Abstract
In everyday conversation, we make many rapid choices between competing concepts and words in order to convey our intent. This process is termed semantic control, and it is thought to rely on information transmission between a distributed semantic store in the temporal lobes and a more discrete region, optimized for retrieval and selection, in the left inferior frontal gyrus. Here, we used diffusion tensor imaging in a group of neurologically normal young adults to investigate the relationship between semantic control and white matter tracts that have been implicated in semantic memory retrieval. Participants completed a verb generation task that taps semantic control (Snyder & Munakata, 2008; Snyder et al., 2010) and underwent a diffusion imaging scan. Deterministic tractography was performed to compute indices representing the microstructural properties of the inferior fronto-occipital fasciculus (IFOF), the uncinate fasciculus (UF), and the inferior longitudinal fasciculus (ILF). Microstructural measures of the UF failed to predict semantic control performance. However, there was a significant relationship between microstructure of the left IFOF and ILF and individual differences in semantic control. Our findings support the view put forth by Duffau (2013) that the IFOF is a key structural pathway in semantic retrieval.
Keywords: Semantic memory, Cognitive control, Lexical access, White matter, Structural connectivity, Broca’s area
Introduction
Communication requires the constant access and retrieval of conceptual knowledge to comprehend and produce language. Complicating matters is the fact that we know a vast amount of information about any one thing; sorting through that knowledge to access the meaningful information best suited for the task at hand is a complex computation that we nonetheless constantly and seamlessly compute. Semantic control is the ability to quickly generate conceptual associations and then choose the best term from competing associations to effectively communicate concept information (Snyder, Banich, & Munakata, 2011; Whitney, Kirk, O’Sullivan, Lambon Ralph, & Jefferies, 2011).
Research on the neural basis of semantic memory has implicated a distributed left-lateralized network consisting of the inferior frontal gyrus (IFG), the anterior temporal lobe, the posterior lateral temporal cortex, and the temporo-parietal junction/angular gyrus (reviewed in Binder & Desai, 2011). The left IFG (LIFG) overlaps with the area known as Broca’s area (Broca, 1861). This region is classically thought to support speech production. Early neuroimaging studies implicated the LIFG in the retrieval of semantic information (Abdullaev & Bechtereva, 1993; Buckner et al., 1995). Adding to this, the role of the LIFG was reexamined in an elegant study that found LIFG activation only when the task demanded selection among competing concepts (Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997), shedding further light on the role of the LIFG in semantic cognition. Further evidence has demonstrated that the LIFG plays an important role in semantic control, the process that allows for the resolution of semantic interference generated by closely related concepts being simultaneously activated (Badre, Poldrack, Paré-Blagoev, Insler, & Wagner, 2005; Novick, Kan, Trueswell, & Thompson-Schill, 2009; Novick, Trueswell, & Thompson-Schill, 2010). Activations in the LIFG have been continually found in conjunction with the controlled retrieval of semantic information (Snyder et al., 2011), cementing its role in semantic control, which is a twofold process whereby control mechanisms in the LIFG facilitate both retrieval and selection among competing concepts.
Discerning where concepts are stored and organized has proven to be a more difficult nut to crack. One dominant hypothesis, termed the “hub and spoke” model, proposes that the anterior temporal lobes (ATLs) are hubs for the storage of amodal concepts (Patterson, Nestor, & Rogers, 2007), such that conceptual information is abstracted from the sensory attributes of the stimulus. Thus, the concept “cat” can be accessed from sight, sound, or text. This model has been critiqued for its reliance on findings from patients with semantic dementia. This type of dementia is characterized by cell loss in the ATLs; however, patients also have both gray and white matter atrophy throughout the brain, making it difficult to claim that deficits are solely the result of damage to the ATLs (Garrard & Hodges, 2000; Hodges, Patterson, Oxbury, & Funnell, 1992; Patterson, Nestor, & Rogers, 2007). There is also conflicting evidence from individuals with focal lesions in the ATLs, who in stark contrast to the findings from individuals with semantic dementia (who have widespread degeneration), have semantic knowledge that is largely unimpaired (Bi et al., 2011; Simmons & Martin, 2009). Moreover, a meta-analysis of the neuroimaging findings on semantic processing found that the area most commonly activated in semantic tasks was the left angular gyrus, located near Wernicke’s area, not the ATL (Binder, Desai, Graves, & Conant, 2009). One potential explanation for the absence of findings in the ATLs is that the signal-to-noise ratio is poor due to their location proximal to the nasal sinuses. It should be noted that the posterior middle temporal gyrus (pMTG), just inferior to the angular gyrus, has also been implicated in semantic cognition (Binder & Desai, 2011; Chao, Haxby, & Martin, 1999). Thus, although the exact location and organization of semantic information is controversial, it is clear that a network of left-lateralized cortical regions in and near the temporal lobes plays a fundamental role in the storage of conceptual knowledge.
Taken together, these findings provide a cortical framework for semantic control, whereby ventral frontal regions – namely the LIF G – are responsible for retrieving and selecting the appropriate information from more posterior, left-lateralized stores of conceptual knowledge. Linking computations performed in anatomically discrete regions requires examination of the fiber pathways connecting these regions. Two association fiber pathways stand out as candidate regions based on their location and prior research findings. The first is the inferior fronto-occipital fasciculus (IFOF). The IFOF is a long-range ventral white matter tract that diffusion tensor imaging (DTI) in humans revealed connects the ventral occipital lobe, lateral occipital lobe, and posterior temporal lobe with portions of the lateral and ventral frontal lobe (Catani & Thiebaut de Schotten, 2008; Duffau, Herbet, & Moritz-Gasser, 2013). The functionality of the IFOF is poorly understood, in part, because it has not been identified in non-human primates (Catani & Thiebaut de Schotten, 2008; Catani, 2007; Schmahmann & Pandya, 2006). The fact that the IFOF is uniquely human suggests that it may support a uniquely human aspect of cognition, like language. Indeed, Duffau and colleagues (2005; 2008) reported that intraoperative electrical stimulation of the IFOF during awake neurosurgery caused semantic naming errors. Two other studies tested groups of patients with brain damage and found that damage to the IFOF correlated with semantic retrieval impairments in picture-naming tasks and matching tasks (Han et al., 2013; Harvey & Schnur, 2015). This compelling work, as well as the anatomical termination of the IFOF in a region implicated in semantic control (the IFG), suggests that this tract likely facilitates information exchange essential for semantic retrieval.
A second tract implicated in semantic control is the uncinate fasciculus (U F), a curved white matter pathway connecting the anterior and medial temporal lobes to the lateral orbitofrontal cortex (OFC; Catani & Thiebaut de Schotten, 2008). It has been hypothesized that the UF is part of the ventral language pathway, supporting object naming by relaying sensory information about objects, processed in the ventral temporal cortex, to language regions in the inferior-lateral frontal lobes (Papagno et al., 2014a). Evidence for this view is mixed. On the one hand, individuals with semantic dementia have altered microstructure in the UF (reviewed by Von Der Heide, Skipper, Klobusicky, & Olson, 2013), and removal of portions of the UF during neurosurgery can cause severe and lasting deficits in one’s ability to retrieve proper names (Papagno, 2011). On the other hand, resection of the left UF does not cause notable or lasting problems with other aspects of semantic memory (Papagno et al., 2014). Furthermore, Duffau and colleagues (2009) found the stimulation of the UF during awake neurosurgery does not disturb semantic processing (Duffau, Gatignol, Moritz-Gasser, & Mandonnet, 2009). Based on this important work done with direct stimulation, Duffau (2013) proposed a model of semantic processing whereby the IFOF is the direct ventral pathway facilitating the retrieval of semantic information, while the UF is part of an indirect, perhaps supportive but not primary pathway in semantic cognition (Duffau et al., 2013). This would account for the proper naming findings mentioned earlier, as well as DTI findings showing that performance of healthy older adults on a variety of semantic memory tasks correlates with the microstructural properties of the left UF, as well as the left IFOF (De Zubicaray, Rose, & McMahon, 2011).
In the present study, we tested the hypothesis that the IFOF and UF are involved in semantic control as part of the ventral semantic pathway. To do this, we tested a group of healthy young adults and examined whether individual differences in the microstructural properties of these tracts predicted their semantic control performance. The value of testing healthy young adults is that you can observe how the brain functions without concerns that neuroplasticity or strategic behavioral changes that accompany brain damage or normal aging are occluding the interpretation of findings.
