Abstract
Rationale
Diastolic dysfunction is a common feature in many heart failure patients with preserved ejection fraction and has been associated with altered myocardial metabolism in hypertensive and diabetic patients. Therefore, metabolic interventions to improve diastolic function are warranted. In mice with a germline cardiac-specific deletion of acetyl CoA carboxylase 2 (ACC2), systolic dysfunction induced by pressure-overload was prevented by maintaining cardiac fatty acid oxidation (FAO). However, it has not been evaluated whether this strategy would prevent the development of diastolic dysfunction in the adult heart.
Objective
To test the hypothesis that augmenting cardiac FAO is protective against angiotensin II (AngII)-induced diastolic dysfunction in an adult mouse heart.
Methods and Results
We generated a mouse model to induce cardiac-specific deletion of ACC2 in adult mice. Tamoxifen treatment (20mg/kg/day for 5 days) was sufficient to delete ACC2 protein and increase cardiac FAO by 50% in ACC2 flox/flox-MerCreMer+ mice (iKO). After 4 weeks of AngII (1.1mg/kg/day), delivered by osmotic mini-pumps, iKO mice showed normalized E/E′ and E′/A′ ratios compared to AngII treated controls (CON). The prevention of diastolic dysfunction in iKO-AngII was accompanied by maintained FAO and reduced glycolysis and anaplerosis. Furthermore, iKO-AngII hearts had a ~50% attenuation of cardiac hypertrophy and fibrosis compared to CON. In addition, maintenance of FAO in iKO hearts suppressed AngII-associated increases in oxidative stress and sustained mitochondrial respiratory complex activities.
Conclusion
These data demonstrate that impaired FAO is a contributor to the development of diastolic dysfunction induced by AngII. Maintenance of FAO in this model leads to an attenuation of hypertrophy, reduces fibrosis, suppresses increases in oxidative stress, and maintains mitochondrial function. Therefore, targeting mitochondrial FAO is a promising therapeutic strategy for the treatment of diastolic dysfunction.
Keywords: cardiac metabolism, lipids, ACC2, myocardium, hypertrophy
Subject Codes: [107] Biochemistry and metabolism, [140] energy metabolism, [145] genetically altered mice, [90] Lipid and lipoprotein metabolism, [105] Contractile function
1. Introduction
Heart failure (HF) is a clinical syndrome that affects approximately 6 million Americans with a striking additional ~500,000 new diagnoses each year [1]. Although HF has been traditionally synonymous with reduced ejection fraction (HFrEF), approximately 50% of all HF patients have preserved ejection fraction (HFpEF) [2]. Although the risk factors (i.e. aging, hypertension, diabetes) and clinical symptoms overlap for HFrEF and HFpEF, the pathophysiological manifestations of the diseases can be quite distinct [3]. As a result, effective pharmacological interventions for patients with HFpEF remain elusive [4]. Despite a lack of consensus on whether diastolic abnormalities are a signature of HFpEF, nearly two-thirds of these patients present with diastolic dysfunction [5,6]. In this regard, further investigations into the pathology of diastolic dysfunction may identify underlying contributing factors and reveal new potential treatment strategies.
The development of diastolic dysfunction is a common feature in the aging population and is associated with an increased risk of HF, including HFpEF [7,8]. We recently demonstrated that acute treatment of rapamycin in aged mice increased fatty acid oxidation (FAO) and reversed diastolic dysfunction [9]. This finding led to the intriguing hypothesis that impaired FAO was an inciting factor in the declination of diastolic function and that restoring FAO in the diseased heart could correct the functional abnormality. Previously, we demonstrated that cardiac-specific deletion of ACC2 (ACC2H−/−) in mice subjected to pressure-overload via transverse aortic constriction, had sustained systolic function and myocardial energetics, as a result of preserved cardiac FAO [10]. These findings indicated that targeting FAO, specifically at the level of fatty acid entry into the mitochondria, was a potential metabolic therapy in which to prevent systolic dysfunction in the pathologically hypertrophied heart. However, it has not been determined whether targeting cardiac FAO, via ACC2 deletion, in an adult heart would be protective against the development of diastolic dysfunction.
To establish the therapeutic benefit of enhancing myocardial FAO in an adult heart with diastolic dysfunction, we generated a mouse model employing an inducible deletion strategy to target ACC2 in mice subjected to chronic angiotensin II (AngII) infusion. We selected the chronic AngII model based on its known systemic effects of hypertension as well as the predominant phenotype of diastolic dysfunction [11–14]. Our results show that deletion of ACC2 in an adult mouse is a safe and effective means of preserving myocardial FAO and preventing diastolic dysfunction that results from chronic exposure to AngII. Furthermore, sustained FAO during chronic AngII stimulation leads to an attenuation of pathological hypertrophy and fibrosis and is accompanied by suppressed elevations of oxidative stress and maintained mitochondrial function. These collective findings indicate that impaired myocardial FAO is a contributor to the development of diastolic dysfunction and that targeting of mitochondrial FAO is a viable therapy to restore diastolic function, and potentially, delay the transition to failure.
