Abstract
Objective
Controlled and uncontrolled studies with primary intratympanic (IT) or combined intratympanic and systemic application of glucocorticosteroids for idiopathic sudden hearing loss (ISSHL) were analyzed by means of a meta-analysis in an attempt to establish optimal local drug delivery protocols.
Study Design
A total of 25 studies with 28 treatment groups between January 2000 and June 2014 were selected that adequately described drug delivery protocols. Cochlear drug levels were calculated by a validated computer model of drug dispersion in the inner ear fluids based on the concentration and volume of glucocorticoids applied, the time the drug remained in the middle ear, and on the specific timing of injections. Various factors were compared with hearing outcome, including baseline data, individual parameters of the application protocols, calculated peak concentration (Cmax), and total dose (Area under the curve, AUC).
Results
There was no dependence of hearing outcome on individual parameters of the application protocol, Cmax or AUC. Final hearing threshold was notably independent of delay of treatment.
Conclusion
During primary IT or combined steroid therapy of ISSHL, the tendency towards early treatment having a positive effect on hearing improvement is thought to be a “sham effect”, likely related to spontaneous recovery. Change in pure tone average (PTA) may not be an adequate outcome parameter to assess effectiveness of the intervention, as it depends on the degree of initial hearing loss. Final PTA provides a better alternative.
Keywords: computer model, intratympanic, meta-analysis, pharmacokinetics, steroids, sudden hearing loss
Introduction
There is an increasing interest in the use of local drug therapy to treat inner ear diseases. Following the wide acceptance of intratympanic applications of gentamicin for the treatment of Menière’s disease, intratympanically applied glucocorticosteroids (“steroids”) have become commonly-applied drugs for the therapy of patients with either Menière’s disease or idiopathic sudden sensorineural hearing loss (ISSHL) (1–4). Drug application protocols and the choice of steroid vary considerably between studies. Variations in parameters such as the applied drug concentration, the applied volume and timing of injections with different treatment protocols makes it difficult to directly compare the outcome in studies, especially with respect to the actual dose of steroid delivered (2).
The aim of this meta-analysis was to quantitatively compare different dosing regimens, application protocols and steroids used. Therefore, baseline data (e.g. initial hearing loss, start of therapy and age), and specific parameters of the treatment protocols, including the applied concentration, volume, number and frequency of injections etc., were correlated with the reported hearing outcome of the studies. A similar approach was successfully used to interpret human studies of intratympanic gentamicin treatment for Menière’s disease using a wide variety of dosing protocols (5). In that study, larger hearing losses were associated with protocols producing the highest estimated perilymph gentamicin concentrations.
To assess the impact of differences in application protocols on inner ear drug concentration, the maximum intracochlear drug concentration (Cmax) and total dose over the duration of treatment (AUC) were calculated with a validated computer simulation program for intracochlear drug distribution. The simulations were based on the specific application protocols in published controlled and uncontrolled clinical studies, with model parameters based on pharmacokinetic data derived from experiments in animals and from clinical studies. The calculated doses were compared to the mean change in hearing thresholds reported in the respective studies.
Here we discuss results of studies on primary intratympanic or combined therapy of ISSHL. A separate analysis will focus on clinical studies with intratympanic steroids used as secondary therapy for ISSHL.
Methods
Data Sources and Study Selection
The databases PubMed and GoogleScholar were searched for clinical studies on intratympanic or combined (intratympanic and systemic) steroid treatment for ISSHL as a primary therapy. Prospective and retrospective, randomized and non-randomized, controlled and non-controlled studies were included. Only treatment groups including single injection or continuous application, e.g. via a round-window-catheter were considered. Studies using the MicroWick for drug delivery were not included, as currently available pharmacokinetic details of this drug delivery system e.g. contact time of drug with the round window membrane, are insufficient for reliable simulation of the procedure.
Further inclusion criteria were: 1) IT dexamethasone or methylprednisolone used (since pharmacokinetic data were only available for these two drugs), 2) mean hearing loss (pure tone average, PTA) before start of local treatment ≥ 90 dB HL in order to exclude patient populations with only profound hearing loss, and 3) delay between onset of ISSHL and start of treatment ≥ 5 weeks (35 days).
Published studies between January 2000 and June 2014 were selected based on detailed description of treatment protocols with respect to drug concentration, application time, dosing regimen (i.e. start, number and time intervals of treatment), and outcome parameters (change of hearing thresholds between before and after treatment, PTA hearing gain) permitting analysis with the computer model. If relevant information was not provided in the publications, the authors were contacted.
Calculation of inner ear drug distribution
A validated computer model of drug distribution in the inner ear fluids (Washington University Cochlear Fluids Simulator, Version 3.082, http://oto2.wustl.edu/cochlea/) was used to calculate the distribution of steroids with time in the perilymph of scala tympani for different application protocols.
Parameter settings
The computer model takes into account the length and variation of cross-sectional areas of the fluid and tissue spaces of the cochlear and vestibular spaces of the human inner ear. The compartments included in the model (with total volume given in μL) are scala tympani (40.5), scala media (7.7), scala vestibuli (including the vestibule) (84.9), spiral ligament (16.5), spiral ganglion (10.3) and the organ of Corti (2.3). Each was derived from 3D reconstruction of a segmented human inner specimen obtained by non-destructive OPFOS imaging (6).
Cochlear drug levels were calculated based on the applied drug (methylprednisolone; diffusion coefficient 0.784 * 10−9 m2/s (7) or dexamethasone phosphate; diffusion coefficient 0.77 * 10−9 m2/s (8)), the concentration and volume of steroids applied, the time the drug remained in the middle ear, and on the specific timing of injections, i.e. intervals and total number. For intratympanic injections, it was assumed that middle ear concentration was constant and did not vary with time, as application times were brief. Similarly, the co-administration of the drug in hyaluronic acid gel was ignored as the quantitative influence on round window permeability and middle ear residence time is currently unknown, so no modifications in the simulations were made relative to normal injections. No entry location other than the round window was used even though significant entry at the stapes occurs for other substances (9,10). Direct entry of steroids at the stapes has not yet been demonstrated or quantified. Since there is only very limited data on steroid concentrations in the inner ear fluids after systemic application, calculations are based only on the local drug application protocols and do not include estimates of perilymph concentrations resulting for systemic drug application.
Pharmacokinetic parameters used for the simulations were: Round window membrane permeability: Methylprednisolone: 7.8 * 10−9 m/s (7), dexamethasone: 50 * 10−9 m/s (11), perilymph elimination half time: Methylprednisolone: 27 min (7), dexamethasone: 22.5 min (12), and flow rate of cerebrospinal fluid entering at the cochlear aqueduct: 3 nl/min (12).
Drug time courses were quantified in terms of the maximum concentration (Cmax) and the area under the curve (AUC) of the drug in the 4 kHz – 500 Hz cochlear segment, calculated as 8.6 mm − 20.3 mm along scala tympani measured from base (13). This refers to the frequency range pure tone average measures are typically based on (PTA0.5-4kHz).
In each case, a 24h time period after the start of injection was simulated. Distances furthest from the application site follow the slowest time courses and are most susceptible to drug accumulation with repeated applications. If Cmax at the apical turn was less than 1/1000 of scala tympani maximum concentration in a 24h period it was assumed that the drug was completely eliminated during this period. Since time intervals between two injections in the treatment protocols evaluated were at least one day, a relevant drug accumulation effect could thus be ruled out. While Cmax remained the same for multiple injections, total dose (AUC) in perilymph was calculated by multiplying the AUC for a single injection by the number of injections.
Statistical evaluation
Perilymph concentration and dose in the cochlea (Cmax and AUC), parameters of the delivery protocol, and demographic data were compared with changes of hearing thresholds (PTA hearing gain) and final outcome (Final PTA). For statistical evaluation (linear regression analysis), the software GraphPad Prism 5.02 (GraphPad Software, La Jolla, USA) was used.