As a control fiber pathway, we examined the inferior longitudinal fasciculus (ILF). The ILF runs inferior to the IFOF, connecting occipital and posterior ventral temporal regions to the amygdala and temporal pole (Catani & Thiebaut de Schotten, 2008; Duffau et al., 2013). The ILF has been implicated in high-level vision (Shinoura et al., 2010; Tavor et al., 2014a; Thomas, Humphreys, Jung, Minshew, & Behrmann, 2011), as well as mapping sound or word-form to meaning (Wong, Chandrasekaran, Garibaldi, & Wong, 2011). Stimulation of the posterior ILF during awake neurosurgery causes alexia but not semantic impairments (Mandonnet, Gatignol, & Duffau, 2009; Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007).
We used DTI to examine such microstructural properties. DTI is a technique for imaging white matter in vivo and relies on diffusion-weighted MR imaging (DW-MRI), which provides information about the degree of water molecule diffusion within any given voxel of the brain. White matter is made up of axons, in which the direction and degree of diffusion is restricted due to presence of axonal membranes and myelin sheaths (Alexander, Lee, Lazar, & Field, 2007; Mori, Crain, Chacko, & van Zijl, 1999). By using DW-MRI, we can measure variations in the degree of diffusion restriction, as well as the direction of diffusion. We also used deterministic tractography, which employs reconstruction algorithms to visualize white matter tracts and allows for the extraction of specific measures of microstructure within a tract.
To assess semantic control, participants performed a verb generation task. In this task, a noun was presented visually and participants were required to generate a related verb. Prior findings have implicated the LIFG in performance of this task (Snyder et al., 2010; Thompson-Schill et al., 1998). Latent semantic analysis (LSA) was used as a measure of semantic relatedness between provided word pairs. LSA is a measure of association strength or semantic relatedness between a pair of words, and has been shown to capture real world semantic knowledge and human behavior (Landauer, Foltz, & Laham, 1998). While the verb generation task typically uses reaction times (RTs) as a measure of task performance (Snyder et al., 2010), we chose to use LSA as a variable of interest and measure of semantic control. LSA extracts information for word co-occurrence and context and represents the semantic relatedness between two words, relying on the principle that the usage of words in text reflects semantic similarity between two words (Wolfe & Goldman, 2003). We believe that LSA measures capture the essential nature of semantic control - choosing appropriate conceptual knowledge for the task at hand – in a way that is superior to reaction time.
In accordance with its terminations in areas functionally implicated for semantic control, we predicted that white matter microstructure of the IFOF would predict individual differences in performance on the verb generation task as indexed by the semantic relatedness of the noun-verb pairs. Drawing on work implicating the UF in retrieval and selection under restricted conditions (Alm, Rolheiser, Mohamed, & Olson, 2015; Harvey, Wei, Ellmore, Hamilton, & Schnur, 2013; Papagno, 2011), we predicted that variation in UF microstructure would predict performance in semantic retrieval conditions that are especially taxing to the selection and retrieval process. We hypothesized that variation in the white matter microstructure of the ILF, would not predict individual differences in semantic control.
Methods
Participants
Thirty-six healthy adults (15 male, 21 female) from the greater Temple University community between the ages of 18 and 28 years (M = 21.39, SD = 2.43) participated in the study. Six participants (two female) were excluded from further analyses; two were found to be substantial outliers with respect to behavioral performance, three were found to be substantial outliers with regard to white matter indices, and one was removed due to a malfunction during image acquisition. This resulted in a study sample of 30 participants (18 female). All participants were native English speakers, right-handed (as ascertained by self-report), and had normal or corrected-to-normal vision. Participants had no history of psychological or neurological disorders and no MRI contraindications as ascertained by self-report. Participants were compensated for their participation, and informed consent was obtained in accordance with the guidelines set forth by the Institutional Review Board of Temple University.
Study design and materials
Study participation took place in two phases. During one session, DTI data, as well as high-resolution anatomical scans, were acquired at Temple University Hospital. In the other session, participants completed computerized tasks in the laboratory on Temple’s campus. Participants were tested individually in a well-lit room. Computerized tasks were programmed in E-Prime (Version 2.0 Professional) and presented on HP computers. Vocal responses were recorded using a Frisby FMC-220 microphone and Windows Sound Recorder software.
Verb generation task
For the present study, we used a verb generation task that was previously used as a proxy for semantic control (Snyder et al., 2011; Thompson-Schill et al., 1998; Thompson-Schill, 2003). In this task, participants are shown concrete nouns and are required to generate semantically-related verbs that describe the noun. For instance, participants are presented with the noun “ball,” to which they might generate the verbs “hit,” “throw,” or “kick”. This task is an ideal paradigm for examining semantic control, because it requires the activation of associated semantic knowledge and the selection and retrieval of the best term for the task at hand.
Our task was previously used by Snyder and Munakata (2008). Materials for the verb generation task were 100 concrete nouns. The nouns were manipulated in a 2 × 2 design with two noun manipulations: selection demand (e.g., high selection demand, such as “ball” vs. low selection demand, such as “scissors”) and retrieval demand, defined by the association strength between given nouns and possible verbs (e.g., high retrieval demand, such as “hedge” vs. low retrieval demand, such as “cat”). There were 25 nouns in each of the four conditions. Each word stimulus was visually presented in the center of the computer screen, and the task was to rapidly generate a verb associated with the noun by speaking aloud. Participants were told that the verb could be either something the noun does or something you do with the noun. For instance, in the example above, “hit,” “throw,” and “kick” all represent actions that can be performed with a ball. An example was given during the presentation of the instructions, and five practice trials preceded the task. Word pairs were removed from analysis if the response was not determined to be a verb or if any part of the given noun was used in the response (e.g., hammer/hammered). Participants were instructed to press the space bar after each verbal response. This button press served as a proxy measure of RT. Trials were self-paced. A fixation cross was presented on screen for 1,000 ms between each word. Nouns were presented in four blocks of 25 nouns with nouns randomized within blocks, and blocks randomized between conditions. Participants were offered a self-paced break between blocks.
Verbal intelligence
A subset of participants (N = 17, eight female) were administered the word-reading portion of the Wide Range Achievement Test, version 4 (WRAT4; Wilkinson & Robertson, 2006). This test requires participants to read aloud words that vary in their frequency in the English Lexicon. The WRAT has been found to be a reliable measure of reading proficiency and verbal intelligence (Robertson, 2009).
Image acquisition
MRI scanning was conducted at Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a conventional twelve-channel, phased-array head coil. DTI data were collected using a diffusion-weighted, echo-planar imaging (EPI) sequence with whole brain coverage. Imaging parameters included 55 axial slices, 2.5-mm slice thickness, TR = 9,900 ms, TE = 95 ms, FOV = 240 mm2, b values of 0 and 1,000 s/mm2, 64 non-collinear directions. These parameters yielded a DTI scan lasting approximately 11 minutes.
In addition to DWIs, high-resolution anatomical images (T1-weighted 3D MPRAGE) were also collected for each participant with the following parameters: 160 axial slices, 1-mm slice thickness, TR = 1,900 ms, TE = 2.93 ms, inversion time = 900 ms, flip angle = 9°, FOV = 256 mm2. These anatomical images were co-registered to the diffusion images and used to draw regions of interest (ROIs).
DTI pre-processing
The diffusion-weighted data were pre-processed using FSL (Smith et al., 2004) to correct for eddy currents and subject motion using an affine registration model. The b-vector matrix was adjusted based on rigid body registration, ensuring a valid computation of the tensor variables. Non-brain tissue was removed using FSL’s (Smith et al., 2004) automated brain extraction tool (BET), and a standard diffusion tensor fitting model was then applied to the data. The diffusion tensor fitting provided estimates of fractional anisotropy (FA) and mean diffusivity (MD), as well as three eigenvectors and eigenvalues. These estimates were computed on individual voxels using a three-dimensional Gaussian distribution model that yielded a single mean ellipsoid for each voxel.
