. 1999;371(2):301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- 51.Cighetti G, Allevi P, Debiasi S, Paroni R. Inhibition of in vitro lipid peroxidation by stable steroidic nitroxyl radicals. Chem Phys Lipids. 1997;88(2):97–106. doi: 10.1016/s0009-3084(97)00052-2. [DOI] [PubMed] [Google Scholar]
- 52.Cimato AN, Piehl LL, Facorro GB, Torti HB, Hager AA. Antioxidant effects of water- and lipid-soluble nitroxide radicals in liposomes. Free Radic Biol Med. 2004;37(12):2042–2051. doi: 10.1016/j.freeradbiomed.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Nilsson UA, Olsson LI, Carlin G, Bylund-Fellenius AC. Inhibition of lipid peroxidation by spin labels. Relationships between structure and function. J Biol Chem. 1989;264(19):11131–11135. [PubMed] [Google Scholar]
- 54.Wisniewska ALA, Subczynski WK. Reoxidation of the Reduced Nitroxide Radical Spin Label TEMPONE: Involvement of Oxygen and Oxygen-Derived Active Forms. Curr Top Biophys. 1992;16:85–91. [Google Scholar]
- 55.Halliwell B, Gutteridge JMC. Iron and lipid peroxidation, Free Radicals in Biology and Medicine. Oxford University Press; New York, NY, USA: 1999. pp. 296–298. [Google Scholar]
- 56.Bhosale P, Bernstein PS. Quantitative measurement of 3′-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Anal Biochem. 2005;345(2):296–301. doi: 10.1016/j.ab.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Ehrenberg B, Anderson JL, Foote CS. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem Photobiol. 1998;68(2):135–140. [PubMed] [Google Scholar]
- 58.Skovsen E, Snyder JW, Lambert JD, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B. 2005;109(18):8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 59.Subczynski WK, Hyde JS. Concentration of oxygen in lipid bilayers using a spin-label method. Biophys J. 1983;41(3):283–286. doi: 10.1016/S0006-3495(83)84439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subczynski WK, Hyde JS. Diffusion of oxygen in water and hydrocarbons using an electron spin resonance spin-label technique. Biophys J. 1984;45(4):743–748. doi: 10.1016/S0006-3495(84)84217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McConnell HM. Molecular motion in biological membranes. In: Berliner LJ, editor. Spin Labelling Theory and Applications. Academic Press; New York, NY, USA: 1976. pp. 525–560. [Google Scholar]
- 62.Martinez CG, Jockusch S, Ruzzi M, Sartori E, Moscatelli A, Turro NJ, Buchachenko AL. Chemically induced dynamic electron polarization generated through the interaction between singlet molecular oxygen and nitroxide radicals. J Phys Chem A. 2005;109(45):10216–21. doi: 10.1021/jp0530404. [DOI] [PubMed] [Google Scholar]
- 63.Zang LY, Zhang ZY, Misra HP. EPR studies of trapped singlet oxygen (1O2) generated during photoirradiation of hypocrellin A. Photochem Photobiol. 1990;52(4):677–83. doi: 10.1111/j.1751-1097.1990.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 64.Darmanyan AP, Tatikolov AS. Singlet oxygen quenching by stable nitroxy radicals. Journal of Photochemistry. 1985;32:157–163. [Google Scholar]
- 65.Vever-Bizet C, Dellinger M, Brault D, Rougee M, Bensasson RV. Singlet molecular oxygen quenching by saturated and unsaturated fatty-acids and by cholesterol. Photochem Photobiol. 1989;50(3):321–5. doi: 10.1111/j.1751-1097.1989.tb04165.x. [DOI] [PubMed] [Google Scholar]
- 66.Doleiden FH, Fahrenholtz SR, Lamola AA, Trozzolo AM. Reactivity of cholesterol and some fatty acids toward singlet oxygen. Photochem Photobiol. 1974;20(6):519–21. doi: 10.1111/j.1751-1097.1974.tb06613.x. [DOI] [PubMed] [Google Scholar]
- 67.Broniec A, Klosinski R, Pawlak A, Wrona-Krol M, Thompson D, Sarna T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic Biol Med. 2011;50(7):892–8. doi: 10.1016/j.freeradbiomed.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen G, Djuric Z. Carotenoids are degraded by free radicals but do not affect lipid peroxidation in unilamellar liposomes under different oxygen tensions. FEBS Lett. 2001;505(1):151–154. doi: 10.1016/s0014-5793(01)02671-0. [DOI] [PubMed] [Google Scholar]
- 69.Krinsky NI. Actions of carotenoids in biological systems. Annu Rev Nutr. 1993;13:561–587. doi: 10.1146/annurev.nu.13.070193.003021. [DOI] [PubMed] [Google Scholar]
- 70.Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62(6 Suppl):1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 71.Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr Rev. 1998;56(9):257–265. doi: 10.1111/j.1753-4887.1998.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 72.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385(1):20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz JL. The dual roles of nutrients as antioxidants and prooxidants: their effects on tumor cell growth. J Nutr. 1996;126(4 Suppl):1221S–1227S. doi: 10.1093/jn/126.suppl_4.1221S. [DOI] [PubMed] [Google Scholar]
- 74.PPalozza P. Evidence for pro-oxidant effects of carotenoids in vitro and in vivo: implications in health and disease. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in health and disease. Marcel Dekker; New York: 2004. pp. 127–149. [Google Scholar]
- 75.Palozza P, Calviello G, Bartoli GM. Prooxidant activity of beta-carotene under 100% oxygen pressure in rat liver microsomes. Free Radic Biol Med. 1995;19(6):887–892. doi: 10.1016/0891-5849(95)00094-e. [DOI] [PubMed] [Google Scholar]
- 76.Young AJ, Phillip DM, Lowe GM. Carotenoid Antioxidant Activity. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in health and disease. Marcel Dekker; New York: 2004. pp. 105–126. [Google Scholar]