2. Materials and Methods
2.1. Animal Model
Studies were approved by the University of Washington Institutional Animal Care and Use Committee. Mice were kept on a 12-hour light/dark cycle with water and food ad libitum. ACC2 flox/flox-MerCreMer+ (ACC2−f/f−MCM+) mice were mated with ACC2f/f to produce both study and control littermates (see supplemental methods for additional details). At 12–14 weeks of age, both ACC2f/f−MCM+ and ACC2f/f (CON) mice received an intraperitoneal injection of tamoxifen (20mg/kg) for 5 days, which was sufficient to cause ACC2 deletion in ACC2f/f−MCM+ (iKO). Four weeks after the last injection of tamoxifen, male CON and iKO mice were implanted with an osmotic mini-pump (Alzet, Durect Corporation, #2004) containing angiotensin II (AngII; 1.1mg/kg/day) or saline (vehicle) for 4 weeks, which resulted in similar increases in both systolic and diastolic blood pressures in both groups (Figure S1).
2.2. Transthoracic Echocardiography
Murine trans-thoracic echocardiography was conducted in mice using a Vevo 770 High-Resolution Imaging System (VisualSonics, Toronto, Ontario, Canada) machine and a 30 mHz probe [15]. Isoflurane (1%) was administered during the procedure. Serial echocardiography was performed on mice at baseline (before tamoxifen) and at 4 and 12 weeks post tamoxifen injections. At the end of the 4 week AngII treatment, echocardiography was performed and diastolic function was assessed via Tissue Doppler Imaging (TDI).
2.3. Isolated Heart Perfusion and Nuclear Magnetic Resonance (NMR) Spectroscopy
Langendorff, isolated mouse heart experiments were conducted as previously described [10,16]. Hearts were perfused with 13C-labeled substrates (1,6-13C glucose and U-13C fatty acids) to determine substrate utilization. 13C NMR spectroscopy was performed on lyophilized heart extracts [10,17]. Substrate utilization, glycolysis, and anaplerosis were calculated by isotopomer analysis. (See Online Data Supplement for expanded methods.)
2.4. Electron Microscopy
Myocardial tissue sections were prepared and imaged on an electron microscope (JEOL USA 1230, Peabody, MA). Mitochondrial area was measured in 5 random fields from each heart by imaging software (Image J, NIH). (See Online Supplement for expanded methods.)
2.5. Organ Weight and Histological Assessment
Body weight, tibia length (TL), and heart weight (HW) were measured in mice at 4 weeks post-mini-pump implantation. Hearts were harvested, rinsed briefly in 1X PBS, blotted dry, and weighed. HW was normalized to TL to assess changes in hypertrophy. For histology, hearts were arrested in diastole by KCl (30 mmol/L) and perfusion fixed with 10% neutral buffered formalin. Longitudinal sections of hearts were stained with Masson’s Trichrome. Percentage of fibrosis was determined using an imaging processing software (Image J, NIH).
2.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from frozen LV tissue using the RNeasy Kit (Qiagen, Valencia, CA). Omniscript reverse synthase and random hexamers were used for cDNA synthesis according to manufacturer’s guidelines. Real Time PCR was performed using SYBR green (Bio-Rad, Hercules, CA). Results of mRNA levels were normalized to 18S rRNA levels and reported as fold-change over control. Primer sequences appear in Supplemental Table 1.
2.7. Biochemical Assays and Immunoblotting
Protein carbonyls were measured in heart lysates using the Protein Carbonyl ELISA kit (Cell BioLabs, Inc, San Diego, CA). Hydrogen peroxide was measured in frozen heart lysates using the Amplex Red Assay kit (Invitrogen, Grand Island, NY). Aconitase activity was measured in frozen heart lysates using a commercially available kit (Cayman Chemical, Ann Arbor, MI). Western blotting was performed on heart lysates. Protein extracts were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes and probed with the following antibodies: anti-ACC (Cell Signaling Technology, Danvers, MA), anti-sarcomeric actin (Sigma Aldrich, St. Louis, MO).
2.8. Respiratory Complex Activity
Respiratory complex activity (Complex I, II, III, IV and citrate synthase) were assessed in lysates from frozen cardiac tissue according to previously published methods using a spectrophotometer (Evolution 220, Thermo Scientific, Waltham, MA) [18]. All assays were performed at 37°C. Activity was calculated by the difference in the change of absorbance in the presence and absence of specific inhibitors and normalized to protein concentration.
2.9. Statistical Analysis
All data are presented as means ± standard error (SEM). Statistical analysis was tested with a one-way analysis of variance (ANOVA) or two-way ANOVA with a Bonferroni’s post hoc. Two group comparisons were made using Student’s t-test. All analyses were performed using GraphPad Prism 6.0. Statistical significance was considered at P<0.05 level.
