Abstract
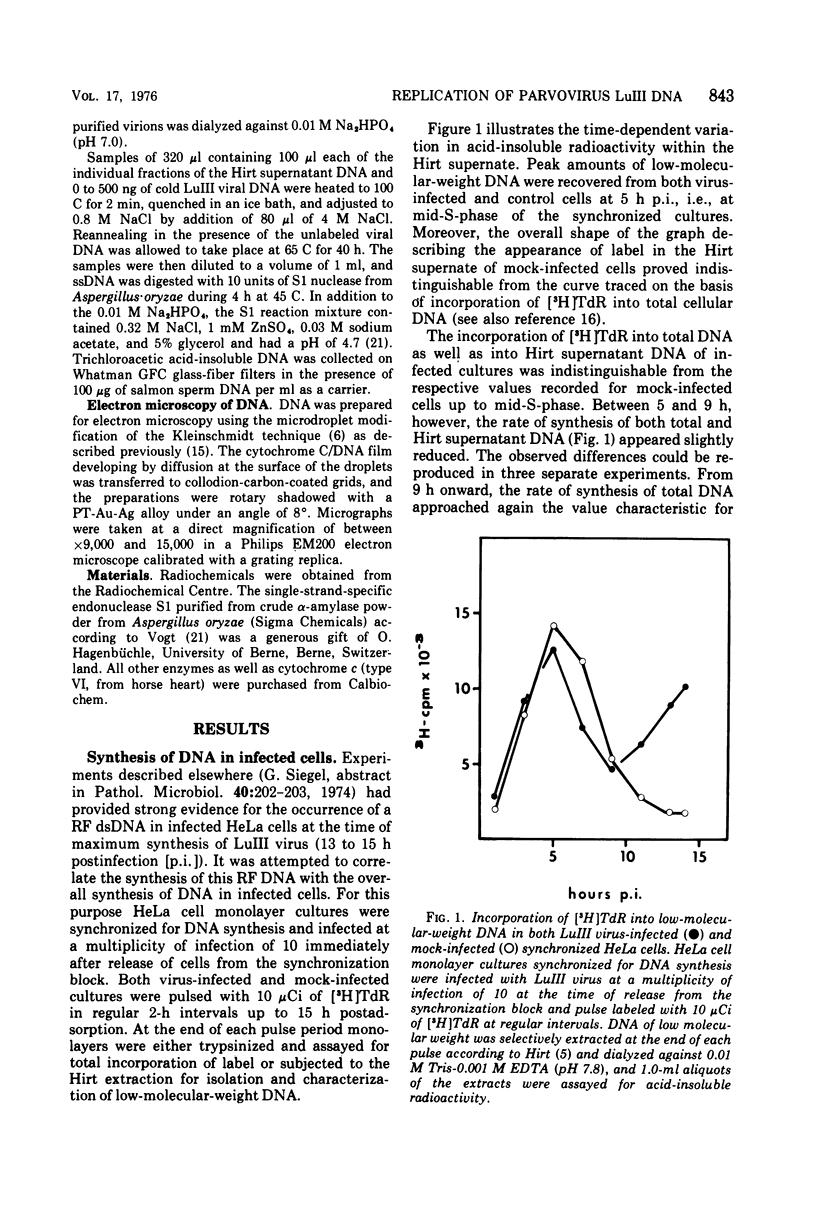
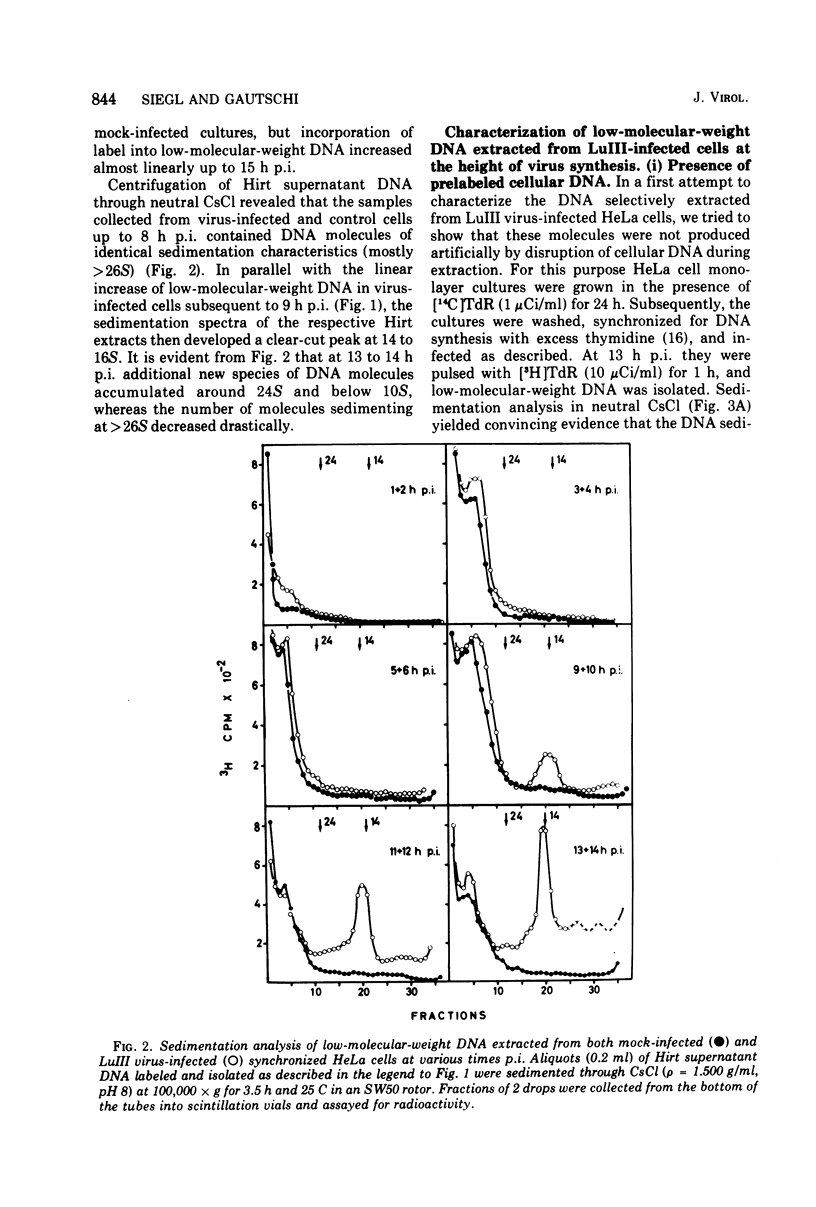
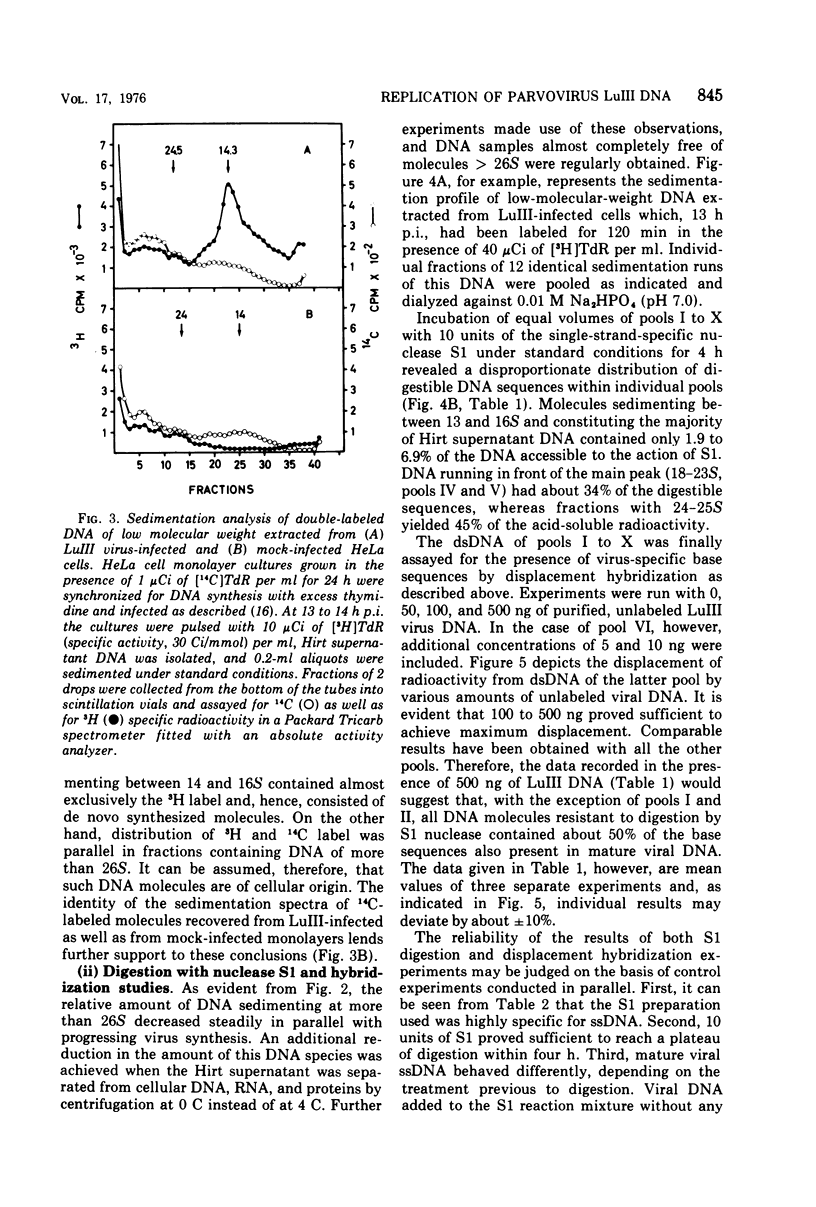
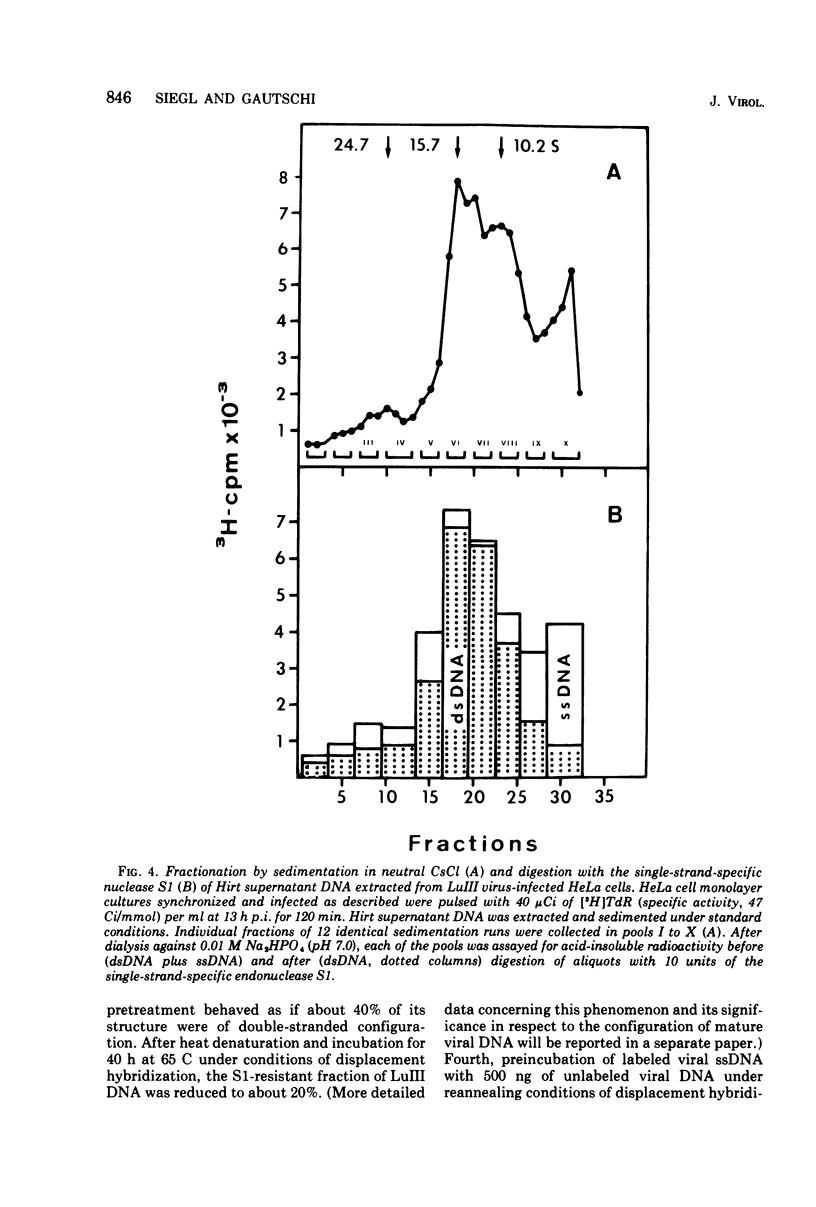
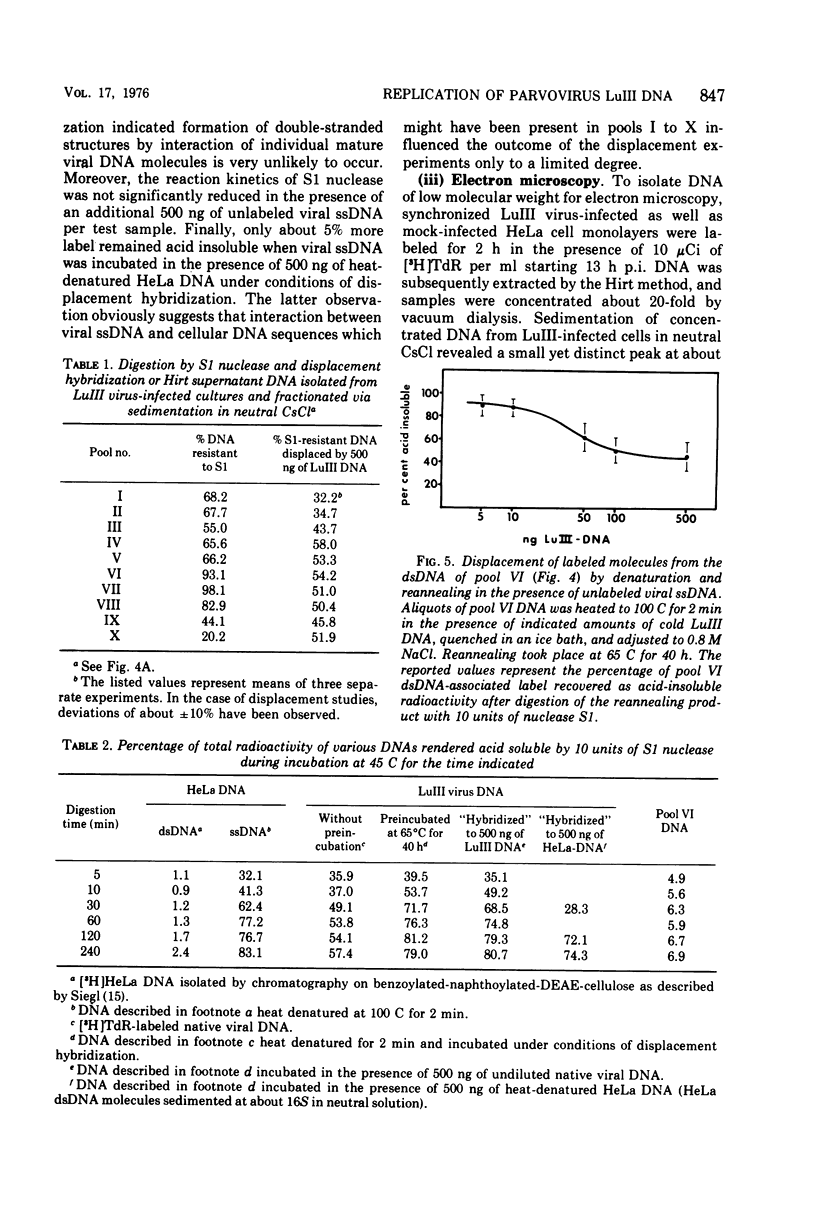
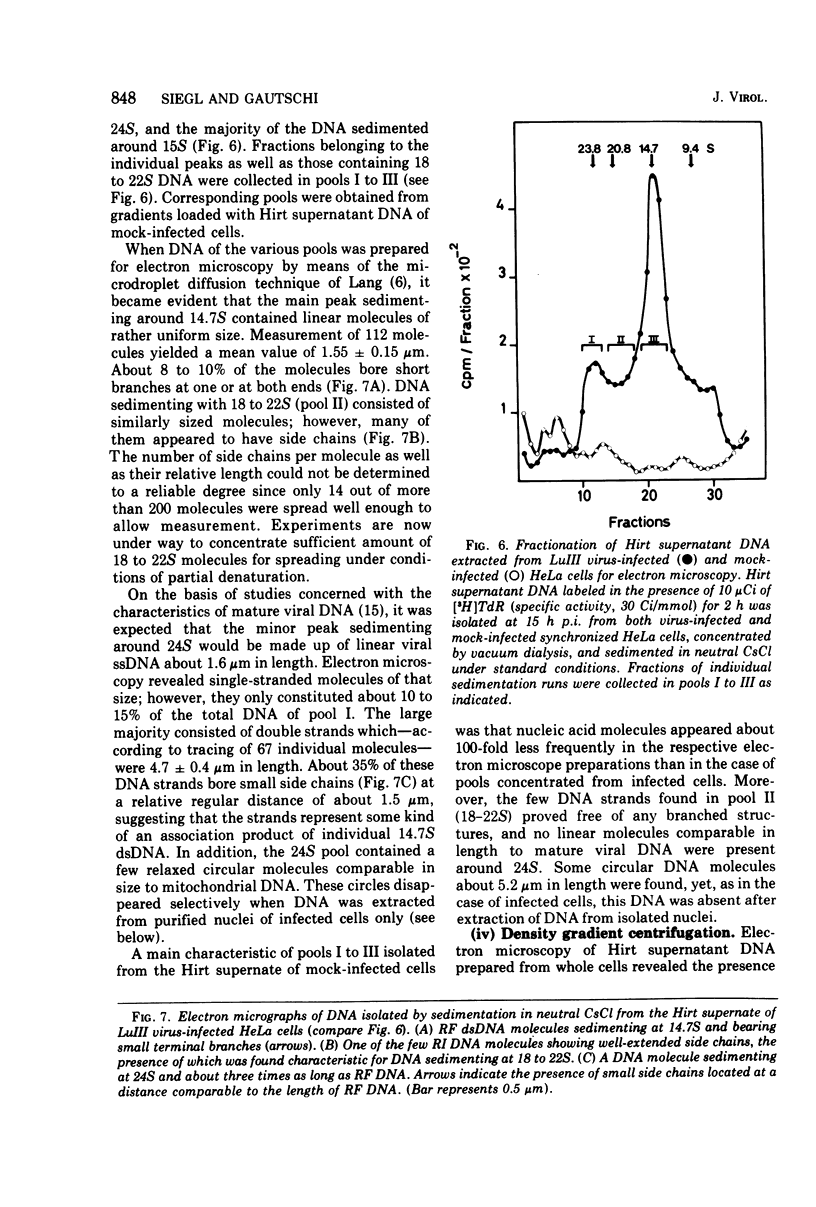
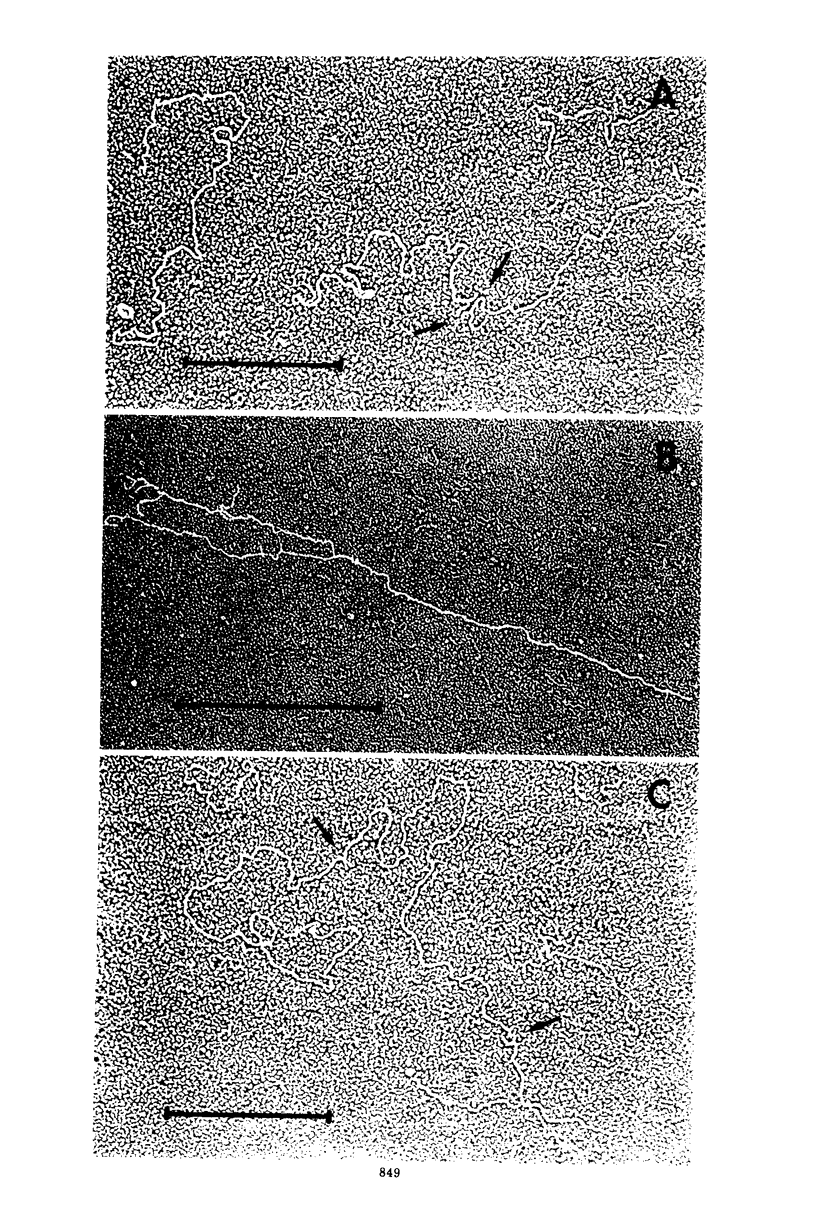
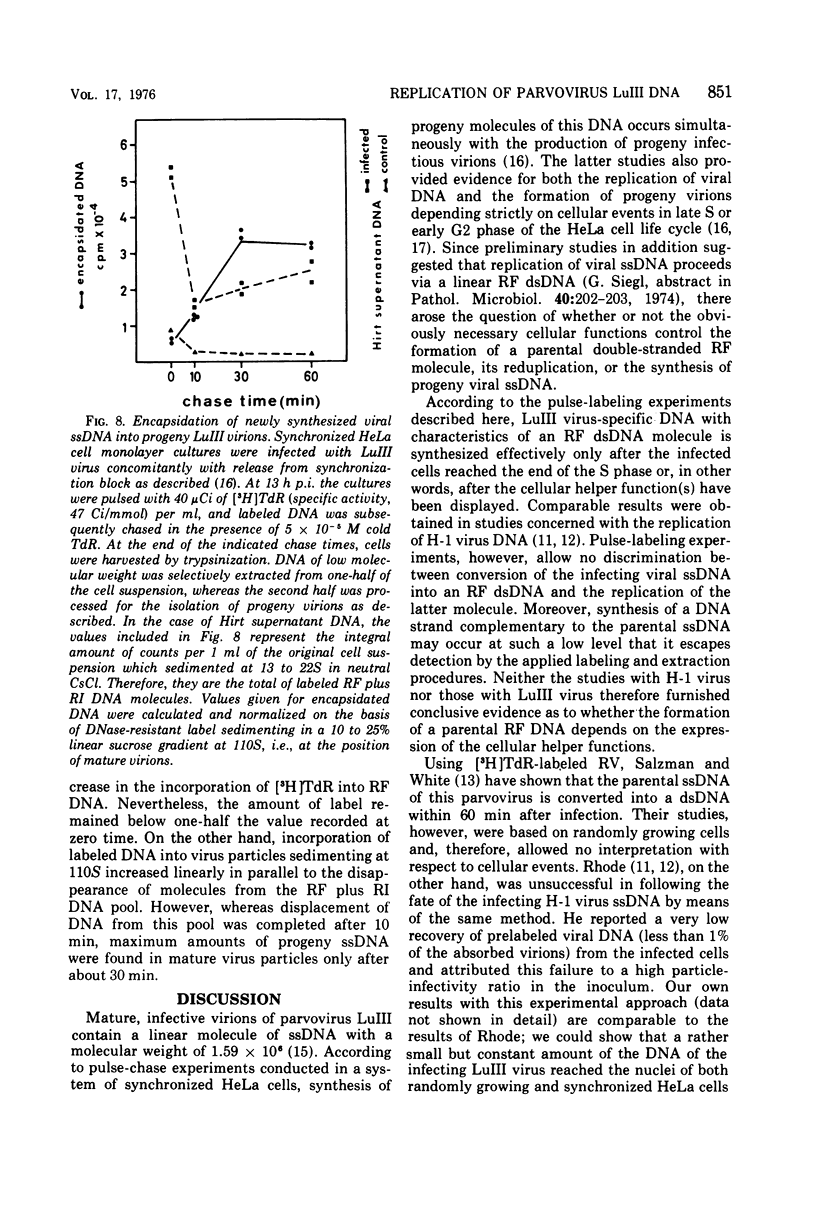
The replication of the single-stranded DNA (ssDNA) of parvovirus LuIII was studied in synchronized HeLa cells. After infection of the cells in early S phase, synthesis of a replicative