Abstract
Age-related cataract is associated with oxidative stress and death of lens epithelial cells (LECs) whose survival is dependent on functional mitochondrial populations. Oxidative stress-induced depolarization/damage of LEC mitochondria results in increased reactive oxygen species (ROS) levels and cell death suggesting the need for a LEC mechanism to remove mitochondria depolarized/damaged upon oxidative stress exposure to prevent ROS release and LEC death. To date, a mechanism(s) for removal of depolarized/damaged LEC mitochondria has yet to be identified and the importance of eliminating oxidative stress-damaged mitochondria to prevent LEC ROS release and death has not been established. Here, we demonstrate that Parkin levels increase in LECs exposed to H2O2-oxidative stress. We establish that Parkin translocates to LEC mitochondria depolarized upon oxidative stress exposure and that Parkin recruits p62/SQSTM1 to depolarized LEC mitochondria. We demonstrate that translocation of Parkin results in the elimination of depolarized/damaged mitochondria and that Parkin clearance of LEC mitochondria is dependent on its ubiquitin ligase activity. Importantly, we demonstrate that Parkin elimination of damaged LEC mitochondria results in reduced ROS levels and increased survival upon oxidative stress exposure. These results establish that Parkin functions to eliminate LEC mitochondria depolarized/damaged upon oxidative stress exposure and that elimination of damaged mitochondria by Parkin is important for LEC homeostasis and survival. The data also suggest that mitochondrial quality control by Parkin could play a role in lens transparency.
Keywords: Oxidative stress, Reactive oxygen species, Mitochondria, Lens cell survival, Cataract
Introduction
The eye lens is composed of a single layer of organelle-containing lens epithelial cells (LECs) that overlies a core of transparent organelle-free lens fiber cells [1]. The homeostasis and survival of LECs is essential for the transparency of the entire lens [1-4] since damage to LECs [5, 6] and their sub-cellular components [7-10] has been suggested to result in cataract formation. Despite advances in surgical techniques, cataract remains a significant cause of world blindness and it has been estimated that any therapy that could delay the onset of cataract by just ten years could half the number of cataract surgeries required annually [11], improving the quality and reducing the cost of visual healthcare.
A key contributor to LEC death and cataract formation is exposure of the lens to oxidative stress [12-16]. Oxidative stress exposure results in damage to a wide-array of LEC components including LEC mitochondria that are particularly sensitive to oxidative stress exposure [7, 12, 13, 17, 18]. Oxidative stress damage to LEC mitochondria is characterized by mitochondrial depolarization [12], increased production of mitochondrial reactive oxygen species (ROS) [12, 17, 18] and LEC death [12, 17].
To prevent oxidative stress-induced damage to LEC mitochondria and thereby prevent LEC death, multiple anti-oxidant [3, 12, 17, 19, 20] and chaperone systems [3, 21-24] function to defend LEC mitochondria against depolarization/damage and thereby prevent increased ROS levels. However, despite the presence of these protective systems, high-level or chronic exposures of LECs to oxidative stress results in increased ROS levels and LEC death [12, 17] and long-term exposure of the lens to hyperbaric oxygen-oxidative stress results in cataract formation in animal models [7, 25-27]. These data suggest the need for an LEC mechanism(s) to remove damaged mitochondria to prevent increased ROS levels and LEC death. However, to date, no LEC mechanism for removal of damaged mitochondria has been identified and the effect of removing damaged LEC mitochondria on ROS levels or survival has not been established.
Evidence for the existence of a mechanism that could function to remove damaged LEC mitochondria was recently provided through the identification of mitochondria contained within autophagolysosomes of embryonic chick and adult human LECs [28]. This observation suggests that the selective autophagy process called mitophagy that eliminates mitochondria [29-32], could function to eliminate LEC mitochondria damaged upon oxidative stress exposure.
One protein that functions to remove damaged mitochondria in multiple cell-types is Parkin, Parkin operates in conjunction with the actions of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (Pink1), mitochondrial-processing protease (MPP), and presenillin-associated rhomboid-like protein (PARL) [29-32]. Under normal conditions, Pink1 is cycled through the inner mitochondrial membrane where it is cleaved by several proteases including MPP and inner membrane PARL [29]. In depolarized/damaged mitochondria, unprocessed Pink1 is retained on the outer mitochondrial membrane where it phosphorylates Parkin [33, 34]. Phosphorylation of Parkin by PINK1 results in its conversion into an active phospho-ubiquitin-dependent E3 ligase that ubiquitinates itself [35] and multiple mitochondrial outer membrane (OMM) proteins [35-39]. These ubiquitinated mitochondrial proteins are then recognized by ubiquitin-binding adaptor proteins such as p62/sequestosome 1 (p62/SQSTM1) [40-43]. p62/SQSTM1 contains an LC3B-interacting region (LIR) that recruits LC3B-labeled autophagosomes to engulf and degrade the damaged mitochondria [41-43]. Consistent with a potential role for Parkin in the removal of LEC mitochondria damaged upon oxidative stress exposure, DNA miccroarray [44] and RNA sequencing analysis [45] revealed that Parkin, Pink1, MPP, PARL and p62/SQSTM1 are expressed by LECs.
Here, we demonstrate that Parkin levels increase in LECs exposed to H2O2-oxidative stress. We establish that Parkin translocates to LEC mitochondria depolarized upon oxidative stress exposure and that Parkin recruits p62/SQSTM1 to depolarized LEC mitochondria. We demonstrate that translocation of Parkin results in the elimination of depolarized/damaged LEC mitochondria resulting from oxidative stress exposure and that Parkin elimination of damaged LEC mitochondria is dependent on its ubiquitin ligase activity. Importantly, we demonstrate that Parkin elimination of damaged LEC mitochondria results in reduced LEC ROS levels and increased LEC survival upon oxidative stress exposure. These results establish that Parkin functions to eliminate LEC mitochondria damaged upon oxidative stress exposure and that elimination of damaged mitochondria by Parkin is important for LEC homeostasis and survival. The data also provide evidence that LEC mitochondrial quality control by Parkin could play an important role in the maintenance of lens transparency.
Materials and Methods
Cell culture of human lens epithelial cells
Human lens epithelial cells (SRA 01/04) [46] were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 15% FBS (Invitrogen), gentamicin (50 units/ml; Invitrogen), penicillin-streptomycin antibiotic mix (50 units/ml; Invitrogen) and fungizone (5 μl/ml; Invitrogen) at 37 °C in the presence of 5% CO2.
Preparation of chicken primary lens epithelial cells
Primary chicken LEC cultures were prepared from the lenses of Embryonic day 10 (E10) White Leghorn embryonated chicken eggs (Charles River Laboratories, Storrs, CT) using the method of Menko et al. [47]. Briefly, primary lens cells were isolated from chicken lenses by trypsinization and agitation. Cells were plated onto glass bottom dishes coated with mouse laminin (Invitrogen) and cultured in Medium 199 (Invitrogen) supplemented with 10% FBS (Invitrogen) and penicillin-streptomycin antibiotic mix (50 units/ml; Invitrogen).