3. Results
3.1. Inducible deletion of ACC2 increases cardiac fatty acid oxidation in adult mouse hearts and does not affect cardiac function or mitochondrial morphometry
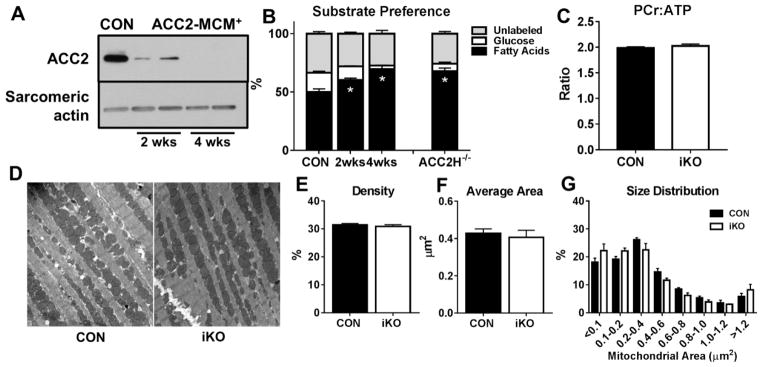
Two weeks after the completion of tamoxifen injections, marked reduction of ACC2 protein was observed and was sufficient to cause a significant increase in the contribution of fatty acids to oxidative metabolism (Figures 1A,1B). After 4 weeks, complete deletion of ACC2 caused a further increase of FAO that was similar to hearts with a cardiac-specific deletion of ACC2 at birth (ACC2H−/−; Figures 1A,1B). The increased dependence on fatty acid over carbohydrates (glucose and lactate) did not alter myocardial energetics (Figure 1C) or alter fractional shortening, LV posterior wall thickness, or LV internal dimensions in iKO mice 6 days (Table S2) or 4 weeks post tamoxifen injections (Figures S2A–S2E), indicating that tamoxifen, Cre-recombinase activation, or acute increases of FAO in adult myocardium does not alter cardiac function (Figures S3A, S3B). Electron microscopic analysis revealed similar mitochondrial density, average mitochondrial area, and size distribution pattern in iKO hearts (Figures 1D–1G) while RT-PCR analysis showed similar expression of genes involved in mitochondrial biogenesis and FAO between CON and iKO hearts (Figure S4). These results demonstrate that deletion of ACC2 in an adult mouse can significantly elevate myocardial FAO while cardiac function and mitochondrial morphometry are well-maintained.
Figure 1. Inducible cardiac-specific deletion of ACC2 increases FAO in adult mouse hearts and does not affect mitochondrial morphometry.
A) Representative Western blots of ACC2 in heart lysates from CON and iKO, 2 and 4 weeks post tamoxifen injections. B) Contribution of 13C labeled substrates to tricarboxylic acid (TCA) cycle determined by 13C NMR spectroscopy in extracts from isolated perfused hearts. Mice with cardiac-specific deletion of ACC2 (ACC2H−/−) are shown for comparison (n= 8–11 per group). C) Phosphocreatine to ATP (PCr:ATP) ratio in isolated hearts perfused with mixed substrate buffer assessed by 31P NMR spectroscopy (n=3 per group). D) Representative images from electron microscopy (10000X). E) Mitochondrial density determined by the percentage of mitochondrial area per field area (n= 3 hearts, 5 fields each). F) Average mitochondrial area of all mitochondria measured (n= 3 hearts, 5 fields each). G) Percentage of mitochondria distributed by area (n= 3 hearts, 5 fields each). * P < 0.05 vs. CON.
3.2. Angiotensin II-induced diastolic dysfunction is prevented in ACC2-iKO mice
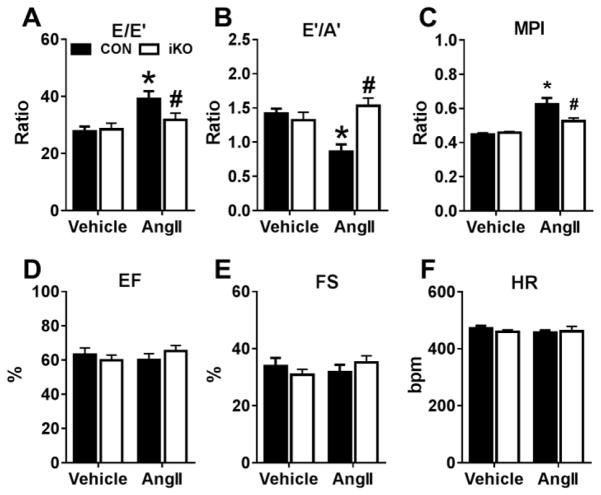
In mice treated with AngII (1.1 mg/kg/day) for 4 weeks, tissue Doppler imaging showed a significant increase in the E/E′ ratio and a significant decrease in the E′/A′ ratio in CON hearts (Figures 2A, 2B), suggestive of diastolic dysfunction. Conversely, no significant changes in these measures of diastolic dysfunction were observed in iKO hearts after AngII treatment (Figures 2A, 2B). Furthermore, myocardial performance index (MPI) was significantly elevated in CON hearts (Figure 2C), primarily due to prolonged isovolumic relaxation time (IVRT), indicating impaired ventricular relaxation (Supplemental Table S3). Of note, the change in MPI and IVRT was absent in iKO hearts. AngII treatment for 4 weeks did not negatively affect systolic function as ejection fraction, fractional shortening, and heart rate in CON and iKO mice were similar to both vehicle groups (Figures 2D–2F). These findings reveal that deletion of ACC2 in an adult mouse protects against the development of diastolic dysfunction in the AngII-treated heart.