Results
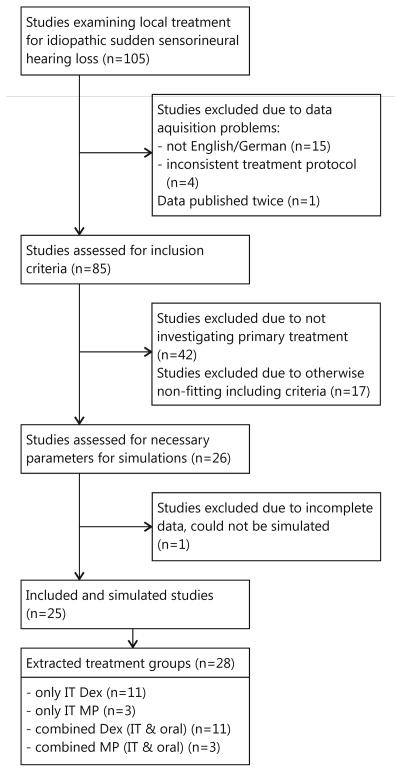
Twenty-five studies with 28 treatment groups for a total of 1275 patients were included in the meta-analysis (figure 1). Characteristics, extracted parameters of the application protocols, and calculated drug concentrations are listed in tables 1 and 2.
Figure 1.
Flowchart summarizing the selection of studies included in the current analysis. IT=intratympanic, Dex = dexamethasone, MP = methylprednisolone.
Table 1.
Individual study groups characteristics and pure tone audiometry outcome measures for primary therapy of ISSHL.
| Study | Type of study | Treatment | Number of patients | Age | Delay onset-treatment | PTA type | PTA frequencies | Timepoint of measured final PTA after start of treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [days] | [days] | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Ahn et al. 2008 (31) | prospective | Combined | 60 | 48.6 | 15.4 | 6.5 | 3.9 | 4 PTA | 500, 1000, 2000, 3000 | 104 | n.a. |
|
| |||||||||||
| Alimoglu et al. 2011 (59) | retrospective | only IT | 43 | n.a. | n.a. | < 30 | n.a. | 4 PTA | 500, 1000, 2000, 4000 | n.a. | n.a. |
|
| |||||||||||
| Arastou et al. 2013 (60) | prospective | Combined | 36 | 45.4 | 14.8 | 18.97 | 23.6 | 5 PTA | 250, 500, 1000, 2000, 4000 | 26 | n.a. |
|
| |||||||||||
| Arslan et al. 2011 (61) | prospective | Combined | 85 | 47.8 | 13.1 | 7.3 | 5.5 | 4 PTA | 500, 1000, 2000, 4000 | 15 | n.a. |
|
| |||||||||||
| Bae et al. 2013 (54) | retrospective | only IT | 94 | 53.6 | 9.9 | 7.3 | 8.3 | 4 PTA | 500, 1000, 2000, 3000 | 40 | n.a. |
|
| |||||||||||
| Bae et al. 2013 (54) | retrospective | Combined | 197 | 50.4 | 15.2 | 6 | 6.7 | 4 PTA | 500, 1000, 2000, 3000 | 40 | n.a. |
|
| |||||||||||
| Battaglia et al. 2008 (62) | prospective | only IT | 17 | 60 | n.a. | 11 | 14 | 3 PTA | 500, 1000, 2000 | 43 | n.a. |
|
| |||||||||||
| Battaglia et al. 2008 (62) | prospective | Combined | 16 | 57 | n.a. | 4 | 3 | 3 PTA | 500, 1000, 2000 | 43 | n.a. |
|
| |||||||||||
| Battaglia et al. 2014 (63) | prospective | Combined | 80 | 57 | 15 | 7.3 | 8 | 4 PTA | 500, 1000, 2000, 4000 | 71 | n.a. |
|
| |||||||||||
| Burkart et al. 2013 (64) | retrospective | Combined | 23 | 47 | 13.7 | 4.2 | 1.9 | 4 PTA | 500, 1000, 2000, 4000 | 90 | n.a. |
|
| |||||||||||
| Dallan et al. 2011 (65) | prospective | only IT | 10 | 56.4 | 14.7 | 7.3 | 2.1 | 4 PTA | 500, 1000, 2000, 3000 | 31 | n.a. |
|
| |||||||||||
| Dispenza et al. 2011 (55) | prospective | only IT | 25 | 47 | n.a. | 9.4 | n.a. | 4 PTA | 500, 1000, 2000, 4000 | 202 | n.a. |
|
| |||||||||||
| Gouveris et al. 2011 (66) | retrospective | Combined | 76 | n.a. | n.a. | n.a. | n.a. | 5 PTA | 500, 1000, 2000, 4000, 8000 | n.a. | n.a. |
|
| |||||||||||
| Gundogan et al. 2013 (56) | prospective | Combined | 37 | 52.32 | 12.94 | 4.7 | 4 | 4 PTA | 500, 1000, 2000, 3000 | 42 | n.a. |
|
| |||||||||||
| Han et al. 2009 (67) | prospective | only IT | 34 | 56.5 | 12.8 | 3.4 | 1.9 | 4 PTA | 500, 1000, 2000, 3000 | 66 | n.a. |
|
| |||||||||||
| Hong et al. 2009 (68) | prospective | only IT | 32 | 56.9 | n.a. | 3.4 | n.a. | 4 PTA | 500, 1000, 2000, 3000 | 98 | n.a. |
|
| |||||||||||
| Jun et al. 2012 (69) | retrospective | Combined | 30 | 47.1 | 14.8 | 5.2 | 11 | 4 PTA | 500, 1000, 2000, 3000 | 90 | n.a. |
|
| |||||||||||
| Kakehata et al. 2006 (70) | prospective | only IT | 10 | 57.7 | n.a. | 5.5 | n.a. | 5 PTA | 250, 500, 1000, 2000, 4000 | n.a. | n.a. |
|
| |||||||||||
| Kakehata et al. 2011 (71) | retrospective | only IT | 19 | 56.2 | 9.7 | 4.8 | n.a. | 5 PTA | 250, 500, 1000, 2000, 4000 | 36 | n.a. |
|
| |||||||||||
| Kara et al. 2010 (72) | prospective | only IT | 29 | 38.9 | 3.15 | 7.03 | 0.8 | 3 PTA | 500, 1000, 2000 | 15 | n.a. |
|
| |||||||||||
| Labatut et al. 2013 (73) | prospective | only IT | 26 | 53 | n.a. | 2 | n.a. | 4 PTA | 500, 1000, 2000, 4000 | 103 | n.a. |
|
| |||||||||||
| Lautermann et al. 2005 (74) | prospective | Combined | 13 | n.a. | n.a. | 2.5 | n.a. | 6 PTA | 500, 750, 1000, 2000, 3000, 4000 | n.a. | n.a. |
|
| |||||||||||
| Lim et al. 2013 (75) | prospective | only IT | 20 | 53.3 | 15.3 | 10.1 | 8.1 | 4 PTA | 500, 1000, 2000, 3000 | 21 | n.a. |
|
| |||||||||||
| Lim et al. 2013 (75) | prospective | Combined | 20 | 47.8 | 14.2 | 9.6 | 7.5 | 4 PTA | 500, 1000, 2000, 3000 | 21 | n.a. |
|
| |||||||||||
| Park et al. 2011 (76) | prospective | Combined | 44 | 45.36 | 12.36 | 3.52 | 3.07 | 4 PTA | 500, 1000, 2000, 3000 | 108 | n.a. |
|
| |||||||||||
| Rauch et al. 2011 (77) | prospective | only IT | 62 | n.a. | n.a. | n.a. | n.a. | 4 PTA | 500, 1000, 2000, 4000 | 72 | n.a. |
|
| |||||||||||
| Suzuki et al. 2012 (57) | retrospective | Combined | 102 | 57 | 19.4 | 5.5 | 4.6 | 5 PTA | 250, 500, 1000, 2000, 4000 | 52 | n.a. |
|
| |||||||||||
| Zhang et al. 2012 (58) | prospective | only IT | 35 | 53.5 | 16.7 | 6.3 | 7 | 5 PTA | 250, 500, 1000, 2000, 4000 | 15 | n.a. |
IT = intratympanic, SD = standard deviation, n.a. = not available, PTA = pure tone average.
