Abstract
Context
Cancer-related fatigue (CRF) persists months after treatment completion. Although a CRF biomarker has not yet been identified, validated self-report questionnaires are used to define and phenotype CRF in the discovery of potential biomarkers.
Objectives
The purposes of this study are to identify CRF subjects using three well-known CRF phenotyping approaches utilizing validated self-report questionnaires and to compare the biologic profiles that are associated with each CRF phenotype.
Methods
Fatigue in men with non-metastatic prostate cancer receiving external beam radiation therapy (EBRT) was measured at: baseline (T1), midpoint (T2), endpoint (T3), and one year post-EBRT (T4) using the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) and Patient Reported Outcomes Measurement Information System-Fatigue (PROMIS-F). Chronic fatigue (CF) and non-fatigue (NF) subjects were grouped based on three commonly used phenotyping approaches: 1) T4 FACT-F <43; 2) T1-T4 decline in FACT-F score >3 points; 3) T4 PROMIS-F T-score >50. Differential gene expressions using whole genome microarray analysis were compared in each of the phenotyping criterion.
Results
The study enrolled 43 men, where 34-38% had CF based on the 3 phenotyping approaches. Distinct gene expression patterns were observed between CF and NF subjects in each of the three CRF phenotyping approaches: 1) Approach 1 had the largest number of differentially expressed genes, and 2) Approaches 2 and 3 had 40 and 21 differentially expressed genes between the fatigue groups, respectively.
Conclusion
The variation in genetic profiles for CRF suggests that phenotypic profiling for CRF should be carefully considered because it directly influences biomarker discovery investigations.
Keywords: cancer-related fatigue, radiation therapy, prostate cancer, transcriptome profiles, fatigue phenotypes
Introduction
Cancer-related fatigue (CRF) is often the most commonly reported distressing side effect of cancer and cancer therapy, affecting anywhere from 50-90% of oncology patients (1). CRF negatively reduces health-related quality of life and increases mortality among cancer patients (2). The management of CRF is challenging for health care clinicians because the concept is poorly defined and its etiology is unknown.
Efforts to understand the etiology of CRF remain challenging because the CRF phenotype has not been well characterized. This lack of a well characterized CRF phenotype stems from the lack of a consistent definition and a standard phenotyping approach (3). In a recent review of 47 articles exploring the biology of CRF, the lack of consensus among researchers in defining CRF was confirmed because CRF was measured using multiple approaches (3). Although most researchers operationally defined CRF using a variety of multi-item and single-item self-report questionnaires, a number defined CRF using clinical guidelines (e.g., National Cancer Institute Common Toxicity Criteria) or conducting diagnostic clinical interviews. Further, studies in these reviewed articles used various scoring rubrics and cut-off scores to determine the presence or absence of CRF when attempting to phenotype CRF for biomarker discovery (3). This lack of consistency and consensus in defining CRF and in characterizing the CRF phenotype creates confusion among researchers who are trying to advance the science of CRF to understand its biologic underpinnings. To advance our science in CRF, the authors would like to refocus the conversation on the need for a clear, well-defined CRF phenotype by presenting three commonly used CRF phenotyping approaches and highlighting the strengths of each phenotyping approach, with the hope of provoking further discussion on the issue.
A clear, well-defined phenotyping approach has been successful with other symptoms, notably pain and depression. For example, a well characterized pain phenotype led to the development of better therapeutic strategies using effective anti-nociceptive therapies (4, 5). Moreover, a well described phenotype for depression led to the inclusion of new depressive disorder classifications in the Diagnostic and Statistical Manual, 5th edition (DSM-V) and an array of effective personalized and targeted treatments (6). A well characterized CRF phenotype is an essential step in the process of identifying biologically-relevant therapeutic targets and developing precise and effective personalized management. Therefore, the purposes of this study are to identify subjects with persistent fatigue following cancer therapy. Considering that there is no gold standard approach to phenotype CRF, we used three well-known CRF phenotyping approaches utilizing validated self-report questionnaires to categorize subjects with persistent fatigue, and compared the biologic profiles that are associated with each phenotyping approach. Instead of identifying specific genes associated with CRF, the main goal of the study was to demonstrate that different approaches to phenotype CRF are associated with different transcriptome profiles. In addition, we aimed to determine biological pathways associated with these distinct transcriptome profiles generated by different phenotyping approaches.
Methods
Subjects
Men with non-metastatic prostate cancer, who were receiving androgen deprivation therapy and scheduled to receive EBRT, were enrolled under a National Institutes of Health (NIH) institutional review board-approved study (NCT00852111). Patients were enrolled from the radiation oncology clinic of the Hatfield Clinical Research Center, NIH, Bethesda, Maryland from May 2009 to December 2010. Fatigue was measured and blood was drawn at four time points: baseline or before EBRT (T1), midpoint (T2), endpoint (T3), and one year post EBRT (T4). Subjects were excluded from the study if they had progressive disease causing significant fatigue; experienced major psychiatric illness within five years; had uncorrected hypothyroidism or anemia; took sedatives, steroids, or non-steroidal anti-inflammatory agents; or had a second malignancy. After obtaining informed consent, demographic information and medical history were obtained by patient interview and medical records review.
Fatigue questionnaires
Fatigue was measured by the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) scale and the Patient Reported Outcomes Measurement Information System-Fatigue subscale (PROMIS-F). FACT-F is a 13-item measure with scores that range from 0-4 for each item (0= the worst; 4= the best) with 52 as the maximum possible score. The lower the FACT-F score, the higher the fatigue intensity (7). FACT-F has good test-retest reliability (r = 0.90) and internal consistency reliability (α = 0.93 and 0.95) on initial and test-retest administration, suggesting that it can be administered as an independent, unidimensional measure of fatigue; it has been used extensively in individuals with cancer [7]. In addition, a FACT-F score of 43 best divides fatigue scores of cancer patients and the general population (8). PROMIS-F is a 7-item questionnaire that was developed from more than 1000 datasets from multiple disease populations including cancer, heart disease, rheumatoid and osteoarthritis, psychiatric conditions, spinal cord injury, and chronic obstructive pulmonary disease. Initial testing of psychometric properties showed an internal consistency reliability coefficient of 0.81(9). The PROMIS measures are reported on a T-score metric that is anchored to the mean score of a healthy American general population (10). The T-score metric has a mean of 50 and a standard deviation (SD) of 10, which improves the interpretability of scores (11). A higher PROMIS T-score represents more of the concept being measured or greater fatigue. We selected to use both FACT-F and PROMIS-F because they have previously been reported to be highly correlated (r = 0.95, p < 0.001) (12), hence fatigue scores generated by these fatigue questionnaires, measured the same concept. Both FACT-F and PROMIS-F items required subjects to recall their fatigue experience in the past 7 days.
All study measures were obtained in an outpatient setting during participants’ clinical visits. Questionnaires were administered to subjects before any clinical procedures were carried out in order to avoid extraneous influences on their responses. Subjects were subdivided into chronic fatigue (CF) and non-fatigue (NF) groups using three commonly used phenotyping approaches. Approach 1: FACT-F score <43 at T4; Approach 2: decline in FACT-F score >3 points from T1 to T4; and Approach 3: PROMIS-F T-score >50 at T4.
Sample preparation and microarray
About 2.5 ml of blood was collected from each subject in a RNA PAXGene tube at each study time-point (Qiagen, Frederick, MD). Samples were stored at -80°C until further processing. RNA extraction and Affymetrix microarray chips (HG U133 Plus 2.0, Santa Clara, CA) were processed as previously described (13). Affymetric GeneChip Command Console (AGCC, 3.0V) was used to scan images during data acquisition.
Data analysis
Affymetrix .CEL files containing raw intensity data were imported into Partek Genomics Suite 6.6 (Partek Inc., St. Louis, MO), log transformed, and normalized using the robust multiarray average (RMA) algorithm. Because the chips were processed on different days, Partek batch removal analysis of variance (ANOVA) was used to eliminate differences due to batch variation. Principal component analysis (PCA) was performed using normalized signal intensities from Affymetrix microarray data. ANOVA with false discovery rate (FDR) correction was used to identify differentially expressed genes between fatigue groups (FDR < 5%).
Principal component analysis (PCA) is a multivariate statistical method performed to reduce complexity of multidimensional data and to identify overall patterns in the data. Dimensionality of a dataset is reduced into linearly uncorrelated variables, principal components (PC), which explain most of the variation in the data. Each PC, a new variable created from linear combinations of the original variables (genes), does not correlate with others. Each enrolled subject is represented as a dot in the ellipsoid plots (Figure 1). The closer the two dots are, the greater the similarity in gene expression profiles between two subjects. Gene ontology (GO) enrichment analysis was performed to identify overrepresented functional and biologically meaningful GO categories of the differentially expressed genes. A chi-square test was performed to compare the proportion of the gene list in the group to the proportion of the background in the group. A high enrichment score indicates a more overrepresented functional group, a FDR corrected p-value <0.05 is considered significant.
Figure 1.
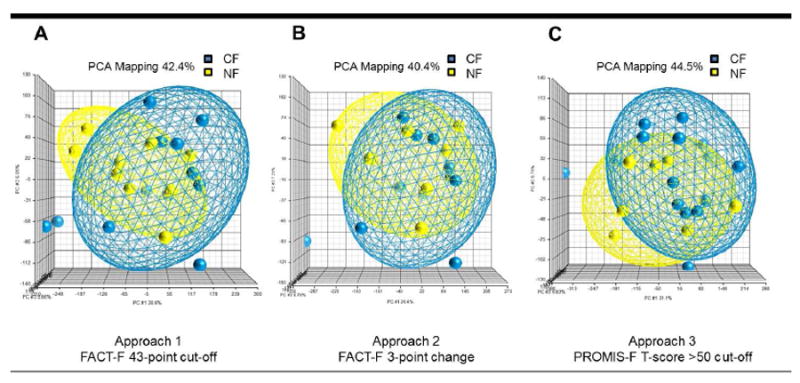
Principal component analysis showing differences in transcriptome profiles between chronic fatigue (CF) and non-fatigued (NF) men following external beam radiation therapy for prostate cancer using three different fatigue phenotyping approaches. Samples were repeatedly grouped into CF (blue) and NF (yellow) groups using three phenotyping approaches: Approach 1 was based on the FACT-F 43-point cut-off (a), Approach 2 was based on FACT-F 3-point change (b), and Approach 3 was based on PROMIS-F T-score >50 cut-off (c) criteria. Each dot represents a subject, each ellipsoid represents a phenotyping approach, and each axis corresponds to one principal component.
To assess fatigue changes over time, a two-way repeated-measures ANOVA was employed. For this analysis the between-subject factors were defined as fatigue levels, while the within-subject factor was defined by study time points. The sphericity assumption was tested with Mauchly’s test, and fatigue differences at each time point were determined by non-directional Student’s t-test with Bonferroni corrections for multiple comparisons. Statistical analyses regarding fatigue symptoms were analyzed with SPSS statistics software version 20 (IBM SPSS, Purchase, NY). Transcriptome profile analysis and GO enrichment analysis were performed using Partek Genomic Suite 6.6.
Results
Subject demographics and fatigue symptoms
A total of 43 men were included in this study. The majority (62%) of these men were Caucasian with a mean age of 66.10 ± 1.20. These 43 men were categorized into CF and NF based on the three phenotyping approaches (Table 1). Approach 1 defined fatigue as FACT-F score < 43 at T4. This approach employs cross-sectional comparisons of the subject’s fatigue score with the mean FACT-F score of the US general population, which is 43 (8). Of the 43 men, 34 subjects had FACT-F scores at T4 and 38.2% of these subjects were categorized as CF. Approach 2 defined fatigue as a decline in FACT-F score ≥3 points from T1 to T4. A 3-point longitudinal change in FACT-F score has been found to be the cut off threshold for clinically important difference in a study comparing multiple fatigue instruments (14). Of the 43 subjects recruited, 29 had FACT-F scores at both T1 and T4 and 34.5% of these subjects had CF. Approach 3 used a different questionnaire and defined fatigue as PROMIS-F T-score >50 at T4. This approach is another cross-sectional phenotyping method that compares the PROMI-F T-score of the subjects to that of the average PROMIS-F score of the healthy US general population, which is 50 (10). Thirty-five of the subjects had PROMIS-F scores at T4 and 34.3% of the subjects had CF. In all three phenotyping approaches, the CF subjects did not differ from NF subjects in demographic characteristics including age, weight, body mass index, and Gleason scores (Table 1). PSA levels were low (≤ 0.2 ng/mL) in both CF and NF subjects one year after EBRT suggesting that disease progression was not a contributing factor to differences in transcriptome profiles generated by the three phenotyping approaches.
Table 1.
Characteristics of sample.
| Total subjects | Approach 1 FACT-F 43 cut-off |
Approach 2 FACT-F 3-point change |
Approach 3 PROMIS T-score >50 cut-off |
||||
|---|---|---|---|---|---|---|---|
| N = 43 | CF (n=13) | NF (n=21) | CF (n=10) | NF (n=19) | CF (n=12) | NF (n=23) | |
|
| |||||||
| Age | 66.1±1.2 | 63.6 ±2.3 | 66.1±1.6 | 65.7±2.4 | 67.0±1.6 | 62.5±2.4 | 66.2±1.5 |
| Race/Ethnicity | |||||||
| Caucasian | 62.5% | 76.9% | 66.7% | 70% | 78.9% | 66.7% | 69.6% |
| African American | 25% | 15.4% | 23.8% | 20% | 15.8% | 25% | 21.7% |
| Asian | 5.4% | 0% | 9.5% | 0% | 5.3% | 0% | 9.5% |
| Hispanic | 7.1% | 9.1% | 0% | 11.1% | 0% | 20% | 0% |
| Height (cm) | 175.6±1.1 | 176.4 ±1.8 | 175.8 ±1.5 | 175.2 ±1.7 | 175.0±1.6 | 176.7 ±2.2 | 176.5 ±1.5 |
| Weight (kg) | 92.4±2.2 | 94.4 ±4.2 | 90.4±3.1 | 96.6±4.2 | 88.2±3.2 | 93.3±4.1 | 91.9±3.1 |
| Body Mass Index | 30.1±0.7 | 30.8±1.5 | 29.2±0.9 | 32.0±1.8 | 28.8±1.0 | 30.4±1.5 | 29.4±0.9 |
| Albumin (g/dL) | 4.0±0.05 | 4.2±0.1 | 4.0±0.06 | 4.1±0.08 | 4.1±0.07 | 4.2±0.09 | 4.1±0.06 |
| Gleason Score | 7.8±0.1 | 7.±0.2 | 7.7±0.2 | 8.0±0.3 | 7.5±0.2 | 7.9±0.2 | 7.6±0.2 |
| Clinical T Stage | T1c – T3b | T1c – T3b | T1c – T3b | T1c – T3b | T1c – T3a | T1c – T3b | T1c – T3b |
| Karnofsky Performance Scale | 89.8±0.2 | 90.0±0.0 | 89.5±0.5 | 90.0±0.0 | 89.4±0.6 | 90.0±0.0 | 89.6±0.4 |
| PSA at baseline | 20.6±4.0 | 10.3±7.9 | 5.6±1.4 | 11.9±10.4 | 6.6±1.5 | 11.0±8.2 | 5.3±1.3 |
| PSA one year after EBRT | 0.2±0.1 | 0.05±0.01 | 0.2±0.2 | 0.05±0.01 | 0.2±0.2 | 0.05±0.01 | 0.2±0.1 |
FACT-F: Functional Assessment of Cancer Therapy – Fatigue; PROMIS: Patient Reported Outcomes Measurement Information System – Fatigue; cm: centimeter; kg: kilogram; g/dL: gram per deciliter; PSA: prostate specific antigen (ng/mL); EBRT: external beam radiation therapy; CF: chronic fatigue; NF: non-fatigued. Values are mean ± standard error.
Genome-wide gene expression comparison of three phenotyping approaches
Top up- and down-regulated genes in CF subjects compared to NF subjects for each of the fatigue phenotyping approach are shown in Table 2, where different gene expression profiles are observed for each of the 3 fatigue phenotyping approaches in the same study subjects. For example, Approach 1 had the most differentially expressed genes (244 genes) between CF and NF subjects. Approach 2 had 40 genes and Approach 3 had 21 differentially expressed genes between fatigue groups.
Table 2.
Most differentially expressed genes of the same prostate cancer men with chronic fatigue following external beam radiation therapy characterized using three different fatigue phenotyping approaches.
| Approach 1 FACT-F 43-point cut-off |
Approach 2 FACT-F 3-point change |
Approach 3 PROMIS-F T-score >50 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Gene ID | Fold change | P value | Gene ID | Fold change | P value | Gene ID | Fold change | P value | |
|
|
|||||||||
| Top up-regulated genes | PI3 | 1.92 | 1.64E-4 | LEF1 | 1.67 | 1.01E-5 | MARCKSL1 | 1.40 | 1.30E-6 |
| IL18RAP | 1.83 | 2.25E-4 | CLTB | 1.48 | 1.37E-6 | FBLN5 | 1.40 | 1.15E-5 | |
| FAM84B | 1.68 | 2.40E-4 | LEF1-AS1 | 1.45 | 2.68E-6 | IGLV1-44 | 1.38 | 2.01E-5 | |
| IGLL3P | 1.68 | 5.58E-5 | FBLN5 | 1.45 | 1.77E-5 | SLC35F2 | 1.26 | 2.03E-5 | |
| ACTB | 1.63 | 1.66E-4 | FDFT1 | 1.41 | 3.14E-5 | ACTR1A | 1.25 | 1.91E-5 | |
|
| |||||||||
| Top down-regulated genes | NUDT6 | -1.40 | 3.04E-5 | CENPN | -1.31 | 1.74E-5 | VEPH1 | -1.29 | 2.38E-6 |
| ZC2HC1C | -1.37 | 7.48E-5 | GALNT2 | -1.30 | 8.52E-6 | SLC22A9 | -1.28 | 1.33E-5 | |
| RRAD | -1.34 | 4.32E-6 | SIM1 | -1.30 | 7.58E-6 | QSOX1 | -1.26 | 6.64E-6 | |
| CDC25C | -1.32 | 3.48E-5 | YPEL2 | -1.28 | 1.51E-6 | UBE2Z | -1.22 | 4.40E-6 | |
| OLIG2 | -1.32 | 6.91E-5 | MTBP | -1.27 | 1.58E-8 | GOLGA3 | -1.19 | 1.23E-5 | |
Top five up-regulated and down-regulated genes in chronic fatigue (CF) men following external beam radiation therapy for prostate cancer compared to non-fatigued (NF) subjects characterized using three different fatigue phenotyping approaches. False discovery rate adjusted p < 0.05. FACT-F: Functional Assessment of Cancer Therapy – Fatigue; PROMIS: Patient Reported Outcomes Measurement Information System – Fatigue.
Principal components (PCs) obtained from the transcriptome data were arranged to account for the variation in data between fatigue phenotyping approaches. The top three PCs that were able to capture most of the variance in the data sets were visualized in scatter plots shown in Figure 1. In Approach 1, 42.4% of the variance of the dataset was represented by the first PC that accounted for 30.6% (X-axis) of the variation; the second and the third PCs accounted for 6.05% (Y-axis) and 5.66% (Z-axis), respectively (Figure 1a). In Approach 2, 40.4% of the variance was represented by the first PC, which accounted for 26.4% (X-axis), second PC = 7.21% (Y-axis), and third PC = 6.79% (Z-axis) (Figure 1b). And in Approach 3, 44.5% explains the total variation in the dataset (Figure 1c). Differences across whole genomes between CF and NF subjects appear dissimilar among the three fatigue phenotyping approaches of the same study subjects.
Variations in differentially expressed genes (FDR adjusted p < 0.05) among the three fatigue phenotyping approaches resulted in different GO enrichment analysis results. In Approach 1, 46.01% of the differentially expressed genes (GO enrichment score = 29.54; p < 0.05) were associated with biological adhesion, 8.24% of genes were associated with component organization or biogenesis (GO enrichment score = 5.29; p < 0.05), 7.58% of genes were associated with immune system process (GO enrichment score = 4.87; p < 0.05), and 5.62% of genes were associated with multicellular organismal process (GO enrichment score = 3.61; p < 0.05) (Figure 2a).
Figure 2.
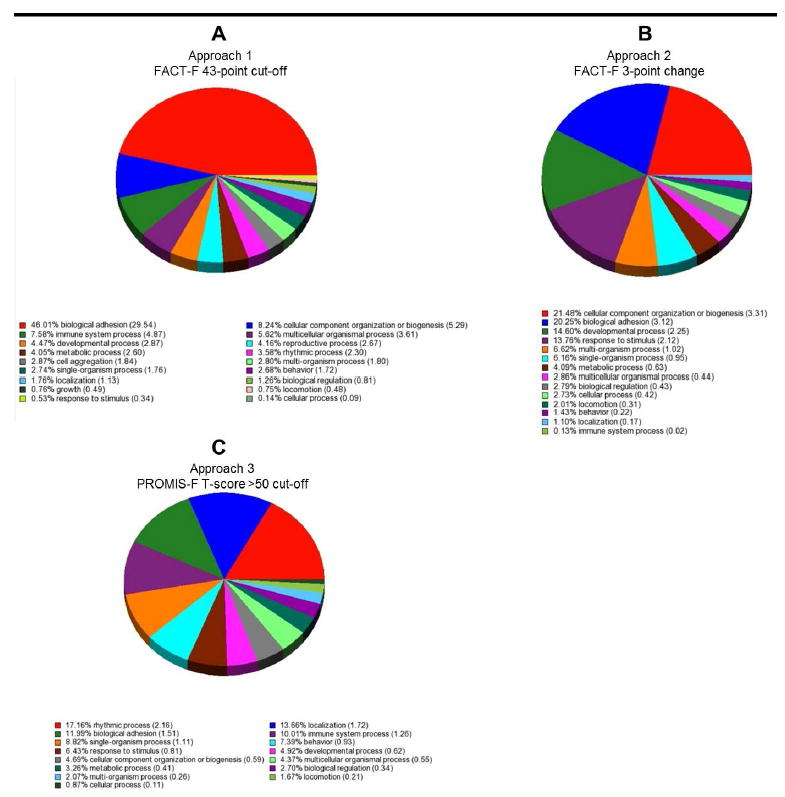
Gene Ontology (GO) enrichment analysis of differentially expressed genes (False Discovery Rate adjusted p < 0.05) in chronic fatigue (CF) compared to non-fatigued (NF) men at one year following external beam radiation therapy for prostate cancer using three different fatigue phenotyping approaches: (a) Approach 1: FACT-F 43-point cut off, (b) Approach 2: FACT-F 3-point change, and (c) Approach 3: PROMIS-F T-score >50 cut off. Pie charts represent the distribution of the number of genes in each enriched GO category. Gene ontology (GO) enrichment analysis was performed to identify overrepresented functional and biologically meaningful GO categories of the differentially expressed genes.
In Approach 2, 21.48% of the differentially expressed genes were associated with cellular component organization or biogenesis (GO enrichment score = 3.31; p < 0.05) and 20.25% of genes were associated with biological adhesion (GO enrichment score = 3.12; p < 0.05) (Figure 2b). Approach 3 had the least homology compared to the other two phenotyping approaches. Nearly 17% of differentially expressed genes in Approach 3 were associated with rhythmic process (GO enrichment score = 2.16), specifically, with circadian rhythm (GO enrichment score = 4.31; p < 0.05) (Figure 2c).
Discussion
Fatigue is a common, debilitating, and costly side effect of many cancer treatment regimens. It is often defined as a “subjective sense of tiredness” that persists over time, interferes with activities of daily living, and is not relieved by adequate rest (15, 16). Understanding the etiology of this distressing symptom is critical to identify biomarkers that can serve as therapeutic targets to develop optimal management. Investigation of mechanisms underlying CRF requires a thoughtful phenotyping approach to ensure its clinical relevance. The three most commonly used and clinically-relevant phenotyping approaches for CRF described in this manuscript reflect distinct genomic profiles representing separate and specific functional and biological pathways. The intention of this study is not to recommend which CRF phenotyping approach is superior over another, but to highlight the strengths of each of the common CRF phenotyping approach, in order to provoke a continued conversation on the need to carefully select an optimal CRF phenotyping approach that can appropriately meet the desired clinical or research outcomes. In the following section, we will discuss the advantage of each fatigue characterization approach in order to help researchers determine the optimal method that can better serve their purpose.