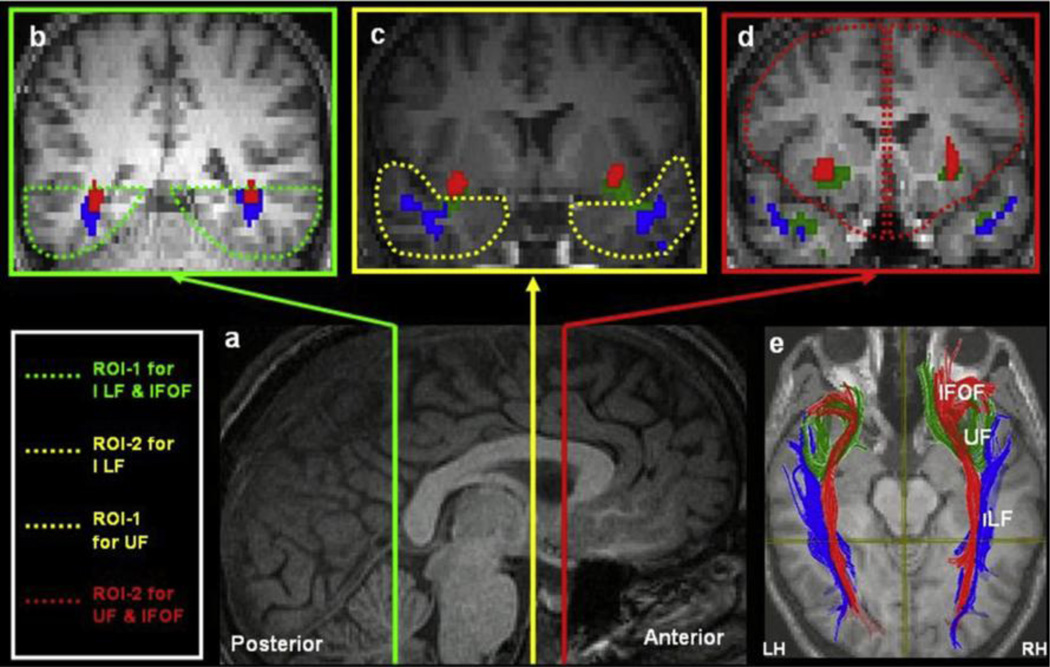
Whole brain deterministic tractography was performed in subject native space using the Diffusion Toolkit and TrackVis software packages (Wang, Benner, Sorensen, & Wedeen, 2007). This software uses a fiber assignment continuous tracking (FACT) algorithm (Mori et al., 1999) to determine the branching and curving of the fiber tracts. For a given voxel, this algorithm estimates the orientation of the principal eigenvector in that voxel and then uses nearest-neighbor interpolation to step along that direction. Step length was fixed at 0.1 mm, and an angle threshold of 35 degrees was used to determine the termination point of the fiber tracts. A spline filter was used to smooth the tractography data. A multiple ROI-based axonal tracking approach (Thomas et al., 2011) was then used to delineate bilateral IFOF, UF, and ILF. All ROIs were drawn in subject native space. For tracing the IFOF, one ROI was drawn in the ventral occipito-temporal cortex inferior to the lateral ventricles, while the other ROI was comprised of the portion of the frontal lobe located anterior to the rostrum of the callosum. For the UF, an ROI was drawn in the portion of the temporal cortex that is anterior to the point at which the fornix descends to the mammillary bodies, along with the same frontal ROI used for delineating the IFOF. Finally, for the ILF, the ventral occipito-temporal ROI (used for defining the IFOF) and the anterior temporal lobe ROI (used for defining the UF) were used. ROIs were drawn using the high-resolution anatomical T1 images, in accordance with methods outlined by Thomas and colleagues (2011) and used previously in our lab (Alm et al., 2015). Figure 1 outlines the ROIs and parameters used to delineate the tracts of interest (Thomas et al., 2011). A Boolean AND term was used to select only the fibers that passed through both of these seed ROIs. FA, MD, axial diffusivity (AD), and radial diffusivity (RD) indices were subsequently extracted from the tracts of interest, averaging along the length of each tract.
Fig. 1.
The regions of interest for extraction of the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and uncinate fasciculus. Reproduced with permission (Thomas et al., 2011)
FA is the most commonly used white matter index across DTI studies; it is a scalar value ranging from 0 to 1 that represents the degree to which diffusion is constrained along any axis within a given voxel. We chose to also focus on the indices of AD, RD, and MD. AD represents the principal eigenvalue, or the principal direction of diffusion within a voxel. RD is an average of the second and third eigenvalues, while MD is an average of all three eigenvalues and indexes the overall degree of diffusion, regardless of direction (Beaulieu, 2011). We included these measures because they are thought to reveal important information about the properties of diffusion and white matter microstructure beyond what just FA can provide (Alexander et al., 2011, 2007).
Latent semantic analysis (LSA) and statistical analyses
The primary behavioral measure of interest used in the present investigation was latent semantic analysis (LSA; Landauer et al., 1998) strength. Association strength for each noun-verb pair was operationalized using LSA, which is a means of extracting information about the semantic nature of words from large corpuses of text. For our study, LSA was calculated using the University of Colorado LSA Website (http://lsa.colorado.edu). A pairwise function was used wherein submitting pairs of text generates a cosine between the pair of words. In this case, a cosine was generated for each noun-verb pair. The “general reading up to first-year college” corpus was used as the semantic space from which the cosine (0–1) for each pair was generated. This is the method used previously by Snyder and Munakata (2008). The LSA cosine for each pair was obtained as a measure of conceptual association strength between the two words. Within each task condition, and collapsed across all conditions, LSA cosines for each pair were averaged to obtain a mean LSA score for each participant.
Statistical analyses were performed using SPSS (Version 21.0). Regression analyses were used to examine the relationship between the microstructure of tracts of interest and performance on the verb generation task. LSA score or RT was the dependent measure in the analysis. Bilateral white matter indices (FA, MD, RD, and AD), entered for each hemisphere separately, and sex were the independent variables in each model. We controlled for multiple comparisons by adjusting family-wise error rate using a Bonferroni correction to adjust for simultaneous predictors in each regression model (critical p = .05/3; Mundfrom, Perrett, Schaffer, Piccone, & Roozeboom, 2006). All reported p-values are Bonferroni-corrected.
Results
Behavioral results
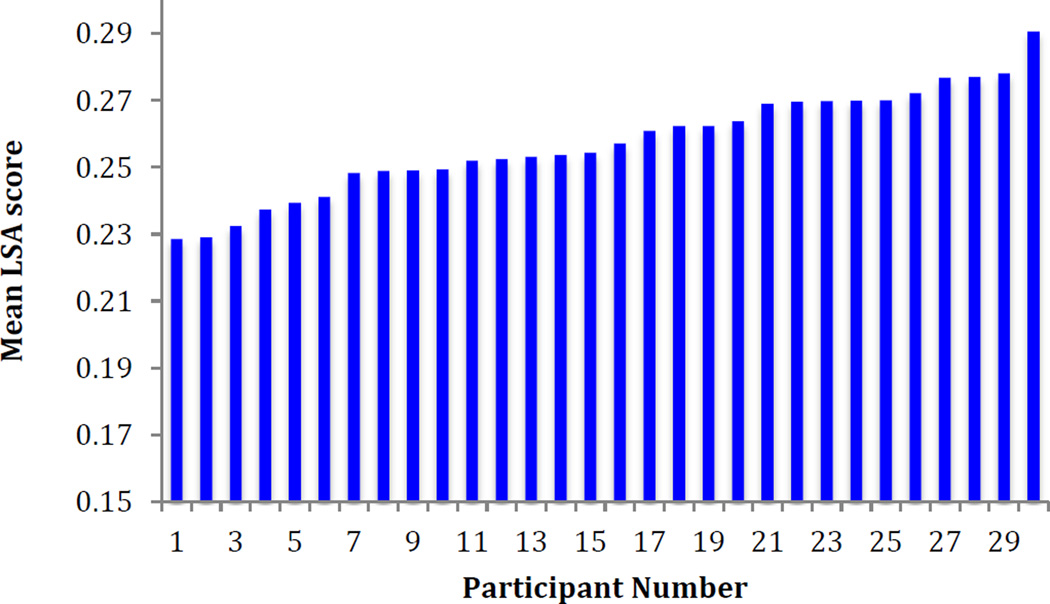
The current study employed a button press as a measure of RT, while previous studies using this task have assessed RT from participant vocal onset (Snyder & Munakata, 2008). However, many participants were not compliant with the instructions to press the button as quickly as possible. We therefore used a different approach to capture RT by measuring the time from the post-stimulus button press (which advanced the experiment to the next word) to the vocal onset of response to the new stimulus. This approach provided a more uniform measure of RT. RT was not available for three participants, because their button press responses could not be recovered (N = 27). Consistent with prior findings (Snyder et al., 2010), RTs were significantly slower for high selection as compared to low selection demand and low association as compared to high association demand conditions (t(26) = 4.30, p < .001; t(26) = 5.87, p < .001 respectively). Mean RTs for all conditions are presented in Table 1. To examine LSA scores, trials in which RTs were faster or slower than 3 standard deviations from the mean were trimmed, in accordance with Snyder and Manukata (2008)). Mean RTs were correlated with mean LSA scores (see Fig. 2 for individual variability and mean LSA scores). There was a significant relationship such that faster RTs correlated with higher LSA scores (r(26) = −0.567, p = .002). Inspection of the LSA scores revealed that there was enough consistent variability to examine individual differences. Since LSA provides a clean measure of semantic coherence between nouns and verbs, we used this as our index of measure of semantic control.
