Abstract
The Hedgehog (Hh) signaling pathway not only plays a key part in controlling embryonic development, but in the adult stomach governs important cellular events such as epithelial cell differentiation, proliferation, gastric disease and regeneration. In particular, Sonic Hedgehog (Shh) signaling has been well studied for its role in gastric physiology and pathophysiology. Shh is secreted from the gastric parietal cells and contributes to the regeneration of the epithelium in response to injury, or the development of gastritis during Helicobacter pylori infection. Dysregulation of the Shh signaling pathway leads to the disruption of gastric differentiation, loss of gastric acid secretion and the development of cancer. In this chapter, we will review the most recent findings that reveal the role of Shh as a regulator of gastric physiology, regeneration and disease.
INTRODUCTION
Using a mutagenesis screen in Drosophila, Nusslein-Volhard and Wieschaus first described the Hedgehog (Hh) gene [1], and this was followed by the identification of three Hedgehog homologs that are greatly conserved from the fruit fly to humans. The three homologs are Sonic Hedgehog (Shh), Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh). In the stomach, Hedgehog is known to control cellular functions including proliferation, differentiation and acid secretion. Therefore, identifying the role of Shh as a regulator of adult stomach physiology and differentiation is important for understanding the pathogenesis of gastric diseases. Shh also affects the division of adult stem cells. The regulation of adult stem cells through Shh signaling has linked this pathway in the development of cancer [2], of which gastric cancer is the focus of this review.
BIOCHEMISTRY OF THE HEDGEHOG SIGNALING PATHWAY
Initial studies of Hedgehog processing were performed in invertebrate systems, but Hedgehog processing in the mammalian system, in particular the stomach, has been shown to be protease- and acid-dependent [3]. Primary parietal cell cultures and tissue extracts from mouse and human stomachs, demonstrated that Shh is processed by pepsin A [3]. Interestingly, processed Shh in human gastric cancer tissue is absent as a consequence of hypochlorhydria due to the parietal cell atrophy [4]. The unprocessed full length Shh precursor protein is also biologically active [5]. Full length Shh binds to Hedgehog receptor Ptch and induces target gene expression in the developing chick neural tube and rescues the eye developmental defect in Drosophila Hedgehog mutants [5].
Shh processing is also regulated by gastrin [4,6]. The regulatory role of gastrin is indicated by studies using gastrin deficient mice demonstrating a significant decrease in Shh protein expression, and that the reinfusion of gastrin restores Shh expression [4]. In addition, using isolated canine parietal cell and mouse organ cultures to identify the mechanism that gastrin regulates Shh, El-Zaatari et al. demonstrated that intracellular calcium release and protein kinase C (PKC) activation stimulate Shh gene expression [6]. Based on previous reports that intracellular calcium release stimulates acid secretion in parietal cells, gadolinium-, thapsigargin-, and carbachol-mediated release of intracellular calcium also induced Shh expression [6].
Once Hedgehog protein has been secreted, it is believed to travel in a paracrine fashion towards the target cell [16]. Hedgehog then binds to, and stimulates the transmembrane receptor Patched 1 (Ptch1) [7], which causes a release of the inhibitory effect that Ptch1 has on G-protein coupled receptor Smoothened (Smo) [9]. Smo is then capable of travelling up the cilium tip via microtubular transport [10-11] where it promotes the dissociation of the glioma-associated oncogene homolog 1 (Gli1) [9,12]. The Gli family of transcription factor proteins (Gli1, Gli2, and Gli3) travel into the nucleus where they bind to the DNA consensus site (5′-GACCACCCA-3′) [12]. Gli1 is a powerful transcriptional activator, while Gli2 and Gli3 possess both transcriptional activation and repression capabilities [14]. The Gli transcription factor family effects the expression of genes that help control cell development and differentiation (cyclin D1, D2, N-myc, Wnt, PdgfRα, Igf2, FoxM1, Hes1), cell survival (Bcl2), self-renewal (Bmi1, Nanog), angiogenesis (Vegf), epithelial-mesenchymal transition (Snail1, Sip1, Elk1, Msx2), invasiveness and feedback inhibition or activation of the Hedgehog signaling cascade [14].