Transfection of human and primary chick lens epithelial cells
For transient transfection, SRA 01/04 were plated onto 35 mm2 glass bottom tissue culture dishes (CellVis, Mountain View, CA) in 2 mls of media or 12 well glass bottom dishes (Matek, Ashland MA) in 1 ml of antibiotic free media and transfected with YFP-wt-Parkin or mutant YFP-C431N-Parkin vectors that were gifts from Dr. Richard Youle, [48] (Addgene, Cambridge, MA; Plasmid #23955 and #46924 respectively) using Lipofectamine 2000® transfection reagent (Invitrogen) according to the manufacturer’s instructions. For transient transfection the efficiency was approximately 50% in SRA 01/04 cells transfected with either vector. Transient transfection was confirmed by visualizing YFP in transfected cells using a Zeiss LSM 700 Confocal microscope.
Primary chicken LECs were plated onto 12 well glass bottom dishes in 1 ml of media , allowed to adhere and reverse transfected with YFP-wt-Parkin or mutant YFP-C431N-Parkin vectors using Lipofectamine 2000® (Invitrogen) transfection reagent according to the manufacturer’s instructions. Transfection efficiency was approximately 60% in primary chicken LECs transfected with either vector. Transient transfection was confirmed by visualizing YFP in transfected cells using a Zeiss LSM 700 Confocal microscope.
For both SRA 01/04 and primary chick LECs, cells were treated 48 h post transfection.
Creation of stable overexpressing SRA 01/04 cell lines
SRA 01/04 cells were transfected as described above with either YFP-wt-Parkin or mutant YFP-C431N-Parkin and incubated for 48 h. After 48 h cells were passaged into media containing 1.4 mg/ml Geneticin for selection of transfected cells. Clonal populations of cells remaining after a number of passages were transferred to 96 well plates and grown in the presence of Geneticin. Three cell lines were created for each vector and stored in media + 10% DMSO in liquid nitrogen. YFP-wt-Parkin or mutant YFP-C431N-Parkin transcripts levels were evaluated relative to a stable GFP overexpressing SRA 01/04 cell line by semi-quantitative RT-PCR using the SuperScript® III one-step RT-PCR system with Platinum Taq polymerase (Invitrogen) according to the manufacturer’s instructions. Total RNA was purified from cells using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. 200 ng of total RNA was assayed following isolation from cell lines. Parkin transcripts were amplified for 30 PCR cycles with a 52 °C annealing temperature and the primer sequences: Forward primer – CTGTGCAGAATTGTGACCT and reverse primer – GCAAAGCTACTGATGTTTCC. GAPDH was amplified as the internal control transcript for 20 PCR cycles with a 60 °C annealing temperature using forward primer – CCACCCATGGCAAATTCCATGGCA and reverse primer - TCTAGACGGCAGGTCAGGTCCACC. Parkin protein levels were also evaluated by western blot analysis using a Parkin-specific antibody (Santa Cruz Biotech, Dallas, TX).
Oxidative stress treatment of lens epithelial cells
To examine the effect of oxidative stress on Parkin mediated clearance of mitochondria, SRA 01/04 LECs that were transiently or stably transfected with YFP-wt-Parkin or mutant YFP-C431N-Parkin vectors were plated onto 35 mm2 tissue culture dishes in 2 mls of media or 12 well glass bottom dishes in 1 ml of media. Cells were transferred to serum free media and treated with indicated amounts of H2O2 for indicated times. The volume of H2O2 added was proportional to the volume of media i.e the stock concentration was consistent. Following treatment, cells were fixed and stained as detailed below or cells were lysed in NP40 lysis (50mM Tris-HCl pH 7.4, 150mM NaCl, 1mM EDTA, 1% NP40, 0.25% sodium deoxycholate) buffer supplemented with a protease inhibitor cocktail (Sigma, St Louis, MO). Following lysis, samples were briefly sonicated and centrifuged for 5 mins at 10,000 rpm. The supernatant was stored at −20 °C.
Analysis of Parkin protein levels in human lens cells following exposure to oxidative stress
SRA 01/04 LECs were seeded onto 6 well plates at a density of 2 × 105 cells per well. Cells were incubated in serum free medium for 2 h and then exposed to 200 μM H2O2 (or PBS as a control) for 2 h in serum free medium as previously described [12, 19]. Cells were washed once in PBS and returned to serum-supplemented medium (15% serum) for a 24 h recovery. Cells were harvested after 24 h and protein isolated. For protein isolation, cells were washed with ice cold PBS and directly lysed on the plate in NP40 lysis buffer containing 1:1000 protease inhibitor cocktail. The lysates were sonicated for 10 sec × 2 and centrifuged at 10,000 × g for 5 min; the supernatant containing the total cell extract was stored at −20 °C until used. Parkin protein levels were analyzed by western blot analysis as described below.
Isolation of cytosolic and mitochondrial proteins
SRA 01/04 cells (used between passage 62 and 70) were treated with H2O2 as described above, washed once in PBS and harvested by trypsinization. Cells were pelleted and the pellet frozen at −80 °C to weaken the cell membrane. Mitochondria were isolated using a mitochondrial isolation kit for cultured mammalian cells (Abcam, Cambridge, MA) following the manufacturer’s instructions. The cytosolic and mitochondrial protein fractions were stored at −80 °C until used. Protein concentrations were determined by Bradford protein assay. Western blot analysis was carried out as previously described [44].
Immunocytochemistry
SRA 01/04 lens cells or primary chick LECs were plated onto 35 mm2 glass bottom tissue culture dishes or 12 well glass bottom dishes and incubated overnight in complete media. Cells were treated with indicated amounts of H2O2 for indicated times. Following exposure to H2O2, immunostaining was conducted by fixing cells with 3.7% formaldehyde in PBS, permeabilizing cells with 0.25% Triton X-100 in PBS and blocking with 1% BSA. For co-localization of Parkin and mitochondria following oxidative stress treatment, cells were fixed and stained with a Parkin-specific rabbit antibody (Santa Cruz) and a TOMM20-specific mouse antibody (Santa Cruz) and counterstained with nuclear stain DAPI (Molecular probes, ThermoFisher). To co-localize mitochondria and p62/SQSTM1, SRA 01/04 LECs were transiently transfected with YFP-wt-Parkin or YFP-C431N-Parkin and treated with H2O2 or CCCP as described above. Following treatment, cells were immunostained by incubating with a TOMM20-specific rabbit antibody (Santa Cruz) and a p62/SQSTM1-specific mouse monoclonal antibody (BD Pharmingen). Following overnight incubation in primary antibody cells were washed three times with PBS, and subsequently incubated with Alexa Fluor 555 goat anti-rabbit secondary and Alexa Fluor 680 anti-mouse secondary antibody (Invitrogen) for 1 h at room temperature at 1:2000 dilutions. Cells were washed with three times with PBS, counterstained with 300 nM DAPI and stored in PBS until imaged. Immunofluorescent staining was visualized using a Zeiss LSM 700 Confocal microscope using the 40 X objective. For SRA 01/04 LECs, at least 100 cells were examined and representative images presented, for primary chick LECs that form lentoid type structures, at least 10 fields of view were examined and representative images presented.
Detection of ROS production
For ROS detection, SRA 01/04 cells stably overexpressing YFP-wt-Parkin or YFP-C431N-Parkin were plated onto 35 mm2 glass bottom dishes and incubated overnight in complete media. Cells were treated with 0, 200 or 300 μM H2O2 for 24 h in serum free media. Following exposure to H2O2 cells were washed once in PBS and incubated with 20 μM dihydroethidium (DHE, Molecular Probes, ThermoFisher) for 20 mins at 37 °C. ROS staining was examined using a LSM 700 Confocal microscope, at least 100 cells were examined and representative images presented.