Table 2.
Extracted parameters of the application protocols for individual study groups, calculated inner ear drug concentrations, and hearing threshold (PTA) before and after treatment.
| Study | Drug used | Drug concentraion |
Application time |
Total # of injections |
Frequency of injections |
Duration of treatment |
Cmax | AUC | PTA before | PTA hearing gain | Final PTA | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [μg/ml] | [min] | [days] | [days] | [μg/ml] | [μg*s/ml] | [dB HL] | [dB] | [dB HL] | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||||||||
| Ahn et al. 2008 (31) | Dex | 5000 | 30 | 3 | 2 | 5 | 0.021 | 16.56 | 74.3 | 27.8 | 22.04 | n.a. | 52.26 | n.a. |
|
| ||||||||||||||
| Alimoglu et al. 2011 (59) | Dex | 4000 | 30 | 6 | 3.5 | 19 | 0.017 | 26.5 | 61.08 | 22.97 | 13.57 | n.a. | 47.51 | n.a. |
|
| ||||||||||||||
| Arastou et al. 2013 (60) | Dex | 4000 | 20 | 4 | 3.5 | 12 | 0.011 | 11.83 | 70.7 | 26.8 | 22.6 | 3.7 | 47.7 | n.a. |
|
| ||||||||||||||
| Arslan et al. 2011 (61) | MP | 125000 | 15 | 5 | 2 | 9 | 0.069 | 91.55 | 65.7 | 22 | 21.8 | 18.4 | 44.2 | 25.5 |
|
| ||||||||||||||
| Bae et al. 2013 (54) | Dex | 5000 | 30 | 4 | 3.5 | 12 | 0.021 | 22.09 | 61 | 19.9 | 19.3 | n.a. | 41.5 | n.a. |
|
| ||||||||||||||
| Bae et al. 2013 (54) | Dex | 5000 | 30 | 4 | 3.5 | 12 | 0.021 | 22.09 | 67.9 | 25.8 | 19.2 | n.a. | 47.2 | n.a. |
|
| ||||||||||||||
| Battaglia et al. 2008 (62) | Dex | 12000 | 20 | 3 | 7 | 15 | 0.034 | 26.63 | 82 | 28 | 31 | n.a. | 51 | 25 |
|
| ||||||||||||||
| Battaglia et al. 2008 (62) | Dex | 12000 | 20 | 3 | 7 | 15 | 0.034 | 26.63 | 75 | 23 | 40 | n.a. | 35 | 21 |
|
| ||||||||||||||
| Battaglia et al. 2014 (63) | Dex | 10000 | 20 | 3 | 7 | 15 | 0.028 | 22.19 | 84.8 | 18 | 34.1 | 26.6 | 50.6 | 27.8 |
|
| ||||||||||||||
| Burkart et al. 2013 (64) | Dex + Hyaluronic acid | 4800 | 30 | 4 | 2.5 | 10 | 0.02 | 21.2 | 81.1 | 16.6 | 48 | n.a. | 33 | n.a. |
|
| ||||||||||||||
| Dallan et al. 2011 (65) | MP | 20000 | 30 | 1 | n.a. | 1 | 0.022 | 5.85 | 67.2 | 31 | 26.4 | 22 | 40.9 | 38.1 |
|
| ||||||||||||||
| Dispenza et al. 2011 (55) | Dex | 4000 | 20 | 4 | 7 | 22 | 0.011 | 11.83 | 65 | n.a. | 29.55 | 8.98 | 35.45 | n.a. |
|
| ||||||||||||||
| Gouveris et al. 2011 (66) | Dex + Hyaluronic acid | 4000 | 20 | 3 | 2 | 5 | 0.011 | 8.88 | 69 | n.a. | 13.53 | n.a. | 55.47 | n.a. |
|
| ||||||||||||||
| Gundogan et al. 2013 (56) | MP | 62500 | 30 | 4 | 3 | 10 | 0.069 | 73.14 | 80.7 | 22.81 | 44.05 | 21.53 | 36.65 | n.a. |
|
| ||||||||||||||
| Han et al. 2009 (67) | Dex | 5000 | 38 | 4 | 3.5 | 12 | 0.026 | 27.91 | 76.3 | 15 | 25.8 | 17.8 | 50.5 | n.a. |
|
| ||||||||||||||
| Hong et al. 2009 (68) | Dex | 5000 | 30 | 8 | 1 | 8 | 0.021 | 44.17 | 77.5 | 27.6 | 26.26 | 17.97 | 51.23 | n.a. |
|
| ||||||||||||||
| Jun et al. 2012 (69) | Dex | 5000 | 30 | 4 | 1 | 4 | 0.021 | 22.09 | 81 | 16.6 | 25.7 | 21.4 | 55.2 | 23.8 |
|
| ||||||||||||||
| Kakehata et al. 2006 (70) | Dex | 4000 | 30 | 8 | 1 | 8 | 0.017 | 35.34 | 79.5 | n.a. | 40.5 | n.a. | 39 | n.a. |
|
| ||||||||||||||
| Kakehata et al. 2011 (71) | Dex | 4000 | 30 | 8 | 1 | 8 | 0.017 | 35.34 | 77.7 | 18.2 | 39.7 | 18.4 | 38.8 | n.a. |
|
| ||||||||||||||
| Kara et al. 2010 (72) | Dex | 4000 | 20 | 5 | 1 | 5 | 0.011 | 14.79 | 79.93 | 4.05 | 31.38 | 5.02 | 48.55 | n.a. |
|
| ||||||||||||||
| Labatut et al. 2013 (73) | MP | 40000 | 30 | 4 | 3.5 | 12 | 0.044 | 46.81 | 81 | 21 | 32 | 21 | 49 | 30 |
|
| ||||||||||||||
| Lautermann et al. 2005 (74) | MP | 32000 | 20 | 5 | 1 | 5 | 0.024 | 31.24 | 70 | 7 | 11 | 15 | 59 | n.a. |
|
| ||||||||||||||
| Lim et al. 2013 (75) | Dex | 5000 | 30 | 4 | 3 | 10 | 0.021 | 22.06 | 58.9 | 31.2 | 12.1 | 14.6 | 46.8 | 28.2 |
|
| ||||||||||||||
| Lim et al. 2013 (75) | Dex | 5000 | 30 | 4 | 3 | 10 | 0.021 | 22.09 | 56.8 | 28.3 | 21.9 | 26.2 | 34.9 | 25.3 |
|
| ||||||||||||||
| Park et al. 2011 (76) | Dex | 5000 | 20 | 6 | 3 | 16 | 0.014 | 22.19 | 73.12 | 17.01 | 34.74 | 25.65 | 38.38 | 23.01 |
|
| ||||||||||||||
| Rauch et al. 2011 (77) | MP | 40000 | 30 | 4 | 3.5 | 12 | 0.044 | 46.81 | n.a. | n.a. | 30.8 | n.a. | n.a. | n.a. |
|
| ||||||||||||||
| Suzuki et al. 2012 (57) | Dex | 4000 | 30 | 4 | 7 | 22 | 0.017 | 17.67 | 80.9 | 20.1 | 27 | 22.1 | 53.9 | 27 |
|
| ||||||||||||||
| Zhang et al. 2012 (58) | Dex | 5000 | 30 | 4 | 2 | 7 | 0.021 | 22.09 | 70.8 | 25 | 23.2 | 18.8 | 47.6 | n.a. |
Dex = dexamethasone, MP = methylprednisolone, SD = standard deviation, n.a. = not available, PTA = pure tone average.