HEDGEHOG SIGNALING AND GASTRIC FUNCTION, DIFFERENTATION AND REGENERATION
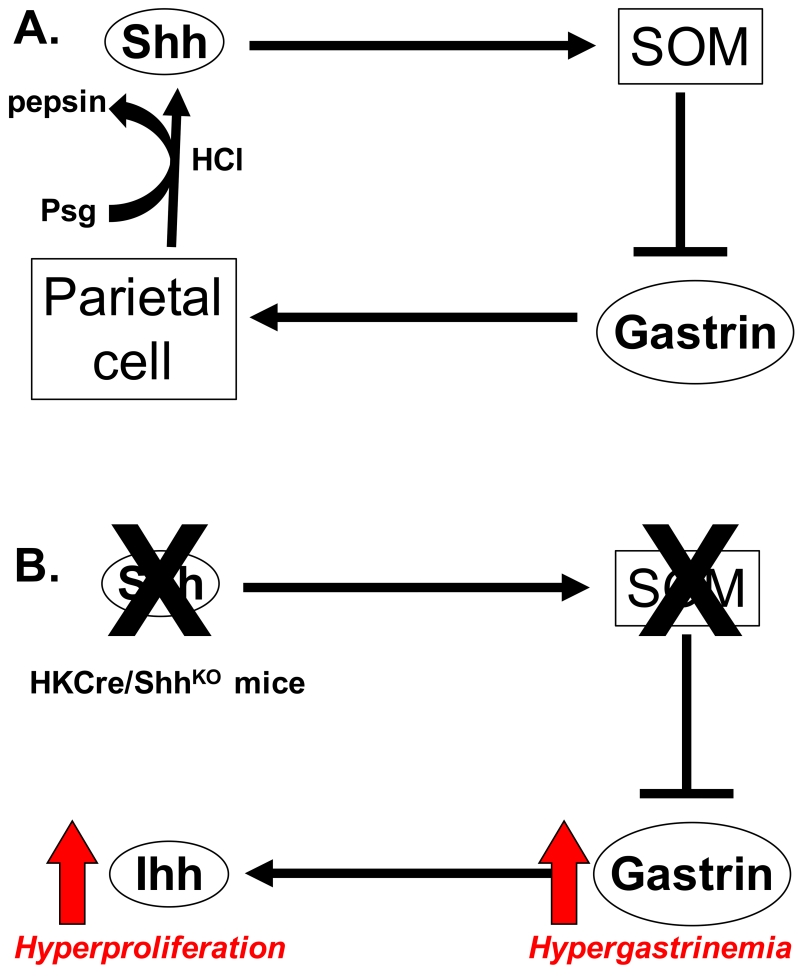
The development of a mouse model expressing a parietal cell-specific deletion of Shh (HKCre/ShhKO mice) has allowed us to assay changes in gastric epithelial cell differentiation, function and regeneration [15-18]. Compared to pathology of the control animals, HKCre/ShhKO mice demonstrated an age-dependent increase in number of surface pit mucous cells reminiscent of foveolar hyperplasia [15]. Interestingly, the phenotype observed in HKCre/ShhKO mouse was comparable to that observed in mice over-expressing the TGFα gene [19] and in patients with Menetrier’s disease [20], without the development of parietal cell atrophy. Loss of parietal cell-expressed Shh was accompanied by hypergastrinemia and increased Ihh within the surface mucous pit epithelium. Somatostatin is a known inhibitor of gastrin and when HKCre/ShhKO mice were infused with the somatostatin analogue octreotide, circulating gastrin concentrations normalized and Ihh expression decreased [15]. Our existing understanding of hypergastrinemia is expanded because, besides the proposed role as a morphogen for the gastric epithelium, Shh may also be a regulator of the gastrin-gastric acid negative feedback mechanism (Figure 1).
Figure 1. Shh as a regulator of gastric physiology and function.
(A) Gastrin-induced acid secretion facilitates Shh processing and secretion that is protease-dependent. Shh induces somatostatin (SOM) secretion, which in turn further inhibits the gastrin production from G-cells and subsequent acid secretion from the parietal cells.
(B) Loss of parietal cell-secreted Shh results in loss of the production of SOM and the subsequent increase in serum gastrin concentrations (hypergastrinemia). Hypergastrinemia induces Ihh expression in the stomach leading to hyperproliferation of the gastric epithelium.
We proposed that increased Ihh gene expression observed in HKCre/ShhKO mice might be due to hypergastrinemia. We studied hypergastrinemic HKCre/ShhKO mice bred with gastrin-deficient (GKO) mice (PC-ShhKO/GKO) [16]. The PC-ShhKO/GKO mice did not exhibit evidence of hyperproliferation, and gastrin infusion caused increased expression of Ihh and proliferation within the surface epithelium. In vivo gastrin-induced proliferation in PC-ShhKO/GKO mice was blocked by Hedgehog signaling inhibitor cyclopamine [16]. In addition, in vitro gastrin-induced proliferation of fundic organoids derived from PC-ShhKO/GKO mouse stomach, was blocked by a smoothened inhibitor [16]. We concluded that Ihh signaling regulates gastrin-induced proliferation of epithelial cells in stomachs of adult mice.