Cell-Viability Assay
Cell viability was assayed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) kit containing the tetrazolium compound MTS according to the manufacturer’s protocols. MTS color change was monitored using a SpectraMax M5e microplate reader (Molecular Devices, Sunnyvale, CA) set at an absorbance reading of 492 nm.
SRA 01/04 cells stably overexpressing either YFP-wt-Parkin or YFP-C431N-Parkin plated in 96-well plates at a density of 10,000 cells per well and treated with increasing concentrations of H2O2 for 24 h in serum-free media. H2O2 treatments were analyzed in sets of eight identical treatments, and cell viability was monitored by using the MTS assay described above. Mean absorbance and standard deviations for each treatment were determined. Differences among treatments were determined using Student’s t-test assuming equal variance where p < 0.01 was considered statistically significant.
Results
Increased levels of Parkin are detected in lens epithelial cells exposed to H2O2-oxidative stress
In order to establish the levels of Parkin protein in LECs exposed to oxidative stress, we exposed human LECs (SRA 01/04) to varying levels of H2O2, and examined Parkin protein levels by immunoblotting with a Parkin-specific antibody (Fig. 1). Levels of H2O2 (0-300 μM) and incubation conditions (2 hours in serum-free media, followed by 2 hours in serum free media containing H2O2 followed by a 24 h recovery in serum-containing media) were chosen based on preliminary data showing that these levels resulted in mitochondrial membrane depolarization in LECs (data not shown) and to parallel those employed in previous studies on lens epithelial cell responses to H2O2-treatment [49, 50]. Our previous studies demonstrated that treatment of LECs with oxidative stress using similar conditions resulted in loss of lens epithelial cell mitochondrial function [12], generation of ROS in LECs [12] and lens epithelial cell death [12, 19].
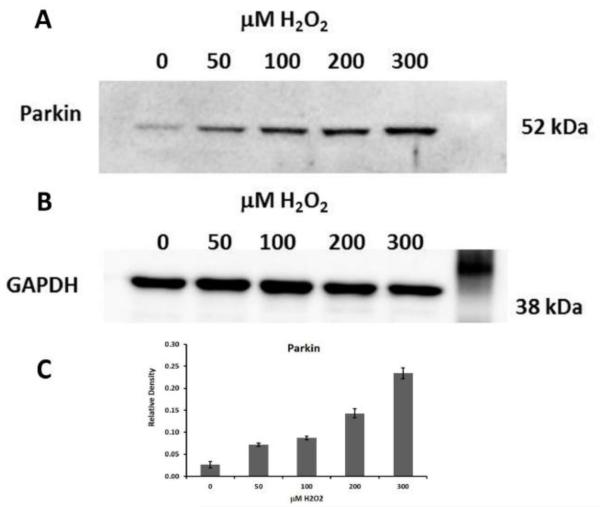
Fig 1.0. Parkin levels increase in lens epithelial cells exposed to H2O2-oxidative stress.
A. Immunoblot analysis of Parkin protein levels in 15 μg of total protein extract isolated from SRA 01/04 lens epithelial cells. SRA 01/04s were treated with indicated amounts of H2O2 in serum free media for 2 h and allowed to recover in complete media for 24 h. B. Immunoblot for GAPDH is shown as a control for equal protein loading. C. Densitometric analyses of the immunoblots plotted as Parkin levels as a ratio to GAPDH loading control.
The analysis revealed that Parkin protein levels were increased in H202-treated LECs relative to untreated cells (Fig. 1A). Parkin levels increased in parallel with the levels of H2O2 up to the maximum 300 μM level of H2O2 used (Fig. 1A). By contrast, GAPDH levels remained constant at all levels of H2O2 examined (Fig. 1B). These results provide evidence that Parkin protein levels increase in lens epithelial cells exposed to H2O2.
Parkin translocates to mitochondria of lens epithelial cells upon H2O2-oxidative stress exposure
Previous studies demonstrated that treatment of HEK293 [40] and HeLa cells [51] with the mitochondrial electron transport uncoupler Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) resulted in translocation of Parkin to the resulting depolarized mitochondria. Since Parkin levels increased in LECs upon H2O2-oxidative stress exposure, and since H2O2-oxidative stress exposure results in mitochondrial depolarization in LECs [12], we sought to determine if exposure of LECs to H2O2-oxidative stress results in translocation of Parkin to the resulting depolarized LEC mitochondria.
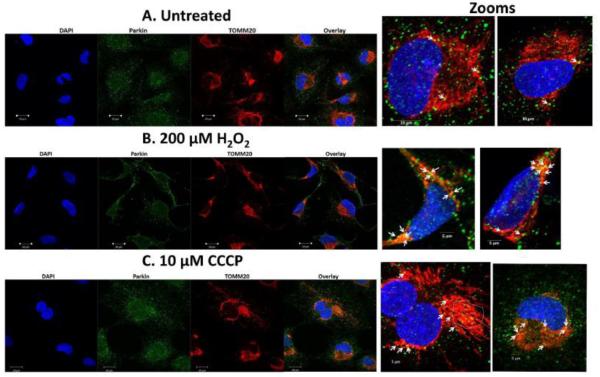
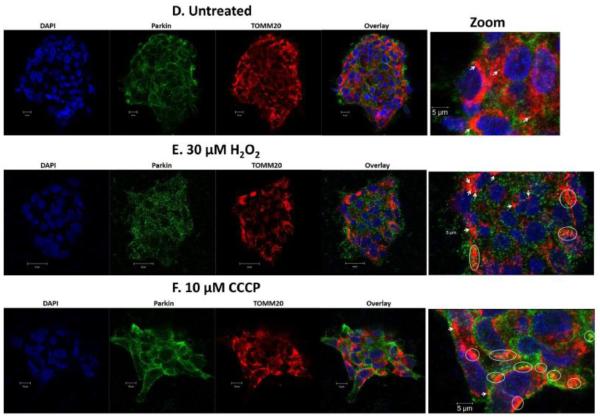
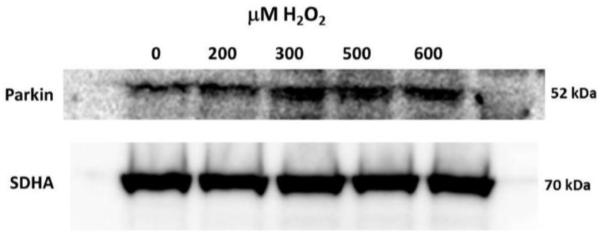
Human SRA 01/04 LECs (Fig. 2B) and primary chick LECs (Fig. 2E) were treated with H2O2 in the absence of serum for 5 hours, co-stained with specific antibodies for Parkin (green) and TOMM20 (red) (a mitochondrial outer membrane marker) and imaged by confocal microscopy. Images were compared with those obtained for untreated SRA 01/04 (Fig. 2A) and primary chick LECs (Fig. 2D) and with those of the same cells treated with 10 μM CCCP for one hour (Figs. 2C and 2F). Comparison of the overlay images reveals increased numbers of distinct yellow puncta consistent with translocation of Parkin to the mitochondria of the H2O2-treated SRA 01/04 (Fig. 2B) and primary chick LECs (Fig. 2E). As a positive control, increased numbers of yellow puncta were also observed in SRA 01/04 (Fig. 2C) and primary chick LECs (Fig. 2F) treated with the mitochondrial uncoupler CCCP. By contrast, fewer yellow puncta were observed in the untreated SRA 01/04 (Fig. 2A) and primary chick LECs (Fig. 2D). Parkin translocation to the mitochondria upon H2O2-exposure was further confirmed by western blotting of protein fractions of purified mitochondria obtained from SRA 01/04 LECs treated for 4 h with a range of H2O2 concentrations (0-500 μM) (Fig. 3). As expected, levels of Parkin detected in LEC mitochondria protein fractions increased with increasing levels of H2O2-oxidative stress exposure (Fig. 3).
