Abstract
The mechanisms underlying the association between chronic psychological stress, development of metabolic syndrome (MetS), and behavioral impairment in obesity are poorly understood. The aim of the present study was to assess the effects of mild chronic psychological stress on metabolic, inflammatory, and behavioral profiles in a mouse model of diet-induced obesity. We hypothesized that (1) high-fat high-fructose diet (HFHF) and psychological stress would synergize to mediate the impact of inflammation on the central nervous system in the presence of behavioral dysfunction, and that (2) HFHF and stress interactions would impact insulin and lipid metabolism. C57Bl/6 male mice underwent a combination of HFHF and two weeks of chronic psychological stress. MetS-related conditions were assessed using untargeted plasma metabolomics, and structural and immune changes in the gut and liver were evaluated. Inflammation was measured in plasma, liver, gut, and brain.
Our results show a complex interplay of diet and stress on gut alterations, energetic homeostasis, lipid metabolism, and plasma insulin levels. Psychological stress and HFHF diet promoted changes in intestinal tight junctions proteins and increases in insulin resistance and plasma cholesterol, and impacted the RNA expression of inflammatory factors in the hippocampus. Stress promoted an adaptive anti-inflammatory profile in the hippocampus that was abolished by diet treatment. HFHF increased hippocampal and hepatic Lcn2 mRNA expression as well as LCN2 plasma levels. Behavioral changes were associated with HFHF and stress. Collectively, these results suggest that diet and stress as pervasive factors exacerbate MetS-related conditions through an inflammatory mechanism that ultimately can impact behavior. This rodent model may prove useful for identification of possible biomarkers and therapeutic targets to treat metabolic syndrome and mood disorders.
Keywords: Predatory stress, Depression, Metabolic syndrome, Insulin, Cholesterol, Lipocalin-2, Metabolomics, Biliverdin
1. Introduction
Evolutionarily, acute stress and energetic balance are part of a successful pathway required for human survival; however chronic stress can modulate body composition by depleting non-adipose tissues or increasing adiposity via hypothalamus-pituitaryadrenal (HPA) axis alterations (Depke et al., 2008; Kuo et al., 2008). Accumulating evidence suggests that the interaction between stress exposure and diet imbalance can be an important connection between mood disorders and the global epidemic of obesity, but the mechanisms underlying this association are poorly understood (Basic et al., 2012; van Reedt Dortland et al., 2013; Blakemore and Buxton, 2014; Qi et al., 2014). Specific types of lipid contents in meals can stimulate the intestine–brain–liver neural axis that acts to regulate insulin sensitivity and circulating excess of nutrients (Wang et al., 2008). Furthermore, western diet associated with high-fat and high-fructose (HFHF) consumption can significantly impact fat storage contributing to the inflammatory status present in obesity (Appelhans et al., 2013; Bluher, 2013; Tchkonia et al., 2013).
Metabolic syndrome (MetS) is a set of diverse conditions including obesity, imbalance of cytokines, dyslipidemia, hepatic steatosis, and gut disorders, and is associated with an increased risk for psychiatric diseases (Capuron et al., 2008; Aschbacher et al., 2014; Bruce-Keller et al., 2014; Muller, 2014). Psychological stress, depression and negative life events are factors associated with increased risk of acute myocardial infarction (Xu et al., 2011). A relationship between anxiety, depression, and MetS in patients with type 2 diabetes (T2D) has been reported (Kahl et al., 2015). Furthermore, patients with chronic liver disease and depression disorders may be at higher risk for other medical complications and impaired quality of life (Sharma and Fulton, 2013; Davidson and Martin, 2014; Karagozian et al., 2014; Posadas-Romero et al., 2014).
Despite the accumulated evidence supporting a role for the immune system and cytokine signaling in the development of obesity and depression, it is not fully understood how HFHF, metabolic imbalance, and inflammation interact in obese states during a response to chronic stress (Ogden et al., 2012; Tekola-Ayele et al., 2013; Gallus et al., 2014; Wu et al., 2014). Chronic inflammation is a key factor in depressive and obesity states (Miller and Raison, 2015; Ramirez and Sheridan, 2016). Changes in central Lipocalin-2 (Lcn2) are associated with depression and anxiety (Mucha et al., 2011; Ferreira et al., 2013). The increased secretion of tumor necrosis factor (TNF) and other cytokines in the presence of HFHF diet or during psychological stress exposure can trigger additional changes and immune responses in metabolically active tissues such as gut, liver, and the adipose tissue that ultimately can affect the brain functions (Spruss et al., 2009; Jan et al., 2011; Milanski et al., 2012; Li et al., 2013a; Skrzypiec et al., 2013; Bailey, 2016).
The high degree of complexity related to obesity and stress described above demonstrates a critical need to use unbiased methodologies to assess how diet and stress can modulate centrally and peripherally metabolic, behavioral and immune outcomes in obesity. High-fat and high-carbohydrates consumption including fructose from beverages is much higher nowadays compared to past decades (Vos et al., 2008) and is associated with an increase in obesity (Dissard et al., 2013). Because diet and stress are two important exposures impacting human health in western society, a HFHF diet was used in this study to promote disturbances in metabolic profiles associated with obesity. We hypothesized that HFHF diet and chronic psychological stress would synergize to adversely mediate the impact of peripheral inflammation on the central nervous system and result in behavioral dysfunction. Secondly, we also expect an interaction between HFHF consumption and chronic psychological stress that could directly affect metabolic factors implicated in MetS, such as insulin resistance and dyslipidemia, both of which are often associated with mood disorders. For this purpose, HFHF intake was combined with two weeks of chronic psychological predatory stress to assess metabolic, inflammatory, and behavioral responses in wild type C57Bl/6 mice. MetS-related conditions were assessed using an untargeted metabolomics approach, and structural and immune changes in the gut and liver were evaluated. The results from this will assist in developing a well-characterized, animal model for screening interventions developed to prevent or treat MetS in westernized societies where chronic stress is an endemic problem.
2. Materials and methods
2.1. Animals
Male C57Bl/6 mice (n = 60), 7 weeks of age, were acquired from The Jackson Laboratory (Bar Harbor, ME). Each animal was singly housed in a colony room with a 12/12 h light-dark cycle (lights off at 7:00 p.m.) at an ambient temperature of 22–23 °C. Additional female C57Bl/6 mice, 4 weeks of age (n = 16), were acquired from the Jackson Laboratory for the Female Urine Sniffing Test (FUST). Adult male Long Evans rats (n = 30; 400+ grams upon arrival) purchased from Harlan (Indianapolis, IN, USA) served as stimulus (predator) animals. Rats were pair-housed and had free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. Rats were housed in a separate room from mice and maintained on a 12/12 h reverse light/dark cycle (lights off at 07:00 a.m.) at an ambient temperature of 22–23 °C. Upon arrival at the animal facility, mice and rats were allowed seven days of acclimatization prior to experimentation during which they were all fed standard rodent diet (Lab Diet 5001). All studies and animal protocols were approved and guided by the Institutional Animal Care and Use Committee at Emory University (#2002041).
2.2. High-fat high-fructose (HFHF) diet
Mice received regular drinking water and standard chow diet (13% kcal from fat, #7001, Harlan-Teklad, Madison, Wisconsin) or high-fat diet (42% kcal from fat, TD.88137, Harlan-Teklad, Madison, Wisconsin) plus 30% (wt/vol) fructose solution (Sigma-Aldrich #F012, Sigma-Aldrich, St. Louis, MO) available ad libitum throughout the duration of the study (Fig. 1). The combination of high fat diet (Table S1, Table S2) and fructose was chosen because it mimics the diet in developed countries with high rates of obesity and MetS (Charlton et al., 2011; Dissard et al., 2013; Bostick et al., 2014). HFHF diet consisted of 21.2% fat (61.85% saturated fat, 27.3% monounsaturated and 4.7% polyunsaturated), 48.5% carbohydrate, and 17.3% protein by weight (18.8 kJ/g). Control diet (Control) consisted of 4.4% fat, 39.5% carbohydrate, and 25.2% protein by weight (12.6 kJ/g).
Fig. 1.
Schematic and study timeline. Control groups consumed standard chow diet (4% kcal from fat and water) and HFHF groups consumed high-fat diet (42% kcal from fat + 30% fructose w/v) for 10 weeks. Groups (n = 14–15): Control diet/No Stress (Control NS), Control diet/Stress (Control S), High-fat high-fructose/No stress (HFHF NS) and High-fat high-fructose Stress (HFHF S). Predatory stress (PS): 15 min of daily predatory stress OR no stress (NS) for 2 weeks. Behavior: Marble burying and female urine test were performed to assess anxiety and anhedonia-like behavior; sociability test was used to evaluate social interaction. At 10 weeks mice were euthanized, and tissues were harvested.
2.3. Experimental design
Mice were randomly assigned to one of the following groups (n = 14–15 per group): Control Diet/No Stress (Control/NS), Control Diet/Stress (Control/S), High-fat high-fructose/No Stress (HFHF/NS) and High-fat high-fructose Stress (HFHF S). The animals were kept on their respective diets for 10 weeks, i.e. the entire duration of the study (Fig. 1). Beginning at week 6, mice assigned to the stress group underwent daily predatory stress for 14 consecutive days. At the end of week 8, blood samples were collected via tail vein, and high-resolution metabolomics (HRM) analysis of plasma was conducted to evaluate global metabolic changes associated with HFHF diet and stress. From weeks 8–9, all mice underwent a battery of behavioral assessments, which took place in a designated behavioral testing suite between 10 am and 4 pm. During the duration of the study, food and drink consumption and changes in body weight were assessed. At the end of week 10, animals were weighed, then euthanized under isoflurane by cervical dislocation. Trunk blood was collected in EDTA vacutainers, immediately placed on ice, and centrifuged at 2000g for 15 min at 4 °C. Plasma was collected and stored at −80 °C. Liver, gut, and brain were harvested. The liver, retroperitoneal, gonadal, and mesenteric adipose tissue deposits were weighed, and the small intestine and colon lengths were measured. Tissue samples were frozen in liquid nitrogen and stored at −80 °C.
2.4. Predatory stress (PS) model
Mice were subjected to predatory stress as described by Barnum et al. (2012) with slight modifications. Prior to experimentation, both rats and mice were allowed to habituate to the testing room on two consecutive days for 30 min. Predatory stress involved placing a mouse inside a 5-inch diameter clear plastic hamster ball (Super Pet, Elk Grove Village, IL; material # 100079348) and then placing that ball into the center of the home cage of a large and aggressive retired breeder male Long-Evans rat daily for 15 min for 14 days. The aggressor rat was allowed to freely investigate the plastic ball for the entire duration of one session. The hamster ball was not secured when placed inside the rat cage, thereby allowing the rat to further agitate the mouse subject. During the stress session, mice were exposed to the sight/sound/ smell of the rat through the holes in the hamster ball but never allowed to make direct physical contact. After each session, mice were returned to their home cages until the next session on the following day. Stressed mice were paired with a different rat for each session to avoid familiarity and possible habituation.
2.5. Caloric intake and body weight
Twice a week, mice were provided with pre-weighed portions of either the control diet and regular water or the high-fat diet and 30% fructose solution. Each day between 10 am and 12 pm, the weight of food and drink present in each mouse’s cage was measured. Residual spillage was not considered. At the end of each week, mice were weighed, and caloric intake was calculated by multiplying the average grams of food and fructose consumed in a day by kJ per gram of food and fructose. At week 8 caloric efficiency was obtained by dividing caloric intake (kJ) by changes in body weight (g).
2.6. Plasma analyses
To test for alterations in metabolism due to HFHF diet and its interaction with the stress exposure, we performed untargeted HRM profiling of plasma collected on week 8 to evaluate the effect of HFHF diet on the plasma metabolome. Sample preparation and instrumental parameters have been described elsewhere (Park et al., 2012; Go et al., 2015a,b). Briefly, 50 µL of plasma were treated with 100 µL of acetonitrile to precipitate proteins, and triplicate 10 µL aliquots were analyzed by hydrophilic interaction liquid chromatography (HILIC) (Accucore, 100 mm × 2.1 mm, 2.6 µm; Thermo Scientific, San Jose CA) and C18 (Accucore, 100 mm × 2.1 mm, 2.6 µm; Thermo Scientific) chromatography with acetonitrile/formic acid gradient interfaced to a Q-Exactive HF high-resolution mass spectrometer (Thermo Scientific). Positive ionization was utilized for HILIC to maximize detection of polar and semi-polar metabolites, while negative ionization was used for C18 to improve capture of fatty acid and lipid species. Metabolites were detected as mass-to-charge ratio (m/z) features over the scan range 85–1275, which were then extracted and aligned using apLCMS (Yu et al., 2013) with modifications by xMSanalyzer (Uppal et al., 2013). Feature identification was accomplished by searching for common adducts at a mass accuracy threshold of ±5 parts-per-million (ppm) using the Kyoto Encyclopedia for Genes and Genomes (KEGG) (Kanehisa et al., 2012) and METLIN (Smith et al., 2005). Prior to statistical analysis, feature tables were averaged, log2 transformed, and filtered for triplicate relative standard deviation (RSD) <50% and for features exhibiting >25% missing values (per group basis), resulting in 6383 unique features detected with HILIC and 4635 detected by C18.
Plasma collected immediately following sacrifice was used to assess metabolic dysregulation and inflammation. Metabolic markers measured in plasma included glucose, cholesterol, triglycerides, leptin, and insulin. Cholesterol and glucose were measured using the spectrophotometric Cholesterol Quantitation Kit (# MAK043, Sigma-Aldrich, St. Louis, MO) and Glucose Assay Kit (# ab65333, abcam, Cambridge, MA). Plasma insulin and leptin were measured using the Mouse Metabolic Kit (Multi-spot Assay System # K15124C-1, Meso Scale Discovery, Rockville, MD). LCN2, Lipopolysaccharide binding protein (LPB) and C-Reactive protein (CRP) were measured using commercially available Enzyme-linked Immunosorbent Assay (ELISA) kits (Mouse Lipocalin-2/ NGAL Quantikine ELISA Kit, #MLCN20, R&D Systems Minneapolis, MN), (LBP, soluble mouse ELISA kit # ALX-850-305-KI0, Enzo Life sciences, Farmingdale, NY) and, (MOUSE C-REACTIVE PROTEIN Kit, # E-90CRP, Immunology Consultants Laboratory, Inc. Portland, OR). Inflammatory cytokines TNF, IL-1, and IL-6 were measured using Mouse Proinflammatory 7-Plex Ultra-Sensitive (Kit #K15012C-1, Meso Scale Discovery, Rockville, MD). Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the fasting plasma insulin concentration (µU/mL) and fasting plasma glucose concentration (mg/dL). Plasma triglyceride levels were determined by ELISA (Charles River Lab, Wilmington, MA).
2.7. Behavioral tests
2.7.1. Marble-burying test
The marble-burying test was used to measure the extent to which mice subjected to either HFHF diet or predatory stress developed anxiety-like behavior (Nasser et al., 2014). Mice were placed in a plastic tub (50.5 × 39.4 × 19.7 cm) that contained 6 in. of lightly pressed bedding as described previously (Barnum et al., 2012). Within each tub 20 marbles were evenly arranged in 5 rows of 4 marbles each. The mouse was placed into the tub and allowed to explore for 30 min after which the number of marbles covered with bedding to at least two-thirds of their height was considered buried.
2.7.2. Female urine sniffing test (FUST)
FUST was performed as described by Malkesman et al. (2010) to determine the extent to which HFHF diet and predatory stress elicited depressive-like behavior. Experimental male mice were habituated to a cotton-tipped applicator taped to their home cages for 1 h before the test. Next, the cotton tips were infused with 60 µL of water, and the duration of sniffing the tip was recorded for 3 min. Forty-five minutes following the water trial, new cotton tips were infused with fresh urine from female mice in estrus of the same strain, and sniffing duration was also recorded for a period of 3 min. A reduction in time spent sniffing female urine indicates anhedonia or depressive-like behavior in experimental male mice. Determination of estrous cycle stage was performed as described by Byers et al. (2012).
2.7.3. Sociability test
Three-chambered sociability test was performed to assess social avoidance, a symptom of depression (Lo et al., 2016). The apparatus (acrylic, 60 × 40 × 22 cm) (UGO BASILE s.r.l., Varese, Italy) consisted of a center compartment with removable doors that led to a side chamber on each side. A mesh cup was placed at the center of each side chamber. Each test animal was first placed in the center compartment and allowed to acclimate for 10 min. Following habituation, a stranger mouse was placed into one of the mesh cups to serve as a social stimulus whereas the other mesh cup remained empty. The doors to the two side chambers were then removed, and the experimental animal was allowed to freely explore all three chambers for 10 min. The total duration of active contact made by the test mouse with each of the mesh cups was recorded. Active contact was defined as sniffing in an area 3–5 cm around the cup. Preference for novel object was calculated as [(time spent exploring empty cup)/(total time spent exploring empty cup and novel mouse)] × 100%. Preference for novel mouse was calculated as [(time spent exploring novel mouse)/(total time spent exploring empty cup and novel mouse)] × 100% (Lo et al., 2016). EthoVision XT Software (Noldus Information Technology, Wageningen, The Netherlands) was used to track and analyze animal behavior activity.
2.8. Intestinal tissue collection and microscopy
Small intestine and colon from a subset of mice (n = 6) were harvested and flushed with PBS, and tissue segments were frozen in Cryo-Embedding Compound. Remaining tissue was flash-frozen and stored at −80 °C. Frozen sections (5–10 µm) were fixed in acetone (Sigma-Aldrich; catalog #) on slides and used for histochemical and immunofluorescent analyses. For fluorescent imaging of tight junction proteins, specimens were first permeabilized and blocked in 0.25% Triton X-100 (EK Industries) in TBS with 5% normal donkey serum (NDS; Millipore), then incubated overnight with 7 µg/mL anti-ZO-1, anti-OCLN, or anti-CLDN2 primary antibodies (Catalog # 61–7300, 71–1500 and, 32–5600, Invitrogen Corporation, Camarillo, CA) in 0.25% TX-100 in TBS with 1% NDS at 4 °C. Specimens were then incubated with 10 µg/mL appropriate secondary antibodies in 0.25% TX-100 in TBS with 1% NDS 1–2 h at room temperature (Table S5). Coverslips were applied with Vectashield mounting medium with DAPI (Vector Laboratories, Inc.). Images were obtained using a Nikon Eclipse 90i microscope with a DS-Fi1 (Nikon) camera and Nikon NIS-Elements AR 3.10.
2.9. Immunoblot analysis
Tight junction protein levels in intestinal tissue were evaluated by immunoblot. After RNA and protein extraction (as described above), ice-cold methanol (Millipore) was added to the organic phase to pellet the protein. The pellet was washed with methanol and then resuspended in 1% SDS (Fisher Scientific) solution. Ten micrograms total protein were run on precast 4–20% gradient SDS-PAGE gels (Bio-Rad) then transferred onto PDVF membranes (Millipore) and blocked in 5% powdered milk (Bio-Rad) in TBS. Blots were probed with 2 µg/mL anti-ZO-1, 3 µg/mL anti-OCLN, 3 µg/mL anti-CLDN2, or 1:400 anti-β-actin primary antibodies and appropriate HRP-conjugated secondary antibody (1:1000) (Table S5).
2.10. Liver analysis
Liver tissue from the left lobe was fixed in 4% paraformaldehyde/PBS and cryoprotected in 30% sucrose solution. Tissue was placed in OCT and frozen under cooled isopentane and stored at −80 °C. Liver samples were sectioned (8–10 µm) and stained with Oil red-O (#150678, Abcam, Cambridge MA) for assessment of lipid content according to the manufacturer’s instructions. Images were obtained using a Nikon Eclipse 90i microscope with a DS-Fi1 (Nikon) camera and Nikon NIS-Elements AR 3.10 software, magnification 40×.
2.11. RNA and protein extraction and cDNA synthesis
RNA was isolated from the hippocampus, hypothalamus, colon, small intestine, and liver. Proteins were isolated from small intestine and colon tissues. Frozen samples were homogenized in TRIzol reagent (Life Technologies) on the TissueLyser II (QIAGEN) with one 5 mm stainless steel bead (QIAGEN) for 2 cycles of 2-min duration at 20 Hz. One-fifth volume chloroform was added to the tissue lysates to enable phase separation. The upper aqueous phase was homogenized by passage through QIAshredderTM columns (QIAGEN), and RNA was isolated from the aqueous phase using Qiagen RNeasy mini (Valencia, CA, USA) protocol for animal tissue. Total RNA yield was determined by absorbance at 260 nm, and purity was determined by the 260/280 nm ratio using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was treated with 1/5 concentration DNAse I (0.64 µL per 4 µg RNA; Life Technologies) in 25 mM MgCl2 (Sigma) for 30 min at 37 °C, then 10 min at 75 °C. DNAse-treated RNA was reverse transcribed to cDNA using SuperScript II Reverse Transcriptase (Life Technologies), dNTPs (Life Technologies), and random hexamers (Integrated DNA Technologies, Coralville) according to the manufacturer’s protocol.
2.12. Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was performed as previously described by Barnum et al. (2008) with some modifications. Briefly, qPCR was performed using an ABI Prism 7900 HT Fast Real-time PCR System (Applied Biosystems Inc., Foster City, CA) in 384-well plate. Each sample was run in triplicate as a 10 µL-reaction consisting of 25 ng cDNA, SYBR green PCR Master mix (SYBR Green; Life Technologies), and 150 nM of each forward and reverse PCR primers. Oligonucleotide primers (Integrated DNA Technologies, Coralville) were validated in a reaction with titrated template and by examination of dissociation curves. mRNA levels were measured in triplicate as threshold of Cycles (Ct) values and normalized to the values for housekeeping genes β-actin (Actb), Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh) and cyclophilin E (Cyclo). The fold change in Ct values with diet and/or predatory stress was calculated. Relative gene expression measured by previously validated primers (Table S6) for Lipocalin-2 (Lcn2), Tight junction protein 1 (Tjp1), Occludin (Ocln), Claudin-2 (Cldn2), Tnf, Il-1B, Il-6, Il-10, Suppressor of cytokine signaling 1 and 3 (Socs1 and Socs3), Peroxisome proliferator-activated receptor gamma (Pparγ), Sterol regulatory element-binding protein 1c (Srebp-1c), Fatty Acid Synthase (Fasn), Inducible nitric oxide synthase (iNOS), Toll-like receptor 2 (Tlr2), Toll-like receptor 4 (Tlr4) (Integrated DNA Technologies, Coralville, IA). Transcript abundance were quantified using the 2−ΔΔCt method as described previously (Livak and Schmittgen, 2001) relative to the geometric means of Gapdh, Actb, and Cyclo. All cDNA was stored at −20 °C until time of assay.
2.13. Inflammatory cytokines & receptors PCR array
Tissue was processed using Qiagen RNeasy mini kit as described previously (Barnum et al., 2012). In brief, reverse transcription of RNA was carried out using SABiosciences RT2 First Strand Kit. qPCR was performed using an ABI Prism 7900HT Fast Detection System (Applied Biosystems). Each 10 µl-reaction was performed in the 384-well format Mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR Array (SABiosciences; catalog # CAPM13007-PAMM-052Z).
2.14. Statistical analyses
Two-way ANOVA was used to assess differences in effects of stress and diet at specific time points. Bonferroni’s post hoc test was used where applicable. Inflammatory Cytokines & Receptors PCR Array data were analyzed using the RT2 Profiler TM PCR Array Data Analysis software on the SABiosciences website http://www.sabiosciences.com/pcrarraydataanalysis.php and are expressed as fold change. All data expressed as Mean ± standard error of the mean (SEM). Pearson’s correlation coefficient (r) was used to analyze the association between variables. The calculations were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA); a p-value threshold <0.05 was used. HRM profiling data were analyzed in R (R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria) with metabolite differential expression evaluated using a linear model for microarray data (LIMMA) based on two-way ANOVA analysis (Smyth, 2005), which included diet, stress, and diet: stress interaction comparisons. Significant features were selected using a false discovery rate (FDR) threshold ⩽5% to correct for multiple hypothesis testing (Benjamini and Hochberg, 1995). Identification of enriched metabolic pathways was completed using Mummichog (Li et al., 2013b).
3. Results
3.1. High fat high fructose diet (HFHF) and stress altered metabolome and plasma metabolic parameters in a manner consistent with metabolic syndrome
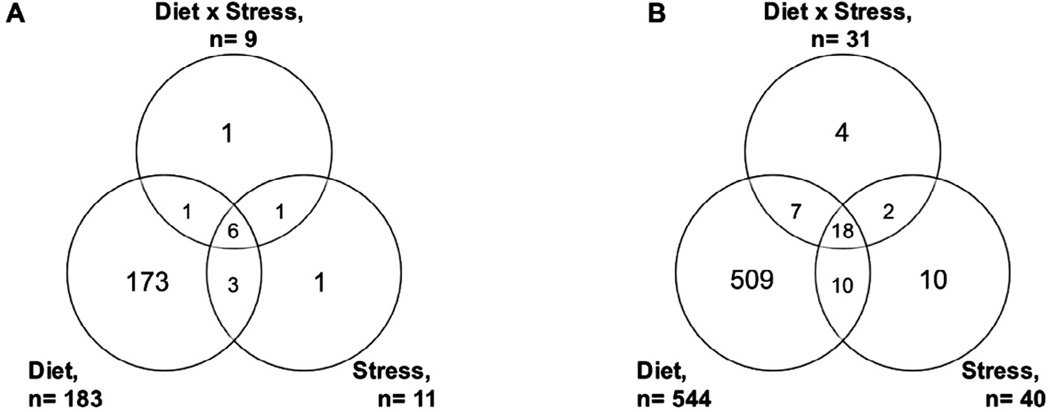
Diet composition of micro- and macronutrients can impact body function and homeostasis of core metabolic processes (Jones et al., 2012). Comparison of control and HFHF diet identified 183 metabolic features from HILIC-pos (Fig. 2A) and 544 from C18-neg at FDR threshold ⩽5% (Fig. 2B). Metabolic features were associated with stress (51 combined from HILIC-pos and C18-neg) and interaction of diet: stress (40 combined). Identification of stress and diet: stress associated metabolites included glucose (p = 0.004), caprylic acid (p = 0.0045), phosphocholine (p = 0.003), hydroxymelatonin (p < 0.0001), acylcarnitine C18:1 (p < 0.0001), and biliverdin (p = 0.0006). Metabolic pathways significantly associated with HFHF diet were consistent with altered dietary patterns, including co-factor metabolism, amino acid metabolism, and fatty acid/lipid-related metabolic pathways (Table 1). Annotation of individual metabolites, such as methionine (p < 0.0001), carnitine (p < 0.0001), and linoleate (p < 0.0001) further supported diet-related alterations to the metabolome. Metabolic changes were also consistent with obesity-related disease risk, including decreased bilirubin (p < 0.0001), altered eicosanoid levels including leukotriene A4 (p < 0.0001), leukotriene B4 (p = 0.001), prostaglandin (PG) E3 (p < 0.0001), and 15-epilipoxin B4 (p < 0.0001), and metabolites related to oxidative stress, such as cysteine (p < 0.0001), gamma-glutamylcysteine (p < 0.0001), and oxidated linoleic acid derivatives. The remaining m/z features resulted in no probable matches in the METLIN and KEGG databases, indicating possible uncharacterized metabolites, conjugates, or dietary compounds.
Fig. 2.
High resolution metabolomics (HRM) reveals metabolic pathways associated with HFHF diet consistent with altered dietary patterns, including co-factor metabolism, amino acid metabolism, and fatty acid/lipid-related metabolic pathways. Number of significant overlapping and distinct differentially-expressed features detected at false discovery rate (FDR) threshold ⩽5% when comparing diet, stress, and diet:stress interactions using two-way ANOVA of HFHF diet and stress at false discovery rate (FDR) threshold ⩽5% for HILIC-pos (A) and C18-neg (B). HFHF diet was associated with the greatest change to the metabolomic profile and impacted a large number of lipid- and fatty acid-related metabolites. Interaction between diet and stress (n = 40 significant features) suggests influence of diet on stress response. Serum was collected from n = 8– 13 mice/group.
Table 1.
Metabolic pathways significantly associated with HFHF diet-related metabolites.
| Metabolic pathway | Number of metabolites present |
P-value1 |
|---|---|---|
| HILIC w/positive ionization | ||
| Urea cycle/amino group metabolism | 8 | 0.00063 |
| Carnitine shuttle | 6 | 0.00073 |
| Vitamin E metabolism | 5 | 0.0012 |
| Vitamin B3 (nicotinate and nicotinamide) | 4 | 0.0012 |
| Lipoate metabolism | 2 | 0.0029 |
| Sialic acid metabolism | 4 | 0.0049 |
| Vitamin B5 – CoA biosynthesis from pantothenate | 2 | 0.0061 |
| Arginine and Proline Metabolism | 4 | 0.015 |
| Lysine metabolism | 3 | 0.02 |
| Phosphatidylinositol phosphate metabolism | 3 | 0.027 |
| Glycosphingolipid metabolism | 3 | 0.032 |
| C18 w/negative ionization | ||
| Fatty acid activation | 8 | 0.00067 |
| De novo fatty acid biosynthesis | 8 | 0.00072 |
| Fatty Acid Metabolism | 5 | 0.0011 |
| Vitamin E metabolism | 9 | 0.0014 |
| Phosphatidylinositol phosphate metabolism | 8 | 0.0015 |
| Glycerophospholipid metabolism | 13 | 0.0017 |
| Linoleate metabolism | 8 | 0.0024 |
| Dynorphin metabolism | 3 | 0.0046 |
| Omega-3 fatty acid metabolism | 3 | 0.0046 |
| Glycosphingolipid metabolism | 9 | 0.0072 |
| Ubiquinone Biosynthesis | 3 | 0.0082 |
| Carnitine shuttle | 5 | 0.0089 |
| Squalene and cholesterol biosynthesis | 5 | 0.013 |
Mummichog derived p-values are determined using a permutation approach that compares metabolite-pathway distribution from randomly sampled m/z features to distribution for the diet associated metabolites.
3.2. Stress attenuated weight gain associated with HFHF consumption without change in adiposity
Our results show that HFHF-fed animals ate less than control mice (Table S3). However, HFHF mice displayed greater total caloric intake (F1,54 = 138.30, p < 0.0001) and decreased caloric efficiency (F1,53 = 18.00, p < 0.0001 (Fig. S1A, Table S4) as compared to mice consuming the control diet and water. There was no significant difference in the final amount of total kilojoules ingested or caloric efficiency when stress was the parameter assessed (Table S4). As expected, HFHF-fed mice presented increased weight gain (F1,54 = 48.76, p = 0.0001); however, stress reduced the body weight gain in the presence of HFHF intake (F1,54 = 15.49, p = 0.0002) (Fig. S1B). HFHF diet was associated with induced hepatomegaly (F1,52 = 11.16, p = 0.001) (Fig. S1C). Gonadal and retroperitoneal adipose deposits were enlarged in HFHF-fed mice as compared to the control diet groups (Fig. S1D, E). Interestingly, the mesenteric adipose tissue mass decreased with the HFHF diet intervention (Fig. S1F) (F1,47 = 9.22, p = 0.003).
3.3. Stress and HFHF diet promoted metabolic dysregulation
We found that plasma insulin was increased by diet (F1,31 = 15.71, p = 0.0004) and stress (F1,31 = 4.38, p = 0.04) (Table 2). Furthermore, HFHF diet and stress interacted to increase insulin concentration (F1,31 = 4.789, p = 0.03). HOMA-IR was significantly elevated in the HFHF animals (F1,32 = 18.39, p = 0.0002) and further increased when diet was combined with stress (F1,30 = 4.97, p = 0.03). There was no significant difference in fasting blood glucose concentrations. HFHF diet caused an increase in plasma leptin concentration (F1,35 = 190.10, p < 0.0001). Diet (F = 1,35 = 268.80, p < 0.0001) and stress (F = 1,35 = 4.63, p = 0.03) promoted significant elevation in plasma cholesterol. Triglycerides measured after 10 weeks of diet manipulation did not differ between the experimental groups.
Table 2.
Stress and HFHF consumption enhances metabolic parameters. Insulin concentration and HOMA-IR were greater in HFHF S mice than in the other experimental groups. Plasma cholesterol was affected by diet in association with psychological stress. Diet intervention increased plasma leptin. There were no differences in glucose or triglyceride levels between the experimental groups.
| Control NS | Control S | HFHF NS | HFHF S | |
|---|---|---|---|---|
| Glucose (mg/dL) | 92.9 ± 3.4a | 91.5 ±3.6a | 96.0 ±5.1a | 97.2 ± 8.5a |
| Insulin (µU/mL) | 10.9 ± 2.6a | 8.4 ± 1.3a | 50.2 ± 10.8ab | 111.1 ± 35.7c |
| HOMA-IR | 2.40 ± 0.7a | 2.0 ± 0.2a | 7.9 ± 1.2b | 14.0 ± 2.9c |
| Leptin (ng/mL) | 1.86 ± 0.16a | 2.79 ±0.5a | 26.0 ±3.1b | 27.72 ± 2.0b |
| Triglycerides (mg/dL) | 37.4 ± 6.5a | 35.9 ±4.2a | 50.8 ± 7.1a | 39.0 ± 3.9a |
| Cholesterol (mg/dL) | 39.83 ±4.3a | 38.94 ±3.05a | 169.15 ± 16.7b | 209.31 ± 7.8c |
Serum was collected from n = 14–15 mice/group, and data are plotted as mean SEM for each group. Different letters indicate significant differences in mean values. Mean values with the same superscript letters were not statistically significant. p-values were indicated for two-way analysis of variance with post hoc Bonferroni test (p < 0.05).
Abbreviations: HOMA-IR (homeostasis model assessment for insulin resistance) = (FPI X FPG)/22.5. FPI-Fasting plasma insulin concentration (µU/ml), FPG-fasting plasma glucose (mg/Dl); Control, Control diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress.
3.4. HFHF diet leads to endotoxemia and peripheral inflammation
HFHF diet increased LBP levels without an additional effect of stress (F = 1,31 = 9.55, p = 0.004) (Table 3). There were positive correlations between LBP and plasma LCN2 (r = 0.58, p = 0.0007) and between LBP and liver TNF levels (r = 0.66, p = 0.001). Plasma cyto-kine levels were assessed to investigate the contribution of stress and diet to the inflammatory profile. Diet was the main factor to impact inflammatory response, increasing circulating TNF (F1,36 = 7.90, p = 0.007), IL-6 (F1,32 = 20.48, p < 0.0001) and LCN2 (F1,35 = 7.43, p = 0.05) concentrations (Table 3). No significant differences were noted in CRP plasma levels (Table 3).
Table 3.
HFHF diet leads to an inflammatory response affecting plasma and liver cytokines. HFHF diet mice presented increased plasma TNF, IL-6, Lcn2, and LBP levels and elevated hepatic TNF.
| Control NS | Control S | HFHF NS | HFHF S | |
|---|---|---|---|---|
| Plasma TNF (pg/mL) | 4.46 ± 0.4a | 4.48 ± 0.3a | 5.43 ± 0.3ab | 6.08 ± 0.7b |
| Plasma IL-6 (pg/mL) | 2.84 ± 0.6a | 3.57 ± 0.4a | 9.51 ± 2.5b | 7.05 ± 0.8ab |
| Plasma Lcn2 (ug/mL) | 10.69 ± 1.7a | 10.97 ± 2.4ab | 16.92 ± 2.4b | 15.81 ±2.5ab |
| Plasma LBP (ug/mL) | 12.58 ± 0.7a | 14.31 ± 0.9ab | 18.57 ± 1.6b | 15.99 ± 1.3ab |
| Plasma CRP (ng/mL) | 3.10 ±0.1a | 3.39 ± 0.23a | 3.39 ± 0.2a | 3.44 ± 0.20a |
| Liver TNF (pg/mL) | 2.32 ± 0.4a | 3.11 ± 0.4ab | 4.48 ± 0.7b | 3.25 ± 0.3ab |
Serum was collected from n = 14–15 mice/group, and data are plotted as mean SEM for each group. p-values were indicated for two-way analysis of variance with post hoc Bonferroni test (p < 0.05). Different letters indicate significant differences in mean values. Mean values with the same superscript letters were not statistically significant.
Abbreviations: Control, Control Diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress; TNF, Tumor necrosis factor; IL-6, interleukin; LBP, LPS binding protein; Lcn2, Lipocalin 2; CRP, C-reactive protein.
3.5. HFHF diet and stress disrupted the organization of tight junction proteins in the small intestine
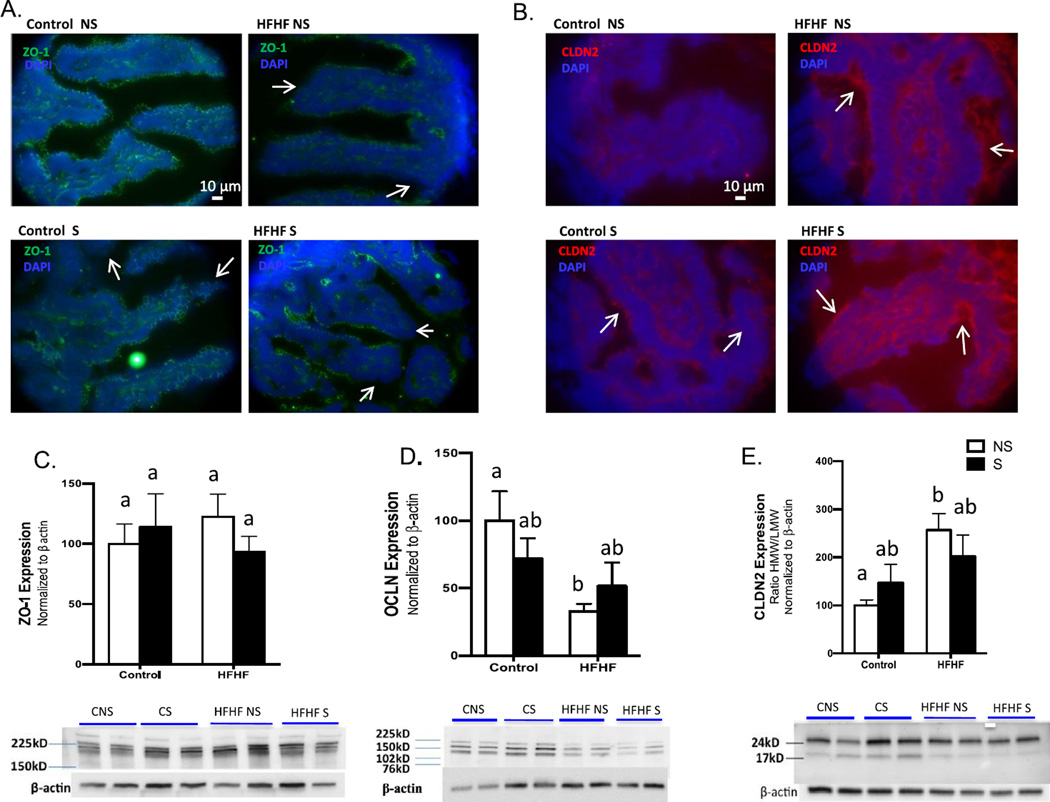
Immunohistochemical analysis revealed structural changes and disruption of ZO-1 networks in the small intestine promoted by HFHF diet and stress (Fig. 3A). HFHF S mice displayed increased CLDN2 lumenal surface localization in the small intestine (Fig. 3B). ZO-1 protein levels were unaffected by diet or stress (Fig. 3C); however, HFHF diet without stress significantly reduced abundance of OCLN protein (F1,17 = 13.83, p = 0.0390) (Fig. 3D). CLDN2 protein levels were unaffected by diet and stress, but the ratio of 25 kDa to 20 kDa forms of the protein increased significantly with HFHF diet without stress (F1,18 = 7.899, p = 0.0154) (Fig. 3E), indicating an increase in the abundance of phosphorylated CLDN2 (Van Itallie et al., 2012). In addition, HFHF NS mice displayed decreased Il-10 expression compared to the control diet groups (F1,13 = 7.313, p = 0.01) (Fig. S2).
Fig. 3.
HFHF diet and stress impact the organization of tight junction proteins in the small intestine. Cryosections of small intestine were probed for ZO-1 (A) and CLDN2 (B) by immunostaining. HFHF diet and stress promote structural changes and disrupt the ZO-1 network in the small intestine (white arrows) (A). HFHF and stress increase CLDN2 lumenal surface localization in the small intestine (white arrows) (B). ZO-1 protein levels are unaffected by diet and stress (C), HFHF diet without stress significantly reduces abundance of OCLN protein (D); CLDN2 protein levels are unaffected by diet and stress, but ratio of high molecular weight to low molecular weight forms increases significantly with HFHF diet without stress (E). Images were obtained using a Nikon Eclipse 90i microscope with a DS-Fi1 (Nikon) camera and Nikon NIS-Elements AR 3.10 software. 40× mag - FITC 100 ms 1×) (CLDN2 40× mag – FITC 100 ms 1 ×). Protein expression in small intestine was measured by Western blot, band intensity calculated using densitometric analysis (Image Studio Lite), and values were normalized to β-actin. Data were analyzed by two-way ANOVA, with Bonferroni’s correction for multiple comparisons. Bar height indicates mean of samples from n = 6 mice per group, error bars indicate standard error of the mean (S.E.M.). Means with different letters are significantly different from each other (p<0.05). Abbreviations: ZO-1, Zonula occludens; CLDN2, Claudin-2; OCLN, Occludin; Control, Control diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress.
3.6. HFHF-fed mice displayed increased liver discoloration consistent with hepatic lipid accumulation
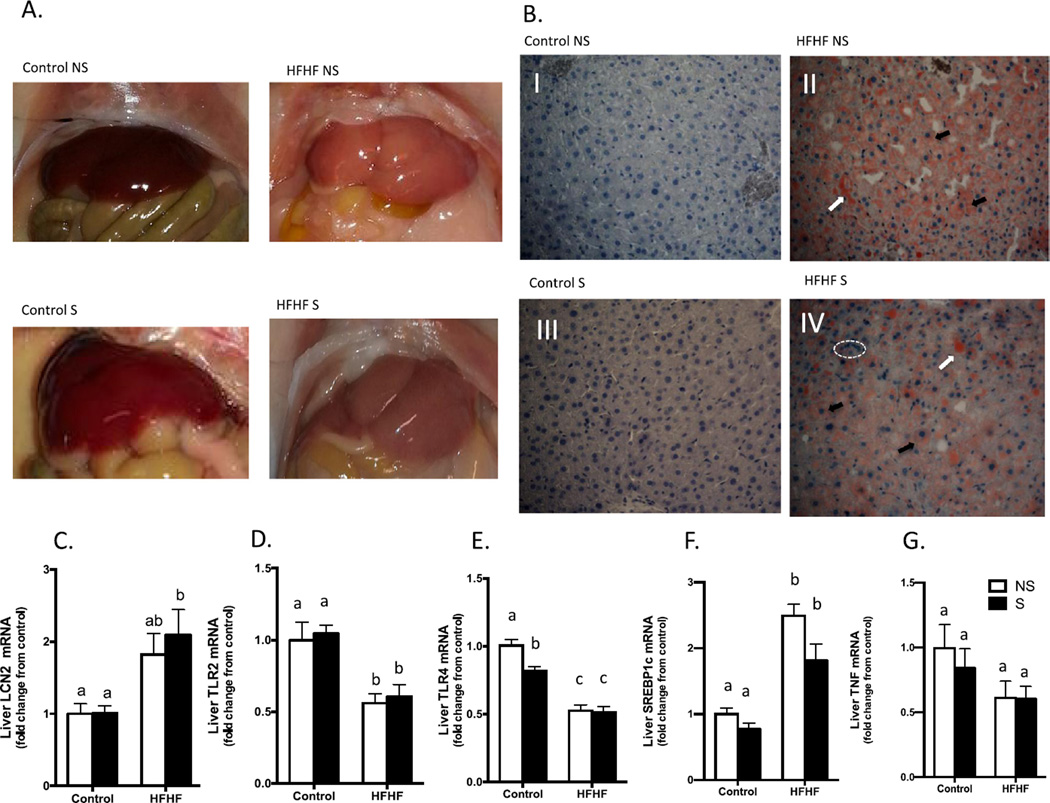
Macroscopic examination of livers revealed discoloration in HFHF diet and stress groups as compared to control diet and no stress groups (Fig. 4A). Thus, we assessed lipid accumulation to investigate whether ten weeks of HFHF diet or stress promoted non-alcoholic fatty liver disease (NAFLD) in our experimental model. Liver sections were stained with Oil Red O and revealed markedly increased lipid deposition in HFHF-fed groups. Histological changes included microvesicular lipid droplets, ballooning of hepatocytes, and cell infiltration (Fig. 4B). The mild discoloration observed in control diet stress mouse livers was not associated with changes in lipid deposition (Fig. 4A).
Fig. 4.
HFHF diet and stress modulate lipogenesis and inflammation in the liver. Gross morphologic changes in liver and gut from HFHF animals compared to the control groups (A). Light microscopic images of liver stained for lipids (B) reveal hepatocytes with normal appearance for the Control diet groups; and HFHF groups displaying lipid droplets (white arrows), cell infiltration (dashed area) and microvacuolization (black arrows). Oil Red O-stained and hematoxylin-counterstained liver sections, magnification 40×. Images were obtained using a Nikon Eclipse 90i microscope with a DS-Fi1 (Nikon) camera and Nikon NIS-Elements AR 3.10 software. HFHF S mice presented increased Lcn2 expression (C). HFHF diet decreases liver Tlr2 and Tlr4, (D, E) and stress reduces hepatic Tlr4 in the control diet group (E). Srebp1c RNA expression increases with HFHF treatment (F). There is no difference in hepatic Tnf mRNA expression (G). Bar height indicates mean of samples from an n = 6 mice per group, error bars indicate standard error of the mean (S.E.M.). Means with different letters are significantly different from each other (p < 0.05). Data were analyzed by two-way ANOVA followed by Bonferroni post hoc. Tissues were analyzed by qPCR using primers directed against murine Lcn2, Tlr2, Tlr4, Srebp1c, and Tnf. For each animal, the Ct values were normalized to the Ct values for Gapdh and Ppia. The relative expression level of the target gene (fold change) was expressed as 2−ΔΔCt, when compared with the mean DCt (threshold cycle) of the control group. Abbreviations: qPCR, quantitative real-time reverse-transcription polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TLR, Toll like receptor; SREBP-1c, sterol regulatory element binding protein-1c; Lcn2, neutrophil gelatinase-associated lipocalin 2; TNF, Tumor necrosis factor; Control, Control diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress.
3.7. HFHF diet and stress jointly modulate inflammation in mouse liver
Lcn2 mRNA expression (Fig. 4C) increased after 10 weeks of HFHF diet (F1,17 = 13.48, p = 0.001). Tlr2 expression (Fig. 4C) (F1,19 = 23.48, p = 0.0001) decreased with HFHF diet, and Tlr4 (Fig. 4C) decreased with diet (F 1,20 = 100.2, p < 0.001) and stress (F1,20 = 6.26, p = 0.02). Srebp-1c is associated with modulation of insulin sensitivity and influences genes related to lipid metabolism in the liver. Diet treatment (F1,20 = 57.94, p < 0.0001) raised Srebp-1c mRNA (Fig. 4C) in the liver after 10 weeks of high fat and fructose intake. Despite the high concentration of liver TNF found in the HFHF NS animals, there was no significant difference in hepatic Tnf mRNA among the experimental groups (Fig. 4C). In addition, HFHF S mice displayed decreased iNOS expression in relation to the control diet NS group (F1,19 = 5.10, p = 0.03) (Data not shown). HFHF-fed groups displayed decreased liver Socs1 (F1.20 = 26.69, p < 0.0001) and Socs3 (F1,19 = 15.96, p = 0.0008) mRNA expression compared to control groups (Data not shown). Fasn and Pparγ gene expression did not significantly change in any of the experimental groups (Data not shown).
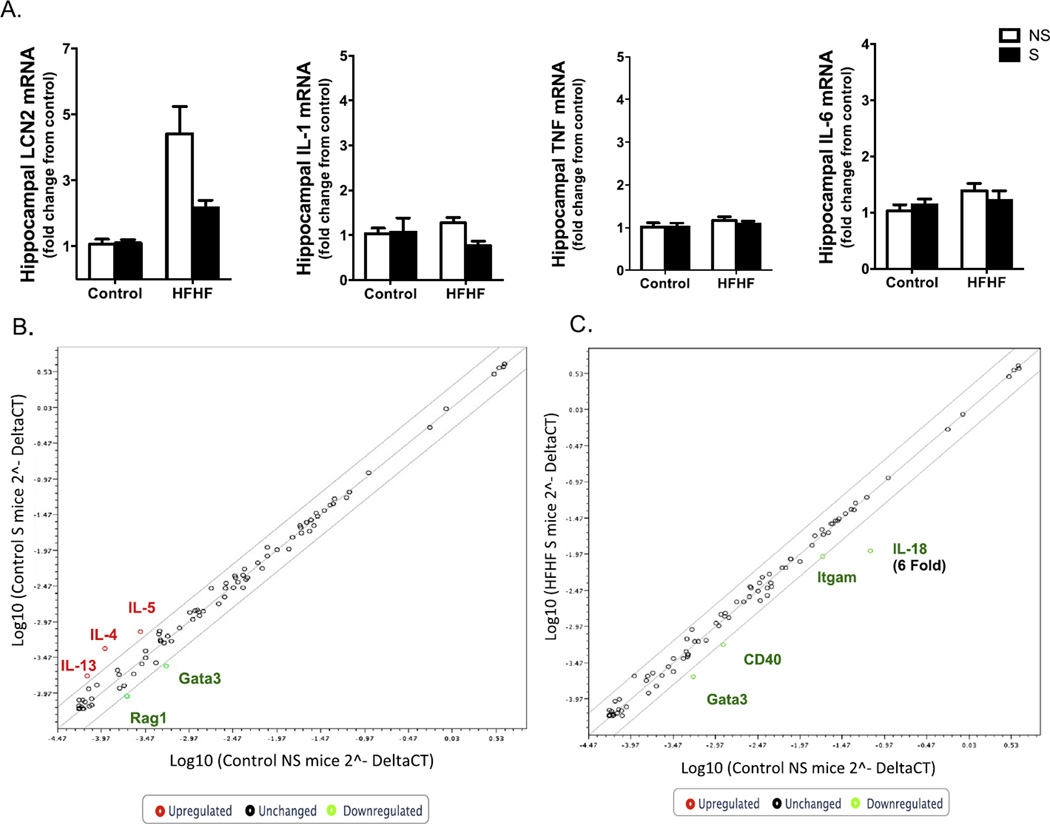
3.8. Stress and HFHF diet disrupt hippocampal gene expression
HFHF diet increased hippocampal mRNA expression of Lcn2 without impacting Tnf, Il-1β, or Il-6 expression (F1,19 = 9.98, p < 0.005) (Fig. 5A). Chronic psychological stress did not lead to alterations in the expression of these cytokines in the hippocampus. No significant changes in cytokines were observed in the hypothalamus with diet or stress exposure (data not shown). In addition, a RT2 Profiler PCR Array was used to investigate further the effects of HFHF diet and stress on the mRNA expression of cytokines and receptors in the hippocampus in this animal model. Of the 83 inflammation-related genes assessed, stress increased Il-4, Il-5, and Il-13 gene expression and decreased Gata3 greater than 2-fold (Fig. 5B). HFHF diet in combination with stress decreased 3 genes greater than 2-fold (Itgam, Cd40, and Gata3), and Il-18 decreased 6-fold in the HFHF S group (Fig. 5C).
Fig. 5.
Stress and HFHF diet modulate gene expression in the mouse hippocampus. HFHF diet increases Lcn2 gene expression in the mouse hippocampus without impacting Il-6, Il-1β, or Tnf mRNA expression (A). Bar height indicates mean of samples, error bars indicate standard error of the mean for an n = 6–8 mice per group. Means with different letters are significantly different from each other (p < 0.05). Data were analyzed by two-way ANOVA followed by Bonferroni post hoc. Tissues were analyzed by qPCR using primers directed against murine Lcn2, Il-1b, Il-6, Tnf, Ppia and Gapdh genes. For each animal, the Ct values were normalized to the Ct values for Gapdh and Ppia. The relative expression level of the target gene (fold change) was expressed as 2−ΔΔCt, when compared with the mean DCt (threshold cycle) of the control group. RT2 Profiler PCR array analysis of inflammation-related genes showed that stress decreases the multitasking and neuromodulatory genes Gata3 and Rag1 more than two-fold and increases Il-4, Il-5, and Il-13 expression more than twofold in the hippocampus of Control S mice relative to Control NS group (B). HFHF diet in combination with stress decreased the expression of hippocampal immunomodulatory factors and Il-18 relative to Control NS mice (6-fold) (C). The hippocampus was dissected and examined for potential changes in inflammation. Samples were pooled together from an n = 6–7 mice per group and compared between the 4 groups (Control NS, Control S, HFHF NS and HFHF S mice). Abbreviations: qPCR, quantitative real-time reverse-transcription polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Lcn2, neutrophil gelatinase-associated lipocalin 2; IL, interleukin; TNF, Tumor necrosis factor; Rag1, Recombinase activation gene 1; Gata 3, trans-acting T-cell-specific transcription factor Gata3; Itgam, Integrin alpha M; CD40, costimulatory molecule CD40, Control, Control Diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress.
3.9. HFHF diet and stress induce behavioral deficits
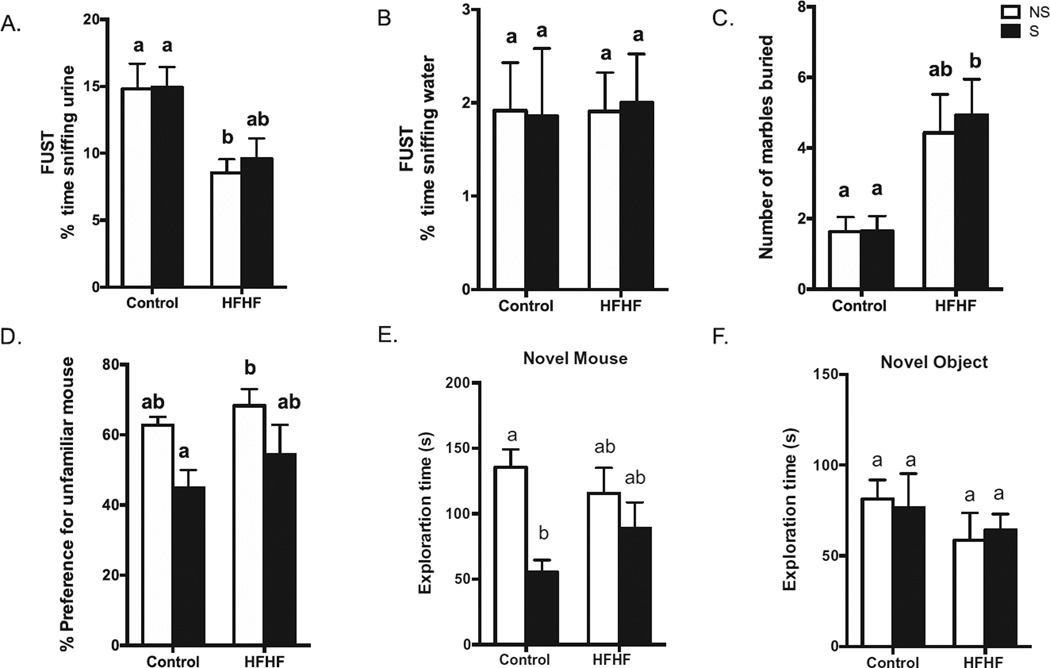
HFHF-fed mice exhibited anhedonia during FUST. All tested mice spent more time sniffing urine than water in the FUST paradigm (Fig. 6A, B). HFHF NS animals spent less time sniffing a cotton tip dipped with urine from a female in estrus than Control NS (F1,49 = 15.04, p < 0.0003) (Fig. 6B). In the marble-burying test, HFHF S mice exhibited more signs of anxiety-like behavior and buried more marbles in a 30 min session (Fig. 6C) (F1,25 = 15.00, p < 0.0007) compared to Control NS and Control S mice. The Control S group showed a decrease in preference for the unfamiliar mouse compared to the HFHF NS mice (Fig. 6D) (F1,24 = 8.88, p = 0.006) and spent less time exploring the unfamiliar mouse compared to Control NS (F1,24 = 10.67, p = 0.003) (Fig. 6E). There was no difference in the time exploring the novel object between the groups (Fig. 6F).
Fig. 6.
HFHF diet and stress induce behavioral deficits. HFHF NS mice spent more time sniffing female urine than control diet mice; PS did not affect urine-sniffing time (A). There was no difference in the percentage of water sniffing time (B). HFHF mice showed increased anxiety-like behavior and buried more marbles in a 30-min session compared to Control mice (C). Stress impacted sociability, decreasing the percentage of preference and time of exploration for an unfamiliar mouse (D, E). Data are expressed as mean ± SEM of time spent sniffing water or urine from same strain female in estrus (A), number of marbles buried (B), or duration (seconds) of time exploring the novel object (empty cup) or novel mouse (unfamiliar mouse). Means with different letters are significantly different from each other (p < 0.05). Female urine test (n = 14–15/group), marble burying (n = 14–15/group), Sociability test (n = 6–7/ group). Abbreviations: PS, predatory stress; Control, Control diet; HFHF, High-fat high-fructose diet; NS, No stress, S, stress.
4. Discussion
The assessment of how chronic psychological stress impacts the pathophysiological responses associated with obesity, including those related to mental health, is a complex task. In this study chronic psychological stress in the presence of HFHF diet promoted increased insulin resistance and plasma cholesterol, disruption of intestinal tight junctions, and modulation of immune factors in the hippocampus. Stress increased IL-4 and IL-5 mRNA expression within the hippocampus, and this profile was abolished by HFHF diet. HFHF promoted increased hippocampal and hepatic Lcn2 mRNA expression as well as increased LCN2 plasma levels. Anxiety and depressive-like behavior were associated with dietary intervention and stress in our model.
The bi-directional relationship between the immune system and metabolic disorders in obesity status can lead to disease-relevant biological and psychological consequences (van Reedt Dortland et al., 2013). The assessment of biomarkers is likely to improve our understanding of how different tissues can interact to bring about behavioral changes in the presence of stress and unhealthy diet. Herein, HFHF-altered metabolites were associated with amino acids, fatty acids, de novo lipogenesis (DNL), and acyl-carnitine pathways. These altered pathways suggest dysregulation of energetic metabolism via beta-oxidation in the presence of adipose tissue expansion and ectopic lipid deposition (Ronnett et al., 2005). In agreement with this metabolic profile, we demonstrate that the neuroendocrine adaptations necessary to supply the homeostatic demands of stress were associated with a decrease in weight gain in HFHF-fed mice without an effect on adiposity. This phenotypic outcome is desirable because the metabolic improvement associated with depletion of adipose tissue can compromise the assessment of possible synergistic effects of diet-stress interactions (Finger et al., 2012; Bruder-Nascimento et al., 2013). In addition, the reduction of mesenteric adipose tissue associated with HFHF diet in the present study may be related to the lipid accumulation observed in the liver. In agreement with these data, Asterholm et al. (2014) revealed that high-fat diet can lead to inappropriate inflammatory responses associated with impairment of mesenteric tissue expansion, ectopic hepatic lipid deposition and gut barrier disorders. The significance of mesenteric adipose tissue reduction associated with the nutritional regimen used here merits further investigations (Wernstedt Asterholm et al., 2014).
It has been reported that excessive flux of free fatty acids (FFA) to ectopic sites and inflammation are risk factors for the development of insulin resistance by interfering with insulin, TLR, and PPAR signaling pathways (Consitt et al., 2009; Gregor and Hotamisligil, 2011). In addition, there is evidence that diet stimulation alters gene expression and neuronal activity in the hypothalamic arcuate nucleus. Some studies suggest that the development of metabolic syndrome is associated with dysfunction in the arcuate nucleus and receptors in the insulin pathway in the brain (Singh et al., 2012). In this regard, the agouti-related protein neurons though neuromodulatory activity may be involved in the peripheral substrate utilization and regulation of storage vs. utilization of carbohydrates or lipids (Denis et al., 2014).
While the relationship between inflammation and obesity in T2D is firmly established, it is still not completely understood how stress increases metabolic vulnerability to insulin resistance (Uchida et al., 2012; Li et al., 2013a; Roberts et al., 2015). The present study shows diet and stress interactions promote disturbances in biliverdin and acylcarnitine C18:1, two metabolites associated with insulin resistance. Indeed, this association was confirmed in posterior assessments of insulin levels two weeks after stress exposure. To the best of our knowledge, our results reveal for the first time evidence for the synergetic effect of HFHF diet and chronic psychological stress affecting the biliverdin pathway. Biliverdin is reduced to bilirubin by biliverdin reductase (BVR), an enzyme that has multiple functions affecting cell signaling and immune responses (Fabris et al., 2009; Kapitulnik and Maines, 2009). BVR modulates the insulin/IGF-1 signaling cascade and, more specifically, regulates serine-threonine- and tyrosine-kinase activity. BVR can be activated by factors elevated in obesity and stress such as reactive oxygen species and cytokines (Lerner-Marmarosh et al., 2005; Kapitulnik and Maines, 2009). Barone et al. reported impairment of the biliverdin pathway associated with insulin resistance in human brains of Alzheimer’s disease (AD) patients and in a mouse model of AD (Barone et al., 2011, 2016). Both MetS and obesity have been identified as risk factors for AD. Our data suggest more in-depth assessment of disturbances in the heme-derived biliverdin pathway may reveal new insights related to mechanisms underlying stress and diet interactions in vulnerability to T2D and age-related neurodegeneration.
Emerging new evidence suggests that stress can cause long term alterations in blood lipid profiles in humans (Catalina-Romero et al., 2013; Shaibani et al., 2015). Interestingly, our results indicate that stress did not affect circulating cholesterol levels in mice fed control diet. In contrast, stress potentiated the hyperc-holesterolemia associated with HFHF diet. Recent studies show a link between changes in cholesterol metabolism and neurodegenerative states and dementia (Di Paolo and Kim, 2011). High fat consumption can positively impact the association between increased plasma and hippocampal cholesterol (Stranahan et al., 2011). In addition, Feng et al. demonstrated that forced swim stress elevates cholesterol content in the brain (Feng et al., 2015). Another study using 1 h of restraint in association with forced swim found significant increases in blood cholesterol with stress in rats (Devaki et al., 2013). Herein we show that 15 min a day of psychological stress for 14 days is sufficient to affect circulating cholesterol levels in an obesogenic environment. This change persisted two weeks beyond the psychological stress exposure. Some studies suggest that cholesterol changes influence neurological disorders associated with protein aggregation like prion disease and Alzheimer’s disease-related dementia (Hannaoui et al., 2014). In light of this connection, our findings suggest that further investigations of the interactions between stress and HFHF consumption on cholesterol metabolism could shed new insight into the relationship between cardiovascular disease, MetS, cognitive decline, and dementia.
The metabolic vulnerability and inflammation associated with conditions present in MetS may share common risk factors with mood disorders. In particular, an increased inflammatory state is recognized to be one of the main mechanisms promoting depression (Maes et al., 2008; Muller, 2014; Byrne et al., 2015; Miller and Raison, 2015). Our results demonstrate that increased pro-inflammatory lipid signaling (evidenced by changes in prostaglandins (PGs), leukotrienes, and alterations in antioxidant metabolites) was detectably associated with insulin resistance and NAFLD, both of which are known risk factors for cardiovascular disease (CVD). Human and animal studies (Tappy and Le, 2015) reveal controversial findings related to the impact of fructose on health outcomes; however recent studies strongly suggest that humans are sensitive to the adverse effects of sustained sugar consumption and show an association between fructose intake and NAFLD (Jin and Vos, 2015), CVD (Stanhope et al., 2015) and endotoxemia (Jin et al., 2014).
Unexpectedly, our findings demonstrated changes in plasma dynorphin (Dyn) in response to HFHF diet but not to the stress intervention (Land et al., 2008; Knoll and Carlezon, 2010). Dyn is an endogenous ligand for kappa opioid receptors involved in modulation of the addiction/reward system (Sauriyal et al., 2011). Central Dyn is extensively associated with anxiety, depression, and anhedonia in animal models of stress (Bilkei-Gorzo et al., 2008; Wittmann et al., 2009). Interestingly and in contrast with other studies (Finger et al., 2012), we demonstrate that diet but not stress is associated with anxiety and anhedonic-like behavior in our animal model. Nevertheless, despite Dyn being implicated in interactions between the nervous and immune systems, there is little information on how peripheral Dyn is associated with mood disorders. Further investigations in this area are warranted.
Our results show HFHF diet promotes endotoxemia and imbalance in peripheral TNF, IL-6, and Lcn2 in association with anxiety and anhedonic-like behavior. In obesity, these pro-inflammatory changes are associated with metabolically active organs and tissues such as liver, gut, and adipose tissue in response to increased FFA and circulating LPS (Guo et al., 2010; Ji et al., 2011; Bluher, 2013; Miyake and Yamamoto, 2013; Ortega et al., 2015). Recent human studies have positively correlated increased circulating NGAL with anxiety and depression. This association seems particularly apparent in states of chronic metabolic imbalance such as in late life depression or chronic heart failure (Naude et al., 2013, 2014, 2015).
Our study revealed dysregulation of tight junctions consistent with increased gut permeability in association with systemic inflammation in HFHF-fed mice. These findings are in line with previous data that implicate increased gut permeability and endotoxemia as triggers for maladaptive immune responses present in obesity (Cani et al., 2008; Moreira et al., 2012). Claudins are a large family of proteins, some of which, such as CLDN2, increase paracellular permeability through the formation of cation-selective channels (Amasheh et al., 2002). Interestingly, even two weeks after the last stress session, HFHF S mice displayed increased small intestine lumenal surface localization of CLDN2. This was supported by immunoblot analysis indicating an increase in the higher molecular weight form of CLDN2, which has been identified as the phosphorylated form that localizes to the plasma membrane to increase permeability (Van Itallie et al., 2012). It was reported that psychological stress affects mast cell function mediated by corticotrophin releasing hormone (Vicario et al., 2010). There is no consistent relationship between cortisol levels and the anthro-pometric and metabolic parameters in obesity (Abraham et al., 2013); however, intestinal mast cells can be activated by high fat intake thereby modulating the intestinal release of mediators such as PGs, leukotrienes, and cytokines (Ji et al., 2011; Abraham et al., 2013). PGs and inflammatory cytokines can interfere with HPA activation to promote direct or indirect regulation of gut permeability (Keita and Soderholm, 2010; Vicario et al., 2010; Zimomra et al., 2011). In addition, although not explored in this study, fat consumption and stress are likely to promote changes in micro-biota that could trigger a “leaky gut” inflammatory syndrome, which would be expected to disrupt the gut-brain axis and impact behavior (Kelly et al., 2015; Bailey, 2016).
In line with recent reports, we observed significantly increased liver Lcn2 expression in the context of obesity and hepatic steatosis (Auguet et al., 2013; Ye et al., 2014; Asimakopoulou et al., 2016). More specifically, the reduction of RNA expression of inflammatory markers associated with increased Lcn2 expression suggests some regulatory hepatic mechanism in response to 10 weeks of HFHF consumption (Alwahsh et al., 2014; Asimakopoulou et al., 2014). On the other hand, we observed potentially regulatory effects of stress on hepatic TLR4 expression in mice under control diet after 15 days of stress exposure. These data contrast with work from Frank et al. (2013) who reported anti-inflammatory actions of glucocorticoids during stress exposure, but sensitization of the immune response after the stressor had ended (Frank et al., 2013). Glucocorticoids can regulate the TLR4 pathway via interaction with transcription factors NF-κB and AP-1 and thereby interfere with the transcription of pro-inflammatory cytokines (Ratman et al., 2013).
Interestingly, our study demonstrates that either peripheral inflammation-associated HFHF intake or 15 min of stress exposure was sufficient to change the expression of a subset of inflammatory cytokines in the hypothalamus or hippocampus.
Surprisingly, our data contrast with previous studies showing increased hippocampal Lcn2 expression in HFHF-fed mice but not in stressed animals (Mucha et al., 2011; Skrzypiec et al., 2013). An increased expression of Lnc2 in the blood brain barrier was reported in response to LPS administration, perhaps an adaptive response to limit the spread of bacterial activity into the brain (Ip et al., 2011). In our studies, this central elevation in Lcn2 expression occurs in a more physiologically relevant model in the presence of peripheral inflammation and metabolic endotoxemia. The impact of increased Lcn2 in the hippocampus has been suggested to involve regulation of microglia activation and changes in neuronal morphology and plasticity associated with depression and anxiety (Mucha et al., 2011; Ferreira et al., 2013). Collectively, our results support a model in which elevated central and peripheral Lcn2/NGAL mediate the impact of chronic inflammatory peripheral disease on the central nervous system in association with behavioral dysfunction (Gouweleeuw et al., 2015).
In previous work, we reported that 28 days of 30 min/day predatory stress exposure was associated with anxiety-like behaviors, neuroinflammation, and microglial activation in different brain regions (Barnum et al., 2012; Burgado et al., 2014); however, the current study revealed that two weeks of 15 min of psychological stress is sufficient to promote increases in hippocampal Il-4, Il-5, and Il-13. Previous studies implicated T cells in a protective role against neuroinflammation and related behavioral changes (Lewitus et al., 2008; Gadani et al., 2012). As highlighted in a recent review, the neuroprotective effects of T cells in responses to stress are related to production of IL-4 that may occur in an evolutionarily adaptive perspective (Miller and Raison, 2015). IL-4 can stimulate astrocytes to produce Brain-Derived Neurotrophic Factor (BNDF), promote protective phenotypes in microglia, and increase neurogenesis in the hippocampus (Gadani et al., 2012; Brachman et al., 2015). Our current data and the results from our previous study (Barnum et al., 2012) suggest that mild stress and continuous prolonged chronic stress exposure can each modulate differently the delicate balance in brain immunity. IL-4 and IL-5 play important roles in health and disease, making it difficult to target these cytokines in the context of mood impairment (Marshall et al., 1998; Marin et al., 2009).
Furthermore, our analyses showed that HFHF S mice display decreased expression of immunomodulatory factors in the hippocampus and reduction in Il-18 mRNA expression. IL-18 is involved in regulation of gene expression in the hippocampus (Alboni et al., 2014). It has been suggested that down-regulation of IL-18 is associated with peripheral adaptations related to the development of MetS (de Wit et al., 2008; Nyima et al., 2016). Previous studies reported that IL18r1−/− mice can exhibit low physical activity, increased weight gain, ectopic lipid deposition, and high anxiety-like behavior (Eisener-Dorman et al., 2010; Lindegaard et al., 2013). To our knowledge, the current study is the first report of the synergic effect of diet and stress on central Il-18 RNA expression. We speculate that the central decrease of Il-18 gene expression in the HFHF S mice may contribute to the energetic imbalance and increased anxiety-like behavior observed in these mice, raising the interesting possibility that targeting IL-18 may be beneficial in reducing the effects of stress and diet-induced depression and cognitive decline.
In summary, our findings revealed that: 1) short-term chronic psychological stress in a mouse model of obesity promotes long-lasting central and systemic changes that can be measured weeks after the stress intervention; 2) these pervasive stressors (diet and stress) exacerbate MetS conditions accompanied by central responses through an inflammatory mechanism; 3) metabolomics analyses represent a useful analytical strategy to investigate interactions between diet and chronic psychological stress; 4) psychological stress and HFHF diet can contribute synergistically to insulin resistance and hypercholesterolemia; 5) stress and HFHF diet can modulate immune factors in the brain, liver, and hippocampus; 6) LCN2 may have a pivotal role in the systemic and hippocampal responses to the metabolic and inflammatory profile associated with HFHF diet consumption. Collectively, these results suggest that this rodent model is useful for identification of possible biomarkers and therapeutic targets to control MetS and mood disorders.
Similar to humans, the hepatic metabolism of rodents has a central role in fatty acid and lipids synthesis (Bergen and Mersmann, 2005). It is necessary to point out however, that differences between rodent and human metabolism need to be considered when obesity and stress, as dynamic and multifactorial conditions, are assessed. Nonetheless, diet-related effects in this study should be viewed within the context of an animal model useful for assessing potential effects of HFHF and stress on metabolic homeostasis. The HFHF diet used in this study was not intended to be a perfect replica of human diet; but rather the strategy was to use a diet that rodents would willingly eat in conjunction with an ethologically relevant stress paradigm to enable assessment of diet × stress synergistic or opposing interactions. Although not investigated in the present study, it has been shown that stress and diet are two important elements that can impact epigenetic phenomena (Stankiewicz et al., 2013; Martinez et al., 2014). Those two exposures result in tissue- and organ-specific changes associated with development of metabolic conditions in humans such as obesity and its comorbidities (Martinez et al., 2014).
In conclusion, we show that a wide range of metabolic and inflammatory conditions demonstrated in our animal model of diet-induced obesity can effectively contribute directly or indirectly to behavioral changes. HFHF diet in combination with chronic stress exposure can modulate the delicate balance in brain immunity, abolishing the adaptive anti-inflammatory phenotype associated with mild stress. More specifically, HFHF diet and stress interactions can be key factors underlying the increased metabolic vulnerability to conditions associated with atherosclerosis, NAFLD, and CVD. We have identified several inflammatory factors that could serve as potential biomarkers or therapeutic targets in obesity and stress-related conditions. Future studies should evaluate the extent to which duration or intensity of stress in association with HFHF diet influences the variability and robustness of the behavioral outcomes linked to these conditions.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Frank Anania and Raymond J. Tesi for useful discussions and Jaegwon Chung for technical assistance with QPCR. The present study was supported in part by the Emory Multiplexed Immunoassay Core (EMIC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. Other funding support for this study was obtained from NIH/NIMHS 1R43MH105048-01A1 (MGT and RJT).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2016.08.021.
Contributor Information
Maria Elizabeth de Sousa Rodrigues, Email: mdesou2@emory.edu.
Mandakh Bekhbat, Email: mandakh.bekhbat@emory.edu.
Madelyn C. Houser, Email: mcrawf4@emory.edu.
Jianjun Chang, Email: jchan47@ emory.edu.
Douglas I. Walker, Email: Douglas.Walker@emory.edu.
Dean P. Jones, Email: dpjones@ emory.edu.
Claudia M.P. Oller do Nascimento, Email: claudia.oller@unifesp.br.
Christopher J. Barnum, Email: cjbarnum@me.com.
Malú G. Tansey, Email: malu.tansey@emory.edu.
References
- Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman LK. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity. 2013;21:E105–E117. doi: 10.1002/oby.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Montanari C, Benatti C, Sanchez-Alavez M, Rigillo G, Blom JM, Brunello N, Conti B, Pariante MC, Tascedda F. Interleukin 18 activates MAPKs and STAT3 but not NF-kappaB in hippocampal HT-22 cells. Brain Behav. Immun. 2014;40:85–94. doi: 10.1016/j.bbi.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwahsh SM, Xu M, Seyhan HA, Ahmad S, Mihm S, Ramadori G, Schultze FC. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J. Gastroenterol. 2014;20:1807–1821. doi: 10.3748/wjg.v20.i7.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Bleil ME, Waring ME, Schneider KL, Nackers LM, Busch AM, Whited MC, Pagoto SL. Beverages contribute extra calories to meals and daily energy intake in overweight and obese women. Physiol. Behav. 2013;122:129–133. doi: 10.1016/j.physbeh.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K, Kornfeld S, Picard M, Puterman E, Havel PJ, Stanhope K, Lustig RH, Epel E. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulou A, Borkham-Kamphorst E, Henning M, Yagmur E, Gassler N, Liedtke C, Berger T, Mak TW, Weiskirchen R. Lipocalin-2 (LCN2) regulates PLIN5 expression and intracellular lipid droplet formation in the liver. Biochim. Biophys. Acta. 2014;1842:1513–1524. doi: 10.1016/j.bbalip.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Asimakopoulou A, Borkham-Kamphorst E, Tacke F, Weiskirchen R. Lipocalin-2 (NGAL/LCN2), a “help-me” signal in organ inflammation. Hepatology. 2016;63:669–671. doi: 10.1002/hep.27930. [DOI] [PubMed] [Google Scholar]
- Auguet T, Terra X, Quintero Y, Martinez S, Manresa N, Porras JA, Aguilar C, Orellana-Gavalda JM, Hernandez M, Sabench F, Lucas A, Pellitero S, Del Castillo D, Richart C. Liver lipocalin 2 expression in severely obese women with non alcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes. 2013;121:119–124. doi: 10.1055/s-0032-1331696. [DOI] [PubMed] [Google Scholar]
- Bailey MT. Psychological stress, immunity, and the effects on indigenous microflora. Adv. Exp. Med. Biol. 2016;874:225–246. doi: 10.1007/978-3-319-20215-0_11. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Eskow KL, Dupre K, Blandino P, Jr, Deak T, Bishop C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemiparkinsonian rat: role for interleukin-1beta. Neuroscience. 2008;156:30–41. doi: 10.1016/j.neuroscience.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnum CJ, Pace TW, Hu F, Neigh GN, Tansey MG. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J. Neuroinflamm. 2012;9:9. doi: 10.1186/1742-2094-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Cassano T, Arena A, Tramutola A, Lavecchia MA, Coccia R, Butterfield DA, Perluigi M. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radical Biol. Med. 2016;91:127–142. doi: 10.1016/j.freeradbiomed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C, Butterfield DA. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer’s disease and amnestic mild cognitive impairment. J. Alzheimer’s Dis. 2011;25:623–633. doi: 10.3233/JAD-2011-110092. [DOI] [PubMed] [Google Scholar]
- Basic M, Butorac A, Landeka Jurcevic I, Bacun-Druzina V. Obesity: genome and environment interactions. Arhiv za higijenu rada i toksikologiju. 2012;63:395–405. doi: 10.2478/10004-1254-63-2012-2244. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J. Nutr. 2005;135:2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Blakemore AI, Buxton JL. Obesity, genetic risk, and environment. BMJ. 2014;348:g1900. doi: 10.1136/bmj.g1900. [DOI] [PubMed] [Google Scholar]
- Bluher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:163–177. doi: 10.1016/j.beem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metab., Clin. Exp. 2014;63:1000–1011. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder-Nascimento T, Campos DH, Alves C, Thomaz S, Cicogna AC, Cordellini S. Effects of chronic stress and high-fat diet on metabolic and nutritional parameters in Wistar rats. Arq. Bras. Endocrinol. Metab. 2013;57:642–649. doi: 10.1590/s0004-27302013000800010. [DOI] [PubMed] [Google Scholar]
- Burgado J, Harrell CS, Eacret D, Reddy R, Barnum CJ, Tansey MG, Miller AH, Wang H, Neigh GN. Two weeks of predatory stress induces anxiety-like behavior with co-morbid depressive-like behavior in adult male mice. Behav. Brain Res. 2014;275:120–125. doi: 10.1016/j.bbr.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Mitchell SA, Allen NB. Adolescent-onset depression: are obesity and inflammation developmental mechanisms or outcomes? Child Psychiatry Hum. Dev. 2015;46:839–850. doi: 10.1007/s10578-014-0524-9. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina-Romero C, Calvo E, Sanchez-Chaparro MA, Valdivielso P, Sainz JC, Cabrera M, Gonzalez-Quintela A, Roman J. The relationship between job stress and dyslipidemia. Scand. J. Public Health. 2013;41:142–149. doi: 10.1177/1403494812470400. [DOI] [PubMed] [Google Scholar]
- Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61:47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Martin AA. Obesity: cognitive impairment and the failure to ‘eat right’. Curr. Biol. 2014;24:R685–R687. doi: 10.1016/j.cub.2014.06.031. [DOI] [PubMed] [Google Scholar]
- de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, Muller M, van der Meer R. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/ 6J mice. BMC Med. Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis RG, Joly-Amado A, Cansell C, Castel J, Martinez S, Delbes AS, Luquet S. Central orchestration of peripheral nutrient partitioning and substrate utilization: implications for the metabolic syndrome. Diabetes Metab. 2014;40:191–197. doi: 10.1016/j.diabet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Depke M, Fusch G, Domanska G, Geffers R, Volker U, Schuett C, Kiank C. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology. 2008;149:2714–2723. doi: 10.1210/en.2008-0038. [DOI] [PubMed] [Google Scholar]
- Devaki M, Nirupama R, Yajurvedi HN. Chronic stress-induced oxidative damage and hyperlipidemia are accompanied by atherosclerotic development in rats. Stress. 2013;16:233–243. doi: 10.3109/10253890.2012.719052. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat. Rev. Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissard R, Klein J, Caubet C, Breuil B, Siwy J, Hoffman J, Sicard L, Ducasse L, Rascalou S, Payre B, Buleon M, Mullen W, Mischak H, Tack I, Bascands JL, Buffin-Meyer B, Schanstra JP. Long term metabolic syndrome induced by a high fat high fructose diet leads to minimal renal injury in C57BL/6 mice. PLoS One. 2013;8:e76703. doi: 10.1371/journal.pone.0076703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Behavioral and genetic investigations of low exploratory behavior in Il18r1(−/−) mice: we can’t always blame it on the targeted gene. Brain Behav. Immun. 2010;24:1116–1125. doi: 10.1016/j.bbi.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris L, Cadamuro M, Okolicsanyi L. The patient presenting with isolated hyperbilirubinemia. Digestive Liver Dis. 2009;41:375–381. doi: 10.1016/j.dld.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Feng X, Lin YL, Wei LN. Behavioral stress reduces RIP140 expression in astrocyte and increases brain lipid accumulation. Brain Behav. Immun. 2015;46:270–279. doi: 10.1016/j.bbi.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AC, Pinto V, Da Mesquita S, Novais A, Sousa JC, Correia-Neves M, Sousa N, Palha JA, Marques F. Lipocalin-2 is involved in emotional behaviors and cognitive function. Front. Cell. Neurosci. 2013;7:122. doi: 10.3389/fncel.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology. 2012;37:729–741. doi: 10.1016/j.psyneuen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav. Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J. Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallus S, Lugo A, Murisic B, Bosetti C, Boffetta P, La Vecchia C. Overweight and obesity in 16 European countries. Eur. J. Nutr. 2014 doi: 10.1007/s00394-014-0746-4. [DOI] [PubMed] [Google Scholar]
- Go YM, Kim CW, Walker DI, Kang DW, Kumar S, Orr M, Uppal K, Quyyumi AA, Jo H, Jones DP. Disturbed flow induces systemic changes in metabolites in mouse plasma: a metabolomics study using ApoE(—)/(—) mice with partial carotid ligation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015a;308:R62–R72. doi: 10.1152/ajpregu.00278.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Liang Y, Uppal K, Soltow QA, Promislow DE, Wachtman LM, Jones DP. Metabolic characterization of the common marmoset (Callithrix jacchus) PLoS One. 2015b;10:e0142916. doi: 10.1371/journal.pone.0142916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouweleeuw L, Naude PJ, Rots M, DeJongste MJ, Eisel UL, Schoemaker RG. The role of neutrophil gelatinase associated lipocalin (NGAL) as biological constituent linking depression and cardiovascular disease. Brain Behav. Immun. 2015;46:23–32. doi: 10.1016/j.bbi.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaoui S, Shim SY, Cheng YC, Corda E, Gilch S. Cholesterol balance in prion diseases and Alzheimer’s disease. Viruses. 2014;6:4505–4535. doi: 10.3390/v6114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JP, Nocon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. J. Neuroinflamm. 2011;8:124. doi: 10.1186/1742-2094-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Virtue AT, Pansuria M, Liu J, Xiong X, Fang P, Meng S, Wang H, Yang XF. The role of immunogenicity in cardiovascular disease. World Heart J. 2011;3:1–29. [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Sakata Y, Tso P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:315–321. doi: 10.1097/MCO.0b013e3283476e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Vos MB. Fructose and liver function - is this behind nonalcoholic liver disease? Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:490–495. doi: 10.1097/MCO.0000000000000203. [DOI] [PubMed] [Google Scholar]
- Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int. J. Hepatol. 2014;2014:560620. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu. Rev. Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl KG, Schweiger U, Correll C, Muller C, Busch ML, Bauer M, Schwarz P. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. 2015;5:e00306. doi: 10.1002/brb3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Karagozian R, Derdak Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metab., Clin. Exp. 2014;63:607–617. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann. NY. Acad. Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Li L, Li X, Zhou W, Messina JL. Acute psychological stress results in the rapid development of insulin resistance. J. Endocrinol. 2013a;217:175–184. doi: 10.1530/JOE-12-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013b;9:e1003123. doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard B, Matthews VB, Brandt C, Hojman P, Allen TL, Estevez E, Watt MJ, Bruce CR, Mortensen OH, Syberg S, Rudnicka C, Abildgaard J, Pilegaard H, Hidalgo J, Ditlevsen S, Alsted TJ, Madsen AN, Pedersen BK, Febbraio MA. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62:3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(—Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lo SC, Scearce-Levie K, Sheng M. Characterization of social behaviors in caspase-3 deficient mice. Sci. Rep. 2016;6:18335. doi: 10.1038/srep18335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008;29:117–124. [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol. Psychiatry. 2010;67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosom. Med. 2009;71:378–384. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav. Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Milagro FI, Claycombe KJ, Schalinske KL. Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv. Nutr. 2014;5:71–81. doi: 10.3945/an.113.004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA, Prada PO, Saad MJ, Velloso LA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatol. Res. 2013;43:139–146. doi: 10.1111/j.1872-034X.2012.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012;108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18436–18441. doi: 10.1073/pnas.1107936108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N. Immunology of major depression. NeuroImmunoModulation. 2014;21:123–130. doi: 10.1159/000356540. [DOI] [PubMed] [Google Scholar]
- Nasser A, Moller LB, Olesen JH, Konradsen LS, Andreasen JT. Anxiety-and depression-like phenotype of hph-1 mice deficient in tetrahydrobiopterin. Neurosci. Res. 2014;89:44–53. doi: 10.1016/j.neures.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Naude PJ, Eisel UL, Comijs HC, Groenewold NA, De Deyn PP, Bosker FJ, Luiten PG, den Boer JA, Oude Voshaar RC. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J. Psychosom. Res. 2013;75:444–450. doi: 10.1016/j.jpsychores.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Naude PJ, Mommersteeg PM, Gouweleeuw L, Eisel UL, Denollet J, Westerhuis LW, Schoemaker RG. NGAL and other markers of inflammation as competitive or complementary markers for depressive symptom dimensions in heart failure. World J. Biol. Psychiatry. 2015;16:536–541. doi: 10.3109/15622975.2015.1062550. [DOI] [PubMed] [Google Scholar]
- Naude PJ, Mommersteeg PM, Zijlstra WP, Gouweleeuw L, Kupper N, Eisel UL, Kop WJ, Schoemaker RG. Neutrophil gelatinase-associated lipocalin and depression in patients with chronic heart failure. Brain Behav. Immun. 2014;38:59–65. doi: 10.1016/j.bbi.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Nyima T, Muller M, Hooiveld GJ, Morine MJ, Scotti M. Nonlinear transcriptomic response to dietary fat intake in the small intestine of C57BL/6J mice. BMC Genomics. 2016;17:106. doi: 10.1186/s12864-016-2424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- Ortega FJ, Moreno M, Mercader JM, Moreno-Navarrete JM, Fuentes-Batllevell N, Sabater M, Ricart W, Fernandez-Real JM. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin. Epigenet. 2015;7:49. doi: 10.1186/s13148-015-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Lee K, Soltow QA, Strobel FH, Brigham KL, Parker RE, Wilson ME, Sutliff RL, Mansfield KG, Wachtman LM, Ziegler TR, Jones DP. High-performance metabolic profiling of plasma from seven mammalian species for simultaneous environmental chemical surveillance and bioeffect monitoring. Toxicology. 2012;295:47–55. doi: 10.1016/j.tox.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas-Romero C, Jorge-Galarza E, Posadas-Sanchez R, Acuna-Valerio J, Juarez-Rojas JG, Kimura-Hayama E, Medina-Urrutia A, Cardoso-Saldana GC. Fatty liver largely explains associations of subclinical hypothyroidism with insulin resistance, metabolic syndrome, and subclinical coronary atherosclerosis. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2014;171:319–325. doi: 10.1530/EJE-14-0150. [DOI] [PubMed] [Google Scholar]
- Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, Liang L, Curhan GC, Pasquale LR, Wiggs JL, De Vivo I, Chan AT, Choi HK, Tamimi RM, Ridker PM, Hunter DJ, Willett WC, Rimm EB, Chasman DI, Hu FB, Qi L. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ. 2014;348:g1610. doi: 10.1136/bmj.g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive-like behaviors. Brain Behav. Immun. 2016 doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol. Cell. Endocrinol. 2013;380:41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72:203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Kim EK, Landree LE, Tu Y. Fatty acid metabolism as a target for obesity treatment. Physiol. Behav. 2005;85:25–35. doi: 10.1016/j.physbeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sauriyal DS, Jaggi AS, Singh N. Extending pharmacological spectrum of opioids beyond analgesia: multifunctional aspects in different pathophysiological states. Neuropeptides. 2011;45:175–188. doi: 10.1016/j.npep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Shaibani AA, Galal E, Singh RB. Stress, peace and the heart. World Heart J. 2015;7:195–204. [Google Scholar]
- Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obesity. 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- Singh RB, Gupta S, Dherange P, De Meester F, Wilczynska A, Alam SE, Pella D, Wilson DW. Metabolic syndrome: a brain disease. Can. J. Physiol. Pharmacol. 2012;90:1171–1183. doi: 10.1139/y2012-122. [DOI] [PubMed] [Google Scholar]
- Skrzypiec AE, Shah RS, Schiavon E, Baker E, Skene N, Pawlak R, Mucha M. Stress-induced lipocalin-2 controls dendritic spine formation and neuronal activity in the amygdala. PLoS One. 2013;8:e61046. doi: 10.1371/journal.pone.0061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, O’Maille G, