Abstract
In humans, some males experience reductions in testosterone levels, as a natural consequence of aging or in the clinical condition termed hypogonadism, which are associated with impaired cognitive performance and mood disorder(s). Some of these behavioral deficits can be reversed by testosterone treatment. Our previous work in rats reported that sex differences in the expression of the transcription factor Zif268, a downstream target of testosterone, within the medial prefrontal cortex (mPFC) mediates sex differences in social interaction. In the present study, we aimed to examine the effects of gonadectomy (GNX) in male rats on mPFC Zif268 expression, mood and cognitive behaviors. We also examined whether reinstitution of Zif268 in GNX rats will correct some of the behavioral deficits observed following GNX. Our results show that GNX induced a downregulation of Zif268 protein in the mPFC, which was concomitant with impaired memory in the y-maze and spontaneous object recognition test, reduced social interaction time, and depression-like behaviors in the forced swim test. Reinstitution of mPFC Zif268, using a novel adeno-associated-viral (AAV) construct, abrogated GNX-induced working memory and long-term memory impairments, and reductions in social interaction time, but not GNX-induced depression-like behaviors. These findings suggest that mPFC Zif268 exerts beneficial effects on memory and social interaction, and could be a potential target for novel treatments for behavioral impairments observed in hypogonadal and aged men with declining levels of gonadal hormones.
Keywords: depression, gonadectomy, medial prefrontal cortex, memory, social interaction, Zif268
Introduction
The decline in testosterone experienced by individuals suffering from hypogonadism, or as a natural consequence of aging, is associated with cognitive dysfunction and onset of mood disorders (Moffat et al., 2002, Shores et al., 2005, Borst et al., 2014). Animal studies have shown that testosterone depletion, achieved via gonadectomy (GNX), recapitulate impairments in cognitive performance and symptoms of affective disorders as is observed in the human population (Ceccarelli et al., 2001, Frye and Seliga, 2001, Daniel et al., 2003, Kritzer et al., 2007, Aubele et al., 2008). Strategies to treat this pervasive human condition with administration of testosterone have yielded mixed results, but most studies have shown beneficial effects of testosterone (Janowsky et al., 1994, Janowsky et al., 2000, Cherrier et al., 2001, Cherrier et al., 2005, Warren et al., 2008). As such, it is important to examine the behavioral and neuromolecular changes that are produced by loss of gonadal hormones since understanding these hormone-related changes will allow for identification of non-hormonal treatment strategies.
In the present study, we used GNX in adult male rats as an established model of testosterone depletion that produces deficits in cognition and mood-related behaviors. Since in vitro work has demonstrated that Zif268 is a target of gonadal hormones (Kousteni et al., 2003; Chauhan et al., 2004; Fix et al., 2004), we used this model to examine the impact of GNX on Zif268 expression in the brain. This model also allowed us to examine the influence of Zif268 levels on memory and mood-related behaviors. Zif268 (also known as Egr-1) belongs to a family of proteins that are immediate-early gene-encoded and function as inducible transcription factors (Sukhatme et al., 1988, Cao et al., 1990, Lemaire et al., 1990). Zif268 protein is rapidly induced in an activity-dependent manner, and plays a role in maintaining long-term potentiation in the dentate gyrus of the hippocampus (Cole et al., 1989, Wisden et al., 1990; Jones et al., 2001). Similarly, levels of Zif268 protein are upregulated in response to learning-induced neural activity (Nikolaev et al., 1992). Loss-of-function studies have demonstrated the necessity of Zif268 gene expression for normal long-term memory formation (Jones et al., 2001, Bozon et al., 2002). In parallel, gain-of-function studies have shown that overexpression of Zif268 within the dentate gyrus can enhance long-term spatial memory (Penke et al., 2014). Within the neural circuitry that supports learning and memory, Zif268 expression is induced following learning-related stimuli. This circuitry includes, but is not limited to, spatial memory within the hippocampus, fear-related memory retrieval within the amygdala, and working memory within the medial prefrontal cortex (mPFC) (Guzowski et al., 2001, Hall et al., 2001, Thomas et al., 2002).
Due to the well-defined role of Zif268 in learning and memory, most studies that examined the functional role of Zif268 have focused on its action within the hippocampus (Hall et al., 2000; Davis et al., 2000) and the amygdala (Malkani and Rosen, 2000). It is important to note that the mPFC exerts top-down influence over brain regions such as the amygdala to influence anxiety and social recognition (Kim et al., 2011; Fossati et al., 2012; Lungwitz et al., 2014). A previous report from our group demonstrated sex differences in Zif268 mRNA and protein expression within the mPFC, where males exhibited higher levels as compared to females. Further, this sex difference in Zif268 mediated sex differences in social interaction (i.e., higher levels of social interaction in males vs. females). Indeed, upon mPFC downregulation of Zif268 (via antisense oligodeoxynucleotides), males displayed similar levels of social interaction as their female counterparts, which suggested an important role of this gene in sex differences in social interaction (Stack et al., 2010). Accordingly, the first aim of the present study was to design and confirm the functionality of an adeno-associated virus (AAV) intended to upregulate levels of Zif268 protein. The second aim was to delineate the functional role of mPFC Zif268 in memory and mood-related behavioral deficits observed following GNX. To this end we assessed whether restitution of mPFC Zif268 would rescue the behavioral deficits observed following GNX.
To explore the functional role of mPFC Zif268 in GNX-related behavioral deficits, we employed behavioral assays that do not require reinforcement, yet can still tap into various aspects of memory and mood. As such, we used the y-maze to investigate working memory since mPFC integrity is required in this type of memory task (Yang et al., 2014). Furthermore, since Zif268 plays a major role in long-term memory (e.g., Penke et al., 2014), and the mPFC contributes to memory tasks without a spatial component (Barker et al., 2007; Barbosa et al., 2013), another aim of this work was to examine the role of mPFC Zif268 in long-term memory, investigated using the spontaneous object recognition (SOR; also known as: novel object recognition) test (Winters et al., 2010). Also, in order to investigate the impact of GNX on social anxiety-like behavior we employed the social interaction test. Previously, this test identified mPFC Zif268 as a major player in sex differences in social interaction (Stack et al., 2010). Finally, since loss of gonadal hormones can produce a depression-like phenotype (Wainwright et al., 2011; Carrier and Kabbaj, 2012b; Khera M, 2013), we used the forced swim test (FST) to examine the impact of GNX and viral-mediated Zif268 mPFC expression on depression-like behaviors.
Experimental Procedures
Zif268 viral construct
The backbone plasmid pAAV.CBA.IRES.WPRE (kind gift from Dr. David G. Standaert, University of Alabama at Birmingham) was used to construct a Flag-tagged transfer vector. From this plasmid the fragment between EcoR1 and BsrG1 was removed allowing for the removal of almost all of the 1st multiple cloning sites and the IRES2.GFP DNA fragment. The remaining plasmid backbone was re-ligated and underwent a subsequent digestion with NotI and EcoRV, which are present in the 2nd multiple cloning site. The full-length open reading frame (ORF) for rat Zif268 was obtained by high-fidelity PCR, using a plasmid carrying the full-length rat Zif268 ORF as a PCR template (a generous gift from Dr. Jeffrey Milbrandt; Washington University, School of Medicine). Using this template in combination with forward and reverse primers designed to contain EcoRI and Hind III sites. The primers were designed as follows: 5’- GAATCCATGGCAGCGGCCAAGGCC-3’ (Fwd) and 5’-AAGCTTTTAGCAAATTTCAATTGTCCTAGGAG-3’ (Rev). Full-length Zif268 cDNA flanked by EcoRI and HindIII was amplified using Phusion Hot Start Flex DNA Polymerase (New England Biolabs). The amplified PCR product was then sub-cloned into a pCMV-3Tag-1 vector (Agilent Technologies) at the unique NotI and EcoRV restriction sites. The Flag-tagged Zif268 was then excised and re-ligated into the AAV2 expression plasmid; the resulting vector was named AAV2.CBA.Flagx3.ratEGR1.WPRE.rBG. The DNA sequence of this vector was confirmed via sequencing and restriction enzyme mapping (Figure 2A) at the Molecular Cloning Facility at Florida State University, prior to being packaged at the Vector Core Facility at the University of Pennsylvania. The Control AAV was also stereotype II and expressed green fluorescent protein; AAV2.CB7.CI.eGFP.rBG (Cat. No. AV-2-PV1963) and was obtained from the Vector Core at the University of Pennsylvania.
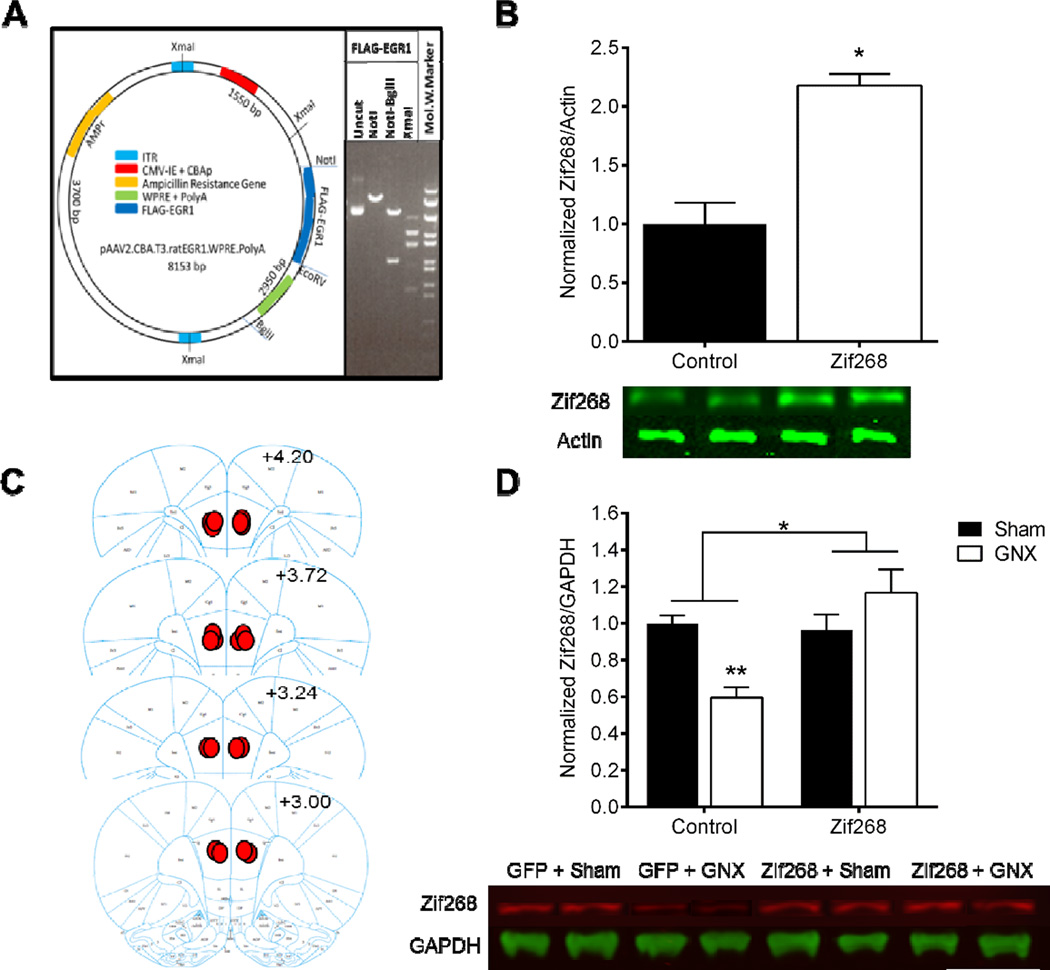
Figure 2. Delivery of AAV-Zif268 construct promotes overexpression of Zif268 in vitro and protects against GNX-induced mPFC Zif268 downregulation in vivo.
Map of the viral vector for AAV2.FLAG.EGR1 (Zif268) with the most relevant restriction sites and vector elements indicated and restriction enzyme digestion of uncut Zif268, cut with NotI, NotI + BglII, or XmaI (lanes 1, 2, 3, & 4, respectively) (A). Western blot analysis of HT22 cell lysates revealed that infection with AAV-Zif268 significantly increased amount of Zif268 as compared to the Control-treated cells (B). A diagram of representative medial prefrontal cortex (mPFC) injection placements based on the atlas of Paxinos and Watson (2007); additional rats’ injections were identified at similar points between the anterior-posterior levels shown here (C). Western blot analysis confirmed GNX-induced down-regulation of Zif268 in the mPFC of animals that received Control virus; Zif268 protein expression was unchanged in Sham and GNX animals that received bilateral injection of AAV-Zif268 (D). Data are presented as means ± SEM. *p<0.05 Control versus AAV-Zif268, **p<0.05 versus all groups, n=2 plates/group in vitro, n=3–4/group in vivo.
In vitro experiments
An immortalized hippocampal neuron cell line (HT22) was a generous gift from Dr. Schubert (Salk Institute, University of California, San Diego; Davis et al., 1994), and was used to validate our Zif268-overexpressing viral construct functionality in vitro. The HT22 cells were maintained in Dulbecco’s Modified Eagle Medium (Gibco) supplemented with 15% fetal bovine serum (HyClone) with 50 units/mL penicillin/50 µg/mL streptomycin (ThermoFisher Scientific), and incubated at 37°C with 10% CO2. HT22 cells were grown on poly-L-lysine-treated Corning dishes (Fisher Scientific) with fresh media exchanges every 48 h. Upon achieving 30–40% confluence, cells were treated with either the AAV2.CB7.CI.eGFP.rBG (Control) or AAV2.CBA.Flagx3.ratEGR1.WPRE.rBG (AAV-Zif268) constructs at a 10−3 dilution. Cells were harvested 7 days post-infection by washing two times with cold phosphate buffered saline (Fisher Scientific); subsequently 200 µL of Lysis buffer (Tris-HCl 100 mM [J.T.Baker]; NaCl 200 mM [Macron Fine Chemicals], 0.5% Triton-X, 5mM EDTA [Sigma], Milli-Q H20) containing protease inhibitor cocktail (Roche) was added to each plate. Once washed, cells were collected in pre-chilled sterilized Eppendorf tubes, re-suspended in 2 mL Lysis buffer, and centrifuged for 1 min at 14,000 rpm for 2 min at 4°C. Protein concentrations were determined via Bradford Assay according to the manufacturer’s instructions (Bio-Rad).
Western blot analysis of cell lysates
After Bradford assay, HT22 cell lysates were mixed with SDS-PAGE buffer (250 mM Tris, 10% w/v SDS, 30% v/v glycerol, 5% v/v 2-mercaptoethanol, 0.02% w/v bromophenol blue, pH 6.8) at a 1:5 dilution. For each sample, 10 µg of total cell lysate was loaded onto a 12% SDS-PAGE gel alongside a pre-stained protein ladder (All Blue, Bio-Rad) and underwent electrophoresis. Subsequently, gels were electrotransferred to a nitrocellulose membrane (Amersham, Protran, GE Life Sciences). Membranes were blocked in 5% milk in Tris-buffered saline with Tween (TBST) for 1 h at room temperature and incubated overnight with rabbit anti-Egr-1 (1:1000, sc-110, Santa Cruz Biotechnology) and mouse anti-Actin (1:1000, MAB1501, Millipore) at 4 °C on a rocking platform. Membranes were rinsed once with TBST and four times with TBS on a rocking platform at room temperature, incubated for 45 min at room temperature with a donkey anti-rabbit secondary antibody and a donkey anti-mouse secondary antibody (1:10,000 dilution, 800LT; Li-Cor Biosciences), rinsed once with TBST and four times with TBS on a rocking platform, and imaged using an Odyssey infrared imaging system (Li-COR Biosciences, Lincoln, NE). Quantification was performed using ImageJ software (NIH).
In vivo experiments
For the experiments described below, animals were transferred to a dimly lit room and allowed to acclimate to the new environment for 15 min prior to the onset of behavioral testing. All behavioral testing occurred during the first 6 h of the light cycle, was recorded with EthoVision software, and was confirmed at a later time by a trained observer blind to experimental conditions. The forced swim test was re-scored with a stopwatch, by a trained observer blind to experimental conditions, due to the variability in the ability of the EthoVision software to accurately track behavior in this assay.
Animals
Adult male Sprague Dawley rats (weighing 250–275 g upon arrival; Charles River Laboratories) were used in the current study. Rats were pair-housed in standard plastic cages (43 x 21.5 × 25.5 cm) and maintained on a 12 h: 12 h light-dark cycle. Water and rat chow (5001, Purina) were available ad libitum except during behavioral testing. All animal protocols were carried out in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Florida State University.
Surgery
Rats were anesthetized with a ketamine (90 mg/kg)/xylazine (5 mg/kg) cocktail delivered intramuscularly. Bupivicaine (0.25% solution) was applied topically as an analgesic and the non-steroidal anti-inflammatory drug Carprofen (5 mg/kg; subcutaneous) (Butler Schein Animal Health Supply) was given at the start of the surgical procedure for management of postoperative pain. Gonadectomy and AAV infusions were conducted in the same surgical procedure.
Gonadectomy and sham surgeries
A 1–2 cm midline incision was made in the scrotum of adult male rats to expose the tunica. The ventral tip of the tunica was pierced and both testes were extracted to expose the vas deferens, which was bilaterally ligated with silk sutures (Ethicon) and the testes were excised, the remaining tissue was placed back into the tunica prior to suturing. For the sham procedure, the same procedure was performed except the testes were not excised and were replaced within the tunica.
Infusion of the AAV constructs into the mPFC
For stereotaxic delivery of the AAV constructs, rats received bilateral microinjection (1 µl per side, infused at a rate of 0.1 µl/min and allowed to diffuse for an additional 10 min before retraction of the injector) of Control or AAV-Zif268 into the prelimbic region of the mPFC. Stereotaxic coordinates for the injection were ±1.0 mm lateral to midline, 2.7 mm anterior to bregma, and 3.0 mm ventral to skull surface. Animals were monitored daily following surgery and given 2 weeks before the onset of behavioral testing to allow for full expression of the viral constructs and washout of endogenous testosterone.
Y-Maze Spontaneous Alternation Test
Spontaneous alternation behavior in a y-maze was evaluated as a measure of working memory. The y-maze was constructed from clear Plexiglass that had three identical arms (46.5 x 15.5 x 31 cm) positioned at equal angles, and was surrounded by a variety of two and three dimensional extra-maze cues. Rats were tested individually in a dimly lit room; they were placed in the center of the arms, after 30 s the barriers to the arms were removed and the rats were given 10 min to freely explore the arms of the y-maze. At the conclusion of each individual test, the arms of the y-maze were cleaned with 70% EtOH. An arm entry was defined as the entry of all four paws into an arm. A correct alternation was defined as successive entries of the three arms, in which three different arms were entered. The maximum number of spontaneous alternations was defined as the total number of arms entered minus two; the percentage of correct alternation behavior was calculated as: (number of correct alternations / maximum alternations) x 100 (Arendash et al., 2006). For example, if the following sequence of arm entries was observed: ABACBCAAB; the animal would have exhibited 9 arm entries, with 7 maximum spontaneous alternations, and 3 correct spontaneous alternations. The percent correct would be 42.86%. Y-maze behavior was recorded by a video camera located above the y-maze, and correct spontaneous alternations were calculated at a later time by a trained observer blind to experimental condition. This protected animals against disturbance by experimenters during the y-maze test.