form (RF) DNA became detectable as early as 9 h postinfection, i.e., after display of the cellular helper function(s) indispensable for the replication of LuIII virus. According to digestion with nuclease S1, hybridization studies, and electron microscopy, RF DNA is a linear, double-stranded molecule comparable in length to mature ssDNA. It sedimented around 15S in neutral solution and banded at 1.714 g/ml in CsCl. Moreover, replication of LuIII DNA obviously includes a further replicative intermediate DNA which sedimented in front of RF DNA and bore single-stranded side-chains. Newly synthesized DNA disappeared from pools containing both RF DNA and replicative intermediate DNA within 5 min and reappeared in progeny virions only after 15 min. Intranuclear accumulation of significant amounts of progeny ssDNA could not be detected. It was postulated, therefore, that newly synthesized ssDNA is immediately enclosed in a stable maturation complex and resists extraction by the method of Hirt (1967).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobson P. R., Helleiner C. W. A replicative form of the DNA of minute virus of mice. Can J Microbiol. 1973 Jan;19(1):35–41. doi: 10.1139/m73-005. [DOI] [PubMed] [Google Scholar]
- Hallauer C., Kronauer G., Siegl G. Parvoiruses as contaminants of permanent human cell lines. I. Virus isolation from 1960-1970. Arch Gesamte Virusforsch. 1971;35(1):80–90. doi: 10.1007/BF01249755. [DOI] [PubMed] [Google Scholar]