Table 1.
Mean latent semantic analysis (LSA) scores, reaction times (RTs, ms), and standard deviations (SDs) for all participants for each task condition. Note that in regards to LSA scores, higher scores are better
| Condition | Mean LSA (SD) | Mean RT (SD) |
|---|---|---|
| Mean LSA/RT | 0.26 (0.02) | 4,076.66 (594.97) |
| High association | 0.37 (0.03) | 3,882.20 (614.86) |
| Low association | 0.15 (0.01) | 4,291.37 (615.26) |
| High selection | 0.24 (0.02) | 4,254.57 (716.51) |
| Low selection | 0.28 (0.03) | 3,900.17 (540.01) |
Fig. 2.
Individual variability in performance on the verb generation task. Numbers on the x-axis represent rank order for each participant. The y-axis indexes LSA score as a proxy for semantic control. Lower scores are indicative of worse semantic control
DTI data
There is some evidence that white matter microstructure varies systematically with age and sex (Gong, He, & Evans, 2011; Ingalhalikar et al., 2014; Lebel et al., 2012). To ensure that age was not driving white matter variability, measures of FA, MD, AD, and RD were correlated with participant age. To examine potential sex differences, t-tests were used. There was no significant relationship between age and any of the white matter indices (p’s > .05), most likely due to the fact that our age range was small. Thus, age was not controlled for in further analyses. There were, however, significant relationships between white matter indices and sex (see Table 2); therefore, sex was controlled for in all further analyses.
Table 2.
Relationship between sex and microstructural properties of the uncinate fasciclus (UF), inferior fronto-occipital fasciculus (IFOF), and inferior longitudinal fasciculus (ILF)
| Left UF | Right UF | |||||
|---|---|---|---|---|---|---|
| Males | Females | p-value | Males | Females |
P- value |
|
| FA | 0.502 | 0.457 | .019* | 0.486 | 0.450 | .015* |
| AD (×10−3 mm2/s) | 1.198 | 1.179 | .163 | 1.196 | 1.147 | .014* |
| MD (×10−3 mm2/s) | 0.747 | 0.769 | .201 | 0.754 | 0.752 | .888 |
| RD (×10−3 mm2/s) | 0.527 | 0.564 | .114 | 0.533 | 0.554 | .292 |
| Left IFOF | Right IFOF | |||||
| FA | 0.577 | 0.546 | .064 | 0.580 | 0.542 | .017* |
| AD (×10−3 mm2/s) | 1.352 | 1.299 | .041* | 1.334 | 1.299 | .047* |
| MD (×10−3 mm2/s) | 0.777 | 0.770 | .583 | 0.762 | 0.774 | .295 |
| RD (×10−3 mm2/s) | 0.493 | 0.505 | .510 | 0.476 | 0.511 | .025* |
| Left ILF | Right ILF | |||||
| FA | 0.541 | 0.540 | .073 | 0.540 | 0.531 | .608 |
| AD (×10−3 mm2/s) | 1.368 | 1.275 | .022* | 1.338 | 1.317 | .403 |
| MD (×10−3 mm2/s) | 0.826 | 0.765 | .026* | 0.805 | 0.780 | .806 |
| RD (×10−3 mm2/s) | 0.559 | 0.518 | .080 | 0.544 | 0.550 | .790 |
FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity
p<.05
Inferior fronto-occipital fasciculus
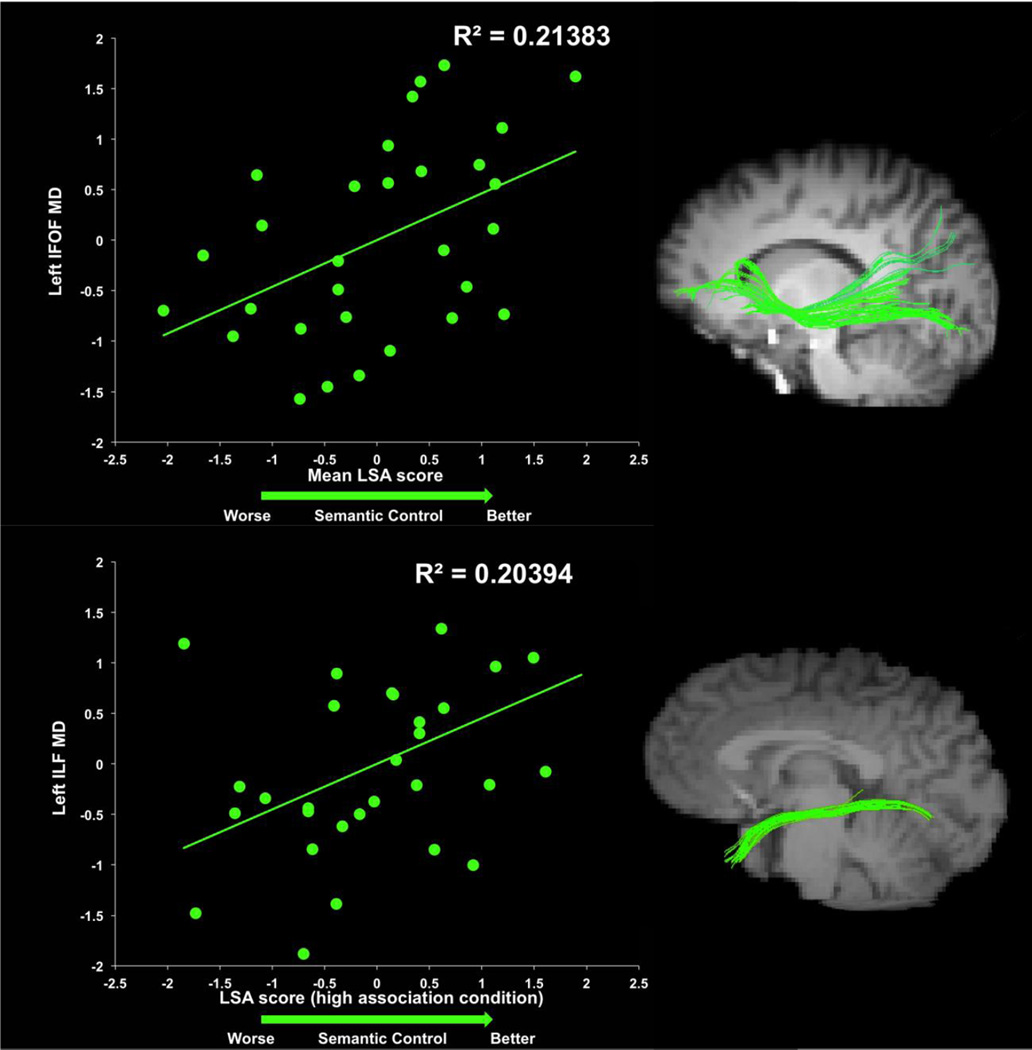
Significant results of the IFOF regression models are listed in Tables 3 and 4. Individual differences in white matter microstructure in the left IFOF significantly predicted LSA performance on the verb generation task (see Fig. 3). Specifically, higher MD in the left IFOF predicted higher mean LSA scores (β = 0.53, t(26) = 2.66, p = .04), as well as higher LSA scores in the high association condition (β = 0.56, t(26) = 2.91, p = .02). Initially, higher AD in the left IFOF also predicted higher LSA scores in the mean condition, high association condition, and low association condition; however, these effects did not survive correction for multiple comparisons (uncorrected p = .03, p = .03 and p = .04, respectively). No other white matter indices revealed significant relationships between IFOF microstructure and LSA or RT (all p’s > .05). These regression models are listed in Supplementary Material Tables S2–S10.