The HKCre/ShhKO mice demonstrate that loss of Shh triggers a number of molecular changes [15] consistent with epithelial-to-mesenchymal transition (EMT) of the gastric epithelium [21]. This included loss of E cadherin expression and translocation of β-catenin to the nucleus [15]. After nuclear translocation, β-catenin binds to DNA-binding proteins Tcf/Lef1 which subsequently regulates target genes including Cyclin D1 [22] important in proliferation. Additionally, laser capture microdissection analysis of the gastric epithelium of HKCre/ShhKO mice demonstrated a significant increase in Snail with a concurrent decrease in E-cadherin [15]. A study in rat kidney epithelial cells shows that Snail, a suppressor of E-cadherin, is a transcriptional target of Hedgehog signaling [23]. As discussed earlier, HKCre/ShhKO mice exhibited hyperproliferation of surface mucous cells [15]. Hyperproliferation of surface mucous cells frequently disrupts differentiation of other cell lineages such as zymogen cells [24]. HKCre/ShhKO mice have a delay in the differentiation of zymogen cells from mucous neck cells whereby zymogen cells located in the base of the gland frequently co-expressed mucous neck cell markers [15]. Aberrant E- cadherin expression causes changes in zymogenic cell morphology and differentiation [24]. We demonstrate that adherens-junction protein E-cadherin may be a downstream target of the Hedgehog signaling pathway [25]. The integrity of adherens- junctions is an important factor in determining epithelial morphology and cell differentiation [26], but also tumor growth, invasiveness and metastasis [27]. Understanding Hedgehog signaling as a regulator of the adherens-junctions advances our knowledge by which Shh may regulate gastric physiology.
Shh secreted from gastric parietal cells is important for epithelial repair following gastric injury. The HKCre/ShhKO mice exhibit impaired gastric regeneration in response to injury. However, in parabiosis experiments a control mouse paired with a mouse expressing a parietal cell-specific deletion of Shh facilitates repair in the HKCre/ShhKO mouse [17]. This suggested for the first time that parietal cell act as an endocrine source of Shh during gastric repair. In addition, the development of a tamoxifen-inducible mouse model expressing a parietal cell-specific deletion of Shh (PC-iShhKO) demonstrated that re-emergence of Shh contributes to gastric regeneration [18]. Studies have indicated that downregulating Shh expression is a mechanism by which Helicobacter pylori (H. pylori) infection triggers gastric cancer, as the outcome of H. pylori infection is parietal cell atrophy and gastric hypochlorhydria. Intestinal metaplastic areas of H. pylori-positive human stomachs have decreased Shh expression [28]. Other studies suggest that early bacterial eradication restores the expression of Shh and the differentiation of the stomach [28-28]. Thus, studies using the PC-iShhKO mice may have clinical suggestions given that removal of H. pylori correlates with re-emergence of Shh and regeneration of the gastric epithelium.
HEDGEHOG SIGNALING AND Helicobacter pylori (H. pylori) INFECTION
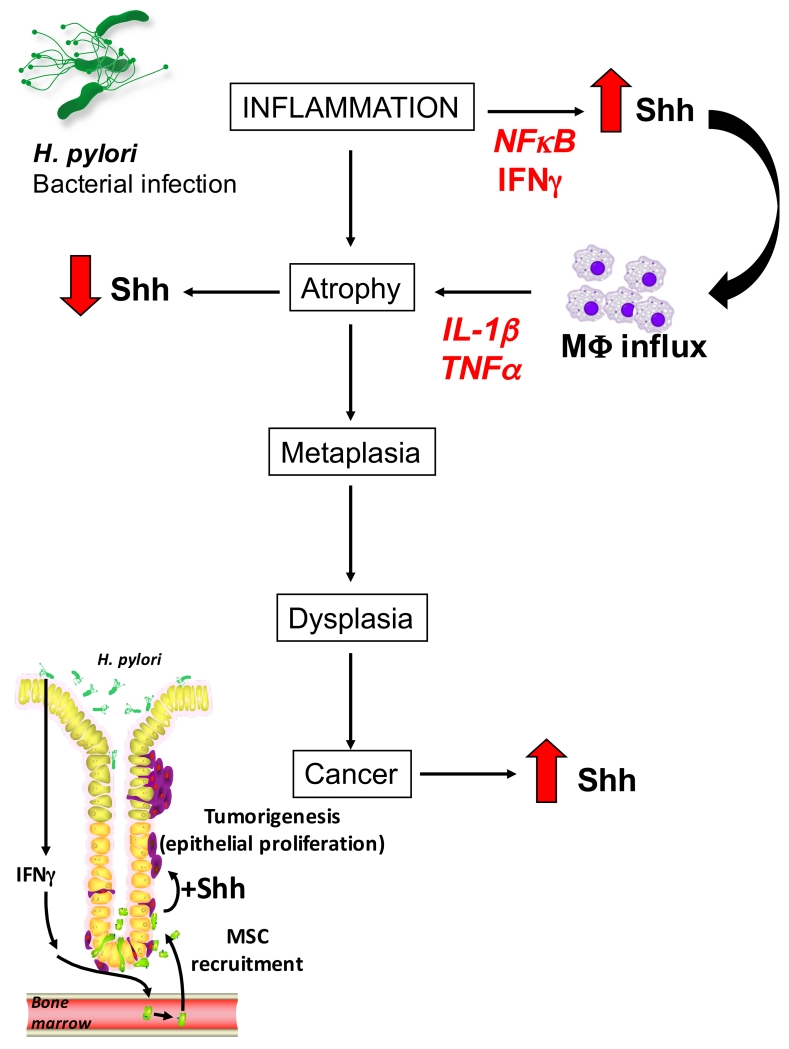
The regulation of Shh during inflammation may be seen as a global increase or decrease in expression during the progression of inflammation to gastric cancer. Data suggests in the innate phase of the host immune response Shh is induced after activation of the NF-κB pathway [29]. During the early stages of infection and as a result of inflammation, pro-inflammatory cytokine interferon-γ (IFNγ) has been shown to stimulate Shh expression [30] (Figure 2). Macrophages recruited to the infection side secrete pro-inflammatory cytokines including TNFα and IL-1β which result in loss of Shh expression [31]. In an in vivo mouse model of H. pylori inflammation parietal cell-expressed Shh is induced within 2 days of infection [32]. In vitro H. pylori infection in gastric organoids also increased Shh expression; an effect that was blocked by inhibiting NFκB signaling. Thus, H. pylori results in increased Shh expression from parietal cells, via activation of the NFκB signaling pathway [33].