Figure 2. Angiotensin II-induced diastolic dysfunction is prevented in ACC2-iKO mice.
A,B) Measures of diastolic function (E/E′ or E′/A′ ratios) measured by Tissue Doppler Imaging. C) Myocardial performance index (MPI) calculated from systolic and diastolic time intervals measured via pulse wave Doppler. D) Ejection fraction (EF), E) Fractional shortening (FS) and F) heart rate (HR) as determined by echocardiography 4 weeks after saline (vehicle) or angiotensin II (AngII) infusion. * P <0.05 vs. vehicle; # P <0.05 vs. AngII, n=6–8 per group.
3.3. Myocardial fatty acid oxidation is preserved in iKO hearts after chronic infusion of angiotensin II
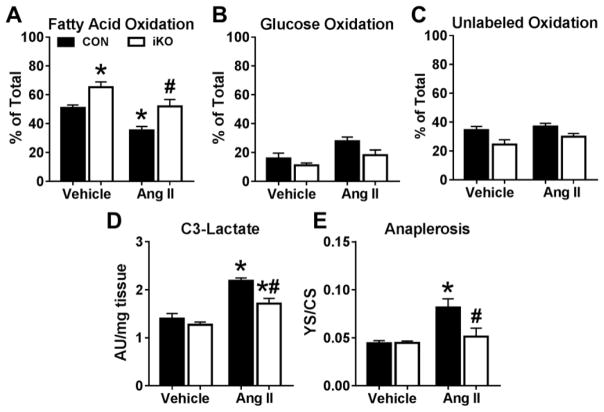
In isolated hearts perfused with 13C labeled substrates, the decreased contribution of fatty acids to oxidative metabolism observed in CON-AngII was normalized in iKO-AngII hearts (Figure 3A). Oxidation of both glucose and unlabeled substrates (lactate and endogenous) was reduced by ~33% (P=0.089) and 20% (P=0.068), respectively, in iKO-AngII versus CON-AngII (Figures 3B, 3C). The enrichment of lactate, a surrogate marker of glycolysis, was significantly attenuated in iKO-AngII hearts (Figure 3D). Isotopomer analysis of tissue extracts obtained from 13C NMR spectroscopy showed significantly reduced anaplerosis in iKO-AngII hearts compared to CON-AngII (Figure 3E). In summary, these data indicate that deletion of ACC2 in an adult heart maintains the dependence on fatty acids for oxidative metabolism, while decreasing the reliance on glycolysis and anaplerosis, consistent with prevention of the fetal metabolic profile in AngII-induced diastolic dysfunction.
Figure 3. Myocardial fatty acid oxidation is preserved in ACC2-iKO hearts after chronic infusion of angiotensin II.
A) Fatty acid oxidation measured in perfused heart tissue extracts by 13C NMR spectroscopy (n=4–6 per group). B) Glucose oxidation, C) Oxidation of unlabeled substrates (lactate and endogenous glycogen and triglycerides), and D) enrichment of 3rd carbon of lactate, normalized to tissue weight, measured in perfused heart tissue extracts by 13C NMR spectroscopy (n=3–5 per group). D) Assessment of anaplerosis (YS) per citrate synthase (CS) flux determined by isotopomer analysis in extracts from hearts perfused with 13C labeled substrates (n=3–5 per group). * P < 0.05 vs. vehicle; # P <0.05 vs. AngII.
3.4. Maintaining fatty acid oxidation attenuates hypertrophy and fibrosis in angiotensin II treated hearts
CON hearts subjected to AngII developed cardiac hypertrophy, assessed by the heart weight to tibia length ratio (HW:TL), which was significantly attenuated in iKO hearts after chronic AngII infusion (Figure 4A). This observation was consistent with reduced concentric hypertrophy as the increase in myocyte width measured in CON was abrogated in iKO cardiomyocytes (Figures 4B–4C). Transcriptional upregulation of the pathological hypertrophy genes, bnp and anp, was also significantly lower in iKO-AngII hearts as compared to CON-AngII (Figures 4D,4E). Analysis of histological sections showed a reduction of fibrosis in iKO-AngII hearts (Figures 4F,4G) that was supported by a reduction in the expression of genes associated with fibrosis (Figures 4H–4J). In total, these data provide further evidence that maintenance of cardiac FAO protects against the pathological remodeling process (i.e., hypertrophy and fibrosis) initiated by chronic administration of AngII.