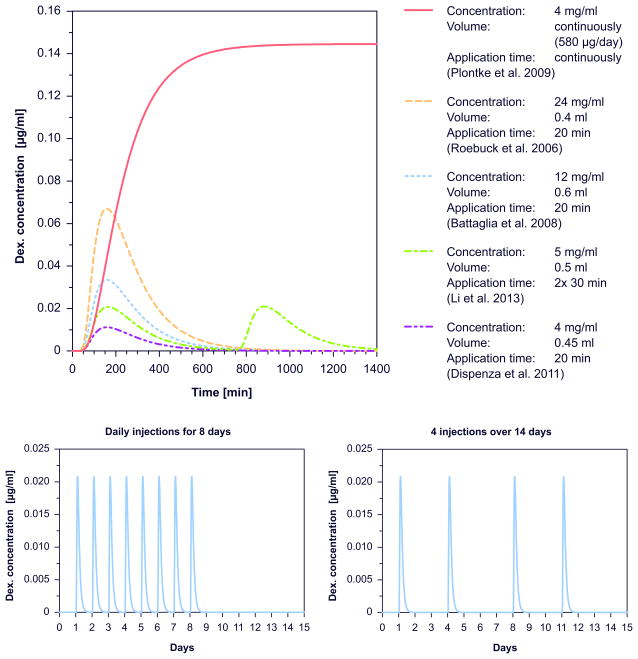
The upper part of figure 2 shows calculated time courses of drug concentration in the scala tympani over 24 hours after injection for five different protocols. Higher drug concentrations used for injection resulted in higher perilymph concentrations. Due to the fast clearance half time of dexamethasone in the inner ear of 22.5 minutes, concentrations declined rapidly within few hours. There was relatively little influence of application time on the concentrations reached, as the differences between protocols were small. In contrast, continuous intratympanic drug application using pumps was calculated to result in much higher inner ear drug concentrations.
Figure 2.
Upper part Examples for time courses of calculated drug concentrations in scala tympani in the 500 – 4000 Hz region after injection for different application protocols according to Dispenza et al. 2011 (55), Li et al. 2013 (78), Battaglia et al. 2008 (62), Roebuck et al. 2006 (79), and Plontke et al. 2009 (80) respectively. Lower part: Example time courses of calculated drug concentrations in scala tympani in the 500 – 4000 Hz region over the total duration of treatment for different treatment protocols: Left: daily injections for 8 days, 5 mg/ml, 0.35 ml, for 30 min application time according to Hong et al. 2009 (68). Right: 4 injections over 14 days, 5 mg/ml, 0.55 ml, for 30 min application time according to Bae et al. 2013 (54).
The lower part of figure 2 shows the time course over the total duration of the therapy protocol for two different injection schemes. Since the drug is almost completely eliminated within 24 hours after a single injection (figure 2) the time courses suggest that there is no accumulation of drug after multiple injections.
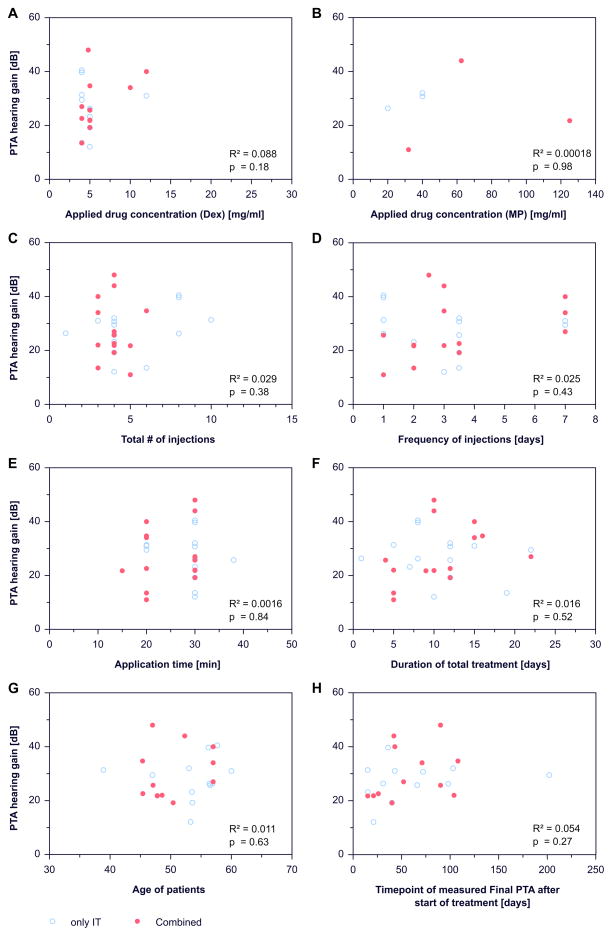
For primary intratympanic or combined therapy of ISSHL there was no dependence of change in hearing threshold on the drug concentration used (figure 3a, 3b), the number of injections (figure 3c), the frequency of injections (figure 3d), the estimated time of drug in the middle ear (time provided in the publications where the patient had to lay down on the contralateral side before standing up) (figure 3e), the total duration of treatment (figure 3f), the age of patients (figure 3g), or on the time of the endpoint measurement (figure 3h). Neither the hearing change (PTA hearing gain) nor the average final hearing threshold (final PTA, data not shown) individually depended on parameters of the application protocol or demographic factors.
Figure 3.
Dependence of change in pure tone average (PTA hearing gain) on: (a) use of dexamethasone formulation for injection, (b) use of methylprednisolone formulation for injection, (c) total number of injections, (d) frequency of injections, (e) application time of the injection, (f) duration of treatment, (g) age of patients and (h) time of endpoint measurement.
There was no dependency of simulated maximum drug concentration (Cmax) and total drug dose (AUC) in the cochlea on hearing outcome for either dexamethasone (figure 4a, 4b) or methylprednisolone (figure 4c, 4d).
Figure 4.
Dependence of change in pure tone average (PTA hearing gain) on the calculated maximum intra-cochlear drug concentrations (Cmax) or total doses (AUC) in the scala tympani within the 500 – 4000 Hz region for dexamethasone and methylprednisolone: (a) dexamethasone Cmax, (b) dexamethasone AUC, (c) methylprednisolone Cmax, (d) methylprednisolone AUC .
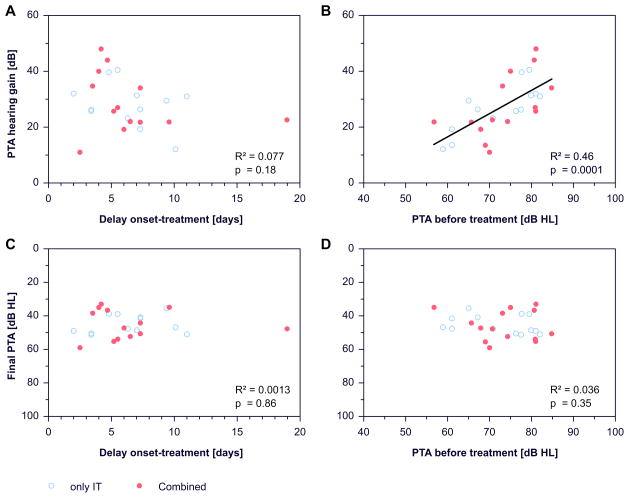
There was a tendency (statistically not significant) for larger hearing gain with earlier start of treatment after onset of ISSHL (figure 5a). However, this tendency was not present when “final PTA” was chosen as outcome parameter (figure 5c).
Figure 5.
Dependence of change in pure tone average (PTA hearing gain) on: (a) the start of treatment (treatment delay) after onset of ISSHL, (b) hearing threshold (PTA) at the beginning of treatment. Dependence of the final hearing threshold (final PTA) on: (c) the start of treatment (treatment delay) after onset of ISSHL, (d) hearing threshold (PTA) at the beginning of treatment.