Table 3.
Summary of the inferior fronto-occipital fasciculus multiple linear regression models predicting individual differences in LSA on the verb generation task collapsed across conditions (mean LSA)
| Inferior fronto-occipital fasciculus | |||||
|---|---|---|---|---|---|
| Dependent variable | Predictor variables | β | t-value | F | R2 |
| LSA | 0.29 | 0.03 | |||
| Gender | 0.16 | 0.76 | |||
| Left FA | −0.07 | −0.24 | |||
| Right FA | 0.08 | 0.26 | |||
| LSA | 2.80** | 0.24 | |||
| Gender | 0.09 | 0.49 | |||
| Left MD | 0.52 | 2.67** | |||
| Right MD | −0.17 | −0.86 | |||
| LSA | 2.25 | 0.21 | |||
| Gender | 0.02 | 0.09 | |||
| Left AD | 0.52 | 2.28 | |||
| Right AD | −0.15 | −0.67 | |||
| LSA | 1.24 | 0.13 | |||
| Gender | 0.13 | 0.64 | |||
| Left RD | 0.39 | 1.67 | |||
| Right RD | −0.23 | −0.91 | |||
LSA latent semantic analysis, FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity, β standardized regression coefficient
p< .05 (after controlling for multiple comparisons)
R2 is a measure of effect size
Table 4.
Summary of the inferior fronto-occipital fasciculus multiple linear regression models predicting individual differences in LSA on the verb generation task for the high association condition
| Inferior fronto-occipital fasciculus | |||||
|---|---|---|---|---|---|
| Dependent variable | Predictor variables | β | t-value | F | R2 |
| LSA | 0.47 | 0.05 | |||
| Gender | 0.21 | 0.96 | |||
| Left FA | −0.12 | −0.39 | |||
| Right FA | 0.11 | 072 | |||
| LSA | 3.45** | 0.28 | |||
| Gender | 0.12 | 0.68 | |||
| Left MD | 0.56 | 2.91** | |||
| Right MD | −0.20 | −1.03 | |||
| LSA | 2.47 | 0.22 | |||
| Gender | 0.06 | 0.31 | |||
| Left AD | 0.53 | 2.33 | |||
| Right AD | −0.16 | −0.74 | |||
| LSA | 1.62 | 0.16 | |||
| Gender | 0.16 | 0.81 | |||
| Left RD | 0.43 | 1.85 | |||
| Right RD | −0.26 | −1.05 | |||
LSA latent semantic analysis, FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity, β standardized regression coefficient
p < .05 (after controlling for multiple comparisons)
R2 is a measure of effect size
Fig. 3.
Scatter plot of standardized residuals from the regression analysis depicting the relationship between individual differences in left inferior fronto-occipital fasciculus (IFOF) and left inferior longitudinal fasciculus (ILF) mean diffusivity (MD) and mean latent semantic analysis (LSA) score. Low LSA scores are indicative of worse semantic control. Variation in left IFOF MD predicted verb generation performance as indexed by mean LSA. Variation in left ILF MD predicted verb generation performance as indexed by LSA in the high association condition
Other tracts: The uncinate fasciculus and inferior longitudinal fasciculus
Results of the UF regression models for mean LSA are listed in Supplementary Material Tables S2–S10. There were no significant effects (p’s > .05). Significant results of the ILF regression models are listed in Table 5. Individual differences in the white matter microstructure of the left ILF predicted performance on the verb generation task (see Fig. 3), such that higher MD in the left ILF predicted LSA scores in the high association condition (β = 0.51, t(26) = 2.58, p = .05). The ILF was the only white matter tract to predict RT, and contrary to all LSA findings, the RT effects were found in the right hemisphere. Specifically, higher FA in the right ILF predicted slower RTs collapsed across conditions (β = 0.61, t(24) = 2.73, p = .04), in the high association condition (β = 0.67, t(29) = 3.12, p = .02), and the low selection condition (β = 0.68, t(24) = 3.11, p = .02). ILF microstructure did not predict RT in any of the other conditions (p’s > .05)
Table 5.
Summary of the inferior longitudinal fasciculus multiple linear regression models predicting individual differences in LSA on the verb generation task for the high association condition
| Inferior longitudinal fasciculus | |||||
|---|---|---|---|---|---|
| Dependent variable | Predictor variables | β | t-value | F | R2 |
| LSA | 0.72 | 0.08 | |||
| Gender | 0.24 | 1.24 | |||
| Left FA | 0.07 | 0.30 | |||
| Right FA | −0.20 | −0.91 | |||
| LSA | 2.74** | 0.24 | |||
| Gender | −0.02 | −0.11 | |||
| Left MD | 0.51 | 2.58** | |||
| Right MD | −0.09 | −0.53 | |||
| LSA | 2.25 | 0.21 | |||
| Gender | 0.03 | 0.16 | |||
| Left AD | 0.45 | 2.28 | |||
| Right AD | −0.12 | −0.66 | |||
| LSA | 1.39 | 0.14 | |||
| Gender | 0.08 | 0.37 | |||
| Left RD | 0.34 | 1.65 | |||
| Right RD | −0.15 | −0.80 | |||
LSA latent semantic analysis, FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity, β standardized regression coefficient
p < .05 (after controlling for multiple comparisons)
R2 is a measure of effect size
Relationship to verbal intelligence scores
Regressions analyses including microstructure of the IFOF, UF, and ILF and gender failed to predict performance on the WRAT4 (all p’s > .05).
Discussion
In this study, participants were asked to verbally recall an action verb related to a common noun, a task that requires selecting between competing activated concepts and retrieving the concept in a goal-directed manner (Snyder et al., 2011; Whitney et al., 2011). The left inferior frontal gyrus, in which Broca’s area is located, and the temporal lobes are gray matter regions that are believed to play a key role in this kind of semantic control (Rogers et al., 2006; Thompson-Schill et al., 1998). The aim of our study was to examine white matter tracts that might also be involved in semantic control. To do this, we tested neurologically normal young adults and found that inter-individual variation in the microstructure of the left IFOF and left ILF predicted performance on our semantic control task, as indexed by semantic relatedness of presented nouns and generated verbs. In contrast, microstructural properties of the left UF, as well as microstructure of all tracts in the right hemisphere were unrelated to LSA scores.
Relationship to existing literature
Duffau and colleagues (2013) drew on evidence from intraoperative recordings to propose that the IFOF is a direct pathway facilitating semantic cognition, while the ILF and the UF constitute an indirect ventral pathway that works in tandem with the IFOF, but is not essential for semantic cognition (Duffau et al., 2013). Studies of lesion populations have also implicated the IFOF in semantic retrieval (Duffau et al., 2005; Duffau et al., 2008; Harvey & Schnur, 2015; Turken & Dronkers, 2011). For instance, one study examined a large group of brain-damaged patients and found that changes in microstructure and lesion volume in the IFOF correlated with performance on picture-naming and picture association matching tasks (Han et al., 2013). Another group tested older adults with left hemisphere stroke and found that IFOF microstructure correlated with picture-naming difficulties (Harvey & Schnur, 2015). In sum, the literature on the IFOF, although small, has consistently linked this white matter tract to semantic retrieval. Our study supports Duffau’s (2013) model, as well as previous findings from lesion populations and picture-naming tasks, and extends the semantic cognition literature by being, to our knowledge, the first study of its kind to investigate the white matter microstructure of pathways facilitating semantic control in a healthy young adult population.
The literature on the UF is less consistent with regard to semantic memory (reviewed by Von Der Heide et al., 2013). Some studies of individuals with semantic dementia or aphasia have implicated the UF in semantic retrieval (Han et al., 2013; Harvey et al., 2013), while other studies suggest the UF plays no role in semantic retrieval (Duffau et al., 2009) or only plays a role in retrieving proper names and unique entities (Mehta et al., 2016; Papagno et al., 2014). Studies reporting that UF microstructure is correlated with semantic cognition have also implicated the IFOF (De Zubicaray et al., 2011) which is geographically proximal to the UF at some points, making it difficult to ascertain whether the UF itself plays an essential role in semantic processing. In the present investigation, we found no relationship between the microstructure of this tract and performance on the verb generation task.