Figure 4. Maintaining myocardial fatty acid oxidation attenuates cardiac hypertrophy and fibrosis in angiotensin II treated hearts.
A) Heart weight (HW) to tibia length (TL) ratios in hearts harvested at the end of the 4 weeks of angiotensin II (AngII) treatment (n=7–10 per group). B) Width and C) Length in isolated cardiomyocytes from mice after 4 weeks of AngII infusion (n=3–4 per group). mRNA levels of the pathological hypertrophy markers, D) brain natriuretic peptide (bnp) and E) atrial natriuretic peptide (anp) determined by RT-PCR (n= 5 per group). F) Representative images from histological sections stained with Masson’s Trichrome. G) Percent fibrosis determined in histological sections (n=3–4 hearts per group). Expression of fibrosis related genes H) collagen type I, alpha 2 (col1a2), I) connective tissue growth factor (ctgf), and J) transforming growth factor, beta 1 (tgfb1) assessed by RT-PCR (n=5 per group). * P <0.05 vs. vehicle; # P <0.05 vs. AngII.
3.5. Sustained fatty acid oxidation in iKO hearts resists against increased oxidative stress due to chronic angiotensin II
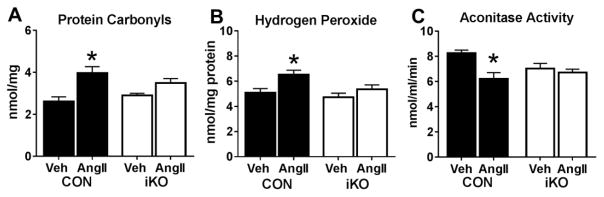
Since increased ROS has been proposed as an underlying mechanism for AngII-induced cardiac damage, [14,19] we evaluated whether changes in oxidative stress were present in AngII treated hearts. As shown in Figure 5A, the elevation of protein carbonyls, an oxidative modification to proteins caused by ROS [20], was mitigated in iKO-AngII hearts compared to CON-AngII. In addition, hydrogen peroxide measured in heart tissue lysates was significantly increased in CON-AngII but remained similar to vehicle treated hearts in iKO-AngII (Figure 5B). Moreover, a significant decrease in aconitase activity, commonly used as a marker of oxidative damage [21,22], was observed in CON-AngII but was unaltered in iKO-AngII (Figure 5C). All told, these data support the concept that maintaining FAO during AngII stress protects against increases of oxidative stress in the myocardium.
Figure 5. Sustained fatty acid oxidation resists against increased oxidative stress.
A) Protein oxidation, an indicator of reactive oxygen species (ROS), assessed by measuring protein carbonyls in tissue lysates from CON and iKO mice after AngII treatment (n=6–7 per group). B) Hydrogen peroxide in tissue lysates measured by Amplex Red assay (n=5 per group). C) Aconitase activity, used as a marker of oxidative damage, measured in tissue lysates using a commercially available kit (n=5 each group). * P <0.05 vs. CON.
3.6. Mitochondrial biogenesis and function are preserved in iKO hearts after AngII infusion
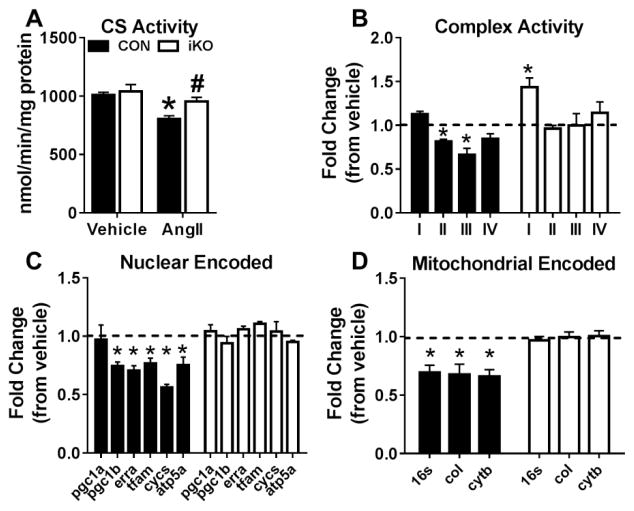
To evaluate whether sustaining cardiac FAO maintained mitochondrial function, we measured citrate synthase (CS) and respiratory complex activities in CON and iKO at the end of the AngII treatment period. Increasing myocardial FAO had no effect on CS activity in the iKO vehicle group (Figure 6A). However, AngII significantly reduced CS activity in CON but not iKO hearts after 4 weeks of AngII treatment (Figure 6A). Moreover, despite significant decreases in Complex II and Complex III activities in CON-AngII; all complex activities in iKO-hearts were comparable to the vehicle group (Figure 6B). Interestingly, Complex I activity increased significantly in iKO hearts but remained unaffected in CON hearts after AngII infusion (Figure 6B). We further interrogated the effects of AngII on the expression of genes related to mitochondrial biogenesis and electron transport chain (ETC) function. Transcription of the nuclear encoded genes related to mitochondrial biogenesis (pgc1b, erra, and tfam) was well-preserved in iKO hearts despite significant decreases in CON hearts receiving AngII (Figure 6C). In addition, genes related to ETC function (cycs, atp5a, coI, and cytb) remained normal in iKO-AngII while all were depressed in CON-AngII hearts (Figure 6D). In total, these data show AngII negatively impacts electron chain transport capacity, as previously reported [23], and that maintaining mitochondrial FAO capacity prevents this deficiency.
Figure 6. Mitochondrial biogenesis and function are preserved in iKO hearts after AngII infusion.
A) Citrate synthase (CS) activity, a mitochondrial matrix enzyme, measured in frozen lysates from CON and iKO mice 4-weeks after saline or AngII mini-pump implantation. B) Respiratory complex activities (Complex I – Complex IV). C) Expression of nuclear encoded genes related to mitochondrial biogenesis and electron transport chain function: pgc1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; pgc1b, peroxisome proliferator-activated receptor gamma coactivator 1-beta; erra, estrogen related receptor alpha; tfam, transcription factor A, mitochondrial; cycs, cytochrome c, somatic; atp5a, ATP synthase, subunit alpha D) Expression of mitochondrial encoded genes: 16s, ribosomal RNA; coI, cytochrome c oxidase, subunit 1; cytb, cytochrome b; For Panels B-D, data is presented as fold change of the AngII group from respective vehicle (dotted line). * P <0.05 vs. CON; # P <0.05 vs. AngII. n=4–6 per group.
4. Discussion
Decreased dependence on fatty acids for energy production is a signature of the metabolic derangements that occur in pathological hypertrophy [10,24–26]. Studies in rodents and humans suggest that decreased myocardial FAO is likely a key pathological mechanism that contributes to the transition to failure [27–30]. Therefore, therapeutic strategies that prevent or promote FAO in the hypertrophied and/or failing heart are highly desirable. In the present study, we generated a mouse model to induce ACC2 deletion in an adult heart, which would more closely mimic pharmacological intervention. In this model, sustained myocardial FAO in the face of chronic AngII infusion normalizes diastolic function, which is accompanied by an attenuation of pathological remodeling (i.e. hypertrophy and fibrosis). In addition, AngII induced elevations in oxidative stress as well as altered mitochondrial function is ameliorated. Since evidence of diastolic dysfunction exists in approximately one-quarter of an apparently healthy older population [8,31] and diastolic dysfunction is observed in approximately half of all heart failure cases [2], the therapeutic potential of enhancing cardiac FAO may be extended to a broader class of cardiac disease etiologies.
A novel finding of the present study is that maintaining FAO during chronic AngII stress protects against the development of diastolic dysfunction. Concordantly, we recently observed that improved diastolic function in aged mice treated with rapamycin was matched by restored FAO and myocardial energetics [32]. Since ATP is critical for the process of cardiomyocyte relaxation, impaired myocardial energetics as a result of decreased FAO is likely a significant contributor to diastolic dysfunction in the hypertrophied heart [33,34]. Our previous work showed that sustaining FAO during transverse aortic constriction protected against the depletion of energy reserves in hearts with a cardiac-specific deletion of ACC2 [10]. The combined observations of maintained in-vivo function, mitochondrial respiratory complex activities and gene transcriptional analysis in iKO hearts treated with AngII strongly suggests a favorable energetic capacity with maintained FAO in the stressed myocardium. Moreover, these changes were achieved by targeting ACC2 in an adult mouse heart, thus highlighting the metabolic therapeutic potential.
Chronic activation of the renin-angiotensin system, particularly through the action of angiotensin II (AngII), has been noted as a major contributor to pathological myocardial remodeling and the progression to heart failure [35]. AngII is a potent vasoconstrictor that causes systemic increases in blood pressure, and as a result, increases cardiac afterload. In addition, AngII has direct intracellular effects via binding to the AngII receptor, a G-protein coupled receptor, which activates a signaling cascade that results in pathological hypertrophy, fibrosis, and reactive oxygen species (ROS) [13,14,36–38], all contributing to the development of diastolic dysfunction. In this study we observed an attenuation of pathological remodeling (i.e., cardiac hypertrophy and fibrosis) as well as resistance against increased oxidative stress in iKO hearts, all supporting maintained diastolic function despite chronic AngII stimulation.