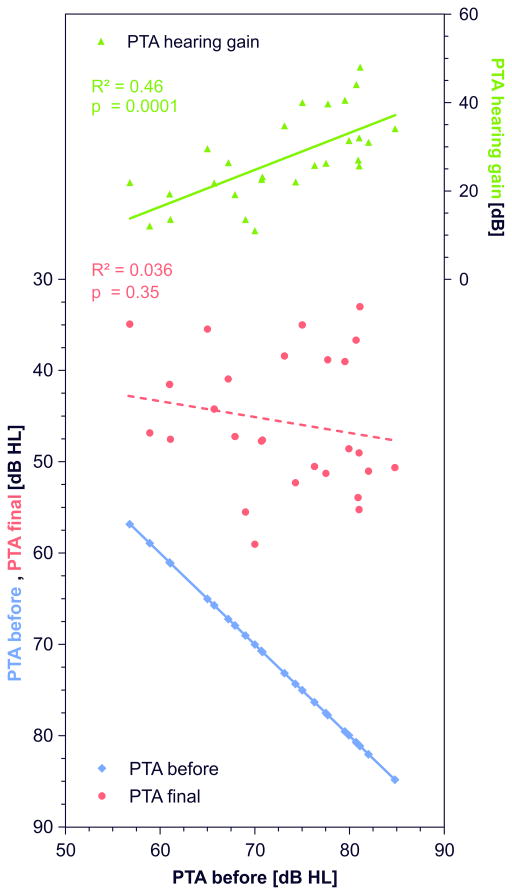
A strong correlation was found between hearing loss before treatment and change of hearing threshold (R²=0.46, p=0.0001, figure 5b) but not between hearing loss before treatment and final PTA (figure 5d).
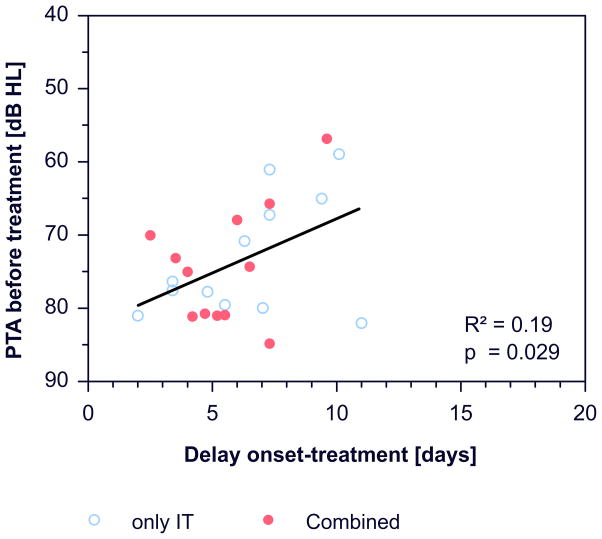
There was a correlation between the delay of starting treatment after onset of ISSHL and hearing loss at the beginning of treatment (R²=0.19, p=0.029, figure 6).
Figure 6.
Dependence of the PTA at the beginning of treatment on the delay of treatment after onset.
Discussion
Individual parameters of the different treatment protocols failed to show an influence on recovery of hearing (figure 3). Calculations of drug concentrations in the inner ear (Cmax or total dose AUC), with a validated computer model using the specific individual parameters of the respective treatment protocols, showed no effect on hearing outcome across studies (figure 4).
There are a number of possible explanations of these findings, as follows: (i) the drug concentrations and total doses reached in the inner ear after intratympanic application in the human may be insufficient (too low) for the treatment of ISSHL; (ii) the dosing regime may have no influence on treatment success so that all treatment protocols are equally effective/ineffective; (iii) the effects of different treatment protocols may be masked by differing patient backrounds across the studies and/or statistical effects due to averaged baseline parameters and averaged outcome results rather than individual patient data, (iv) effects may be masked by a dominance of spontaneous recovery in the patient populations, (v) the patient numbers may not be high enough to detect small changes with statistical significance and thus small effects with respect to treatment parameters and/or (vi) steroid therapy may be ineffective for treating ISSHL.
The concentration of the steroids dexamethasone and methylprednisolone that could potentially influence cochlear function can be estimated from a variety of studies in the literature. One study based on the activation kinetics of the signal cascades in the glucocorticoid receptor and mineralocorticoid receptor (table 3) suggest that the calculated intracochlear drug concentrations achieved by the evaluated treatment protocols could induce drug mediated changes in inner ear tissue (14). An in vivo study on a cochlear implant electrode insertion trauma (EIT) model using an electrode dummy as delivery device for dexamethasone demonstrated that alterations in gene expression induced by the insertion trauma were reduced in comparison to a control group with electrode dummies implanted without incorporated dexamethasone (15). In this study an inner ear dexamethasone steady state concentration of 0.1 μg/ml was achieved in the scala tympani (15,16). In another study using a similar dummy and EIT animal model a down regulation of TNF-a in cochlear tissue was shown in comparison to control group (17).
Table 3.
Relative upregulation of genes depending on the activation of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) (14).
| Calculated Cmax (500 – 4000 Hz) [μg/ml] |
GR dependent genes | MR dependent genes | |
|---|---|---|---|
| Dexamethasone | 0.011 – 0.034 | 95 – 100% | 85 – 95% |
| Methylprednisolone | 0.022 – 0.069 | 85 – 100% | 90 – 100% |
In vitro studies show that dexamethasone concentrations of 0.04 ug/ml were required to modulate ion transport in epithelium of cochlea scalae (18,19) and dexamethasone concentrations of 0.01 μg/ml are needed to impede fibroblast growth (20,21). It was demonstrated in vivo that a dexamethasone concentration of both 0.1 μg/ml and 0.05 μg/ml stable over several weeks reduces fibrosis after EIT and protects hair cells and neural elements in the cochlea in comparison to a control group (22) (Liebau, unpublished data). The same study also could show that a drug concentration of 0.0006 μg/ml leads to an incomplete protection of hair cells and neural elements under equal EIT conditions (22) (Liebau et al., unpublished data). However a reduced protective effect against apoptosis was still present in comparison to control group (22).
For effective immune suppression dexamethasone concentrations above 0.018 μg/ml is thought to be necessary and for methylprednisolone it is 0.051 μg/ml (23). It was demonstrated that an electrode dummy loaded with dexamethasone achieving an inner ear steady state drug concentration of 0.1 μg/ml in the scala tympani was effective to significantly reduce lymphocyte, macrophage, and giant cell infiltration into the cochlea after EIT (16,24).
It is therefore possible that drug concentrations achieved in the PTA range (0.5 – 4 kHz; 8.6 to 20.3 mm from the base of scala tympani) by intratympanic injection may be too low to ensure the biological changes needed for therapeutic success. It is believed that there are much higher drug levels present after intratympanic injections in the basal region of the cochlea, corresponding to the high frequency range (25,26). However, hearing recovery is reported to be the lowest in the high frequency range after ISSHL (27–31).
In our meta-analysis on secondary treatment of ISSHL simulations of application protocols using the round window catheter showed constant drug concentrations above 0.36 μg/ml in the PTA(0.5–4kHz) range for dexamethasone as well as methylprednisolone for the whole duration of treatment. Nevertheless we did not find a higher hearing recovery in patients of studies using the round window catheter in comparison to studies using intratympanic injections for application.
Bird and colleges measured the intra cochlear dexamethasone concentration after 70 minutes after systemic application (iv, 0.17 mg/kg) by sampling the first 20 μl perilymph through the round window and found on average a concentration of 0.12 μg/ml in 9 patients (26). If it is assumed there is a homogenous drug concentration in the scala tympani after systemic application therefore the determined concentration is also representative for the PTA(0.5–4kHz) range (25). Combined application protocols may have produced higher drug levels in the inner ear, however the treatment success in study groups with combined therapy is not superior to study groups with intratympanic injection only (figure 4).
We found that there was no influence of the age of patients on hearing outcome (figure 3g). In prior studies, however, multivariable statistical analysis suggested that increasing age slightly worsened the prognosis for hearing recovery (32–34). Some authors have stated that the highest incidence for ISSHL is around an age of 50 years (35,36). This appears to be merely a statistical effect based on todays life expectancy in industrial countries of about 80 years and the fact that young people (<20 years) are rarely affected by ISSHL. The mean of the remaining range is about 50 years, but cases of ISSHL are distributed throughout this age spectrum (37,38).