- Hallauer C., Siegl G., Kronauer G. Parvoviruses as contaminants of permanent human cell lines. 3. Biological properties of the isolated viruses. Arch Gesamte Virusforsch. 1972;38(4):366–382. doi: 10.1007/BF01262827. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lang D. Individual macromolecules: preparation and recent results with DNA. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):151–158. doi: 10.1098/rstb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Pearson G. D. Intermediate in adenovirus type 2 replication. J Virol. 1975 Jul;16(1):17–26. doi: 10.1128/jvi.16.1.17-26.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétursson G., Weil R. A study on the mechanism of polyoma-induced activation of the cellular DNA-synthesizing apparatus. Synchronization by FUdR of virus-induced DNA synthesis. Arch Gesamte Virusforsch. 1968;24(1):1–29. doi: 10.1007/BF01242898. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. III. Factors affecting H-1 RF DNA synthesis. J Virol. 1974 Oct;14(4):791–801. doi: 10.1128/jvi.14.4.791-801.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., McKerlie L. Growth characteristics of Kilham rat virus and its effect on cellular cellular macromolecular synthesis. J Virol. 1972 Oct;10(4):573–577. doi: 10.1128/jvi.10.4.573-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch. 1973;40(1):119–127. doi: 10.1007/BF01242643. [DOI] [PubMed] [Google Scholar]
- Siegl G. Physicochemical characteristics of the DNA of parvovirus Lu 3. Arch Gesamte Virusforsch. 1973;43(4):334–344. doi: 10.1007/BF01556150. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]