Open Field Test and Spontaneous Object Recognition Test
During the habituation phase, rats were placed in an empty open field box (1 m x 1 m x 1 m) and allowed to investigate it for 10 min, after which they were returned to their home cage. Time spent in the center of the field (70 cm x 70 cm), time spent in the borders of the field (15 cm each), and the total distance traveled during the test was determined in order to assess anxietylike behavior and general locomotor activity. Twenty-four h after the habituation phase, a pair of identical objects was placed in the open field arena (each 35 cm from the edge of the arena); the rat was placed in the opposite side of the open field box facing away from the training objects, and then given 10 min to explore its environment. A total of five animals were not advanced to the test phase; three were excluded due to evidence of a side preference during the training phase, one was excluded due to failure to interact with both objects during the training phase, and one was excluded because the time spent investigating the training objects was two standard deviations away from the mean of the group.
After a 24 h retention interval, one training object and one novel object were placed in the open field box (each 35 cm from the edge of the open field) and the rat was given 10 min to freely explore its environment. To avoid coercion to explore one object over another, rats were released in the center of the arena and were facing the wall. Two sets of identical objects were used for training and testing. One set was comprised of porcelain turquoise piggy banks and the other set was sealed glass mason jars filled with tan and silver sparkling sand. Both sets of objects were similar in height (13 cm - 12 cm high) yet differed in color and shape to allow for discrimination between the two. The objects weighed ≥800 g to discourage their movement during the test. The set of objects used as the training or novel objects was counterbalanced between rats to avoid any effect of innate object bias (Hale and Good, 2005), and the position of the novel object was alternated between corners to protect against any positional biases. Between each trial, the arena and objects were wiped down with a clean cloth and 70% EtOH to remove any odorant cues from the previous subject. A digital camera located directly above the arena recorded the rats’ behavior; videos were analyzed with EthoVision XT (Noldus Information Technology) and confirmed at a later time by a trained observer blind to treatment condition. Percent of novel object preference was calculated as: (time spent exploring a novel object/total exploration time) x 100 (Oliveira et al., 2010), and this percentage was used for statistical analysis. Time with an object was defined as an animal orienting towards an object with ≤ 3 cm between its nose and the object. Data from one animal was removed from analysis due to failure to approach the objects during the object recognition test; one animal was removed because object exploration time was two standard deviations away from the mean of the group.
Social interaction test
Rats were placed in an empty open field box (1 m x 1 m x 1 m), and given 10 min to freely explore it. After this acclimation period, an untreated social target that was matched for age, sex, and body weight was introduced to the open field box in the opposite corner from the experimental target. The experimental target and social target were given 10 min to freely explore each other and the environment. At the conclusion of the behavioral test rats were returned to their respective cages, and the open field box was cleaned with 70% EtOH. Social interaction tests were video recorded and scored with EthoVision, and confirmed by a trained observer blind to experimental conditions. Social interaction was defined as the time the experimental rat spent investigating or engaging in physical activity with the social target with ≤ 5 cm between the nose of the experimental rat and the social target (File and Seth, 2003; Stack et al., 2010). Anogenital investigation was defined as the time the experimental rat spent in close proximity and investigating (e.g., sniffing) the anogenital region of the social target. The olfactory cues obtained by rodents when sniffing the anogenital region are key components in the development of social recognition (Engelmann et al., 1995; Ferguson et al., 2002).
Forced Swim Test
The forced swim test (FST) is a two-day procedure where rats are forced to swim in an inescapable cylinder for 15 min on day 1 and for 5 min on day 2 (Porsolt et al., 1977; Lucki, I, 1997). We used clear cylinders (75 cm high, 20 cm wide) filled with 25°C water to a depth that prevented tails from touching the bottom of the cylinder. FST from both sessions were video recorded, and the video from day 2 (5 min session) was scored by a trained observer blind to experimental condition. Latency to immobility was defined as the time when the animal first assumed a stationary posture not associated with an attempt to escape the cylinder. Immobility was scored when the rat only made the movements necessary to maintain its head above water.
Physiological consequences of GNX
Body weight was recorded pre- and post-operatively throughout the course of the experiment to confirm blunted weight gain in the adult males that received GNX, as has been described previously (e.g., Kakolewski et al., 1968; Sillence et al., 1995). At the time of sacrifice, trunk blood was collected into chilled polyethylene centrifuge tubes containing 0.5M EDTA pH 8.0 (Sigma Aldrich), stored on ice, and centrifuged at 4 °C at 2000 rpm for 20 min. Plasma was collected into sterilized microcentrifuge tubes and stored at −80°C until further processing. Total plasma testosterone levels were determined by coat-a-count iodine-125 radioimmunoassay according to the manufacturer’s instructions (Cruinn Diagnostics, Dublin, Ireland).
mPFC tissue collection, protein isolation, and Western blot analysis
To verify injection placement and level of Zif268 expression at the conclusion of experiments, rats were sacrificed via rapid decapitation one week after the FST. Brains were rapidly removed and flash frozen on methyl butane with dry ice (−20°C) and stored at −80°C until subsequent processing. Coronal cryostat sections were collected at 50 µm, injection placement was recorded (according to Paxinos and Watson, 2007), and 1 mm tissue punches were taken around the injection damage site (Figure 2C). Total protein was extracted from the mPFC at the site of injection as previously described (Stack et al., 2010), using the Tri Reagent protocol (Molecular Research Center, Cincinnati, OH). Ten µg of protein per animal was run on 12% acrylamide gel and subsequently transferred to a nitrocellulose membrane (Whatman, Protran BA83, GE Healthcare). The membrane was cut between 50 and 75 kDa; the 50 kDa and below membrane was incubated with a rabbit anti-GAPDH primary antibody (1:2000, Cat. No. 5174, Cell Signaling).The 75 kDa and above membrane was incubated with a rabbit anti-Egr-1 primary antibody (1:1000; sc-110, Santa Cruz Biotechnology) overnight at 4 °C on a rocking platform. Membranes were rinsed once with TBST and four times with TBS on a rocking platform at room temperature. Membranes were then incubated for 45 min at room temperature with a goat anti-rabbit secondary antibody diluted 1:10,000 (680LT and 800LT; Li-Cor Biosciences), rinsed once with TBST and four times with TBS on a rocking platform, and imaged using an Odyssey infrared imaging system (Li-COR Biosciences, Lincoln, NE). Quantification was performed using ImageJ software (NIH). Data are shown as the ratio of Zif268 to GAPDH in rats’ mPFC, calculated by: the signal intensity of Zif268 – the background signal / signal intensity of GAPDH.
Statistical analysis
Data are reported as mean ± SEM. Western blot analysis of cell lysates was evaluated by unpaired t-test; Western blot analysis of mPFC tissue was evaluated by two-way analysis of variance (ANOVA). Change in body weight over time was evaluated by two-way repeated measures ANOVA. Radioimmunoassay results were examined by two-way ANOVA. All results obtained from behavioral tests were evaluated using two-way ANOVA, with peripheral manipulation (GNX or sham GNX) or central manipulation (Control or AAV-Zif268) as independent factors, followed by Fisher LSD post hoc tests where appropriate. Any value that was more than two standard deviations from the group mean was excluded from analysis. All statistical analyses were performed using GraphPad Prism 6.07 and StatView 5.0.1 (SAS Institute, Inc.) with p values < 0.05 considered statistically significant. Cohen’s d for pair-wise comparisons and Eta squared (η2) for ANOVA results were calculated to estimate effect sizes.
Results
Experiment 1: Delivery of AAV-Zif268 construct promotes overexpression of Zif268 in vitro and protects against GNX-induced mPFC Zif268 downregulation in vivo
In vitro examination of Zif268 expression revealed that HT22 cells treated with the Control virus (M=1.00, SD=0.26) exhibited lower expression of Zif268/Actin levels as compared to AAV-Zif268-treated cells (M=2.18, SD=0.14; t(2)=−5.71, p<0.05, d=5.70; n=2 infected plates/group; Figure 2B). For in vivo expression of Zif268 following GNX, two-way ANOVA revealed a significant main effect of central treatment [F(1,10)=10.825, p<0.01, η2=0.3; n=3–4/group] and a significant interaction between central and peripheral treatment [F(1,10)=13.83, p<0.005, η2=0.38], but no main effect of peripheral manipulation [F(1,10)=1.549, p>0.05, η2=0.043]. Fisher post hoc revealed significantly lower levels of Zif268 protein in GNX/Control-treated animals (M=0.596, SD=0.111) as compared to Sham/Control (M=1.0, SD=0.075; t(10)=3.510, p<0.01, d=0.754), Sham/Zif268 (M=0.965, SD=0.164; t(10)=3.463, p<0.01, d=2.635), and GNX/Zif268 groups (M=1.166, SD=0.221; t(10)=4.956, p<0.001, d=3.26; Figure 2D).
Experiment 2: GNX blunted weight gain and suppressed plasma testosterone
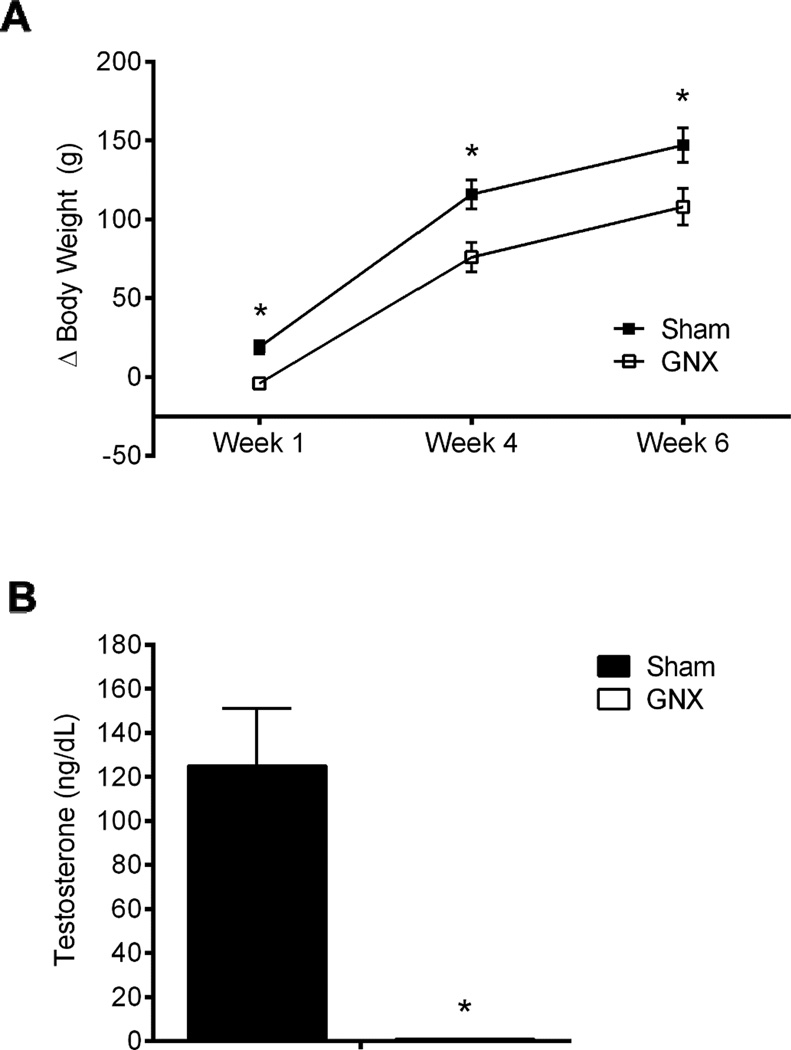
Repeated measures two-way ANOVA showed that peripheral manipulation (i.e., sham or GNX) significantly influenced weight gain (change from pre-surgery weight, Δ) at one, four, and six weeks following surgery [F(1,72)=11.671, p<0.005, η2=0.38, n=10/group]. Central manipulation (Control or AAV-Zif268) did not impact growth rate at any time point examined [F(1,76)=1.468, p>0.05, η2=0.05], nor was there a significant interaction [F(1,72)=0.032, p>0.05, η2=0.0). Since central manipulation did not impact growth rate, we collapsed Control and AAV-Zif268 groups in Figure 3A. In a subset of animals we examined levels of plasma testosterone. Two-way ANOVA revealed a significant effect of GNX to eliminate testosterone in plasma [F(1, 19)=22.66, p<0.001, η2=0.93, n=5–6/group], with no effect of central manipulation on testosterone levels [F(1, 19)=0.39, p>0.05, η2=0.02], and no interaction [F(1, 19)=0.376, p>0.05, η2=0.02], confirming the efficacy of the GNX procedure. Since central treatment did not influence testosterone levels, we collapsed Control and AAV-Zif268 groups in Figure 3B.
Figure 3. GNX blunted weight gain and suppressed plasma testosterone.
There was a significant effect of gonadectomy (GNX) to blunt weight gain throughout the course of the experiment, reported as change from baseline (Δ) (A). Radioimmunoassay examination of plasma testosterone confirms the efficacy of the GNX procedure to diminish levels of peripheral testosterone (B). Data are presented as means ± SEM. *p<0.05 Sham versus GNX, n=10/group body weight, n=5–6/group testosterone assay.
Experiment 3: GNX-induced memory impairment in the y-maze was prevented by mPFC Zif268 reinstitution
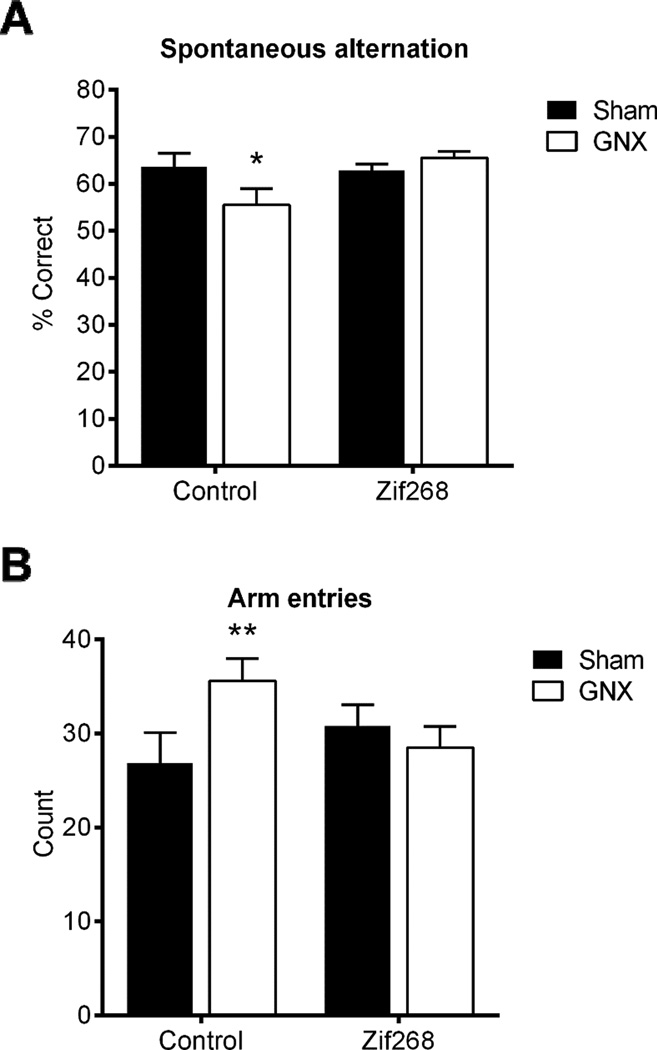
Two-way ANOVA revealed a trend towards a main effect of AAV-Zif268 to improve percentage of correct spontaneous alternations in the y-maze [F(1,32)=3.374, p=0.075, η2=0.08], no main effect of GNX [F(1,32)=1.102, p>0.05, η2=0.03, n=8–10/group], and a significant interaction between central and peripheral manipulations [F(1,32)=4.413, p<0.05, η2=0.11]. Post hoc comparisons revealed a significantly lower percentage of correct spontaneous alternations in GNX/Control (M=55.54.62, SD=10.92) as compared to all other groups, Sham/Control (M=63.5, SD=2.93; t(32)=2.272, p<0.05, d=0.996), Sham/Zif268 (M=62.876, SD=4.167; t(32)=2.187, p<0.05, d=0.888), and GNX/Zif268 (M=65.53, SD=3.901; t(32)=2.808, p<0.01, d=1.218; Figure 4A).
Figure 4. GNX-induced memory impairment in the y-maze was prevented by mPFC Zif268 reinstitution.
GNX/Control exhibited lower percent correct spontaneous alternations in the y-maze as compared to all other groups, with a trend towards a main effect of AAV-Zif268 to improve performance (A). GNX/Control animals exhibited significantly more arm entries in the y-maze test as compared to Sham/Control (B). Data are presented as means ± SEM. *p<0.05 versus all groups, **p<0.05 versus Sham/Control, n=8–10/group.
Two-way ANOVA revealed no main effect of central [F(1,32)=0.394, p>0.05, η2=0.01] nor of peripheral treatment [F(1,32 )=1.614, p>0.05, η2=0.04] to influence number of arm entries. However, there was a significant interaction between central and peripheral treatments [F(1,32)=4.752, p<0.05, η2=0.12]. Post hoc analysis identified that GNX/Control animals (M=35.6, SD=7.531) exhibited significantly more arm entries as compared to Sham/Control (M=26.875, SD=9.078; t(32)=2.44, p<0.05, d=1.05; Figure 4B).
Experiment 4: GNX and mPFC Zif268 expression levels did not influence anxiety-like behaviors in the open field test. GNX-induced impairments in the spontaneous object recognition test was prevented by mPFC Zif268 reinstitution
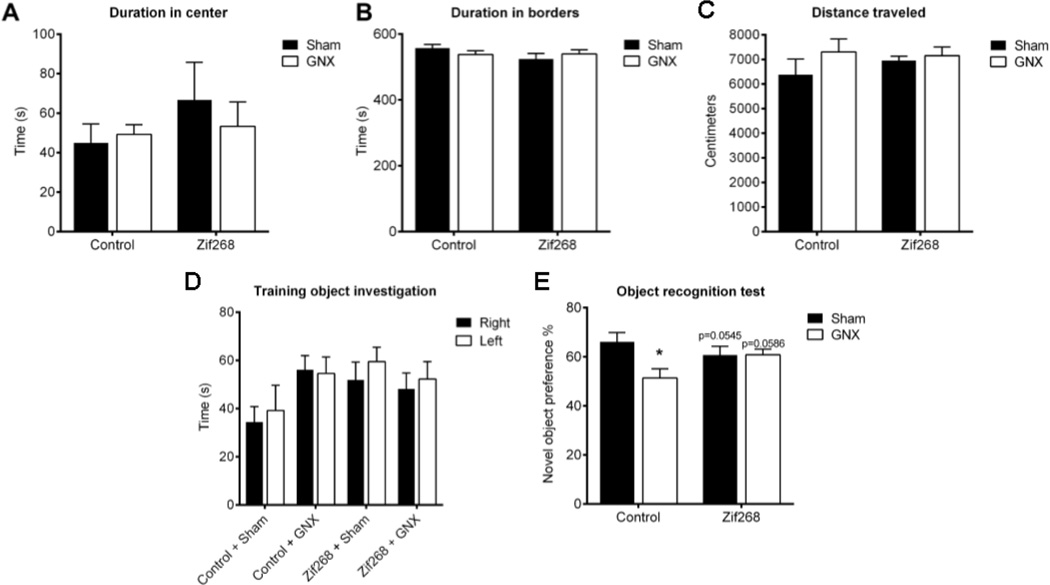
Two-way ANOVA revealed no main effect of peripheral [F(1,30)=0.106, p>0.05, η2=0.003] or central manipulation [F(1,30)=0.055, p>0.05, η2=0.027; n=7–10/group], nor was there an interaction between these variables [F(1,30)=0.3946, p>0.05, η2=0.013] to influence duration in center of the open field arena (Figure 5A). There was no main effect of peripheral [F(1,30)=0.6696, p>0.05, η2=0.0054] or central manipulation [F(1,30)=0.1638, p>0.05, η2=0.05884], nor was there an interaction between these variables [F(1,30)=0.1321, p>0.05, η2=0.0692] to influence time spent in the borders of the open field arena (Figure 5B). We also found no main effect of peripheral manipulation [F(1,30)=1.736, p>0.05, η2=0.053], central manipulation [F(1,30)=0.2533, p>0.05, η2=0.0077], nor an interaction of the two [F(1,30)=0.7379, p>0.05, η2=0.0225] to influence distance traveled during the open field test (Figure 5C). These results indicate that neither GNX nor AAV-Zif268 delivery to the mPFC influenced anxiety-like behavior measured in the open field test, nor do they affect locomotor activity.
Figure 5. GNX and mPFC Zif268 expression levels did not influence anxiety-like behaviors the open field test. GNX-induced impairments in the object recognition test was prevented by mPFC Zif268 reinstitution.
Neither gonadectomy (GNX) nor mPFC AAV-Zif268 altered the amount of time spent in the center of the open field (A), time spent in the borders of the open field (B), or influenced distance traveled during the open field test (C). There were no group differences in time spent investigating the objects on the right or left side of the arena during the training phase of the object recognition test (D). GNX significantly reduced novel object preference in animals treated with the Control virus; and AAV-Zif268 protected against GNX-induced impairments in novel object preference (E). Data are presented as means ± SEM. *p<0.05 versus Sham/Control, n=7–10/group.
During the training session of the SOR each group explored the objects on the left and the right side of the arena equally (Control/Sham: t(12)=0.3854, p>0.05; Control/GNX: t(12)=0.0565, p>0.05; Zif268/Sham: t(18)=0.7884, p>0.05; Zif268/GNX: t(16)=0.679, p>0.05; Figure 5D). During the test session, two-way ANOVA revealed a significant effect of peripheral [F(1,30)=4.602, p<0.05, η2=0.116], but not central manipulation [F(1,30)=0.3578, p>0.05, η2=0.009], as well as a significant interaction [F(1,30)=4.676, p<0.05, η2=0.118; Figure 5E] to influence novel object preference. Post hoc comparisons revealed significantly lower novel object preference in GNX/Control (M=51.36, SD=10.566) vs. Sham/Control (M=66.071, SD=10.08; t(30)=2.88, p<0.01, d=1.42), with trends toward significantly lower preference vs. Sham/Zif268 (M=60.73, SD=11.227; t(30)=2.011, p=0.0545, d=0.86), and vs. GNX/Zif268 (M=60.789, SD=7.032; t(30)1.966, p=0.0586, d=1.05). During the test session, each group explored the objects on the left and right side of the arena equally (Control/Sham: t(12)=1.103, p>0.05; Control/GNX: t(14)=0.4523, p>0.05; Zif268/Sham: t(18)=0.4781, p>0.05; Zif268/GNX: t(16)=0.4915, p>0.05; data not shown). The location of the novel object was counterbalanced across animals within each group. These results demonstrate that AAV-Zif268 within the mPFC protected against GNX-induced impairments in long-term memory formation.
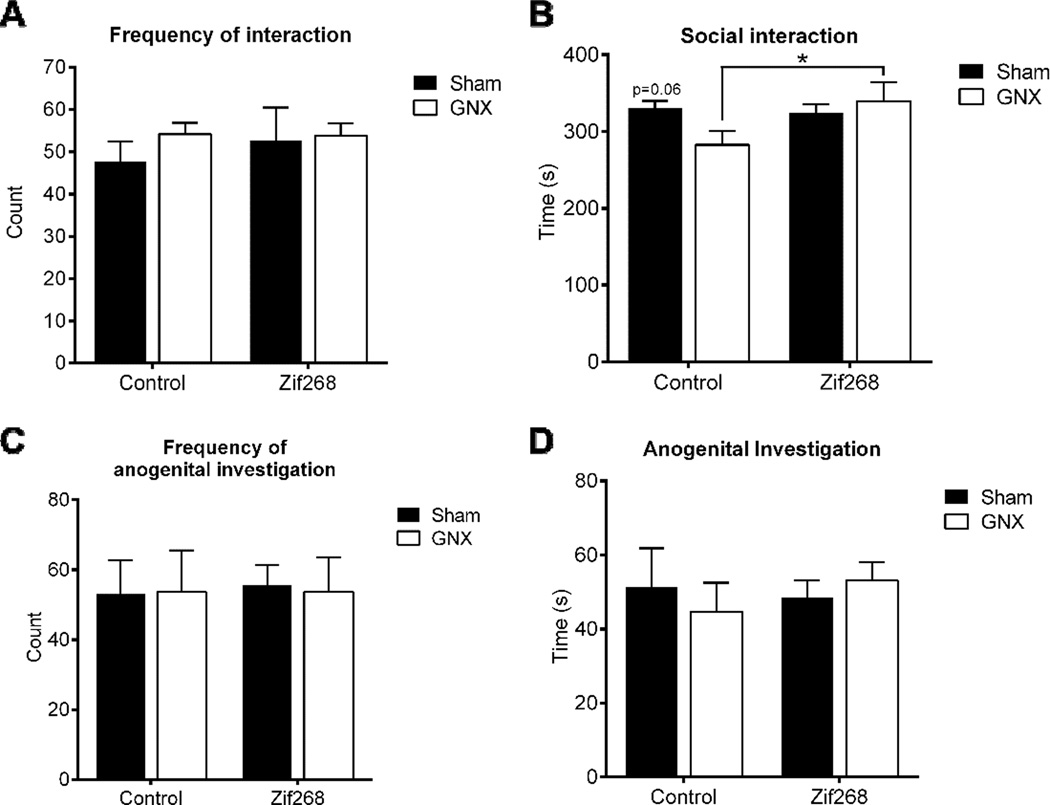
Experiment 5: GNX-induced social interaction impairments were prevented by mPFC Zif268 reinstitution
During the social interaction test, the frequency of social interactions was unaffected by peripheral manipulation [two-way ANOVA, F(1,20)=0.5905, p>0.05, η2=0.03; n=6/group], or by central manipulation [F(1,20)=0.2188, p>0.05, η2=0.01], and there was no significant interaction [F(1,20)=0.2857, p>0.05, η2=0.013; Figure 6A]. Duration of social interaction examined by two-way ANOVA revealed no main effect of peripheral manipulation [F(1, 20)= 0.87, p>.05, η2=0.03] or central manipulation [F(1, 20)= 2.23, p>.05, η2=0.08] to influence time spent engaging in social interaction. However, there was a trend towards a significant interaction between peripheral and central treatments [F(1, 20)=3.46, p=.0776, η2=0.13] to influence social interaction time. To examine this trend further, post hoc comparisons identified significantly lower interaction times in GNX/Control (M=282.717, SD=43.77) vs. GNX/Zif268 (M=339.617, SD=60.491; t(20)=2.371, p<0.05, d=1.078; Figure 6B), and a trend vs. Sham/Control (M=330.133, SD=23.485; t(20)=1.976, p=0.0621, d=1.35). Frequency of anogenital investigation examined via two-way ANOVA revealed no main effect of peripheral [F(1, 20)= 0.004835, p>.05, η2=0.000241] or central manipulation [F(1, 20)= 0.01934, p>.05, η2=0.000965], nor interaction between the two [F(1, 20)= 0.01934, p>.05, η2=0.000965; Figure 6C] to influence frequency of investigation. Consistent with this, duration of anogenital investigation revealed no main effect of peripheral [F(1, 20)= 0.02081, p>.05, η2=0.001003] or central manipulation [F(1, 20)= 0.1519, p>.05, η2=0.007322], nor interaction between the two [F(1, 20)= 0.5681, p>.05, η2=0.027386; Figure 6D] to influence time spent engaging in anogenital investigation. These results indicate that mPFC AAV-Zif268 protects against GNX-induced deficits in social interaction time.
Figure 6. GNX-induced social interaction impairments were prevented by mPFC Zif268 reinstitution.