We recently argued that the UF may serve to adjudicate between competing memory representations at the time of retrieval, and would thus be most implicated in tasks with high levels of competition among stimulus or response choices (Alm, Rolheiser, & Olson, 2016). Our verb-generation task is believed to evoke competition among possible response choices. In order to retrieve an appropriate response, this competition must be resolved, and it is plausible that the resolution may be achieved by involvement of the uncinate. However, the lack of findings in the UF may be related to the nature of the stimuli: While the task did require adjudication between competing verb response choices, the stimuli were common nouns that are very overlearned. It is possible that a more powerful manipulation of semantic competition would have yielded effects in measures of UF microstructure.
The ILF, which runs inferior and parallel to posterior portions of the IFOF, has been implicated in semantic cognition to a lesser degree (see Harvey & Schnur, 2015; Wong et al., 2011). The majority of research on the ILF has implicated this tract in high-level vision, including face recognition (reviewed in Unger, Alm, Collins, Leary, & Olson, 2016). Based on this literature, we intended the ILF to serve as a control tract. Our data however, revealed an unexpected relationship between the ILF and performance on the verb generation task. Specifically, higher MD of the left ILF predicted higher LSA, in congruence with the effect’s found in MD for the left IFOF.
While unexpected, there is some precedent for our findings. ILF microstructure has been correlated with reading proficiency in children (Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012) and reading impairments caused by resection of the ILF during surgery for removal of left occipito-temporal glioma (Zemmoura, Herbet, Moritz-gasser, & Duffau, 2015). While these studies do not specifically address semantic cognition, a reading task by its nature requires access to semantic knowledge. Indeed, the aforementioned studies used the Woodcock Johnson Basic Reading task (Woodcock & Johnson, 2011) and single word reading tasks both involving objects, which would require accessing concept information for the task at hand. There is work that explicitly addresses the role of semantic knowledge in reading comprehension by Graves and colleagues (2014). White matter connectivity between cortical regions implicated in semantic cognition predicted the level to which skilled readers relied on semantic knowledge for a reading task. These tracts connecting the inferior temporal sulcus (ITS) with the posterior middle temporal gyrus (pMTG) overlapped with the ILF (Graves et al., 2014), providing further evidence for the role of the ILF in semantic cognition.
With regard to the current study, which specifically taxes object knowledge, early functional neuroimaging work by Chao, Haxby and Martin (1999) implicated the posterior temporal lobe as a crucial part of a network representing conceptual information. Regions implicated in representing object concepts in the brain include: posterior superior temporal sulcus (pSTS), lateral fusiform gyrus, and posterior middle temporal gyrus (pMTG) (reviewed in Martin, 2007). Anatomically, terminations of the ILF in the posterior temporal lobe converge with these findings. Moreover, a recent study of naming and recognition of concrete entities in patients with brain lesions, found the ILF to be associated with naming impairments for non-unique entities (Mehta et al., 2016). Visual objects form the foundation of concrete semantic knowledge and ILF microstructure has been shown to relate to object processing (Ortibus et al., 2012; Shinoura et al., 2007). Therefore, it is plausible that semantic tasks placing a high load on visual processing rely to some degree on this tract.
The ILF was the only tract that predicted reaction time on the verb generation task. Unlike all other findings, this relationship was found in the right hemisphere and in a different measure of microstructure (FA), whereby lower FA predicted faster RTs. Positive correlations between FA and cognitive performance don’t always mean “better” connectivity or integrity. Higher FA levels in the corpus callosum have been observed post concussion (Shenton et al., 2012). Relatively higher FA levels have also been observed in the uncinate fasciculus in children with conduct disorder as well as adults with antisocial personality disorder (reviewed in Olson et al., 2015). In regards to the ILF specifically, Tavor et al. (2014) reported several negative relationships between FA in certain portions of the ILF and performance on scene and face memory tasks. Thus higher FM/lower MD values may not always be better.
When this relationship is examined by condition, it was the high association and low selection conditions that drove this relationship. These conditions are made up of stimuli that are the most salient and highly imageable (Snyder & Munakata, 2008). Since the ILF, and specifically damage to the right ILF (Shinoura et al., 2010), has been linked to impairment in visual object recognition, this finding may be revealing an interesting dichotic relationship by which faster access to salient visual conceptual knowledge can be facilitated by the ILF in the right hemisphere, in contrast with the IFOF and ILF in the left hemisphere facilitating retrieval of responses that are more semantically related as indexed by higher LSA score. We acknowledge that this idea is highly speculative and requires further testing.
In sum, our findings both support the model put forth by Duffau and colleagues (2013), which implicates the IFOF as a direct pathway and the ILF as playing an indirect role in semantic control processes, and extends the literature by using LSA as a measure of interest for the experimental task.
Limitations
The IFOF, our principal track of interest, is a very large white matter tract connecting the lateral and ventral frontal lobe and the posterior temporal lobe with the ventral occipital lobe. (Catani & Thiebaut de Schotten, 2008). Therefore, it is highly likely that the IFOF supports several cognitive processes. For instance, our laboratory has implicated the IFOF in the processing of facial emotions (Unger et al., 2016). Our goal, however, was not to attribute a single function to the IFOF, but rather to determine whether the IFOF has a role in the selection and retrieval of semantic information in a controlled manner. Our findings are consistent with such a prediction. Additionally, our study was not designed to examine whether different subsections of the IFOF may play different supporting functions. Future studies may wish to localize semantic control regions in the LIFG and use this as a seed region to assess the role of an IFOF sub-tract for its functionality in both linguistic and non-linguistic processes.
Related to this, DTI cannot assess the direction of information flow in cortical networks. It is theoretically important to know whether frontal regions are exerting control on information held in posterior temporal regions, or whether posterior temporal regions are shunting information to the lateral frontal lobe for refinement and selection. Some leverage might be gained by collecting DTI on individuals who performed the verb generation task while ERPs are recorded, since ERPs can provide temporal and spatial specificity with respect to the direction of the frontal-temporal relationships.
Two prior studies (De Zubicaray et al., 2011; Han et al., 2013) reported that higher FA values in the IFOF correlated with better performance on semantic tasks. Our findings differ from these previous findings in that, lower MD predicted better performance. While results like these may seem strange, they are not necessarily unexpected. The relationship between white matter indices and their meaning in terms of neural signaling is not yet well understood. It is common to see significant findings reported in some but not all white matter indices (e.g., Alm et al., 2015; Cherubini et al., 2010; Mcdonald, Ahmadi, Tecoma, Dale, & Halgren, 2008; Tavor et al., 2014a).
Additionally, we reported that higher MD was related to better performance, whereas the previous studies (e.g., De Zubicaray et al., 2011; Han et al., 2013) found that higher FA correlated with better semantic memory performance. Since FA and MD are typically inversely related, this may seem a bit puzzling. First, we note that we studied a young healthy population while De Zubicaray and colleagues (2011) and Han and colleagues (2013) examined older adults, who typically have decreased FA values across the whole brain, and worse semantic memory performance. Besides this difference in sample population, in general, one should be wary when interpreting the directionality of DTI-behavior relationships. Based on these methodologies, we cannot make claims about better or worse white matter connectivity based on the direction of the relationship between a particular microstructural index and performance on a cognitive task. What we have demonstrated here is a significant relationship between the IFOF and ILF microstructure and performance on our semantic control task. We caution against generalized interpretations that higher or lower values of a particular white matter index are directly indicative of enhanced or diminished connectivity. Although it is true that FA and MD are inversely related, it is not always the case that higher FA, and therefore, lower MD signify “better” connectivity. Thicker myelination or increased axonal integrity may not always be equated with better behavioral performance (Scholz, Tomassini, Johansen-berg, & Reading, 2009). For instance, numerous studies have demonstrated that expert musicians (Imfeld et al., 2005) and simultaneous interpreters (Elmer et al., 2011) exhibit lower FA values compared to non-experts in various task-related white matter tracts, suggesting that perhaps extensive training is associated with decreases in FA within task-relevant tracts. Furthermore, Tavor and colleagues (2014) recently examined different subcomponents of the ILF and reported negative correlations between FA in certain subcomponents and performance on face and scene memory tasks. Scholz et al. (2009) offer a possible explanation for such counterintuitive findings by proposing that certain increases in connectivity may lead to behavioral interference or strategy differences among participants, which may result in substandard performance on a given task. It is also possible that decreases in myelination may have positive effects on behavioral performance. For example, processes like synaptic pruning would be related to decreases in the presence of myelin (which may correspond to increased MD), but would also lead to increased neuronal efficiency, and therefore, improvements in performance. The aforementioned work and the inversely related properties of FA and MD provide some precedent; however, our findings in MD are novel and an extension of the literature.
