Abstract
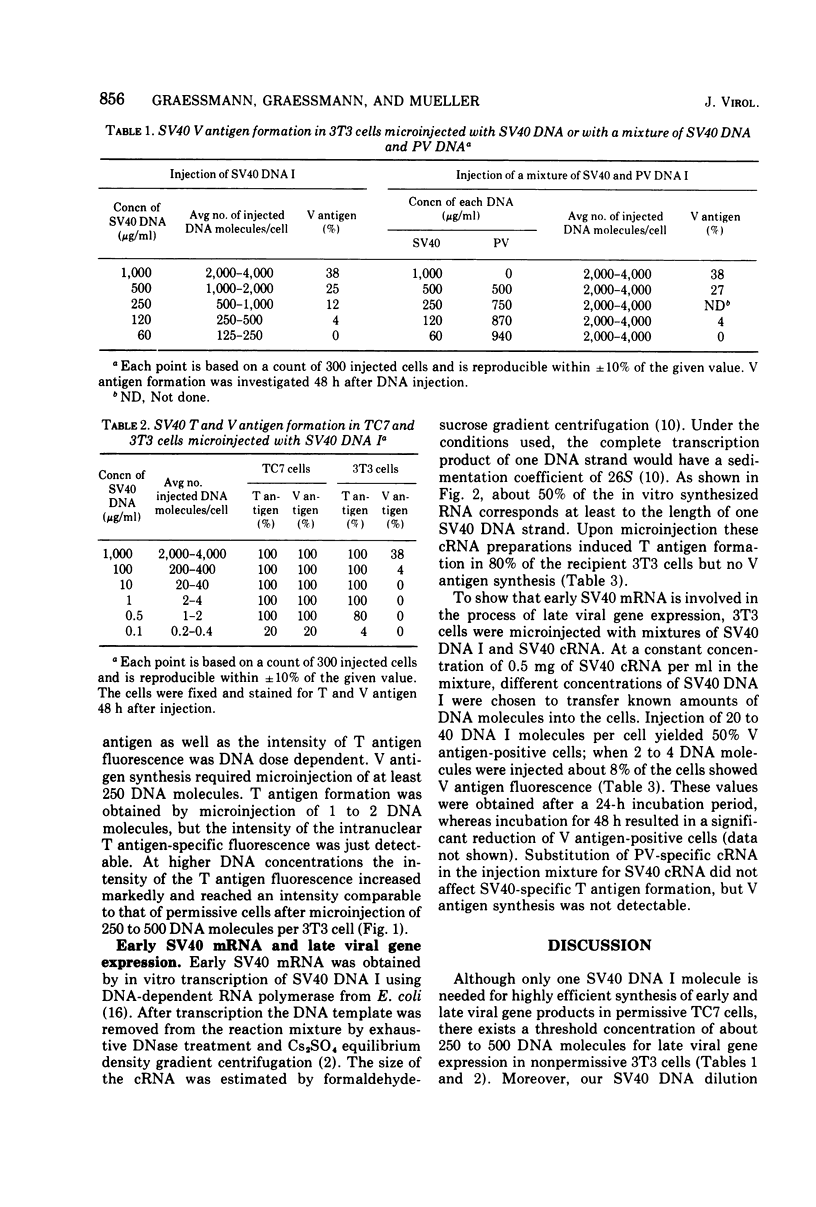
Primary or continuous lines of mouse cells (3T3) are nonpermissive for simian virus 40 (SV40). Abortively infected cells synthesize tumor antigen (T antigen but not viral DNA and virus capsid protein (V antigen). V antigen, however, was obtained when SV40 DNA was injected into 3T3 cells. This late gene expression also appears to be correlated with the quantity of injected DNA molecules per 3T3 cell. T antigen formation can be detected after microinjection of only 1 to 2 DNA molecules, but the intensity of intranuclear T antigen fluorescence is significantly brighter with injection of higher concentrations of viral DNA. In permissive cells (TC7), early and late SV40 gene expression is directly related to the number of injected molecules. Microinjection of 1DNA molecule induced T and V antigen formation with the same efficiency as microinjection of 2,000 to 4,000 molecules. The question of weather late SV40 gene expression is directly related to the quantity of an early virus-specific product was approached by microinjection of early SV40 complementary RNA together with small amounts of viral DNA. V antigen was obtained in a high proportion of recipient 3T3 cells at conditions where microinjection of viral DNA alone induced T but not V antigen synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandner G., Mueller N., Graessmann A., Graessmann M., Niebel J., Hoffmann H. Inhibition by interferon of SV40 tumor antigen formation in cells injected with SV40 cRNA transcribed in vitro. FEBS Lett. 1974 Mar 1;39(3):249–251. doi: 10.1016/0014-5793(74)80122-5. [DOI] [PubMed] [Google Scholar]
- Cowan K., Tegtmeyer P., Anthony D. D. Relationship of replication and transcription of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1927–1930. doi: 10.1073/pnas.70.7.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Regulation mechanism of simian virus 40 late gene expression in primary kidney cells and simian virus 40 transformed 3T3 cells. Virology. 1975 Jun;65(2):591–594. doi: 10.1016/0042-6822(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Gruen R., Graessmann M., Graessmann A., Fogel M. Infection of human cells with polyoma virus. Virology. 1974 Mar;58(1):290–293. doi: 10.1016/0042-6822(74)90162-7. [DOI] [PubMed] [Google Scholar]
- Grässman A. Mikrochirurgische Zellkerntransplantation bei Säugetierzellsn. Exp Cell Res. 1970 Jun;60(3):373–382. doi: 10.1016/0014-4827(70)90530-6. [DOI] [PubMed] [Google Scholar]
- Grässmann A., Grässmann M. Uber die Bildung von Melanin in Muskelzellen nach der direkten Ubertragung von RNA aus Harding-Passey-Melanomzellen. Hoppe Seylers Z Physiol Chem. 1971 Apr;352(4):527–532. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Kedinger C., Gissinger F., Chambon P. Size of the RNA's synthesized by purified calf thymus DNA-dependent RNA polymerases on SV40 DNA. FEBS Lett. 1973 Jan 15;29(2):109–112. doi: 10.1016/0014-5793(73)80537-x. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]