Figure 2. Changes in Shh expression during the progression from gastric inflammation to cancer.
It is accepted that the major cause of chronic inflammation in the normal, acid-secreting stomach is H. pylori colonization. Upon H. pylori infection, Shh is induced by NFκB and IFNγ, and acts as a chemokine for the recruitment of macrophages to the stomach and the development of gastritis. Cytokines including IL- 1β and TNFα, leads to the loss of Shh, possibly as a consequence of parietal cell atrophy. Based on the Correa model for the development of gastric cancer [84], it is known that inflammation caused by H. pylori infection progresses through the development of atrophic gastritis, metaplasia, dysplasia and eventually cancer. The Hedgehog signaling pathway is aberrantly activated during gastric cancer. MSCs recruited from the bone marrow to the tumor site are a major source of Shh and provide a proliferative stimulus for the epithelium.
After induction of Shh during early stages of H. pylori infection, Shh then acts as a macrophage chemoattractant during early gastritis [32}. Bone marrow chimera experiments were achieved with mice that have myeloid cell-specific deletion of the Hedgehog signal transduction protein Smoothened (LysMCre/SmoKO). Macrophage recruitment to the gastric epithelium was measured by fluorescence-activated cell sorting analysis. Control mice that received bone marrow transplants from control mice had an infiltration of macrophages to the gastric mucosa in response to H. pylori infection. In contrast, macrophage infiltration was not observed in H. pylori-infected mice that received bone marrow transplants from LysMCre/SmoKO mice, suggesting that Shh signaling is crucial for macrophage infiltration [32]. Moreover, PC-ShhKO mice (discussed earlier) did not develop gastritis, even after 6 months infection with H. pylori. However, control mice infected with H. pylori for 6 months experienced an inflammatory response characterized by infiltration of CD4(+) T cells and increased levels of IFNγ and IL-1β. These studies demonstrated that Shh acts as a macrophage chemoattractant during initiation of H. pylori-induced gastritis [32] (Figure 2). Decreases in the expression of Shh and Ptch1 have been observed in parietal cells during H. pylori infection of the gastric epithelium [34]. These finding represents changes during the early stages of H. pylori infection that takes place in gastric mucosal cells prior to neoplastic transformation [18].
HEDGEHOG SIGNALING AND GASTRIC CANCER
Gastric cancer is one of the foremost widespread types of cancer. In 2015, an estimated 24,590 people were diagnosed with stomach cancer and 10,720 people were predicted to succumb to this illness by the American Cancer Society. The five-year survival rate for people with gastric cancer was 28%. This percentage rate is low because most gastric cancer diagnoses are made after there has already been metastasis to surrounding organs. This is also indicative of the efficacy of current chemotherapeutics and the high recurrence rates, as well as metastasis events [38]. With regards to the role of the Hedgehog signaling pathway in gastric cancer development, high expression of Shh signaling components correlated with the development of gastrointestinal cancers [39] (Figure 2). Increased Ptch expression coincided with elevated Shh. In vivo data showed that increased Shh promoted cell proliferation, and that tumor growth regressed in a xenograft mouse model treated with the Hedgehog pathway inhibitor cyclopamine [39]. Further characterization of Ptch1 and Gli1 expression within the gastric tumor microenvironment was performed using a collection of human biopsies [40]. In these samples, elevated Shh corresponded to increased Ptch1 and Gli1 in cancerous tissue. Elevated Hedgehog pathway activation was most common in poorly differentiated and high-grade samples, implicating Shh as an inducer of an aggressive cancer phenotype [40].