Fig 2.0. Parkin translocates to lens epithelial cell mitochondria exposed to H2O2-oxidative stress.
SRA 01/04 lens epithelial cells (A-C) or primary chick lens epithelial cells (D-F) were treated with either H2O2-induced oxidative stress (B,E) or the mitochondrial uncoupler CCCP (C,F) and compared to untreated cells (A,D). Cells were treated with H2O2 for 5 h and CCCP for 1 h. Following treatment, cells were immunostained for Parkin (green), the outer mitochondrial membrane protein TOMM20 (red) and counterstained with DAPI (blue). Images were obtained using a confocal fluorescent microscope. All images were obtained using the 40 × objective. In representative images arrows show co-localization of Parkin (green) and TOMM20 (red) resulting in yellow puncta in the overlay image (zoomed images). Areas with large numbers of yellow puncta are outlined.
Fig 3.0. Parkin levels increase in mitochondria of lens epithelial cells exposed to H2O2-oxidative stress.
Immunoblot analysis of Parkin levels in mitochondria isolated from H2O2 treated SRA 01/04 lens epithelial cells. SRA 01/04 cells were equilibrated in serum free media for 2 h and treated with indicated amounts of H2O2 for 4 h. Immunoblot for mitochondrial protein SDHA is shown as a control for equal protein loading.
Parkin ubiquitin ligase activity is required to recruit p62/SQSTM1 to mitochondria of lens epithelial cells exposed to H2O2-oxidative stress
Previous studies suggest that Parkin auto-ubiquitination [52] and its ubiquitination of mitochondrial outer membrane (OMM) proteins results in the recruitment of p62/SQSTM1 to the mitochondria for recruitment of LC3B-labeled autophagosomes [40-43]. To establish if Parkin ubiquitin ligase activity is required to recruit p62/SQSTM1 to lens epithelial cell mitochondria damaged upon exposure to H2O2, wild-type Parkin (Fig. 4) or a mutant form of Parkin that lacks ubiquitin ligase activity (C431N-Parkin, Fig. 5) that were fused with yellow green fluorescent protein (YFP) were transiently transfected into SRA 01/04 LECs. Cells were subsequently treated with either 200 μM H2O2 (Figs 4B & 5B), or 10 μM CCCP (Figs 4C & 5C) as a positive control, while untreated cells served as a negative control (Figs 4A & 5A). Cells were treated for 6 h and co-stained with specific antibodies for p62/SQSTM1 (pseudo green) and TOMM20 (pseudo red) and imaged by confocal microscopy. Cells overexpressing wild-type Parkin or C431N-Parkin were outlined only in images and p62/SQSTM1 and TOMM20 were pseudo colored to make the images easier to interpret.
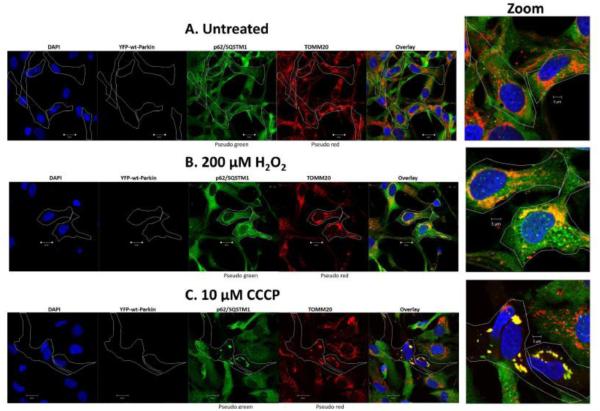
Fig 4.0. Wt-Parkin recruits p62/SQSTM1 to damaged mitochondria in lens epithelial cells exposed to H2O2-oxidative stress.
SRA 01/04 lens epithelial cells (A-C) were transiently transfected with YFP-wt-Parkin and treated with either 200 μM H2O2 (B) or 10 μM CCCP (C) for 6 h and compared to untreated cells (A). Following treatment cells were immunostained for p62/SQSTM1 (pseudo green) and TOMM20 (pseudo red) and counterstained with DAPI (blue). Images were obtained using a confocal fluorescent microscope. All images were obtained using the 40 × objective. Cells overexpressing YFP-Parkin are outlined. In representative images, the overlay images show co-localization of p62/SQSTM1 (pseudo green) and TOMM20 (pseudo red) in YFP-wt-Parkin overexpressing cells (outline), resulting in yellow puncta. Examples of cells with yellow puncta are shown in large zoom for each treatment.
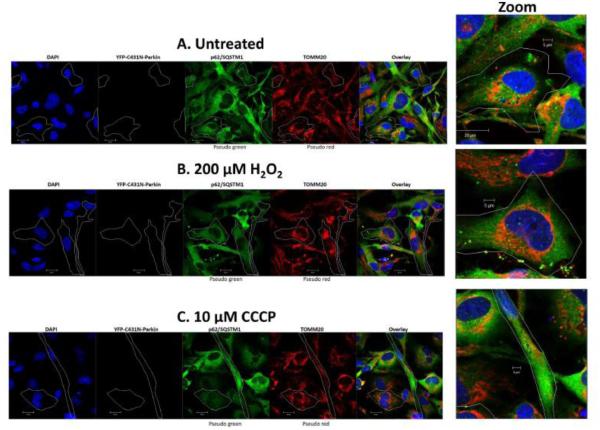
Fig 5.0. Mutant C431N-Parkin fails to recruit p62/SQSTM1 to damaged mitochondria in lens epithelial cells exposed to H2O2-oxidative stress.
SRA 01/04 lens epithelial cells (A-C) were transiently transfected with mutant YFP-C431N-Parkin and treated with either 200 μM H2O2 (B) or 10 μM CCCP (C) for 6 h and compared to untreated cells (A). Following treatment cells were immunostained for p62/SQSTM1 (pseudo green) and TOMM20 (pseudo red) and counterstained with DAPI (blue). Images were obtained using a confocal fluorescent microscope. All images were obtained using the 40 × objective. Cells overexpressing mutant YFP-C431N-Parkin are outlined. In representative images, the overlay images demonstrate that p62/SQSTM1 (pseudo green) fails to co-localize with TOMM20 (pseudo red) in mutant YFP-C431N-Parkin overexpressing cells (outline). Examples of mutant YFP-C431N-Parkin cells are shown in large zoom for each treatment.