Last, our study did not assess other aspects of language, such as syntax and phonology. Tracts that have been specifically linked to sound and phonological processing include the superior longitudinal fasciculus, the arcuate fasciculus, and the middle longitudinal fasciculus (Dick & Tremblay, 2012). Tracts that have been associated with syntactic processing are the arcuate fasciculus and the extreme capsule (Papoutsi, Stamatakis, Griffiths, Marslen-Wilson, & Tyler, 2011; Rolheiser, Stamatakis, & Tyler, 2011; Teichmann et al., 2015). Although we are unaware of any evidence linking the IFOF to these other linguistic processes, future DTI studies should examine these in order to ascertain specificity of function.
Conclusions
We are endowed by experience with a rich tapestry of knowledge that makes up our world and influences our ability to communicate. For every given communicative moment there is a concept – often in the form of a word that is best suited for the task at hand. Selecting that concept from other activated concepts and retrieving it for subsequent use seems like a labor-intensive task. Yet, it is something done constantly and seemingly effortlessly (for some). The neural computations and pathways that facilitate this process have been studied for decades by a variety of methodologies. Using diffusion imaging to examine white matter fiber pathways that may facilitate semantic control is a new frontier that can contribute to the field by helping us understand the network that underlies this process. In the present investigation, we found that individual differences in white matter microstructure in the left IFOF and left ILF are able to predict performance on a semantic control task. This finding, coupled with previous work in the IFOF (Duffau et al., 2005, 2008; Harvey & Schnur, 2015; Turken & Dronkers, 2011), suggests that the IFOF is critical for semantic control and begets further examination of the tract in different linguistic paradigms in healthy populations. The significant effect observed in the ILF extends the small body of work linking the ILF to some aspects of semantic memory (Graves et al., 2014; Harvey & Schnur, 2015; Mehta et al., 2016) and provides a structural framework for cortical regions functionally implicated in object knowledge (Martin, 2007).
Supplementary Material
Acknowledgments
We would like to thank Ashley Unger, Hyden Zhang, Molly Split, Yin Wang, Vanessa Troiani, and Lauren Harris for assistance with participant testing and neuroimaging data analysis. We would also like to thank Hannah Snyder for providing task stimuli, Cibu Thomas for providing tractography ROI parameters, and Dorian Pustina for providing DTI expertise. This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
The authors declare no competing or conflicting financial interests.
References
- Abdullaev YG, Bechtereva NP. Neuronal correlate of the higher-order semantic code in human prefrontal cortex in language tasks. International Journal of Psychophysiology. 1993;14(3):167–177. doi: 10.1016/0167-8760(93)90031-j. http://doi.org/10.1016/0167-8760(93)90031-J. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Field AS. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connectivity. 2011 doi: 10.1089/brain.2011.0071. http://doi.org/10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. http://doi.org/10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Mohamed FB, Olson IR. Fronto-temporal white matter connectivity predicts reversal learning errors. Frontiers in Human Neuroscience. 2015 Jun;9:1–11. doi: 10.3389/fnhum.2015.00343. http://doi.org/10.3389/fnhum.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Olson IR. Inter-Individual Variation in Fronto-Temporal Connectivity Predicts the Ability to Learn Different Types of Associations. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.02.038. http://doi.org/10.1016/j.neuroimage.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack Ra, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. http://doi.org/10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. What makes diffusion anisotropic in the nervous system. In: Jones DK, editor. Diffusion MRI: theory, methods, and applications. New York: Oxford University Press; 2011. pp. 92–109. [Google Scholar]
- Bi Y, Wei T, Wu C, Han Z, Jiang T, Caramazza A. The role of the left anterior temporal lobe in language processing revisited: Evidence from an individual with ATL resection. Cortex. 2011;47(5):575–587. doi: 10.1016/j.cortex.2009.12.002. http://doi.org/10.1016/j.cortex.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011a;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. http://doi.org/10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011b;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. http://doi.org/10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. http://doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph Ma. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cerebral Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. http://doi.org/10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Broca PP. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech) Bulletin de La Société Anatomique. 1861;6:330–357. [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J. Neurosci. 1995;15(1 Pt 1):12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. Retrieved from PM:7823123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M. From hodology to function. Brain. 2007;130(3):602–605. doi: 10.1093/brain/awm008. http://doi.org/10.1093/brain/awm008. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. http://doi.org/10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Spoletini I, Di Paola M, Di Iulio F, Hagberg GE, Spalletta G. Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer’s disease patients. Journal of Alzheimer’s Disease :JAD. 2010;19(4):1273–1282. doi: 10.3233/JAD-2010-091186. http://doi.org/10.3233/J A D-2010-091186. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI, Rose SE, McMahon KL. The structure and connectivity of semantic memory in the healthy older adult brain. NeuroImage. 2011;54(2):1488–1494. doi: 10.1016/j.neuroimage.2010.08.058. http://doi.org/10.1016/j.neuroimage.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529–3550. doi: 10.1093/brain/aws222. http://doi.org/10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: A study using cortico-subcortical electrostimulations. Brain. 2005;128(4):797–810. doi: 10.1093/brain/awh423. http://doi.org/10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language?: A A cerebral stimulation study. Journal of Neurology. 2009;256(3):382–389. doi: 10.1007/s00415-009-0053-9. http://doi.org/10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Duffau H, Herbet G, Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Frontiers in Systems Neuroscience. 2013 Aug;7:44. doi: 10.3389/fnsys.2013.00044. http://doi.org/10.3389/fnsys.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. http://doi.org/10.3171/J N S/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Elmer S, Hänggi J, Meyer M, Jäncke L. Differential language expertise related to white matter architecture in regions subserving sensory-motor coupling, articulation, and interhemispheric transfer. Human Brain Mapping. 2011;32(12):2064–2074. doi: 10.1002/hbm.21169. http://doi.org/10.1002/hbm.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard P, Hodges JR. Semantic dementia: Clinical, radiological and pathological perspectives. Journal of Neurology. 2000;247(6):409–422. doi: 10.1007/s004150070169. http://doi.org/10.1007/s004150070169. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain Connectivity: Gender Makes a Difference. The Neuroscientist. 2011 doi: 10.1177/1073858410386492. http://doi.org/10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Graves WW, Binder JR, Desai RH, Humphries C, Stengel BC, Seidenberg MS. Anatomy is strategy: Skilled reading differences associated with structural connectivity differences in the reading network. Brain and language. 2014;133:1–13. doi: 10.1016/j.bandl.2014.03.005. http://doi.org/10.1016/j.bandl.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y. White matter structural connectivity underlying semantic processing: Evidence from brain damaged patients. Brain. 2013;136(10):2952–2965. doi: 10.1093/brain/awt205. http://doi.org/10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Harvey DY, Schnur TT. Distinct loci of lexical and semantic access deficits in aphasia: Evidence from voxel-based lesion-symptom mapping and diffusion tensor imaging. Cortex. 2015;67:37–58. doi: 10.1016/j.cortex.2015.03.004. http://doi.org/10.1016/j.cortex.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Harvey DY, Wei T, Ellmore TM, Hamilton aC, Schnur TT. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51(5):789–801. doi: 10.1016/j.neuropsychologia.2013.01.028. http://doi.org/10.1016/j.neuropsychologia.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. 115 doi: 10.1093/brain/115.6.1783. (Pt 6 Brain : a journal of neurology 1783–1806 (1992). http://doi.org/10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. NeuroImage White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage. 2005;46(3):600–607. doi: 10.1016/j.neuroimage.2009.02.025. http://doi.org/10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott Ma, Ruparel K, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. http://doi.org/10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph Ma, Cipolotti L, Manes F, Patterson K. Taking both sides: Do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133(11):3243–3255. doi: 10.1093/brain/awq264. http://doi.org/10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- Landauer TK, Foltz PW, Laham D. An introduction to latent semantic analysis. Discourse Processes. 1998 http://doi.org/10.1080/01638539809545028. [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. http://doi.org/10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clinical Neurology and Neurosurgery. 2009;111(7):601–605. doi: 10.1016/j.clineuro.2009.03.007. http://doi.org/10.1016/j.clineuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(3):623–629. doi: 10.1093/brain/awl361. http://doi.org/10.1093/brain/aw1361. [DOI] [PubMed] [Google Scholar]