Frequency of social interactions was unaffected by GNX or by AAV-Zif268 treatment (A). Time spent engaging in social interaction was significantly reduced in GNX/Control animals as compared to GNX/Zif268 counterparts, with a trend towards a reduction versus intact controls (B). Neither GNX nor AAV-Zif268 treatment influenced the frequency (C) or duration (D) of anogenital investigation in the social interaction test. Data are presented as means ± SEM. *p<0.05 versus GNX/Zif268, n=6/group.
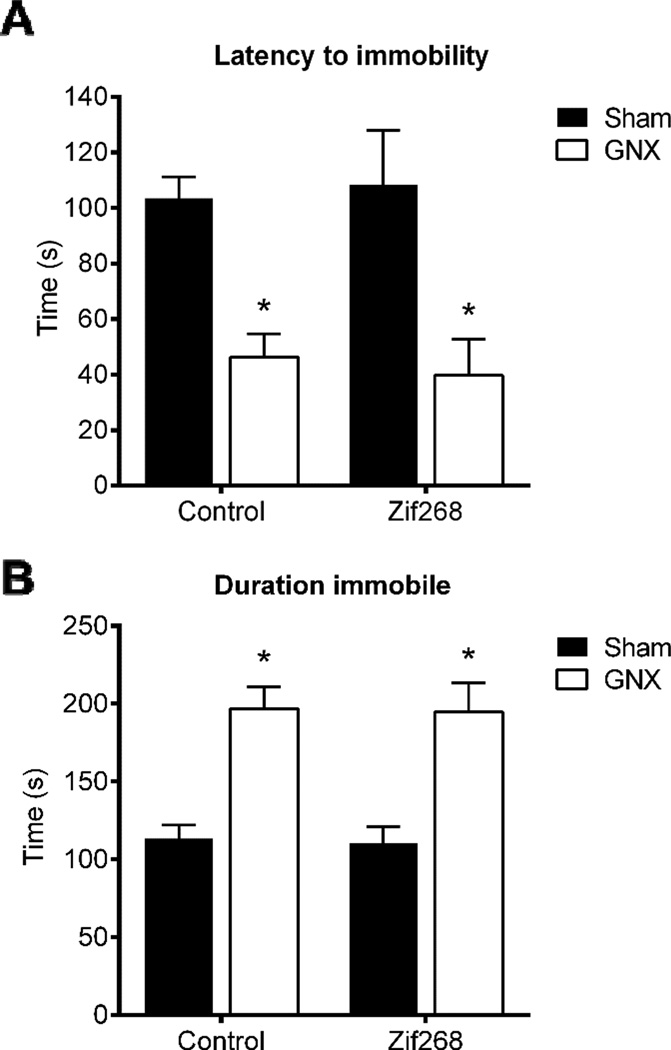
Experiment 6: GNX-induced depression-like behaviors measured in the forced swim test were not influenced by mPFC Zif268 reinstitution
Two-way ANOVA revealed a significant main effect of peripheral manipulation [F(1,20)=23.2, p<0.05, η2=0.53; n=6/group], yet no main effect of central treatment [F(1,20)=0.004, p>0.05, η2=9.35e-5] nor interaction of the two [F(1,20)=0.1877, p>0.05, η2=0.004; Figure 7A] to decrease the latency to become immobile. Similarly, two-way ANOVA revealed a significant main effect of GNX to increase time spent immobile [F(1,20)=38.73, p<.05, η2=0.66], but no main effect of central treatment [F(1,20)=0.03, p>.05, η2=0.0005] nor interaction [F(1,20)=0.0012, p>0.05, η2=2.03e-5; Figure 7B] to influence immobility.
Figure 7. GNX-induced depression-like behaviors measured in the forced swim test were not influenced by mPFC Zif268 reinstitution.
Gonadectomy (GNX) significantly reduced the latency to adopt an immobile posture (A) and increased time spent immobile in the forced swim test (B). There was no effect of mPFC AAV-Zif268 to influence the expression of depression-like behaviors in the forced swim test. Data are represented as means ± SEM. *p<0.05 Sham versus GNX, n=6/group.
Discussion
In the present study, we aimed to examine the effects of GNX-induced decreases in mPFC Zif268 protein expression on cognition and mood, and attempted to rescue some of these behavioral deficits using a new AAV-Zif268 construct. In our hands, GNX males displayed reductions in mPFC expression of Zif268 protein, impaired working memory and long-term memory, reduced social interaction time, and expression of depression-like behaviors. In contrast, GNX males that were infused with AAV-Zif268 into the mPFC were protected from the working memory, long-term memory, and social interaction impairments. Yet, mPFC AAV-Zif268 did not protect against GNX-induced depression-like behaviors in the FST. These findings indicate specificity of Zif268 within the PFC to positively impact memory and social interaction but not depression related behaviors induced by GNX in male rats.
Gonadectomy and reinstitution of Zif268 in the mPFC have no effect on anxiety-like behavior examined in the open field test
Evidence from human and rodent studies has shown that levels of testosterone are inversely correlated with levels of anxiety (Frye and Seliga, 2001; Frye and Edinger, 2004; Carrier and Kabbaj, 2012b; Khera M, 2013; Khakpai F, 2014). In order to examine the role of gonadal hormone depletion and Zif268 expression to influence anxiety-like behaviors, we analyzed behavior in the open field during the habituation phase of the spontaneous object recognition task. We found no effect of GNX to increase anxiety-like behavior in the open field test, as evidenced by similar amounts of time spent in the center and borders of the open field among the experimental groups. One explanation for this lack of effect could be due to the behavioral assay employed. In fact, different dimensions of anxiety can be influenced by peripheral levels of testosterone. For example, GNX has been shown to increase anxiety in Vogel’s drinking conflict model (Svensson et al., 2000) or in the elevated plus maze (Carrier and Kabbaj, 2012b); yet there is no effect of GNX to promote anxiety when assessed by the canopy test (Ray and Hansen, 2004) or open field test (Carrier and Kabbaj, 2012b). Additional aspects of anxiety-like behavior should be included in future investigations. If other measures of anxiety were included (e.g., elevated plus maze) we would have a better understanding of the role of mPFC Zif268 expression to augment GNX-induced anxiety. However, as will be described later, GNX caused an increase in anxiety like-behaviors in the social interaction test, an effect that was rescued by mPFC Zif268 reinstitution in GNX rats, suggesting as we showed before (Stack et al, 2010) that Zif268 might be implicated in anxiety where there is a social context.
Reinstitution of Zif268 in the mPFC protects against gonadectomy-induced working memory and long-term memory deficits
In animal models, loss of endogenous gonadal hormones has been associated with impairments in memory and cognitive abilities (Janowsky et al., 1994; Frye and Seliga, 2001; Kritzer et al., 2001; Kritzer et al., 2007; Aubele et al., 2008; Pintana et al., 2015). The y-maze allows for examination of working memory in rodents, and can be completed in a single trial without any prior training (Morellini F, 2013). We found that Control/GNX animals performed more poorly in the y-maze as compared to intact controls and AAV-Zif268 counterparts. These results support the role of mPFC Zif268 in protecting working memory performance negatively affected by GNX. It is important to note the higher number of arm entries exhibited by Control/GNX animals, which is indicative of higher levels of spontaneous locomotor activity. However, in the OFT we found no difference in locomotor activity among the groups. This disparity in locomotor behavior may be due to differences in the size of the testing apparatus. Alternatively, there is some evidence that higher number of arm entries is associated with poor performance in the y-maze (Kitanaka et al., 2015; Ghafouri et al., 2016). This suggests that the increase in arm entries exhibited by the Control/GNX group may be related to their working memory impairments, but this needs to be confirmed experimentally. Future investigations should employ working memory tasks that do not have a locomotor component, to protect against this potential confound.
Consistent with previous reports that GNX can impair long-term memory, we report that GNX significantly impaired performance in the spontaneous object recognition test with a 24 h testing interval. Our results add to the body of literature demonstrating that the GNX-induced loss of gonadal hormones has powerful effect on performance in memory-related tasks. Within distinct memory-related brain areas (e.g., amygdala and hippocampus), levels of Zif268 are increased following learning-related stimuli (Malkani et al., 2000; Guzowski et al., 2001; Asok et al., 2013). Conversely, knockdown of the Zif268 gene within the hippocampus impairs performance in memory tasks (Jones et al., 2001; Bozon et al., 2003; Davis et al., 2010). Consistent with previous reports that Zif268 is critical for memory formation, we demonstrate that GNX rats that received intra-mPFC AAV-Zif268 displayed intact performance in the spontaneous object recognition test (i.e., long-term memory). Our results show for the first time, that GNX-induced impairments in the spontaneous object recognition test can be prevented by viral-mediated Zif268 expression. Mice with genetically-induced forebrain overexpression of Zif268 exhibit enhanced long-term object-location recognition as compared to controls (Penke et al., 2014). In the present study, we observed no enhancement of novel object preference in males that were gonadally intact and received mPFC AAV-Zif268 construct. We believe this was due to our use of an object recognition task vs. an object location recognition task, as was used by Penke and colleagues (2014), and likely reliant upon the significant overexpression of Zif268 within the dentate gyrus of the hippocampus which plays a key role in spatial memory (Kee et al., 2007; Jessberger et al., 2009). Alternatively, endogenous mechanisms may have limited the level of Zif268 expression and thus limited behavioral performance. One such mechanism is NGF1-A-binding protein 2 (NAB2), which represses the expression of Zif268 within neurons (Russo et al., 1995; Svaren et al., 1996). Further support for this inhibitory role of NAB2 over Zif268 expression comes from the demonstration that Zif268-inducing stimuli, and even Zif268 itself, promotes the expression of NAB2 (Kumbrink et al., 2005). Thus, NAB2 is poised to limit endogenous Zif268 within sham GNX rats treated with AAV-Zif268, and may have limited behavioral performance and overexpression of Zif268 in these sham GNX rats.