Consistent with the oxidative stress-dependent translocation of Parkin to LEC mitochondria and the subsequent recruitment of p62/SQSTM1, comparison of the overlay images shows increased numbers of distinct yellow puncta consistent with co-localization of p62/SQSTM1 to TOMM20-labeled mitochondria in the H2O2-treated and CCCP-treated cells (Figs. 4B&C) that were overexpressing wild-type Parkin. Consistently, in cells overexpressing mutant C431N-Parkin fewer yellow puncta are present in cells treated with either H2O2 or CCCP (Figs. 5B&C) relative to cells overexpressing wild-type Parkin (Fig. 4A&B). Indeed, the levels of yellow puncta found in H2O2 or CCCP treated C431N-Parkin overexpressing cells were similar to that detected in the absence of H2O2- or CCCP-treatment (Fig. 4A and 5A) and were likely due to the presence of endogenous Parkin. The data suggests that Parkin E3-ubiquitin ligase is required to recruit p62/SQSTM1 to damaged mitochondria in LECs. Loss of p62/SQSTM1 could result in an inability to recruit autophagosomes and clear damaged mitochondria.
Parkin ubiquitin ligase activity is required for clearance of damaged mitochondria in lens epithelial cells exposed to H2O2-oxidative stress
To test the requirement for Parkin in the elimination of H2O2-damaged lens epithelial cell mitochondria, we tested the abilities of wild-type Parkin and the mutant form of Parkin that lacks ubiquitin ligase activity (Parkin C431N) [51] to clear damaged mitochondria in human LECs exposed to H2O2-oxidative stress. Wild-type Parkin or C431N-Parkin were transiently transfected into SRA 01/04 (Fig. 6A-F) or primary chick (Fig. 7A-D) LECs. Cells were subsequently treated with either 200 μM H2O2 (Fig. 6A-B), or 10 μM CCCP as a positive control (Fig. 6C-D, Fig. 7A-B) while untreated cells served as a negative control (Fig. 6E-F, Fig 7C-D). 48 h after treatment, the cells were fixed and stained with TOMM20-specific antibody (red) to detect remaining mitochondria.
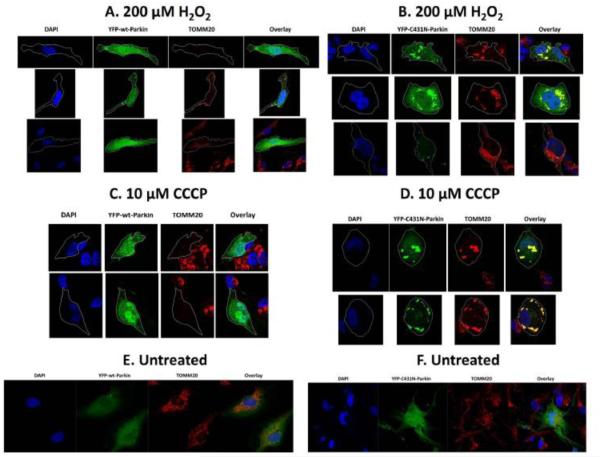
Fig 6.0. Parkin E3-ubiquitin ligase activity is required for clearance of damaged mitochondria in SRA 01/04 lens epithelial cells exposed to H2O2-oxidative stress.
SRA 01/04 lens epithelial cells (A-F) were transiently transfected with either YFP-wt-Parkin (green) or YFP-C431N-Parkin (green) and treated with either 200 μM H2O2 (A&B) or 10 μM CCCP (C&D) for 48 h and compared to untreated cells (E,F). Primary chick lens epithelial cells (G-J) were treated with 10 μM CCCP only (G,H) for 48 h and compared to untreated cells (I,J). Following a 48 h exposure to stress, cells were fixed and immunostained for outer mitochondrial marker protein TOMM20 (red) and counterstained with nuclear stain DAPI. Images were obtained using fluorescent confocal microscope. All images were obtained using the 40 × objective. Outlined cells in YFP-wt-Parkin show absence of TOMM20 stain indicating clearance of mitochondria. Outlined cells in YFP-C431N-Parkin overexpressing SRA 01/04 lens epithelial cells treated with either 200 μM H2O2 or 10 μM CCCP for 48 h show large yellow puncta in the overlay image indicating co-localization of YFP-C431N-Parkin (green) with mitochondria marker TOMM20 (red).
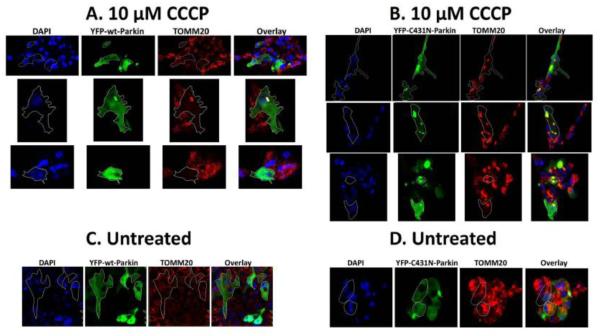
Fig 7.0. Parkin E3-ubiquitin ligase activity is required for clearance of damaged mitochondria in primary chick lens epithelial cells exposed to the mitochondrial uncoupler CCCP.
Primary chick lens epithelial (A-D) were transiently transfected with either YFP-wt-Parkin (green) or YFP-C431N-Parkin (green) and treated 10 μM CCCP (A&B) for 48 h and compared to untreated cells (C,D). Following a 48 h exposure to stress, cells were fixed and immunostained for outer mitochondrial marker protein TOMM20 (red) and counterstained with nuclear stain DAPI. Images were obtained using fluorescent confocal microscope. All images were obtained using the 40 × objective. Outlined cells in YFP-wt-Parkin show absence of TOMM20 stain indicating clearance of mitochondria. Outlined cells in YFP-C431N-Parkin overexpressing primary chick lens epithelial cells treated with10 μM CCCP for 48 h show large yellow puncta in the overlay image indicating co-localization of YFP-C431N-Parkin (green) with mitochondria marker TOMM20 (red).
Mitochondria damaged by H2O2-oxidative stress or CCCP exposures were completely eliminated in all SRA 01/04 cells overexpressing wild-type Parkin and in primary LECs overexpressing wild-type Parkin as evidenced by the absence of TOMM20 (red) in the outlined cells (Fig. 6A&C, Fig. 7A). By contrast, mutant C431N-Parkin translocates to damaged mitochondria as evidenced by yellow puncta that result from co-localization of TOMM20 (red) and Parkin (green), but fails to clear the damaged mitochondria resulting from H2O2-oxidative stress or CCCP treatment (Compare Figs. 6B&D and 7B). Interestingly Lazarou et al., [51] demonstrated delayed or defective mitochondrial translocation by multiple mutant C431X-Parkins, however they were able to rescue mutant C431N-Parkin (and other C431 mutations) translocation by co-transfecting with Parkin mutated in the c-terminal Ring1 domain only. The rescue was a result of self-association of Parkin that activates Parkin HECT-like activity that is required for translocation but not required for its E3-ubiquitin ligase activity. In the present study, we examined translocation and mitochondrial elimination after 48 h of oxidative stress, since we demonstrated induction of Parkin after 24 h of the same H2O2-oxidative stress (Fig 1.0), we believe that the transfected mutant C431N-Parkin self-associates with nascent wild-type Parkin to allow translocation to the mitochondria, but since self-association fails to activate its E3 ligase activity, C431N-Parkin fails to initiate elimination of damaged mitochondria. The data suggests that Parkin E3-ubiquitin ligase is required for elimination of damaged mitochondria in LECs and that loss of Parkin E3-ubiquitin ligase activity results in retention of damaged mitochondria.