The importance of baseline data for the evaluation of treatment success is demonstrated by the strong correlation between hearing improvement and hearing loss before treatment (figure 5b). While hearing improvement (PTA change) significantly correlated with hearing loss before treatment, final PTA was nearly independent on hearing loss before treatment (figure 5b, 5d). Therefore the correlation of higher hearing improvement with higher hearing loss before treatment may only reflects the greater potential of improving in patients with more pronounced hearing loss. There was also a tendency (not significant) for larger hearing gain, i.e. larger change in PTA with earlier onset of treatment (figure 5a). This is in correspondence with arguments often used in communication of doctors with patients when they suggest to start a therapy as soon as possible to get the greatest benefit from the treatment (39–42). However, such an argument appears not to be supported by the present data. The tendency for an apparently better outcome disappeared if instead of „hearing gain“, „final PTA“ was used as an outcome criterion (figure 5c). In addition, a significant correlation across studies was found for an earlier start of therapy in studies with higher initial hearing loss (R²=0.12, p=0.029, figure 6), and hearing gain also significantly correlated with initial hearing loss (figure 5b). We therefore consider the tendency towards a positive effect of early treatment on hearing gain during therapy as a “sham effect”, which is most likely due to spontaneous recovery and not due to the time point of the start of the intervention.
The shorter the delay between onset of ISSHL and measuring PTA before treatment (reference PTA), the higher is the proportion of hearing gain due to spontaneous recovery that is contributing to the treatment success. This influence is greatest when therapy is initiated within the first two weeks after onset since in most patients showing some degree of spontaneous recovery the largest proportion of hearing gain occurs during this time period (35,36,43,44). Additionally, within this time period the influence of spontaneous recovery is increasing in a nearly exponential way (figure 5a). In most patients hearing gain due to spontaneous recovery follows a time course of exponential decrease (45,46).
Figure 7 demonstrates that larger hearing loss at start of therapy is compensated with higher hearing gain. This effect is most likely due to spontaneous recovery. Similar final hearing levels are reached in all groups independent of treatment protocol, although there is a tendency for a poorer prognosis with larger hearing loss before treatment. In figure 2 of their 1977 publication, Mattox and Simmons already have shown on a frequency specific manner that a similar final PTA was reached regardless of hearing loss before treatment and type of treatment. Patients attained higher gain in low frequencies and lesser gain in high frequencies resulting in similar final audiograms independent on initial audiograms but only when considering averaged audiograms in a considerably large patient group (98 patients), compared to many of the currently published uncontrolled or retrospective controlled reports (43).
Figure 7.
Dependence of change in pure tone average (PTA hearing gain) and final hearing threshold (final PTA) on hearing threshold at the beginning of (“PTA before”) treatment (see figure 5b, 5d). PTA hearing gain, final PTA, and PTA before treatment of each group are arranged at the y-axis and study results are sorted by increasing PTA before treatment on the x-axis. Larger hearing loss at start of therapy is compensated by higher hearing gain resulting in similar final hearing thresholds (final PTA) with a tendency for a poorer prognosis with larger hearing loss before treatment.
A number of clinical studies have noticed that patients with profound hearing loss (80 – 120 dB HL) have a very poor prognosis (35,47–50). It seems, that there exists a “boundary” between approximately 80 and 90 dB HL were hearing loss is less likely compensated by recovery and prognosis gets significantly worse. Most patients belonging to this group have “flat” with pancochlear hearing loss or show a profound high frequency loss. It has been described on many occasions that “flat” audiograms have the poorest prognosis and patients with profound high frequency loss often reach only small amounts of recovery (37,43,50). Another negative prognostic factor especially in the latter ones seems to be associated vertigo (43,51–53).
The lack of any correlation between specific parameters of the treatment protocol or calculations of Cmax and dose (AUC) and hearing outcome, i.e. the absence of a clear dose-effect relationship, may call into question the efficacy of intratympanic steroid therapy for the primary therapy of ISSHL.
We did not include systemically applied steroids in combined therapy strategies in our calculation of inner ear drug concentration and doses (Cmax an AUC). We also did not consider other additionally used substances or treatments (e.g. vitamins, hyperbaric oxygen therapy). Nonetheless, since we found a similar final PTA across the groups, there seems to be no clear impact of treatment protocol on the treatment success.
Limitations of the present meta-analysis arise from the limitations of the published data in studies on ISSHL treatment.
Firstly, no uniform scheme for audiological evaluation was used across the studies (i.e. PTA frequencies, time points of measurements). This could distort objective evaluation of hearing outcome in patients and make it difficult to compare them especially when having different types of hearing loss. Standardization of outcome parameters are needed for a better comparability between studies (38).
Secondly, as the hearing gain seems to strongly depend on hearing levels at the beginning of the therapy, final hearing might be considered as a better, more robust outcome measure when comparing studies in meta-analysis.
Thirdly, to avoid “smoothing out” of treatment effects by averaging outcome data, we encourage authors to publish outcome data for individual patients. Such data were only available in very few publications. For more detailed analyses of treatment effects it is of utmost importance to have individual patient data available with different degrees or different types of hearing loss. Impact of patient background on treatment success, such as patient’s age, treatment delay or associated symptoms like vertigo can only be analyzed in an advanced way if the individual attribution to specific patients is not lost by averaging data. Some studies performed multivariable statistical analysis to filter out important factors influencing the prognosis. However, we think it would help advance the field to also have individual patient data available for meta-analyses (34,54–58). Even in times of continuing efforts of cost reductions for print publication, publishers need to understand the importance of providing individual patient data, at least in the form of supplementary digital materia
Acknowledgments
This work was supported by BMBF 01KG1427 (SKP) and by NIH/NIDCD grant DC001368 (ANS).
Footnotes
Disclosures: Stefan K. Plontke is a consultant for Otonomy, Inc., San Diego, USA and chief of the scientific advisory board of AudioCure Pharma GmbH, Berlin Germany. Alec N. Salt is a member of the scientific advisory board of Otonomy, Inc. This work was not sponsored by any of the above companies
Literature
- 1.Vlastarakos PV, Papacharalampous G, Maragoudakis P, et al. Are intra-tympanically administered steroids effective in patients with sudden deafness? Implications for current clinical practice. Eur Arch Otorhinolaryngol. 2012;269:363–80. doi: 10.1007/s00405-011-1738-0. [DOI] [PubMed] [Google Scholar]
- 2.Lavigne P, Lavigne F, Saliba I. Intratympanic corticosteroids injections: a systematic review of literature. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3689-3. [DOI] [PubMed] [Google Scholar]
- 3.Huon LK, Fang TY, Wang PC. Outcomes of intratympanic gentamicin injection to treat Meniere's disease. Otol Neurotol. 2012;33:706–14. doi: 10.1097/MAO.0b013e318259b3b1. [DOI] [PubMed] [Google Scholar]