Despite a similar chronic elevation of blood pressure with AngII in both genotypes, cardiac hypertrophy in iKO hearts was attenuated. This is consistent with our prior observation that maintenance of cardiac FAO, via ACC2 deletion at birth, led to reduced pathological hypertrophy in a model of pressure overload induced by transverse aortic constriction [10]. A reduction in the pathological hypertrophy and fibrosis in iKO hearts likely contributes to the protection of diastolic dysfunction caused by chronic AngII treatment. Although we previously hypothesized that improvement of myocardial energetics by sustaining FAO could decrease pathological remodeling [10], it is also possible that normalization of FAO in the hypertrophied heart could lead to the reduction of toxic lipid intermediates as suggested in models of pathological hypertrophy induced by lipotoxicity [39–41].
As myocardial bioenergetics is dependent upon mitochondria, we directly tested respiratory complexes activities in AngII treated hearts to further evaluate the relationship between sustained cardiac FAO and mitochondrial function. We find that activity of Complex III, as well as Complex II, is significantly decreased after AngII treatment. The impaired Complex III activity is in accordance with observations made in human heart failure tissues [42,43]. Combined with the decrease in citrate synthase activity, this is evidence of impaired mitochondrial function in the AngII-induced hypertrophied heart. Remarkably, respiratory complex and citrate synthase activities are well-maintained in the iKO hearts, suggesting that mitochondrial respiratory function can be maintained by preventing the downregulation of FAO.
Another important finding from the present study addresses the common notion that increasing mitochondrial FAO leads to increased ROS production. However, this supposition arises from early studies in animal models of obesity and/or diabetes [44,45], where it is difficult to account for the individual effects of elevated FAO from the discordant ratio of fatty acid uptake and oxidation. Overall, there is limited direct evidence in the literature that increased FAO directly increases ROS [46]. Observations of increased ROS in isolated mitochondria from diabetic mouse hearts were made with succinate and/or rotenone and not in the presence of acylcarnitines [44]. In fact, it has been reported that ROS production from palmitoylcarnitine/malate is several folds lower than succinate in mitochondria isolated from healthy rats [47]. In this regard, the increased ROS production in obese or diabetic mitochondria is likely a hallmark of dysfunctional electron transport capacity and not a predilection for FAO to increase mitochondrial oxidative stress. In the present study, elevations in cardiac FAO do not result in increased oxidative stress in unstressed hearts, suggesting that cardiac mitochondria are well-tolerant of high FAO capacity. Based on the data obtained from hearts during chronic AngII administration, we propose that maintaining normal myocardial metabolism (i.e., dependence on fatty acids with less reliance on glucose and glycolysis) sustains mitochondrial function and protects against increased oxidative stress in the hypertrophied heart.
Although our data implicate sustaining myocardial FAO as a critical intervention in the hypertrophied heart, the notable reduction in glycolysis observed in targeting ACC2 during cardiac stress is also worth consideration. Studies in small and large animal models as well as in diseased humans have noted beneficial outcomes by enhancing glucose oxidation via inhibition of FAO [48–50]. Partial inhibition of FAO improved the coupling of glycolysis and glucose oxidation and reduced the reliance of the hypertrophied or failing heart on glycolysis. In our models of ACC2 deletion, the coupling of glycolysis and glucose oxidation is also improved, albeit as the result of increased FAO, so the degree of uncoupling between glycolysis and oxidative phosphorylation may be a primary factor in determining the effectiveness of metabolic therapies. Regardless of the approach, promotion of oxidative metabolism in the dysfunctional myocardium is likely an agreeable goal for treatment.
In summary, we demonstrate that decreased cardiac FAO contributes to the development of diastolic dysfunction and that targeting ACC2 in the adult mouse heart is effective in sustaining mitochondrial FAO and maintaining diastolic function during chronic AngII stimulation. In addition, sustaining myocardial FAO in the stressed myocardium protects against pathological remodeling, preserves mitochondrial function, and prevents increases in oxidative stress. Overall, the findings from the current study add to the emerging concept that an impairment of cardiac FAO is a maladaptive response that can be rectified by directly targeting mitochondrial FAO in the hypertrophied heart.
Supplementary Material
Highlights.