Clearance of damaged mitochondria by Parkin results in reduced ROS levels in lens epithelial cells exposed to H2O2-oxidative stress
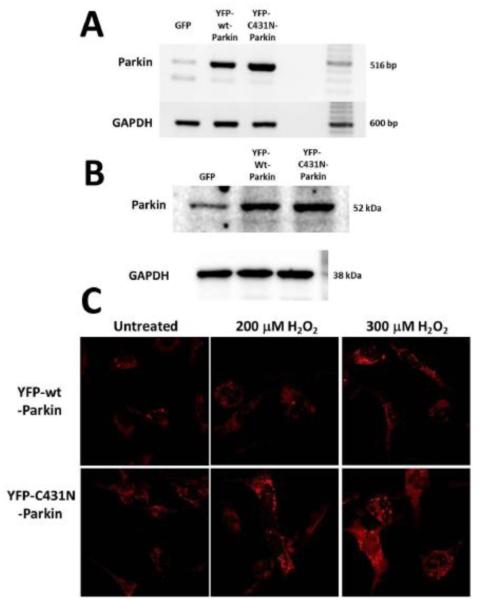
Since LEC mitochondria damaged upon H2O2 exposure release toxic ROS species [12, 17] we sought to determine whether clearance of damaged/depolarized mitochondria in LECs by Parkin could limit ROS levels in LECs exposed to H2O2. To test for a potential role for Parkin in limiting LEC ROS production upon oxidative stress exposure, we created SRA 01/04 LECs that stably overexpress either YFP-wt-Parkin or the mutant YFP-C431N-Parkin. Examination of transcript levels (Fig. 8A) and protein levels (Fig. 8B) demonstrated equal levels of overexpression for the wild-type and mutant forms of Parkin in the stably overexpressing cells. The resulting cells were exposed to H2O2 in the absence of serum. ROS formation in the treated cells was measured using the superoxide-specific detection reagent DHE [53].
Fig 8.0. Clearance of damaged mitochondria by Parkin results in decreased ROS levels in lens epithelial cells exposed to H2O2-oxidative stress.
A. Ethidium bromide stained agarose gels showing Parkin transcript levels and control GAPDH transcript levels in 200 ng RNA isolated from SRA 01/04 lens epithelial cells stably overexpressing GFP2 (control), YFP-wt-Parkin or YFP-C431N Parkin. B. Immunoblot analysis of Parkin protein levels in 15 μg of total protein extract isolated from SRA 01/04 lens epithelial cells stably overexpressing GFP2 (control), YFP-wt-Parkin or YFP-C431N Parkin. Immunoblot for GAPDH is shown as a control for equal protein loading. C. SRA 01/04 lens epithelial cells stably overexpressing either YFP-wt-Parkin or YFP-C431N Parkin were treated with 200 μM or 300 μM H2O2 for 24 h and stained for ROS production using the superoxide specific dye DHE. Images were obtained using live cell fluorescent confocal microscope.
Following exposure to 0, 200 or 300 μM H2O2, SRA 01/04 cells stably overexpressing mutant YFP-C431N-Parkin exhibited increased ROS production relative to SRA 01/04 cells stably overexpressing YFP-wt-Parkin (Fig. 8C) as evidenced by increased intensity of DHE staining. These data suggest that Parkin-mediated mitochondrial clearance functions to limit ROS production in LECs exposed to oxidative stress through its ability to target and clear ROS-producing damaged mitochondria in LECs.
Clearance of damaged lens epithelial cell mitochondria results in increased cell survival of lens epithelial cells exposed to H2O2-oxidative stress
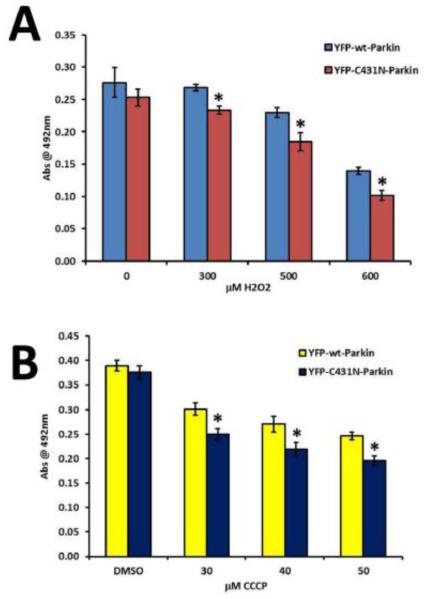
Increased levels of ROS are a key factor in the death of LECs exposed to H2O2-oxidative stress [12, 17]. Since oxidative stress exposed LECs exhibit mitochondrial retention and increased mitochondrial ROS production when overexpressing dysfunctional mutant YFP-C431N-Parkin relative to those overexpressing the functional YFP-wt-Parkin, we hypothesized that Parkin clearance of damaged mitochondria could impact the survival of LECs exposed to H2O2 oxidative stress through their ability to clear damaged mitochondria and thereby prevent ROS release that results in LEC death. To test this hypothesis, we compared the viability of LECs stably overexpressing wild-type Parkin upon exposure to 0, 300, 500 or 600 μM H2O2 in serum free media for 24 h relative to those overexpressing dysfunctional mutant YFP-C431N-Parkin using an MTS cell viability assay. As control, the same cell lines were exposed to the mitochondrial uncoupler CCCP for 24 h in media containing serum.
Consistent with our hypothesis, LEC overexpressing functional YFP-wt-Parkin exhibited increased viability (p<0.01, n=8) relative to the same cells overexpressing the dysfunctional mutant YFP-C431N-Parkin (Fig. 9A) following exposure to H2O2-oxidative stress. Consistently, LEC exposed to increasing levels of CCCP and overexpressing functional YFP-wt-Parkin also exhibited increased viability (p<0.01, n=8) relative to the same cells overexpressing dysfunctional mutant YFP-C431N- (Fig. 9B). These data provide evidence that Parkin-mediated clearance of damaged mitochondria functions to increase survival of LECs exposed to lethal levels of H2O2-oxidative stress.
Fig 9.0. Clearance of damaged mitochondria by Parkin results in increased survival of lens epithelial cells exposed to H2O2-oxidative stress.
Representative graphs depicting viability upon exposure to H2O2-induced oxidative stress or CCCP-induced mitochondrial depolarization in SRA 01/04 lens epithelial cells stably overexpressing either YFP-wt-Parkin or YFP-C431N-Parkin using MTS cell-viability assays. H2O2 (A) treatments were conducted for 24 h in serum-free media, (B) CCCP treatments were conducted in serum containing media for 24 h. Error bars represent standard deviations of eight separate cell-viability assays. Viability levels are shown as mean abs at 492 nm ± standard deviation. Differences among treatments were determined using Students T-test assuming equal variance where p < 0.01 was considered statistically significant. * p<.01 compared to YFP-wt-Parkin overexpressing SRA 01/04s.
Discussion
Age-related cataract is associated with oxidative stress [14-16] and it has been suggested to be associated with death of LECs [5], whose survival is dependent on the presence of functional mitochondrial populations [7, 12, 17]. Oxidative stress-induced depolarization/damage of LEC mitochondria results in increased ROS levels [12, 17, 18] and LEC death [12, 17]. These data suggest the need for an LEC mechanism(s) to remove damaged mitochondria to prevent increased ROS levels and LEC death. However, to date, no LEC mechanism for removal of damaged mitochondria has been identified and the effect of removing damaged LEC mitochondria on ROS levels or survival has not been established.