- 4.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 5.Salt AN, Gill RM, Plontke SK. Dependence of hearing changes on the dose of intratympanically applied gentamicin: a meta-analysis using mathematical simulations of clinical drug delivery protocols. Laryngoscope. 2008;118:1793–800. doi: 10.1097/MLG.0b013e31817d01cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 7.Plontke SK, Mikulec AA, Salt AN. Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124–30. Otol Neurotol. 2008;29:732–3. doi: 10.1097/MAO.0b013e318173fcea. author reply 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salt AN, Hartsock J, Plontke S, et al. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16:323–35. doi: 10.1159/000322504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King EB, Salt AN, Eastwood HT, et al. Direct entry of gadolinium into the vestibule following intratympanic applications in Guinea pigs and the influence of cochlear implantation. J Assoc Res Otolaryngol. 2011;12:741–51. doi: 10.1007/s10162-011-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salt AN, King EB, Hartsock JJ, et al. Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hear Res. 2012;283:14–23. doi: 10.1016/j.heares.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plontke SK, Biegner T, Kammerer B, et al. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–6. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salt AN, Hartsock JJ, Gill RM, et al. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol. 2012;13:771–83. doi: 10.1007/s10162-012-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood DD. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann C, Scholz T, Rochel M, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151:397–406. doi: 10.1530/eje.0.1510397. [DOI] [PubMed] [Google Scholar]
- 15.Takumi Y, Nishio SY, Mugridge K, et al. Gene expression pattern after insertion of dexamethasone-eluting electrode into the guinea pig cochlea. PLoS One. 2014;9:e110238. doi: 10.1371/journal.pone.0110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Jolly C, Braun S, et al. In vitro and in vivo pharmacokinetic study of a dexamethasone-releasing silicone for cochlear implants. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3760-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Jolly C, Braun S, et al. Effects of a dexamethasone-releasing implant on cochleae: A functional, morphological and pharmacokinetic study. Hear Res. 2015;327:89–101. doi: 10.1016/j.heares.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Pondugula SR, Raveendran NN, Ergonul Z, et al. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol Genomics. 2006;24:114–23. doi: 10.1152/physiolgenomics.00006.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Kim KX, Raveendran NN, et al. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am J Physiol Cell Physiol. 2009;296:C544–57. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhmann AG, Berliner DL. Effect of Steroids on Growth of Mouse Fibroblasts in Vitro. Endocrinology. 1965;76:916–27. doi: 10.1210/endo-76-5-916. [DOI] [PubMed] [Google Scholar]
- 21.Fagot D, Buquet-Fagot C, Mester J. Antimitogenic effects of dexamethasone in chemically transformed mouse fibroblasts. Endocrinology. 1991;129:1033–41. doi: 10.1210/endo-129-2-1033. [DOI] [PubMed] [Google Scholar]
- 22.Bas E, Bohorquez J, Goncalves S, et al. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear Res. 2016 doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Czock D, Keller F, Rasche FM, et al. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Farhadi M, Jalessi M, Salehian P, et al. Dexamethasone eluting cochlear implant: Histological study in animal model. Cochlear Implants Int. 2013;14:45–50. doi: 10.1179/1754762811Y.0000000024. [DOI] [PubMed] [Google Scholar]
- 25.Hahn H, Salt AN, Schumacher U, et al. Gentamicin concentration gradients in scala tympani perilymph following systemic applications. Audiol Neurootol. 2013;18:383–91. doi: 10.1159/000355283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird PA, Murray DP, Zhang M, et al. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011;32:933–6. doi: 10.1097/MAO.0b013e3182255933. [DOI] [PubMed] [Google Scholar]
- 27.Lee JB, Choi SJ, Park K, et al. The efficiency of intratympanic dexamethasone injection as a sequential treatment after initial systemic steroid therapy for sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2011;268:833–9. doi: 10.1007/s00405-010-1476-8. [DOI] [PubMed] [Google Scholar]
- 28.Moon IS, Lee JD, Kim J, et al. Intratympanic dexamethasone is an effective method as a salvage treatment in refractory sudden hearing loss. Otol Neurotol. 2011;32:1432–6. doi: 10.1097/MAO.0b013e318238fc43. [DOI] [PubMed] [Google Scholar]
- 29.Yang CH, Wu RW, Hwang CF. Comparison of intratympanic steroid injection, hyperbaric oxygen and combination therapy in refractory sudden sensorineural hearing loss. Otol Neurotol. 2013;34:1411–6. doi: 10.1097/MAO.0b013e3182a1eb83. [DOI] [PubMed] [Google Scholar]
- 30.Raymundo IT, Bahmad F, Jr, Barros Filho J, et al. Intratympanic methylprednisolone as rescue therapy in sudden sensorineural hearing loss. Braz J Otorhinolaryngol. 2010;76:499–509. doi: 10.1590/S1808-86942010000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn JH, Yoo MH, Yoon TH, et al. Can intratympanic dexamethasone added to systemic steroids improve hearing outcome in patients with sudden deafness? Laryngoscope. 2008;118:279–82. doi: 10.1097/MLG.0b013e3181585428. [DOI] [PubMed] [Google Scholar]
- 32.Nosrati-Zarenoe R, Arlinger S, Hultcrantz E. Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol. 2007;127:1168–75. doi: 10.1080/00016480701242477. [DOI] [PubMed] [Google Scholar]
- 33.Narozny W, Kuczkowski J, Kot J, et al. Prognostic factors in sudden sensorineural hearing loss: our experience and a review of the literature. Ann Otol Rhinol Laryngol. 2006;115:553–8. doi: 10.1177/000348940611500710. [DOI] [PubMed] [Google Scholar]
- 34.Hultcrantz E, Nosrati-Zarenoe R. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: analysis of an RCT and material drawn from the Swedish national database. Eur Arch Otorhinolaryngol. 2015;272:3169–75. doi: 10.1007/s00405-014-3360-4. [DOI] [PubMed] [Google Scholar]
- 35.Byl FM., Jr Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. 1984;94:647–61. [PubMed] [Google Scholar]
- 36.Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–74. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Chen CY, Halpin C, Rauch SD. Oral steroid treatment of sudden sensorineural hearing loss: a ten year retrospective analysis. Otol Neurotol. 2003;24:728–33. doi: 10.1097/00129492-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Plontke SK, Bauer M, Meisner C. Comparison of pure-tone audiometry analysis in sudden hearing loss studies: lack of agreement for different outcome measures. Otol Neurotol. 2007;28:753–63. doi: 10.1097/mao.0b013e31811515ae. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee A, Parnes LS. Intratympanic corticosteroids for sudden idiopathic sensorineural hearing loss. Otol Neurotol. 2005;26:878–81. doi: 10.1097/01.mao.0000185052.07513.5a. [DOI] [PubMed] [Google Scholar]
- 40.Tsai YJ, Liang JG, Wu WB, et al. Intratympanic injection with dexamethasone for sudden sensorineural hearing loss. J Laryngol Otol. 2011;125:133–7. doi: 10.1017/S0022215110002124. [DOI] [PubMed] [Google Scholar]
- 41.Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833–40. doi: 10.1056/NEJMcp0802129. [DOI] [PubMed] [Google Scholar]
- 42.Alexander TH, Harris JP, Nguyen QT, et al. Dose Effect of Intratympanic Dexamethasone for Idiopathic Sudden Sensorineural Hearing Loss: 24 mg/mL Is Superior to 10 mg/mL. Otol Neurotol. 2015;36:1321–7. doi: 10.1097/MAO.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 43.Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977;86:463–80. doi: 10.1177/000348947708600406. [DOI] [PubMed] [Google Scholar]