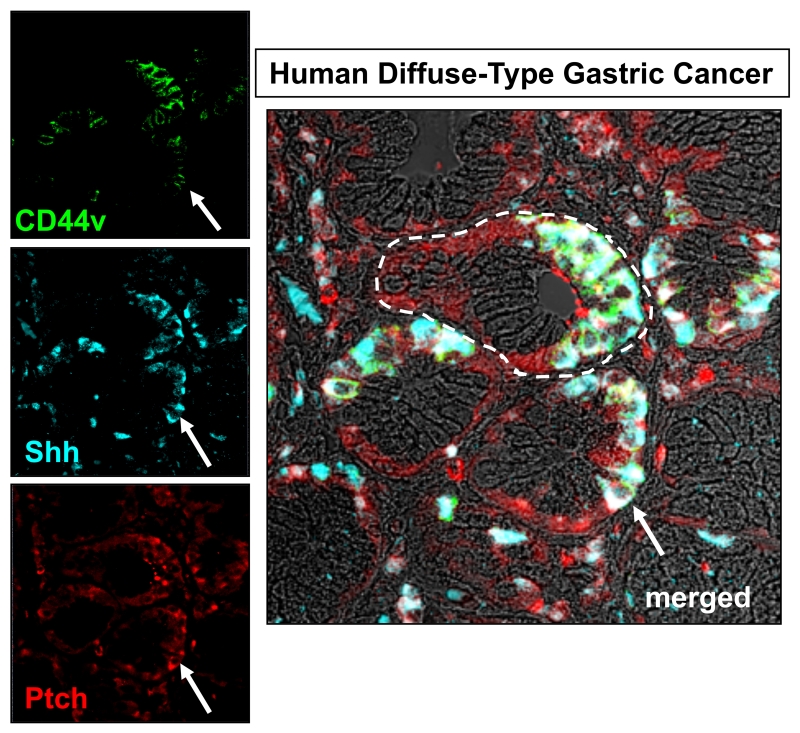
Studies of both human intestinal- and diffuse-type gastric cancer were performed to localize Hedgehog signaling by cell type in the setting of tumor formation [41]. The intestinal phenotype expressed low mRNA abundance of Smo, Gli1 and Gli2, while the diffuse-type phenotype highly expressed Ptch, Smo, Gli1 and Gli2. Diffuse-type gastric cancer samples showed strong Ihh staining in epithelial cancer cells, while Shh was expressed in fibroblastic cells co-staining with vimentin, α-actin and desmin [41]. Immunostaining by our laboratory using tissue collected from a patient with diffuse-type gastric cancer and antibodies specific for Shh, Ptch and cancer stem cell marker CD44 demonstrated co-expression of Shh within CD44-expressing cells within the tumor (Figure 3). Interestingly, Ptch was expressed within the mesenchyme, but also co-localized to CD44+/Shh+cells within the tumor (Figure 3). These data are consistent with studies demonstrating that Hedgehog maintains the malignant transformation phenotype in CD44+ gastric cancer cells that promotes chemotherapy resistance [18]. A recent study by Syu et al. [42] further supports the role of Hedgehog signaling as a driver in the progression of gastric cancer. In this study the investigators developed a mouse model expressing Hedgehog pathway transcription factor GLI2 within Lgr5-expressing stem cells and their descendants in adult mice when treated with doxycycline. Mice developed gastric adenocarcinoma three weeks following induction of the Hedgehog pathway oncogene GLI2A. These data provided further evidence that dysregulated activation of Hedgehog/Gli2 signaling in Lgr5-positive stem cells drives the emergence of gastric adenocarcinoma in mice [42]. Therefore, in contrast to patients with atrophic gastritis that show loss of Shh protein expression, over-expression of the Hedgehog pathway is a driver and prognostic marker in the development of gastric carcinoma [42] (Figure 2).
Figure 3. Expression of Shh, Ptch and CD44v in human diffuse-type gastric cancer.
Immunostaining of tissue collected from a patient with diffuse-type gastric cancer using antibodies specific for CD44 variant isoform (CD44v, green), Shh (blue) and Ptch (red).
The mechanism by which Hedgehog is elevated in gastric cancer and then able to act on cancer cells to induce their proliferation remains largely unknown. However, studies from our laboratory suggest that mesenchymal stem cells (MSCs) may be a source of Hedgehog protein. The permanent engraftment of the bone marrow-derived MSCs in an IFNγ-rich tissue environment results in the differentiation of these cells into cells closely resembling stem cells, whereby they have acquired the capacity to self-renew and become incorporated in the developing tumor [43]. We first investigated the mechanism for recruitment of these MSCs into the gastric epithelium. The MSC population collected from bone marrow of control- or IFNγ-treated mice demonstrated that IFNγ significantly increased MSC proliferation, a response mediated by Hedgehog signaling. While MSC cell lines with intact Shh expression were recruited to the gastric mucosa in response to IFNγ, MSCs lacking expression of Shh were not. Thus, our data suggests that Hedgehog signaling is important in MSC proliferation and recruitment to the stomach in response to IFNγ [44]. Further studies from our laboratory using these ‘transformed’ MSCs secreted Shh responsible for providing a proliferative stimulation of the gastric epithelium which is associated with tumor development [45] (Figure 2).
HEDGEHOG SIGNALING AND POTENTIAL CANCER THERAPEUTICS
The aberrant activation of the Shh signaling pathway has been shown in different types of human cancers including gastric cancer, basal cell carcinoma, malignant gliomas, leukemias, breast, a n d pancreatic cancers [46-50]. Downstream players in the Shh pathway, smoothened (SMO) and glioma-associated oncogene homolog (GLI) family of zinc finger transcription factors, have been targeted for potential cancer therapeutics. Many efforts have been focused on pharmacologically targeting SMO, and to date, two SMO inhibitors (LDE225/Sonidegib and GDC-0449/Vismodegib) have received United States Food and Drug Administration (FDA) approval for treating basal cell carcinoma. Vismodegib is a second generation cyclopamine derivative developed by Roche/Genentech/Curis that binds directly to SMO to prevent GLI activation [80]. Vismodegib, approved by the FDA in January 2012, was the first drug targeting the Shh pathway approved for treating any cancer. Vismodegib is currently used to treat adults for metastatic basal cell carcinoma, or patients with recurrent basal cell carcinoma who are not candidates for surgery or radiation therapy [51]. In particular, GDC-0449/Vismodegib was also used in Phase II trials for treatment of metastatic gastric and esophageal cancers [52].
We also discussed above that the Hedgehog pathway maintains the malignant transformation phenotype in CD44+ gastric cancer cells that promotes chemotherapy resistance [9]. Compared to the CD44- cell population, CD44+ cells showed a resistance to 5-fluorouracil and cisplatin chemotherapy that was negated when Hedgehog signaling was inhibited using Smo shRNA or vismodegib [9]. Importantly, clinical tumor samples from a phase II trial of chemotherapy with or without Vismodegib for advanced gastric cancer were examined for CD44 expression. In the group that only received chemotherapy, increased CD44 expression was associated with a decrease in survival. In the chemotherapy plus Vismodegib group, high CD44 expression was associated with enhanced survival [9]. In another study, Xu et al. [53] showed that Shh and Gli1 signaling enhanced drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. These CD44(+)/Musashi-1(+) cells presented a high level of drug efflux activity. The cells were resistant to doxorubicin-induced apoptosis and also upregulated ATP-binding cassette sub-family G member 2 (ABCG2) expression. These effects were reversed by treatment with Gli inhibitors GANT61 and GDC-0449 or by knocking down the expression of Gli1/Shh. The co-expression of CD44(+)/Musashi- 1(+) could be used as a method of detecting gastric cancer stem cells. Collectively, these studies demonstrate that gastric cancer is a heterogeneous and complicated disease. Thus, combining chemotherapy with Hedgehog inhibition may be effective only in tumors with high CD44 levels.
CONCLUSIONS
Shh is a parietal cell-secreted protein regulating gastric cell proliferation, differentiation, function and epithelial regeneration [7,18]. Intriguingly, Shh acts as a chemokine for monocyte recruitment during H. pylori infection and the development of gastritis [32]. Given the emerging roles of Shh as a regulator of the immune response, adult tissue physiology and regeneration and the development of gastric disease, this has broadened our understanding of this developmental morphogen in the stomach. Understanding the role of the Hedgehog pathway during tumorigenesis will allow for the development of effective therapies for the treatment of gastric disease.
HIGHLIGHTS.
Hedgehog signaling regulates gastric physiology, regeneration and disease.
Sonic Hedgehog is secreted from the gastric parietal cells.
The dysregulation of Sonic Hedgehog signaling leads to the development of cancer
ACKNOWLEDGEMENTS
This work was supported by NIH 2 R01 DK083402-06 grant and The University of Cincinnati, College of Medicine Bridge Funding Program (Y. Zavros). Albert J. Ryan Fellowship, Dean’s Fellowship, NIH5T32GM105526 grant (N Bertaux-Skeirik).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared
REFERENCES
- 1.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila [Internet] Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Wan J, Zhou J, Zhao H, Wang M, Wei Z, Gao H, Wang Y, Cui H. Sonic hedgehog pathway contributes to gastric cancer cell growth and proliferation. [Internet] Biores. Open Access. 2014;3:53–9. doi: 10.1089/biores.2014.0001. ** This manuscript demonstrates gastric cancer cells have the hedgehog signaling molecules SHH, PTCH, GLI1, and GLI2, and are responsive to hedgehog specific inhibition.**
- 3.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma [Internet] Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 4.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. [Internet] J. Biol. Chem. 2007;282:33265–74. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 5.Tokhunts R, Singh S, Chu T, D’Angelo G, Baubet V, Goetz JA, Huang Z, Yuan Z, Ascano M, Zavros Y, et al. The Full-length Unprocessed Hedgehog Protein Is an Active Signaling Molecule [Internet] J. Biol. Chem. 2010;285:2562–2568. doi: 10.1074/jbc.M109.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Zaatari M, Zavros Y, Tessier A, Waghray M, Lentz S, Gumucio D, Todisco A, Merchant JL. Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion. [Internet] Gastroenterology. 2010;139:2061–2071. doi: 10.1053/j.gastro.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant JL. Hedgehog signaling in gut development, physiology and cancer. [Internet] J. Physiol. 2012;590:421–32. doi: 10.1113/jphysiol.2011.220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun JI, Kim HR, Park H, Kim SK, Lee J. Small molecule inhibitors of the hedgehog signaling pathway for the treatment of cancer. Arch. Pharm. Res. 2012;35:1317–33. doi: 10.1007/s12272-012-0801-8. [DOI] [PubMed] [Google Scholar]
- 9.Yoon C, Park DJ, Schmidt B, Thomas NJ, Lee HJ, Kim TS, Janjigian YY, Cohen DJ, Yoon SS. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014;20:3974–3988. doi: 10.1158/1078-0432.CCR-14-0011. ** This paper shows that HH signaling maintains a CSC phenotype in gastric cancer cells that highly express CD44.**
- 10.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. [Internet] PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2009;106:21666–71. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winklmayr M, Schmid C, Laner-Plamberger S, Kaser A, Aberger F, Eichberger T, Frischauf A-M. Non-consensus GLI binding sites in Hedgehog target gene regulation. [Internet] BMC Mol. Biol. 2010;11:2. doi: 10.1186/1471-2199-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coni S, Infante P, Gulino A. Control of stem cells and cancer stem cells by Hedgehog signaling: pharmacologic clues from pathway dissection. [Internet] Biochem. Pharmacol. 2013;85:623–8. doi: 10.1016/j.bcp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Hooper JE, Scott MP. Communicating with Hedgehogs. [Internet] Nat. Rev. Mol. Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 15.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. [Internet] Gastroenterology. 2010;138:550–61. 561.e1–8. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng R, Aihara E, Kenny S, Yang L, Li J, Varro A, Montrose MH, Shroyer NF, Wang TC, Shivdasani RA, et al. Indian Hedgehog Mediates Gastrin-Induced Proliferation in Stomach of Adult Mice. Gastroenterology. 2014;147:655–666.e9. doi: 10.1053/j.gastro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engevik AC, Feng R, Yang L, Zavros Y. The acid-secreting parietal cell as an endocrine source of Sonic Hedgehog during gastric repair. [Internet] Endocrinology. 2013;154:4627–39. doi: 10.1210/en.2013-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao C, Feng R, Engevik AC, Martin JR, Tritschler JA, Schumacher M, Koncar R, Roland J, Nam KT, Goldenring JR, et al. Sonic Hedgehog contributes to gastric mucosal restitution after injury. [Internet] Lab. Invest. 2013;93:96–111. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenring JR, Ray GS, Soroka CJ, Smith J, Modlin IM, Meise KS, Coffey RJ. Overexpression of transforming growth factor-α alters differentiation of gastric cell lineages [Internet] Dig. Dis. Sci. 1996;41:773–784. doi: 10.1007/BF02213134. [DOI] [PubMed] [Google Scholar]
- 20.Larsen B, Tarp U, Kristensen E. Familial giant hypertrophic gastritis (Ménétrier’s disease). [Internet] Gut. 1987;28:1517–21. doi: 10.1136/gut.28.11.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. [Internet] J. Cell Sci. 2003;116:1959–67. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 22.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. [Internet] Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation [Internet] Oncogene. 2005;25:609. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev. Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao C, Ogle SA, Schumacher MA, Schilling N, Tokhunts RA, Orr-Asman MA, Miller ML, Robbins DJ, Hollande F, Zavros Y. Hedgehog signaling regulates E- cadherin expression for the maintenance of the actin cytoskeleton and tight junctions [Internet] AJP Gastrointest. Liver Physiol. 2010;299:G1252–G1265. doi: 10.1152/ajpgi.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga VM. Cell–cell adhesion and signaling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion [Internet] Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 28.Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. [Internet] Am. J. Gastroenterol. 2005;100:581–7. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- 29.El-Zaatari M, Tobias A, Grabowska AM, Kumari R, Scotting PJ, Kaye P, Atherton J, Clarke PA, Powe DG, Farewell V. De-regulation of the sonic hedgehog pathway in the InsGas mouse model of gastric carcinogenesis [Internet] Br. J. Cancer. 2007;96:1855–1861. doi: 10.1038/sj.bjc.6603782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Zaatari M, Zavros Y, Tessier A, Waghray M, Lentz S, Gumucio D, Todisco A, Merchant JL. Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion. [Internet] Gastroenterology. 2010;139:2061–2071.e2. doi: 10.1053/j.gastro.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. [Internet] Gastroenterology. 2010;138:562–72. 572.e1–2. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher MA, Donnelly JM, Engevik AC, Xiao C, Yang L, Kenny S, Varro A, Hollande F, Samuelson LC, Zavros Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. [Internet] Gastroenterology. 2012;142:1150–1159.e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacher MA, Feng R, Aihara E, Engevik AC, Montrose MH, Ottemann KM, Zavros Y. Helicobacter pylori -induced Sonic Hedgehog Expression is Regulated by NFκB Pathway Activation: The Use of a Novel In vitro Model to Study Epithelial Response to Infection [Internet] Helicobacter. 2015;20:19–28. doi: 10.1111/hel.12152. ** .**This work is novel in that it uses a gastric organoid culture system to show a role for HH signaling in H. pylori infection.**
- 34.Oshima R, Iwamoto M, Osawa H, Kaneko Y, Muto H, Sato K, Sugano K. Helicobacter pylori infection induces the loss of sonic hedgehog and patched expression [Internet] Gastroenterology. 2003;124:A589. [Google Scholar]
- 35.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. [Internet] Gastroenterology. 2010;138:562–72. 572, e1–2. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. [Internet] Am. J. Gastroenterol. 2005;100:581–7. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher MA, Donnelly JM, Hollande F, Zavros Y. Sonic Hedgehog Mediates the Development of the Gastric Immune Response to Helicobacter pylori Infection [Internet] Gastroenterology. 2011;140:S-92. [Google Scholar]
- 38.Khosravi Shahi P, Díaz Muñoz de la Espada VM, García Alfonso P, Encina García S, Izarzugaza Perón Y, Arranz Cozar JL, Hernández Marín B, Pérez Manga G. Management of gastric adenocarcinoma [Internet] Clin. Transl. Oncol. 2007;9:438–442. doi: 10.1007/s12094-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 39.Berman DM, Karhadkar SS, Maitra A, Montes de Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours [Internet] Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Chen K, Huang S, Zhang X, Adegboyega PA, Evers BM, Zhang H, Xie J. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. [Internet] Carcinogenesis. 2005;26:1698–705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H, et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. [Internet] Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Syu L-J, Zhao X, Zhang Y, Grachtchouk M, Demitrack E, Ermilov A, Wilbert DM, Zheng X, Kaatz A, Greenson JK, et al. Invasive mouse gastric adenocarcinomas arising from Lgr5+ stem cells are dependent on crosstalk between the Hedgehog/GLI2 and mTOR pathways. Oncotarget. 2016;7:10255–10270. doi: 10.18632/oncotarget.7182. ** This work shows that activation of HH in Lgr5+ stem cells drives tumor development, and thus documents the importance of tissue specific context in defining stem cell responsiveness to stimuli that are oncogenic in nature.**
- 43.Li H, Stoicov C, Rogers A, Houghton J. Stem cells and cancer: evidence for bone marrow stem cells in epithelial cancers [Internet] GSBS Student Publ. 2006 doi: 10.3748/wjg.v12.i3.363. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly JM, Chawla A, Houghton J, Zavros Y. Sonic hedgehog mediates the proliferation and recruitment of transformed mesenchymal stem cells to the stomach. [Internet] PLoS One. 2013;8:e75225. doi: 10.1371/journal.pone.0075225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnelly JM, Engevik A, Feng R, Xiao C, Boivin GP, Li J, Houghton J, Zavros Y. Mesenchymal stem cells induce epithelial proliferation within the inflamed stomach. [Internet] Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G1075–88. doi: 10.1152/ajpgi.00489.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen J, Lee J, Malhotra A, Nahta R, Arnold AR, Buss MC, Brown BD, Maier C, Kenney AM, Remke M, et al. WIP1 modulates responsiveness to Sonic Hedgehog signaling in neuronal precursor cells and medulloblastoma [Internet] Oncogene. 2016 doi: 10.1038/onc.2016.96. doi:10.1038/onc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dagklis A, Pauwels D, Lahortiga I, Geerdens E, Bittoun E, Cauwelier B, Tousseyn T, Uyttebroeck A, Maertens J, Verhoef G, et al. Hedgehog pathway mutations in T-cell acute lymphoblastic leukemia. [Internet] Haematologica. 2015;100:e102–5. doi: 10.3324/haematol.2014.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Memmi EM, Sanarico AG, Giacobbe A, Peschiaroli A, Frezza V, Cicalese A, Pisati F, Tosoni D, Zhou H, Tonon G, et al. p63 sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling [Internet] Proc. Natl. Acad. Sci. 2015;112:3499–3504. doi: 10.1073/pnas.1500762112. ** This study highlights the role of HH signaling in modulating p63 expression during the self-renewal of mammary cancer stem cells.**
- 49.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. [Internet] Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, Seplyarskiy VB, Sharpe HJ, McKee T, Letourneau A, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma [Internet] Nat. Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 51.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. 2012 doi: 10.1056/NEJMoa1113713. http://dx.doi.org/10.1056/NEJMoa1113713. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H-W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. [Internet] Cancers (Basel) 2016;8 doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu M, Gong A, Yang H, George SK, Jiao Z, Huang H, Jiang X, Zhang Y. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. [Internet] Cancer Lett. 2015;369:124–33. doi: 10.1016/j.canlet.2015.08.005. ** *This study shows that expression of CD44 and Musashi-1 may be responsible for resistance to drug treatment in gastric cancer.**