- Martin A. The Representation of Object Concepts in the. Annual Review of Psychology. 2007 doi: 10.1146/annurev.psych.57.102904.190143. http://doi.org/10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mcdonald CR, Ahmadi ME, Tecoma ES, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy, 1869–1876. 2008 doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Inoue K, Rudrauf D, Damasio H, Tranel D, Grabowski T. Segregation of anterior temporal regions critical for retrieving names of unique and non-unique entities reflects underlying long-range connectivity. Cortex. 2016;75:1–19. doi: 10.1016/j.cortex.2015.10.020. http://doi.org/10.1016/j.cortex.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. http://doi.org/10.1002/1531-8249(199902)45:2 <265::AID-ANA21 > 3.0.C 0;2-3. [DOI] [PubMed] [Google Scholar]
- Mundfrom DJ, Perrett JJ, Schaffer J, Piccone A, Roozeboom M. Bonferroni Adjustments in Tests for Regression Coefficients. Multiple Linear Regression Viewpoints. 2006;32(1):1–6. [Google Scholar]
- Novick JM, Kan IP, Trueswell JC, Thompson-Schill SL. A case for conflict across multiple domains: memory and language impairments following damage to ventrolateral prefrontal cortex. Cognitive neuropsychology. 2009;26 doi: 10.1080/02643290903519367. http://doi.org/10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Broca’ s Area and Language Processing: Evidence for the Cognitive Control Connection. Language and Linguistics Compass. 2010;4:906–924. http://doi.org/10.1111/j.1749-818X.2010.00244.x. [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Developmental Cognitive Neuroscience. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L. Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: A diffusion tensor imaging study. Developmental Medicine and Child Neurology. 2012;54(1):38–43. doi: 10.1111/j.1469-8749.2011.04147.x. http://doi.org/10.1111/j.1469-8749.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Current Neurology and Neuroscience Reports. 2011 doi: 10.1007/s11910-011-0219-6. http://doi.org/10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Papagno C, Casarotti A, Comi A, Pisoni A, Lucchelli F, Bizzi A, Bello L. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Structure and Function. 2014a doi: 10.1007/s00429-014-0920-8. http://doi.org/10.1007/s00429-014-0920-8. [DOI] [PubMed] [Google Scholar]
- Papagno C, Casarotti A, Comi A, Pisoni A, Lucchelli F, Bizzi A, Bello L. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Structure and Function. 2014b doi: 10.1007/s00429-014-0920-8. http://doi.org/10.1007/s00429-014-0920-8. [DOI] [PubMed] [Google Scholar]
- Papoutsi M, Stamatakis Ea, Griffiths J, Marslen-Wilson WD, Tyler LK. Is left fronto-temporal connectivity essential for syntax? Effective connectivity, tractography and performance in left-hemisphere damaged patients. NeuroImage. 2011;58(2):656–664. doi: 10.1016/j.neuroimage.2011.06.036. http://doi.org/10.1016/j.neuroimage.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews. Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. http://doi.org/10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MaL. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. http://doi.org/10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. Wide-Range Achievement Test. The Corsini Encyclopedia of Psychology. 2009 [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price CJ. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cognitive, Affective & Behavioral Neuroscience. 2006;6(3):201–213. doi: 10.3758/cabn.6.3.201. http://doi.org/10.3758/C A B N.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rolheiser T, Stamatakis Ea, Tyler LK. Dynamic Processing in the Human Language System: Synergy between the Arcuate Fascicle and Extreme Capsule. Journal of Neuroscience. 2011;31(47):16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. http://doi.org/10.1523/J N E U R O S C I.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York Oxford University Press. 2006;1 http://doi.org/10.1093/acprof:oso/9780195104233.001.0001. [Google Scholar]
- Scholz J, Tomassini V, Johansen-berg H, Reading B. Individual Differences in White Matter Microstructure in the Healthy Brain. Diffusion MRI. Elsevier Inc. 2009 http://doi.org/10.1016/B978-0-12-374709-9.00011-0. [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y, Yagi K. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase: Case Studies in Neuropsychology, Neuropsychiatry, and Behavioural Neurology. 2007;13(2):127–130. doi: 10.1080/13554790701399254. http://doi.org/10.1080/13554790701399254. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Yoshida M, Yamada R, Tabei Y, Yagi K. Deficits in the left inferior longitudinal fasciculus results in impairments in object naming. Neurocase: Case Studies in Neuropsychology, Neuropsychiatry, and Behavioural Neurology. 2010;16(1):135–139. doi: 10.1080/13554790903329174. http://doi.org/10.1080/13554790903329174. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. Journal of the International Neuropsychological Society : JINS. 2009;15(5):645–649. doi: 10.1017/S1355617709990348. http://doi.org/10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23 doi: 10.1016/j.neuroimage.2004.07.051. http://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Banich MT, Munakata Y. Choosing Our Words: Retrieval and Selection Processes Recruit Shared Neural Substrates in Left Ventrolateral Prefrontal Cortex. Journal of Cognitive Neuroscience. 2011 doi: 10.1162/jocn_a_00023. http://doi.org/10.1162/jocn_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hutchison N, Nyhus E, Curran T, Banich MT, O’Reilly RC, Munakata Y. Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16483–16488. doi: 10.1073/pnas.1002291107. http://doi.org/10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. So many options, so little time: the roles of association and competition in underdetermined responding. Psychonomic Bulletin & Review. 2008;15(6):1083–1088. doi: 10.3758/PBR.15.6.1083. http://doi.org/10.3758/PBR.15.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. NeuroImage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. http://doi.org/10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Teichmann M, Rosso C, Martini J-B, Bloch I, Brugières P, Duffau H, Bachoud-Lévi A-C. A cortical-subcortical syntax pathway linking Broca’s area and the striatum. Human Brain Mapping. 2015 Feb;2283 doi: 10.1002/hbm.22769. n/a-n/a. http://doi.org/10.1002/hbm.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex. 2011;47(7):863–873. doi: 10.1016/j.cortex.2010.07.006. http://doi.org/10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where.”. Neuropsychologia. 2003;41(3):280–292. doi: 10.1016/s0028-3932(02)00161-6. http://doi.org/10.1016/S0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. http://doi.org/10.1073/pnas.94.26.l4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. http://doi.org/10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011 Feb;5:1. doi: 10.3389/fnsys.2011.00001. http://doi.org/10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger A, Alm KH, Collins JA, Leary JMO, Olson IR. Variation in White Matter Connectivity Predicts the Ability to Remember Faces and Discriminate Their Emotions. Journal of the International Neuropsychological Society :JINS. 2016;(22):180–190. doi: 10.1017/S1355617715001009. http://doi.org/10.1017/S13556l7715001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013 doi: 10.1093/brain/awt094. http://doi.org/10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen aG, Wedeen VJ. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc. Intl. Soc. Mag. Reson. Med. 2007;15:3720. [Google Scholar]
- Whitney C, Kirk M, 0’ Sullivan J, Lambon Ralph Ma, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. http://doi.org/10.1093/cercor/bhql80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT4) Psychological Assessment Resources, Lutz. 2006 [Google Scholar]
- Wolfe MBW. Use of latent semantic analysis for predicting psychological phenomena: Two issues and proposed solutions. 2003;35(1):22–31. doi: 10.3758/bf03195494. [DOI] [PubMed] [Google Scholar]
- Wong FCK, Chandrasekaran B, Garibaldi K, Wong PCM. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(24):8780–8785. doi: 10.1523/JNEUROSCI.0999-11.2011. http://doi.org/10.1523/JNEUROSCI.0999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock ARW, Johnson MB. Woodcock Johnson TEST OF ACHIEVEMENT. 2011 [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell Ba. PNAS Plus: Development of white matter and reading skills. Proceedings of the National Academy of Sciences. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. http://doi.org/10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmoura I, Herbet G, Moritz-gasser S, Duffau H. New Insights into the Neural Network Mediating Reading Processes Provided by Cortico-Subcortical Electrical Mapping. 2015 Aug;00 doi: 10.1002/hbm.22766. 2014. http://doi.org/10.1002/hbm.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.