The FACT-F score lower than 43 approach effectively categorized fatigue and non-fatigue subjects post cancer treatment as seen by significant differences in mean fatigue scores at completion of EBRT and one year post EBRT completion (Figure 3a). Compared to other criteria, phenotyping CRF using the FACT-F 43-point cut-off score was associated with the most significant changes in gene expression profiles, as well as the largest number of differentially expressed genes between CF and NF subjects. It is likely that this phenotyping approach is a good method to examine cancer treatment-related alterations in transcriptome profiles; however, it may not be able to delineate immediate and chronic transcriptome profile changes associated with fatigue. GO enrichment analysis revealed that most of the differentially expressed genes (46.01% of genes; GO enrichment score = 29.54; p < 0.05) were associated with biological adhesion, a process important for inflammatory responses to cellular insults. This finding suggests that differences in immune responses to radiation-induced insults may have contributed to the differences in fatigue phenotypes.
Figure 3.
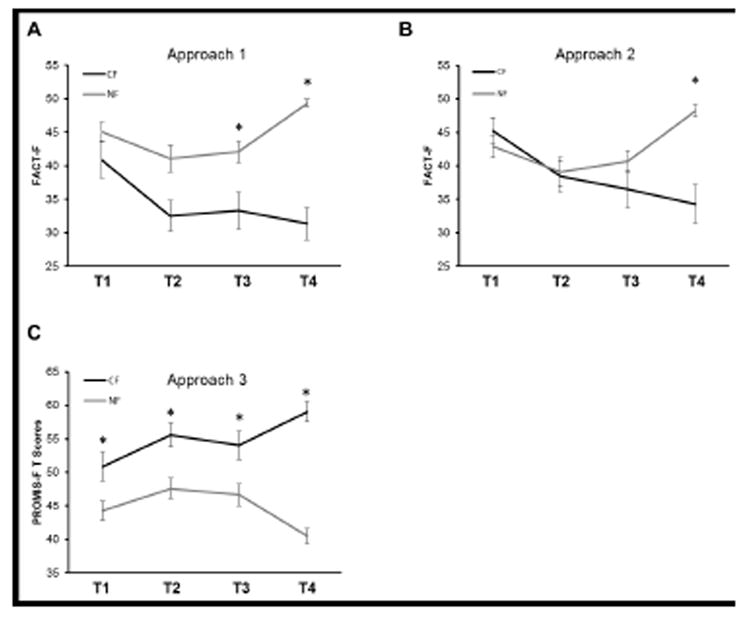
Fatigue score changes of men with prostate cancer as measured at baseline (T1; prior to EBRT), midpoint (T2; day 19-21), endpoint (T3; day 38-42), and one year after EBRT completion (T4). Subjects were subdivided into chronic fatigue (CF) and non-fatigue (NF) groups based on three phenotyping approaches: Approach 1: a FACT-F 43-point cut off at one year (a), Approach 2: 3-point change in FACT-F score from baseline to one-year after EBRT (b), and Approach 3: PROMIS-F a T-score greater than 50 at one year cut off (c) criteria. (a) 38.2% of subjects fell into the CF category using the FACT-F 43-point cut-off criterion (F3,33 = 5.4, p < 0.01). (b) 34.5% of subjects had a change of 3 points and greater from baseline to one year after EBRT (F3,33 = 12.3, p < 0.01). (c) Chronic fatigue was experienced in 34.3% of subjects based on PROMIS >50 T-score cut-off criterion (F3,34 = 8.3, p < 0.01). Mauchly’s test of sphericity was not significant in the repeated-measures ANOVA models (p > 0.1) indicating that the assumption of sphericity was not violated in any of the models. Repeated measures ANOVA and post hoc non-directional Student’s t-test with Bonferroni corrections for multiple comparisons were performed to analyze fatigue changes over time with fatigue levels defined as the between subjects factor and time as the within subjects factor. * Indicates significant differences between CF and NF subjects (p<.05).
The 3-point change in FACT-F score approach between time points has a unique advantage over the other phenotyping approaches, because it considers the longitudinal variability in fatigue experience pre- and post-cancer treatment, by using each subject’s own pre-treatment baseline score as reference point, instead of capturing the mean symptom score of all participants at one given time point. This approach effectively isolated the CF subjects at one time point post treatment (Figure 3b); hence, it may be a good method to isolate the chronic fatigue phenotype one year after treatment completion. Most of the differentially expressed genes detected using this phenotyping approach were associated with cell component biogenesis (21.48%; GO enrichment score = 3.31; p < 0.05) and 20.25% of genes were associated with biological adhesion (GO enrichment score = 3.12; p < 0.05) (Figure 2b). Associating the CF phenotype with biogenesis is a uniquely important finding, because it suggests that the chronic fatigue experience may be a consequence of a cascade of physiological responses to the radiation-induced insult to re-establish homeostasis (18, 19). In addition to biogenesis, differentially expressed genes that are associated with the CF phenotype were also related to cell adhesion processes. Both processes are involved in various immune responses (20-24), suggesting that fatigue phenotype based on Approach 2 may be related to differences in immune response to radiation.
CF subjects categorized by PROMIS-F T-scores higher than 50 had significantly different fatigue scores from NF subjects at T1, T2, T3, and T4 (Figure 3c). The apparent difference between CF and NF subjects at baseline suggests that the fatigue experience described by PROMIS-F is associated with intrinsic differences between two subpopulations within the patient cohort. This finding also suggests that PROMIS-F may be relatively more sensitive to subtle differences in fatigue experiences that are either associated with cancer itself or subjective reporting. Moreover, the significant differences in PROMIS-F scores between CF and NF subjects in all study time points suggest that this phenotyping approach may be the best method to phenotype chronic stable fatigue. Using this phenotyping approach, 17% of the differentially expressed genes were associated with rhythmic process (GO enrichment score = 2.16), specifically, with circadian rhythm (GO enrichment score = 4.31; p < 0.05) (Figure 2c). These findings suggest that it is possible that the fatigue phenotypes detected using Approach 3 may be related to differences in circadian rhythm, which plays an important role in regulation of the immune system (25), and is thought to play an important role in chronic fatigue conditions (25-28).
One major limitation of the study is the small sample size. To address this issue, a post-hoc power analysis was conducted using the mean between-group comparison effect size observed in the current study (Cohen’s d = 1.70, r = 0.65), an n of 9 subjects would be needed to obtain statistical power at 0.80. Therefore, although the sample size in our study is limited, the sample size was sufficient given the observed effect size. Another caveat is that the fold change values of differentially expressed genes were small. For the purpose of this study, we included all differentially expressed genes that satisfied the statistical cut off of FDR-corrected p value of <0.05. Previous studies have shown that using a statistical significance cut off criterion reduces variability and improves reproducibility, whereas including fold-change cut off criteria contributes to interpretations that are biologically meaningful (29-32). Our goal for this paper was to demonstrate that different behavioral phenotypes were associated with different clusters of gene expression patterns. Our future studies aimed at drawing biologically meaningful conclusions related to the mechanisms associated with fatigue will utilize a combination of fold change and statistical cut off criteria.
All of the subjects included in this study were elderly men because of the nature of the cancer that was being investigated. Gender has been shown to influence symptom intensity and subjective reporting of the severity of symptoms such as depression and pain (33, 34). Therefore, it is important that these three fatigue phenotyping approaches are assessed in female subjects in future work. Because we also enrolled only a few non-Caucasian subjects, it was not possible to make a meaningful conclusion (African American: n = 11; Asian: n = 2; Hispanic: n = 3) related to racial differences. Future studies are needed to explore the influence of racial and age differences in phenotyping fatigue and the associated transcriptome profiles of these phenotypes. Further, symptom intensity in many conditions exhibit diurnal rhythmicity (35, 36). Because subjects included in this study were seen in the morning (8 am to 12 noon), future studies are needed to explore the role of circadian rhythm in the fatigue experience and the associated changes in the transcriptome profiles of subjects with fatigue. Future studies should also incorporate regression frameworks to better explore the relationship between fatigue phenotypes and gene expression patterns.
Despite their similarities, the three most commonly used fatigue phenotyping approaches vary in subtle ways that translate into different biological interpretations of the symptom. Each approach has its advantages and should be chosen carefully depending on the purpose of the study. One takeaway message of this study is that it may be premature to settle on one phenotyping approach, however, the scientist needs to be aware that biological pathways of transcriptome profiles are vastly different depending on which phenotyping approach is used. Therefore, the scientist needs to choose the CRF phenotyping approach that can appropriately answer the research question.
Conclusion
Assessing subjective experiences such as fatigue generally requires self-report questionnaires, and patient self-report outcome questionnaires inevitably rely on patients’ own perceptions. Therefore, it becomes extremely important for clinicians to carefully select the appropriate measure and phenotyping approach. We have highlighted the need for a careful characterization of the CRF phenotype. As we have shown, even well-correlated outcome measurements can differ in subtle, yet important ways. Fatigue is a symptom that can originate from multiple pathogenic processes. Defining CRF in a manner that can characterize a clear CRF phenotype is both a necessary and a critical component of supportive care and symptom management.
Acknowledgments
This study is fully supported by the Division of Intramural Research, National Institute of Nursing Research, National Institutes of Health.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Li Rebekah Feng, Email: rebekah.feng@nih.gov, National Institute of Nursing Research, National Institutes of Health, Bethesda, Maryland.
Kristin Dickinson, Email: kristin.filler@nih.gov, National Institute of Nursing Research, National Institutes of Health, Bethesda, Maryland.
Neila Kline, Email: nekline@vassar.edu, Vassar College, Poughkeepsie, New York.
Leorey N. Saligan, National Institute of Nursing Research, National Institutes of Health, Bethesda, Maryland.
References
- 1.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22:1273–9. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 2.Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109:161–71. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saligan L, Olson K, Filler K, et al. The biology of cancer-related fatigue: a review of the literature. Supportive Care in Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. The Lancet Neurology. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double - blind, placebo - controlled phenotype - stratified study. PAIN. 2014;155:2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 6.de Almeida JRC, Phillips ML. Distinguishing between Unipolar Depression and Bipolar Depression: Current and Future Clinical and Neuroimaging Perspectives. Biological Psychiatry. 2013;73:111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining Anchor and Distribution-Based Methods to Derive Minimal Clinically Important Differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. Journal of Pain and Symptom Management. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 9.Hays R, Bjorner J, Revicki D, Spritzer K, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothrock NE, Hays RD, Spritzer K, et al. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) Initiative. Journal of Clinical Epidemiology. 2010;63:1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook KF, Bamer AM, Amtmann D, Molton IR, Jensen MP. Six Patient-Reported Outcome Measurement Information System Short Form Measures Have Negligible Age- or Diagnosis-Related Differential Item Functioning in Individuals With Disabilities. Archives of Physical Medicine and Rehabilitation. 2012;93:1289–1291. doi: 10.1016/j.apmr.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Junghaenel DU, Christodoulou C, Lai J-S, Stone AA. Demographic Correlates of Fatigue in the US General Population: Results from the Patient-Reported Outcomes Measurement Information System (PROMIS) Initiative. Journal of psychosomatic research. 2011;71:117–123. doi: 10.1016/j.jpsychores.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saligan LN, Hsiao CP, Wang D, et al. Upregulation of α-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain, Behavior, and Immunity. 2013;27:63–70. doi: 10.1016/j.bbi.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. Journal of Clinical Epidemiology. 2011;64:507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100:1155–66. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 16.Mock V. Clinical Excellence Through Evidence-Based Practice: Fatigue Management as a Model. Oncology Nursing Forum. 2003;30:787–795. doi: 10.1188/03.onf.787-795. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Lai J-s, Chang C-H, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 18.Morris G, Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metabolic Brain Disease. 2013;28:523–540. doi: 10.1007/s11011-012-9324-8. [DOI] [PubMed] [Google Scholar]
- 19.Barnden LR, Crouch B, Kwiatek R, et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR in Biomedicine. 2011;24:1302–1312. doi: 10.1002/nbm.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buschow SI, van Balkom BWM, Aalberts M, et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- 21.Sorci-Thomas MG, Thomas MJ. High Density Lipoprotein Biogenesis, Cholesterol Efflux, and Immune Cell Function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:2561–2565. doi: 10.1161/ATVBAHA.112.300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 23.Reglero-Real N, García-Weber D, et al. Cellular Barriers after Extravasation: Leukocyte Interactions with Polarized Epithelia in the Inflamed Tissue. 2016 doi: 10.1155/2016/7650260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benvenuto F, Voci A, Carminati E, et al. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Research & Therapy. 2015;6:1–12. doi: 10.1186/s13287-015-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nater UM, Youngblood LS, Jones JF, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 27.Rahman K, Burton A, Galbraith S, Lloyd A, Vollmer-Conna U. Sleep-Wake Behavior in Chronic Fatigue Syndrome. Sleep. 2011;34:671–678. doi: 10.1093/sleep/34.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Supportive Care in Cancer. 2009;18:105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy DJ, Smyth GK. Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics. 2009;25:765–771. doi: 10.1093/bioinformatics/btp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schena M, Shalon D, Heller R, et al. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Tong W, Fang H, et al. Cross-platform comparability of microarray technology: Intra-platform consistency and appropriate data analysis procedures are essential. BMC Bioinformatics. 2005;6:S12–S12. doi: 10.1186/1471-2105-6-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalman MR, Deeter A, Nimishakavi G, Duan Z-H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinformatics. 2012;13:1–4. doi: 10.1186/1471-2105-13-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girgus JS, Yang K. Gender and depression. Current Opinion in Psychology. 2015;4:53–60. [Google Scholar]
- 34.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British Journal of Anaesthesia. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilron I, Ghasemlou N. Chronobiology of chronic pain: focus on diurnal rhythmicity of neuropathic pain. Current Opinion in Supportive and Palliative Care. 2014;8:429–436. doi: 10.1097/SPC.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 36.Smolensky MH, Portaluppi F, Manfredini R, et al. Diurnal and twenty-four hour patterning of human diseases: Cardiac, vascular, and respiratory diseases, conditions, and syndromes. Sleep Medicine Reviews. 2015;21:3–11. doi: 10.1016/j.smrv.2014.07.001. [DOI] [PubMed] [Google Scholar]