Deletion of ACC2 in adult mouse heart increases fatty acid oxidation
Maintaining cardiac fatty acid oxidation during Angiotensin II infusion prevents diastolic dysfunction
Sustained fatty acid oxidation protects mitochondria and reduces myocardial oxidative stress
Mitochondrial fatty acid oxidation is a viable therapy for pathological hypertrophy
Acknowledgments
This study was supported by grants from the American Heart Association (14SDG18590020 to S.C.K), NIH Heart, Lung, and Blood Institute (HL122199, HL110349, HL118989, and HL129510 to R.T.), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES 7231-12-1 to A.B.M.M), NIH Biomedical Research Support Shared Instrumentation Grant (S10RR029021 to 14T HRIM Facility) and NIH Center Grant (P30 EY01730 to Vision Research Center Core).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–32. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–13. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep. 2014;16:501. doi: 10.1007/s11886-014-0501-8. [DOI] [PubMed] [Google Scholar]
- 5.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circulation Heart failure. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circulation Heart failure. 2013;6:944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8:314–27. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolwicz SC, Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circulation research. 2012;111:728–38. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Mello WC, Danser AH. Angiotensin II and the heart : on the intracrine renin-angiotensin system. Hypertension. 2000;35:1183–8. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Okamoto H, Akino M, Onozuka H, Matsui Y, Tsutsui H. Pravastatin attenuates left ventricular remodeling and diastolic dysfunction in angiotensin II-induced hypertensive mice. J Cardiovasc Pharmacol. 2008;51:62–70. doi: 10.1097/FJC.0b013e31815bb629. [DOI] [PubMed] [Google Scholar]
- 13.Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circulation Heart failure. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 14.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circulation research. 2011;108:837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. American journal of physiology Heart and circulatory physiology. 2013;305:H397–402. doi: 10.1152/ajpheart.00088.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolwicz SC, Jr, Tian R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolwicz SC, Jr, Liu L, Goldberg IJ, Tian R. Enhancing Cardiac Triacylglycerol Metabolism Improves Recovery From Ischemic Stress. Diabetes. 2015;64:2817–27. doi: 10.2337/db14-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012;7:1235–46. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 19.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxidants & redox signaling. 2013;19:1085–94. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frohnert BI, Bernlohr DA. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Advances in nutrition. 2013;4:157–63. doi: 10.3945/an.112.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadek HA, Humphries KM, Szweda PA, Szweda LI. Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion. Arch Biochem Biophys. 2002;406:222–8. doi: 10.1016/s0003-9861(02)00446-0. [DOI] [PubMed] [Google Scholar]
- 22.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11168–72. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin JE, Frank BC, Gaspard RM, Duka I, Gavras H, Quackenbush J. Cardiac transcriptional response to acute and chronic angiotensin II treatments. Physiol Genomics. 2004;18:152–66. doi: 10.1152/physiolgenomics.00057.2004. [DOI] [PubMed] [Google Scholar]
- 24.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Molecular and cellular biochemistry. 2008;311:215–24. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 25.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. The Journal of biological chemistry. 2001;276:44390–5. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 26.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–50. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 27.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–42. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 28.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–53. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedi KC, Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth A, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–16. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circulation Heart failure. 2009;2:105–12. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 32.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016 doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, et al. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99:2261–7. doi: 10.1161/01.cir.99.17.2261. [DOI] [PubMed] [Google Scholar]
- 34.Tian R, Nascimben L, Ingwall JS, Lorell BH. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation. 1997;96:1313–9. doi: 10.1161/01.cir.96.4.1313. [DOI] [PubMed] [Google Scholar]
- 35.Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circulation research. 2001;88:961–8. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 36.Mori J, Zhang L, Oudit GY, Lopaschuk GD. Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure. Journal of molecular and cellular cardiology. 2013;63:98–106. doi: 10.1016/j.yjmcc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 37.de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. American journal of physiology Heart and circulatory physiology. 2009;296:H550–8. doi: 10.1152/ajpheart.01176.2008. [DOI] [PubMed] [Google Scholar]
- 38.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. American journal of physiology Heart and circulatory physiology. 2013;304:H1103–13. doi: 10.1152/ajpheart.00636.2012. [DOI] [PubMed] [Google Scholar]
- 39.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. The Journal of clinical investigation. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, et al. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126:1705–16. doi: 10.1161/CIRCULATIONAHA.111.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. The Journal of clinical investigation. 2012;122:3919–30. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarreta D, Orus J, Barrientos A, Miro O, Roig E, Heras M, et al. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovascular research. 2000;45:860–5. doi: 10.1016/s0008-6363(99)00388-0. [DOI] [PubMed] [Google Scholar]
- 43.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovascular research. 2001;52:103–10. doi: 10.1016/s0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 44.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 45.Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001;25:378–88. doi: 10.1038/sj.ijo.0801536. [DOI] [PubMed] [Google Scholar]
- 46.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiological reviews. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 47.Schonfeld P, Wieckowski MR, Lebiedzinska M, Wojtczak L. Mitochondrial fatty acid oxidation and oxidative stress: lack of reverse electron transfer-associated production of reactive oxygen species. Biochimica et biophysica acta. 2010;1797:929–38. doi: 10.1016/j.bbabio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Fragasso G, Rosano G, Baek SH, Sisakian H, Di Napoli P, Alberti L, et al. Effect of partial fatty acid oxidation inhibition with trimetazidine on mortality and morbidity in heart failure: results from an international multicentre retrospective cohort study. Int J Cardiol. 2013;163:320–5. doi: 10.1016/j.ijcard.2012.09.123. [DOI] [PubMed] [Google Scholar]
- 49.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circulation research. 2000;86:580–8. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 50.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovascular research. 2005;66:454–61. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.