- 44.Plaza G, Herraiz C. Intratympanic steroids for treatment of sudden hearing loss after failure of intravenous therapy. Otolaryngol Head Neck Surg. 2007;137:74–8. doi: 10.1016/j.otohns.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Suckfuell M, Lisowska G, Domka W, et al. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss: a double-blind, randomized, placebo-controlled phase II study. Otol Neurotol. 2014;35:1317–26. doi: 10.1097/MAO.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 46.Yeo SW, Lee DH, Jun BC, et al. Hearing outcome of sudden sensorineural hearing loss: long-term follow-up. Otolaryngol Head Neck Surg. 2007;136:221–4. doi: 10.1016/j.otohns.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Lee JD, Park MK, Lee CK, et al. Intratympanic steroids in severe to profound sudden sensorineural hearing loss as salvage treatment. Clin Exp Otorhinolaryngol. 2010;3:122–5. doi: 10.3342/ceo.2010.3.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belhassen S, Saliba I. Intratympanic steroid injection as a salvage treatment for sudden sensorineural hearing loss. J Laryngol Otol. 2014;128:1044–9. doi: 10.1017/S0022215114002710. [DOI] [PubMed] [Google Scholar]
- 49.Fetterman BL, Saunders JE, Luxford WM. Prognosis and treatment of sudden sensorineural hearing loss. Am J Otol. 1996;17:529–36. [PubMed] [Google Scholar]
- 50.Kim SH, Jung SY, Kim MG, et al. Comparison of steroid administration methods in patients with idiopathic sudden sensorineural hearing loss: a retrospective observational study. Clin Otolaryngol. 2015;40:183–90. doi: 10.1111/coa.12342. [DOI] [PubMed] [Google Scholar]
- 51.Cvorovic L, Deric D, Probst R, et al. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008;29:464–9. doi: 10.1097/MAO.0b013e31816fdcb4. [DOI] [PubMed] [Google Scholar]
- 52.Nosrati-Zarenoe R, Hansson M, Hultcrantz E. Assessment of diagnostic approaches to idiopathic sudden sensorineural hearing loss and their influence on treatment and outcome. Acta Otolaryngol. 2010;130:384–91. doi: 10.1080/00016480903161541. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima T, Yanagita N. Outcome of sudden deafness with and without vertigo. Laryngoscope. 1993;103:1145–9. doi: 10.1288/00005537-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Bae SC, Noh HI, Jun BC, et al. Efficacy of intratympanic steroid therapy for idiopathic sudden sensorineural hearing loss: comparison with systemic steroid therapy and combined therapy. Acta Otolaryngol. 2013;133:428–33. doi: 10.3109/00016489.2012.749520. [DOI] [PubMed] [Google Scholar]
- 55.Dispenza F, Amodio E, De Stefano A, et al. Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study. Eur Arch Otorhinolaryngol. 2011;268:1273–8. doi: 10.1007/s00405-011-1523-0. [DOI] [PubMed] [Google Scholar]
- 56.Gundogan O, Pinar E, Imre A, et al. Therapeutic efficacy of the combination of intratympanic methylprednisolone and oral steroid for idiopathic sudden deafness. Otolaryngol Head Neck Surg. 2013;149:753–8. doi: 10.1177/0194599813500754. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki H, Hashida K, Nguyen KH, et al. Efficacy of intratympanic steroid administration on idiopathic sudden sensorineural hearing loss in comparison with hyperbaric oxygen therapy. Laryngoscope. 2012;122:1154–7. doi: 10.1002/lary.23245. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q, Song H, Peng H, et al. Noninvasive intratympanic dexamethasone treatment for sudden sensorineural hearing loss. Acta Otolaryngol. 2012;132:583–9. doi: 10.3109/00016489.2011.649145. [DOI] [PubMed] [Google Scholar]
- 59.Alimoglu Y, Inci E, Edizer DT, et al. Efficacy comparison of oral steroid, intratympanic steroid, hyperbaric oxygen and oral steroid + hyperbaric oxygen treatments in idiopathic sudden sensorineural hearing loss cases. Eur Arch Otorhinolaryngol. 2011;268:1735–41. doi: 10.1007/s00405-011-1563-5. [DOI] [PubMed] [Google Scholar]
- 60.Arastou S, Tajedini A, Borghei P. Combined intratympanic and systemic steroid therapy for poor-prognosis sudden sensorineural hearing loss. Iran J Otorhinolaryngol. 2013;25:23–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Arslan N, Oguz H, Demirci M, et al. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2011;32:393–7. doi: 10.1097/MAO.0b013e318206fdfa. [DOI] [PubMed] [Google Scholar]
- 62.Battaglia A, Burchette R, Cueva R. Combination therapy (intratympanic dexamethasone + high-dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008;29:453–60. doi: 10.1097/MAO.0b013e318168da7a. [DOI] [PubMed] [Google Scholar]
- 63.Battaglia A, Lualhati A, Lin H, et al. A prospective, multi-centered study of the treatment of idiopathic sudden sensorineural hearing loss with combination therapy versus high-dose prednisone alone: a 139 patient follow-up. Otol Neurotol. 2014;35:1091–8. doi: 10.1097/MAO.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 64.Burkart C, Linder T, Gartner M. Intratympanic steroid administration: use in the treatment of profound idiopathic sudden sensorineural hearing loss. HNO. 2013;61:152, 4–8. doi: 10.1007/s00106-012-2557-3. [DOI] [PubMed] [Google Scholar]
- 65.Dallan I, Fortunato S, Casani AP, et al. Intratympanic methylprednisolone as first-line therapy in sudden sensorineural hearing loss: preliminary results from a case-control series. J Laryngol Otol. 2011;125:1004–8. doi: 10.1017/S0022215111001782. [DOI] [PubMed] [Google Scholar]
- 66.Gouveris H, Schuler-Schmidt W, Mewes T, et al. Intratympanic dexamethasone/hyaluronic acid mix as an adjunct to intravenous steroid and vasoactive treatment in patients with severe idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2011;32:756–60. doi: 10.1097/MAO.0b013e31821a3fc3. [DOI] [PubMed] [Google Scholar]
- 67.Han CS, Park JR, Boo SH, et al. Clinical efficacy of initial intratympanic steroid treatment on sudden sensorineural hearing loss with diabetes. Otolaryngol Head Neck Surg. 2009;141:572–8. doi: 10.1016/j.otohns.2009.06.084. [DOI] [PubMed] [Google Scholar]
- 68.Hong SM, Park CH, Lee JH. Hearing outcomes of daily intratympanic dexamethasone alone as a primary treatment modality for ISSHL. Otolaryngol Head Neck Surg. 2009;141:579–83. doi: 10.1016/j.otohns.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Jun HJ, Chang J, Im GJ, et al. Analysis of frequency loss as a prognostic factor in idiopathic sensorineural hearing loss. Acta Otolaryngol. 2012;132:590–6. doi: 10.3109/00016489.2011.652306. [DOI] [PubMed] [Google Scholar]
- 70.Kakehata S, Sasaki A, Oji K, et al. Comparison of intratympanic and intravenous dexamethasone treatment on sudden sensorineural hearing loss with diabetes. Otol Neurotol. 2006;27:604–8. doi: 10.1097/01.mao.0000224092.79635.ee. [DOI] [PubMed] [Google Scholar]
- 71.Kakehata S, Sasaki A, Futai K, et al. Daily short-term intratympanic dexamethasone treatment alone as an initial or salvage treatment for idiopathic sudden sensorineural hearing loss. Audiol Neurootol. 2011;16:191–7. doi: 10.1159/000320269. [DOI] [PubMed] [Google Scholar]
- 72.Kara E, Cetik F, Tarkan O, et al. Modified intratympanic treatment for idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2010;267:701–7. doi: 10.1007/s00405-009-1130-5. [DOI] [PubMed] [Google Scholar]
- 73.Labatut T, Daza MJ, Alonso A. Intratympanic steroids as primary initial treatment of idiopathic sudden sensorineural hearing loss. The Hospital Universitario Ramon y Cajal experience and review of the literature. Eur Arch Otorhinolaryngol. 2013;270:2823–32. doi: 10.1007/s00405-012-2306-y. [DOI] [PubMed] [Google Scholar]
- 74.Lautermann J, Sudhoff H, Junker R. Transtympanic corticoid therapy for acute profound hearing loss. Eur Arch Otorhinolaryngol. 2005;262:587–91. doi: 10.1007/s00405-004-0876-z. [DOI] [PubMed] [Google Scholar]
- 75.Lim HJ, Kim YT, Choi SJ, et al. Efficacy of 3 different steroid treatments for sudden sensorineural hearing loss: a prospective, randomized trial. Otolaryngol Head Neck Surg. 2013;148:121–7. doi: 10.1177/0194599812464475. [DOI] [PubMed] [Google Scholar]
- 76.Park MK, Lee CK, Park KH, et al. Simultaneous versus subsequent intratympanic dexamethasone for idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2011;145:1016–21. doi: 10.1177/0194599811418169. [DOI] [PubMed] [Google Scholar]
- 77.Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071–9. doi: 10.1001/jama.2011.679. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Ren J, Yin T, et al. Intratympanic dexamethasone perfusion versus injection for treatment of refractory sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2013;270:861–7. doi: 10.1007/s00405-012-2061-0. [DOI] [PubMed] [Google Scholar]
- 79.Roebuck J, Chang CY. Efficacy of steroid injection on idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2006;135:276–9. doi: 10.1016/j.otohns.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 80.Plontke SK, Löwenheim H, Mertens J, et al. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009;119:359–69. doi: 10.1002/lary.20074. [DOI] [PubMed] [Google Scholar]