Reinstitution of mPFC Zif268 protects against gonadectomy-induced social interaction impairments
Previous reports have shown that GNX, and the subsequent loss of gonadal hormones, negatively influences social behaviors (Primus and Kellog; 1990; Frye and Seliga, 2001; Vetter-O’Hagen & Spear, 2012). These findings are consistent with the results in the present study, where we report that GNX/Control rats spent significantly less time engaging in social interaction as compared to Zif268-treated GNX counterparts. A recent report indicated that social interaction was sufficient to increase levels of Zif268 within the PFC (El Rawas et al., 2012), which supports our findings that AAV-Zif268 delivery into the mPFC is able to protect against GNX-induced reduction in social interaction. Together, these findings support the conclusion that Zif268 within this brain region has significant influence over anxiety-like behaviors measured in social contexts. Furthermore, our data extend a previous report from our laboratory which demonstrated that a temporary downregulation of mPFC Zif268 levels produced a marked reduction in social interaction in male rats (Stack et al., 2010). One shortcoming of the present investigation is the use of interaction time and anogenital investigation time to examine social behavior. This level of analysis does not allow for conclusions regarding the potential therapeutic potential of AAV-Zif268 in conditions of diminished gonadal hormones. Indeed, microstructural analysis would provide a deeper understanding of the features of the social interaction and the influence of GNX and AAV-Zif268. Future studies would benefit from the use of the social preference-avoidance test (Berton et al., 2006; Haller and Bakos, 2002), the Visible Burrow System (Hardy et al., 2002), or ultrasonic vocalizations (e.g., Blanchard et al., 1991; Portfors, 2007) to fully examine the wide array of behaviors and affective state exhibited when rodents are engaging in social interaction. Yet, the results from the social interaction test show for the first time that Zif268 in the mPFC has robust influence over GNX-induced anxiety-like behaviors in social contexts.
Reinstitution of mPFC Zif268 has no effect on gonadectomy-induced depressive-like behaviors
In humans and rodents alike, loss of endogenous gonadal hormones is associated with depression and a depression-like phenotype in adult male rodents (Wainwright et al., 2011; Carrier and Kabbaj, 2012b; Khera M, 2013). In the present study, we utilized the FST as a well-validated behavioral assay to confirm this effect of GNX in our males and simultaneously screen for potential antidepressant-like effects of mPFC AAV-Zif268. As expected, GNX males displayed higher indices of depression-like behaviors as compared to sham-operated controls. However, we observed no effect of AAV-Zif268 to augment latency to become immobile or to influence time spent immobile in the FST. Which demonstrates the specificity of mPFC Zif268 to exclusively modulate GNX-induced memory-related and social interaction impairments when virally expressed within the mPFC. It is possible that intra-hippocampal delivery of the AAV-Zif268 virus may protect against GNX-induced depression-related behaviors, since a previous study from our laboratory demonstrated that hippocampal expression of ERK1/2, which is upstream of Zif268 (e.g., Davis et al., 2000; Kousteni et al., 2003), mediated GNX-induced mood alterations (Carrier and Kabbaj, 2012a). Consistent with the positive role of gonadal hormones to regulate mood, estrogen action within the hippocampus can also enhance learning and memory through an ERK1/2-dependent manner (Boulware et al., 2013). Interestingly, while GNX reduces levels of testosterone and DHT within the plasma and brain of males, this manipulation has been shown to exert no influence over levels of estrogen (Munetsuna et al., 2009b; Hojo et al., 2009). Thus, future studies are required to delineate the role of testosterone versus estrogen to interact with Zif268 within brain regions that mediate learning and memory.
General Conclusions
The reductions in levels of gonadal hormones is linked to a decline in cognitive performance and with mood disorder within the male population (Moffat et al. 2002; Shores et al., 2005; Borst et al., 2014). Using a rat model of GNX, we report a significant reduction in mPFC Zif268 protein levels in Control/GNX males. However, reinstitution of mPFC Zif268 conferred significant protection against the working memory impairments, long-term memory impairments, and social interaction deficits induced by GNX. However, reinstitution of mPFC Zif268 failed to protect against GNX-induced depression-like behaviors measured in the FST.
Previous in vivo and in vitro data indicate that expression of Zif268 is enhanced by gonadal hormones (Suva et al., 1991; Slade and Carter, 2000; Chauhan et al., 2004; Fix et al., 2004). However, previous reports have shown that the Zif268 promoter region lacks a testosterone response element (Knapska & Knaczmarek, 2004). It is then possible that gonadal hormones regulate Zif268 expression indirectly, through their interactions with the ERK1/2 pathway (Kousteni et al., 2003; Estrada et al., 2003). It remains to be determined whether the observed consequences of GNX on mPFC Zif268 expression is due to reductions in activity at androgen receptors or estrogen receptors. It is possible that testosterone or estrogen underlie many of the protective functions observed in intact animals, since previous reports have identified that both gonadal hormones promote mood-enhancing effects and target common molecular mechanisms for these effects (e.g., ERK1/2) (Carrier et al., 2015). Further support for the potential role of estrogen comes from evidence that androgen status (e.g., intact vs. GNX) did not impact novelty-induced expression of Zif268 within the male hippocampus (Kerr et al., 1996). Future studies are required to delineate the role of specific gonadal hormones to influence expression of Zif268. Are these effects carried out through nuclear androgen or estrogen receptors that can promote Zif268 transcription? Or, are they mediated through more rapid, non-genomic actions of these receptors? One other shortcoming that should be acknowledged, is that we only studied males in the present investigation. Future investigations should include female subjects, since women are at an increased risk for suffering from anxiety disorders as compared to males (Kessler et al., 2005). Further, this increased expression of anxiety-like disorders parallels fluctuations in ovarian hormone levels (Hayward and Sanborn, 2002), which highlights the need to study the impact of ovarian hormone loss and possible rescue of ovariectomy-related deficits by AAV-Zif268 in females.
With regard to potential mechanisms leading to reduced Zif268 expression in the mPFC following GNX and its associated behavioral deficits, the dopaminergic (DA) system is an excellent candidate. In fact, GNX and the resultant loss of peripheral gonadal hormones negatively impacts PFC-dependent functions, specifically functions that are influenced by DA such as memory formation and social behaviors (Stam et al., 1989; Murphy et al., 1996; Zahrt et al., 1997). The negative effect of GNX on these behaviors is likely due to the aberrant DA tone in the PFC that occurs following long-term GNX. It has been shown that DA axon density is robustly increased in response to long-term testosterone-depletion, and this increase in axon density is paralleled by elevated levels of DA in the PFC (Aubele et al., 2011). While increased levels of DA have been implicated in learning (e,g., Chudasama and Robbins, 2004), there is ample evidence that a balance of DA levels are required for normal performance in memory tasks (reviewed in Floresco et al., 2013). Where DA depletion (Brozoski et al., 1979; Mizoguchi et al., 2009) and elevations within the PFC can negatively impact working memory (Murphy et al., 1996). Which is consistent with the negative impact of GNX on DA tone (Kritzer et al., 1999; Aubele et al., 2011). Interestingly, acute administration of cocaine increases DA levels within the PFC (e.g., Tanda et al., 1997). While repeated cocaine administration blunts forebrain levels of Zif268 (Bhat et al., 1992; Ennulat et al., 1994; Mutschler et al., 2000). These results suggest that chronic elevations in PFC DA, like those observed in the GNX condition, may exert negative effects on PFC Zif268 levels. Through this DA-induced disruption of Zif268, viral-mediated expression of Zif268 in GNX rats may rescue social interaction behaviors and memory functions. That being said, additional mechanisms implicating other neurotransmitters, such as serotonin and others, cannot be ruled out.
Taken together, our results demonstrate that Zif268 functions within the mPFC to influence working memory, long-term memory, and social interaction time. It is important to note that while GNX reliably increased indices of depression-like behaviors in the FST, this effect of GNX was not attenuated by mPFC delivery of AAV-Zif268. Our results demonstrate the specificity of Zif268 within the mPFC to influence social anxiety and memory functions. Increasing levels of this immediate early gene may provide a novel, non-hormonal treatment for the cognitive and mood deficits that are observed following decline in gonadal hormones as those observed in hypogonadal and aged men (Janowsky et al., 1994; Cherrier et al., 2007; Borst et al., 2014).
Figure 1.
Experimental timeline.
Gonadectomy negatively impacts PFC Zif268 expression, long-term memory, social interaction, and mood-related behaviors
mPFC AAV-Zif268 expression prevents GNX-induced reductions in Zif268, memory, and social interaction
Zif268 may serve as a non-hormonal treatment for cognitive declines associated with testosterone loss
Acknowledgments
The authors would like to acknowledge the excellent assistance provided by Lindsay Elvir and Hui Wang in the collection of brain tissue and plasma for this study.
Funding:
This work was supported by The Florida State University Department of Biomedical Sciences, Small Grant Project to H. Jourdi, and National Institute of Mental Health grants to M.K. (R01 MH087583 and R01 MH099085).
Abbreviations
- Control
AAV2.CB7.CI.eGFP.rBG
- AAV-Zif268
AAV2.CBA.Flagx3.ratEGR1.WPRE.rBG
- ANOVA
Analysis of variance
- Egr-1
Early growth response 1
- EDTA
Ethylenediaminetetraacetic acid
- FST
Forced swim test
- GNX
Gonadectomy
- g
Gram
- h
Hour
- min
Minute
- SOR
Spontaneous object recognition
- PCR
Polymerase Chain Reaction
- TBST
Tris-buffered saline with Tween 20%
- TBS
Tris-buffered saline
- Zif268
Zinc finger protein 268
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
A.M.D. and M.K. designed the experiments, analyzed the data, and wrote the manuscript; H.J. designed the virus; A.M.D. and K.N.W. performed surgeries; A.M.D. and C.E.S conducted behavioral experiments; A.M.D. conducted cell culture virus confirmation; all authors contributed to editing the manuscript.
The authors declare no conflict of interest.
References
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 2013;106:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cereb Cortex. 2011;21:222–232. doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FF, Santos JR, Meurer YS, Macêdo PT, Ferreira LM, Pontes IM, Ribeiro AM, Silva RH. Differential Cortical c-Fos and Zif-268 Expression after Object and Spatial Memory Processing in a Standard or Episodic-Like Object Recognition Task. Front Behav Neurosci. 2013;7:112. doi: 10.3389/fnbeh.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Cole AJ, Baraban JM. Chronic cocaine treatment suppresses basal expression of zif268 in rat forebrain: in situ hybridization studies. J Pharmacol Exp Ther. 1992;263:343–349. [PubMed] [Google Scholar]
- Blanchard DC, Weatherspoon A, Shepherd J, Rodgers RJ, Weiss SM, Blanchard RJ. “Paradoxical” effects of morphine on antipredator defense reactions in wild and laboratory rats. Pharmacol Biochem Behav. 1991;40:819–828. doi: 10.1016/0091-3057(91)90092-g. [DOI] [PubMed] [Google Scholar]
- Borst SE, Yarrow JF, Fernandez C, Conover CF, Ye F, Meuleman JR, Morrow M, Zou B, Shuster JJ. Cognitive effects of testosterone and finasteride administration in older hypogonadal men. Clin Interv Aging. 2014;9:1327–1333. doi: 10.2147/CIA.S61760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12:570–577. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, Hay RV, Sukhatme VP. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990;10:1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012a;71:642–651. doi: 10.1016/j.biopsych.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav. 2012b;61:678–685. doi: 10.1016/j.yhbeh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The Anxiolytic and Antidepressant-like Effects of Testosterone and Estrogen in Gonadectomized Male Rats. Biol Psychiatry. 2015;78:259–269. doi: 10.1016/j.biopsych.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123:65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Kunz S, Davis K, Roberts J, Martin G, Demetriou MC, Sroka TC, Cress AE, Miesfeld RL. Androgen control of cell proliferation and cytoskeletal reorganization in human fibrosarcoma cells: role of RhoB signaling. J Biol Chem. 2004;279:937–944. doi: 10.1074/jbc.M311325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Davis JB, Maher P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994;652:169–173. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Davis S, Renaudineau S, Poirier R, Poucet B, Save E, Laroche S. The formation and stability of recognition memory: what happens upon recall? Front Behav Neurosci. 2010;4:177. doi: 10.3389/fnbeh.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, Zernig G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front Behav Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, Babb S, Cohen BM. Persistent reduction of immediate early gene mRNA in rat forebrain following single or multiple doses of cocaine. Brain Res Mol Brain Res. 1994;26:106–112. doi: 10.1016/0169-328x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. (2003) [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol 22 Suppl. 2012;3:S487–S491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Ghafouri S, Fathollahi Y, Javan M, Shojaei A, Asgari A, Mirnajafi-Zadeh J. Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Res. 2016;126:37–44. doi: 10.1016/j.eplepsyres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G, Good M. Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgments in adult mice expressing the APPswe Alzheimer’s disease mutation. Behav Neurosci. 2005;119:884–891. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N. Stress-induced social avoidance: a new model of stress-induced anxiety? Physiol Behav. 2002;77:327–332. doi: 10.1016/s0031-9384(02)00860-0. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67:1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J Adolesc Health. 2002;30:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106–5112. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kakolewski JW, Cox VC, Valenstein ES. Sex differences in body-weight change following gonadectomy of rats. Psychol Rep. 1968;22:547–554. doi: 10.2466/pr0.1968.22.2.547. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ. Androgens selectively modulate C-fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience. 1996;74:757–766. doi: 10.1016/0306-4522(96)00219-9. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav Brain Res. 2014;263:9–15. doi: 10.1016/j.bbr.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Khera M. Patients with testosterone deficit syndrome and depression. Arch Esp Urol. 2013;66:729–736. [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Hall FS, Fujii M, Goto A, Kanda Y, Koizumi A, Kuroiwa H, Mibayashi S, Muranishi Y, Otaki S, Sumikawa M, Tanaka K, Nishiyama N, Uhl GR, Takemura M. Memory impairment and reduced exploratory behavior in mice after administration of systemic morphine. J Exp Neurosci. 2015;9:27–35. doi: 10.4137/JEN.S25057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Kumbrink J, Gerlinger M, Johnson JP. Egr-1 induces the expression of its corepressor nab2 by activation of the nab2 promoter thereby establishing a negative feedback loop. J Biol Chem. 2005;280:42785–42793. doi: 10.1074/jbc.M511079200. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Vesque C, Schmitt J, Stunnenberg H, Frank R, Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990;10:3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Lungwitz EA, Stuber GD, Johnson PL, Dietrich AD, Schartz N, Hanrahan B, Shekhar A, Truitt WA. The role of the medial prefrontal cortex in regulating social familiarity-induced anxiolysis. Neuropsychopharmacology. 2014;39:1009–1019. doi: 10.1038/npp.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97:693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Tanaka Y, Maruyama W, Tabira T. Age-related spatial working memory impairment is caused by prefrontal cortical dopaminergic dysfunction in rats. Neuroscience. 2009;162:1192–1201. doi: 10.1016/j.neuroscience.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Morellini F. Spatial memory tasks in rodents: what do they model? Cell Tissue Res. 2013;354:273–286. doi: 10.1007/s00441-013-1668-9. [DOI] [PubMed] [Google Scholar]
- Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SA, Yamazaki T. Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150:4260–4269. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA, Hammer RP. Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–538. doi: 10.1016/s0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- Nikolaev E, Kaminska B, Tischmeyer W, Matthies H, Kaczmarek L. Induction of expression of genes encoding transcription factors in the rat brain elicited by behavioral training. Brain Res Bull. 1992;28:479–484. doi: 10.1016/0361-9230(92)90050-8. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sixth. New York: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- Penke Z, Morice E, Veyrac A, Gros A, Chagneau C, LeBlanc P, Samson N, Baumgärtel K, Mansuy IM, Davis S, Laroche S. Zif268/Egr1 gain of function facilitates hippocampal synaptic plasticity and long-term spatial recognition memory. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130159. doi: 10.1098/rstb.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintana H, Pongkan W, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Testosterone replacement attenuates cognitive decline in testosterone-deprived lean rats, but not in obese rats, by mitigating brain oxidative stress. Age (Dordr) 2015;37:9827. doi: 10.1007/s11357-015-9827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc Natl Acad Sci U S A. 1995;92:6873–6877. doi: 10.1073/pnas.92.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Hansen S. Temperament in the rat: sex differences and hormonal influences on harm avoidance and novelty seeking. Behav Neurosci. 2004;118:488–497. doi: 10.1037/0735-7044.118.3.488. [DOI] [PubMed] [Google Scholar]
- Shores MM, Moceri VM, Sloan KL, Matsumoto AM, Kivlahan DR. Low testosterone levels predict incident depressive illness in older men: effects of age and medical morbidity. J Clin Psychiatry. 2005;66:7–14. doi: 10.4088/jcp.v66n0102. [DOI] [PubMed] [Google Scholar]
- Sillence MN, Reich MM, Thomson BC. Sexual dimorphism in the growth response of entire and gonadectomized rats to clenbuterol. Am J Physiol. 1995;268:E1077–E1082. doi: 10.1152/ajpendo.1995.268.6.E1077. [DOI] [PubMed] [Google Scholar]
- Slade JP, Carter DA. Cyclical expression of egr-1/NGFI-A in the rat anterior pituitary: a molecular signal for ovulation? J Neuroendocrinol. 2000;12:671–676. doi: 10.1046/j.1365-2826.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, de Bruin JP, van Haelst AM, van der Gugten J, Kalsbeek A. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Suva LJ, Harm SC, Gardner RM, Thiede MA. In vivo regulation of Zif268 messenger RNA expression by 17 beta-estradiol in the rat uterus. Mol Endocrinol. 1991;5:829–835. doi: 10.1210/mend-5-6-829. [DOI] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson AI, Söderpalm B, Engel JA. Gonadectomy enhances shock-induced behavioral inhibition in adult male rats: implications for impulsive behavior. Pharmacol Biochem Behav. 2000;65:731–736. doi: 10.1016/s0091-3057(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012;227:224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SR, Lieblich SE, Galea LA. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology. 2011;36:1327–1341. doi: 10.1016/j.psyneuen.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Warren MF, Serby MJ, Roane DM. The effects of testosterone on cognition in elderly men: a review. CNS Spectr. 2008;13:887–897. doi: 10.1017/s1092852900016990. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Implications of animal object memory research for human amnesia. Neuropsychologia. 2010;48:2251–2261. doi: 10.1016/j.neuropsychologia.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, Evan G, Bliss TV, Hunt SP. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain. 2014;7:61. doi: 10.1186/s13041-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]