Here, we sought to establish how mitochondria damaged in LECs upon exposure to H2O2-oxidative stress are eliminated by Parkin and we examined whether their elimination could prevent the release of toxic ROS and result in increased LEC survival upon oxidative stress exposure. We chose H2O2 as an oxidative stress model since previous studies demonstrated that exposure of LECs to H2O2 resulted in depolarization of LEC mitochondria, ROS production and LEC death [12, 17, 18]. The levels of H2O2 chosen for these studies were consistent with those used to examine LEC gene expression [49], homeostasis [12, 19] and survival [12, 19] in previous studies on LEC mitochondrial function.
We specifically tested whether Parkin [29-32] could function to eliminate oxidative stress damaged mitochondria in LECs since it was detected to be significantly expressed in LECs in DNA microarray studies of human LECs [44] and RNA sequencing studies of microdissected embryonic chick LECs [45]. Our data demonstrate that exposure of LECs to as little as 50 μM H2O2 results in the accumulation of increased levels of Parkin protein (Fig. 1). Although further studies will be needed to establish the relationship between increased LEC Parkin levels and H2O2 exposure, these data suggest that increased Parkin transcription and/or increased stabilization of Parkin mRNA and/or protein could be an important cellular response of LECs to the presence of H2O2-oxidative stress. These results are consistent with previous studies that detected increased levels of Parkin upon exposure of dopaminergic neuroblastoma cells to dopamine [54].
Our data also demonstrate that Parkin translocates to damaged LEC mitochondria in transformed and primary LECs exposed to H2O2-oxidative stress (Fig. 2B and 2E) and that wild-type Parkin recruits p62/SQSTM1 to the damaged mitochondria (Fig. 4B&C), while mutant C431N-Parkin that lacks ubiquitin ligase activity fails to recruit p62/SQSTM1. These results provide evidence that Parkin removes damaged mitochondria in LECs by recruiting the autophagy machinery to engulf and degrade the damaged mitochondria since p62/SQSTM1 recruits autophagosomes through its LIR domain interaction with LC3b [41-43]. Consistently, we determined that following recruitment of p62/SQSTM1 to damaged mitochondria (Fig. 4B&C), Parkin eliminates damaged mitochondria from LECs exposed to H2O2 (Fig. 6B) or CCCP (Fig 6C and 7A). In contrast, the mutant form of Parkin (C431N) that lacks E3-ubiquitin ligase activity and fails to recruit p62/SQSTM1 to damaged mitochondria (Figs. 5B&C), subsequently fails to clear damaged mitochondria in LECs exposed to H2O2-treatment (Fig. 6B) or CCCP (Fig. 6C and 7B). Collectively, these data suggest that Parkin ubiquitin ligase activity is required for removal of damaged mitochondria in LECs.
Our data also provide evidence that Parkin functions to limit toxic ROS release in LECs upon H2O2-oxidative stress exposure (Fig. 8C). These data suggest that removal of damaged mitochondria in LECs exposed to oxidative stress prevents the release of ROS and therefore LEC damage caused by increased ROS levels. Importantly, overexpression of functional wild-type but not ubiquitin ligase-deficient mutant Parkin results in increased survival of LECs exposed to H2O2-oxidative stress (Fig. 9A) or the mitochondrial uncoupling agent CCCP (Fig. 9B). These data implicate Parkin-directed elimination of mitochondria in the homeostasis and survival of LECs upon oxidative stress exposure. To our knowledge, this is the first report for Parkin-directed elimination of mitochondria having a role in lens cell survival. Others have demonstrated that Parkin's neuroprotective role is exerted through elimination of damaged mitochondria via linear ubiquitination [55, 56].
It is tempting to speculate that loss of Parkin function could contribute directly to cataract formation. However, more data are needed to support this hypothesis since, to date, there are no reports of increased rates of cataract formation in Parkinson’s patients harboring an established Parkin mutation and there are no reports of cataract formation in Parkin-deficient mice [57-60]. It is possible that cataract could result in Parkin-deficient mice upon long-term exposure to oxidative stress as has been demonstrated for mice deficient for the lens mitochondrial repair enzyme methionine sulfoxide reductase A (MsrA) that only develop cataract after long-term exposure to hyperbaric oxygen oxidative stress [7]. Indeed, Parkin is not the only mediator of mitochondrial clearance found in lens cells and it is likely that other mitochondrial elimination pathways also function to eliminate mitochondria in lens cells and thereby contribute to LEC survival upon oxidative stress exposure. Consistently our RNA sequencing studies [45] reveal that multiple proteins implicated in elimination of mitochondria including BNIP3L/Nix [61-63], BNIP3 [64], SMURF1 [65] and FUNDC1 [66, 67] are expressed by LECs in addition to Parkin.
In conclusion, the present data demonstrate that increased levels of Parkin are a feature of LECs exposed to oxidative stress and that Parkin functions in LECs to remove mitochondria damaged upon oxidative stress exposure. The data provide evidence that elimination of damaged LEC mitochondria is important for the prevention of toxic ROS accumulation in LECs and that elimination of damaged mitochondria by Parkin is important for LEC survival upon oxidative stress exposure. Given the well-defined link between lens oxidative stress, lens mitochondrial damage and cataract formation [5, 7, 8, 14-16], the data suggest that the Parkin-mediated removal of damaged LEC mitochondria could be important for lens homeostasis upon exposure to oxidative stress and likely is important for the maintenance of lens transparency. Since oxidative stress and mitochondrial damage are hallmarks of many age-related diseases, these results provide insight into those mechanisms required for the homeostasis of multiple tissues and the etiology of multiple disease states.
Highlights.
Parkin levels increase in lens epithelial cells exposed to H2O2-oxidative stress.
Parkin translocates to damaged LEC mitochondria and recruits p62/SQSTM1.
Parkin clearance of damaged LEC mitochondria requires E3-ubiquitin ligase activity.
Clearance of damaged LEC mitochondria reduces ROS levels and increases LEC survival.
Parkin levels increase in lens epithelial cells exposed to H2O2-oxidative stress.
Parkin translocates to damaged LEC mitochondria and recruits p62/SQSTM1.
Parkin clearance of damaged LEC mitochondria requires E3-ubiquitin ligase activity.
Clearance of damaged LEC mitochondria reduces ROS levels and increases LEC survival.
Acknowledgments
This work was supported by the National Institutes of Health EY026478 (MK).
Abbreviations
- LEC
Lens epithelial cells
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1250–64. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delamere NA, Tamiya S. Lens ion transport: from basic concepts to regulation of Na,K-ATPase activity. Exp Eye Res. 2009;88(2):140–3. doi: 10.1016/j.exer.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88(2):195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat SP. The ocular lens epithelium. Bioscience Reports. 2001;21(4):537–63. doi: 10.1023/a:1017952128502. [DOI] [PubMed] [Google Scholar]
- 5.Li WC, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. Journal of Cell Biology. 1995;130(1):169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hightower KR. The role of the lens epithelium in development of UV cataract. Curr Eye Res. 1995;14(1):71–78. doi: 10.3109/02713689508999916. [DOI] [PubMed] [Google Scholar]
- 7.Brennan LA, et al. Deletion of mouse MsrA results in HBO-induced cataract: MsrA repairs mitochondrial cytochrome c. Mol Vis. 2009;15:985–99. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, et al. Glutaredoxin 2 (Grx2) gene deletion induces early onset of age-dependent cataracts in mice. J Biol Chem. 2014;289(52):36125–39. doi: 10.1074/jbc.M114.620047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palsamy P, Bidasee KR, Shinohara T. Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim Biophys Acta. 2014;1842(9):1794–805. doi: 10.1016/j.bbadis.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elanchezhian R, et al. Age-related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012;200(1):1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupfer C. The National Eye Institute's low vision education program: improving quality of life. Ophthalmology. 2000;107(2):229–230. doi: 10.1016/s0161-6420(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 12.Marchetti MA, et al. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83(5):1281–6. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Xing K, Lou MF. Glutaredoxin 2 prevents H(2)O(2)-induced cell apoptosis by protecting complex I activity in the mitochondria. Biochim Biophys Acta. 2010;1797(10):1705–15. doi: 10.1016/j.bbabio.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou MF. Redox regulation in the lens. Progress in Retinal and Eye Research. 2003;22(5):657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 15.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80(5):709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Vinson J. Oxidative stress in cataracts. Pathophysiology. 2006;13(3):151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, et al. Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic Biol Med. 2011;51(11):2108–2117. doi: 10.1016/j.freeradbiomed.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks MM, et al. Lenticular mitoprotection. Part A: Monitoring mitochondrial depolarization with JC-1 and artifactual fluorescence by the glycogen synthase kinase-3β inhibitor, SB216763. Molecular Vision. 2013;19:1406–1412. [PMC free article] [PubMed] [Google Scholar]
- 19.Kantorow M, et al. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci U S A. 2004;101(26):9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui H, et al. The effect of up- and down-regulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44(8):3467–3475. doi: 10.1167/iovs.02-0830. [DOI] [PubMed] [Google Scholar]
- 21.Nahomi RB, et al. Chaperone peptides of α-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013;288(18):13022–35. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao YW, et al. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11(5):512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 23.McGreal RS, et al. Chaperone-independent mitochondrial translocation and protection by alphaB-crystallin in RPE cells. Exp Eye Res. 2013;110:10–7. doi: 10.1016/j.exer.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGreal RS, et al. alphaB-crystallin/sHSP protects cytochrome c and mitochondrial function against oxidative stress in lens and retinal cells. Biochim Biophys Acta. 2012;1820(7):921–30. doi: 10.1016/j.bbagen.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padgaonkar VA, et al. The effects of hyperbaric oxygen on the crystallins of cultured rabbit lenses: a possible catalytic role for copper. Exp. Eye Res. 2000;71:371–383. doi: 10.1006/exer.2000.0887. [DOI] [PubMed] [Google Scholar]
- 26.Lim JC, et al. Characterization of the Effects of Hyperbaric Oxygen on the Biochemical and Optical Properties of the Bovine Lens. Invest Ophthalmol Vis Sci. 2016;57(4):1961–73. doi: 10.1167/iovs.16-19142. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Oxygen-induced changes in mitochondrial DNA and DNA repair enzymes in aging rat lens. Mech Ageing Dev. 2010;131(11-12):666–73. doi: 10.1016/j.mad.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Costello MJ, et al. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res. 2013;116:141–50. doi: 10.1016/j.exer.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15(4):403–11. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal. 2011;14(10):1929–38. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondapalli C, et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012;2(5):120080. doi: 10.1098/rsob.120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazlauskaite A, et al. Phosphorylation of Parkin at Serine65 is essential for activation: elaboration of a Miro1 substrate-based assay of Parkin E3 ligase activity. Open Biol. 2014;19(4):130213. doi: 10.1098/rsob.130213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, et al. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;27(24):13354–9. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimura H, et al. Famalial Parkinson disease gene product parkin is a ubiquitin protein ligase. Nat Genet. 2000;25(3):302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 37.Trempe JF, et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science. 2013;340(6319):1451–5. doi: 10.1126/science.1237908. [DOI] [PubMed] [Google Scholar]
- 38.Sarraf SA, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–6. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PloS Biology. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Qin S, Jiang C. Parkin-induced ubiquitination of Mff promotes its association with p62/SQSTM1 during mitochondrial depolarization. Acta Biochim Biophys Sin (Shanghai) 2015;47(7):522–9. doi: 10.1093/abbs/gmv044. [DOI] [PubMed] [Google Scholar]
- 41.Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 42.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393(7):547–64. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JY, et al. Disease-causing mutations in Parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biology. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan LA, et al. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis. 2012;18:1773–86. [PMC free article] [PubMed] [Google Scholar]
- 45.Chauss D, et al. Differentiation state-specific mitochondrial dynamic regulatory networks are revealed by global transcriptional analysis of the developing chicken lens. G3 (Bethesda) 2014;4(8):1515–27. doi: 10.1534/g3.114.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibaraki N, et al. Human lens epithelial cell line. Exp Eye Res. 1998;67:577–85. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- 47.Menko AS, Klukas KA, Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biology. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 48.Narendra D, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goswami S, et al. Spectrum and range of oxidative stress responses of human lens epithelial cells to H2O2 insult. Invest Ophthalmol Vis Sci. 2003;44(5):2084–93. doi: 10.1167/iovs.02-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan LA, Lee W, Kantorow M. TXNL6 is a novel oxidative stress-induced reducing system for methionine sulfoxide reductase a repair of alpha-crystallin and cytochrome C in the eye lens. PLoS One. 2010;5(11):e15421. doi: 10.1371/journal.pone.0015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazarou M, et al. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol. 2013;200(2):163–172. doi: 10.1083/jcb.201210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durcan TM, Fon EA. The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29(10):989–99. doi: 10.1101/gad.262758.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–25. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 54.Yang YX, Muqit MM, Latchman DS. Induction of parkin expression in the presence of oxidative stress. Eur J Neurosci. 2006;24(5):1366–72. doi: 10.1111/j.1460-9568.2006.04998.x. [DOI] [PubMed] [Google Scholar]
- 55.Parkin promotes cell survival via linear ubiquitination. The Embo Journal. 2013;32:1072–1074. doi: 10.1038/emboj.2013.70. E, F. and D. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ller-Rischart AKM, et al. The E3 Ligase ParkinMaintains Mitochondrial Integrity by Increasing Linear Ubiquitination of NEMO. Molecular Cell. 2013;49(5):908–921. doi: 10.1016/j.molcel.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 57.Itier JM, et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12(18):2277–91. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 58.Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopam-inergic neurons. J Biol Chem. 2003;278(44):43628–35. doi: 10.1074/jbc.M308947200. M.S., G., et al. [DOI] [PubMed] [Google Scholar]
- 59.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Von Coelln R, et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc. Natl Acad. Sci. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novak I, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11(1):45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandoval H, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–5. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schweers RL, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104(49):19500–5. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novak I, Dikic I. Autophagy receptors in developmental clearance of mitochondria. Autophagy. 2014;7(3):301–303. doi: 10.4161/auto.7.3.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orvedahl A, et al. Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature. 2011;480(7375):113–7. doi: 10.1038/nature10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14(2):177–85. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 67.Wu W, et al. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 2014;15(5):566–75. doi: 10.1002/embr.201438501. [DOI] [PMC free article] [PubMed] [Google Scholar]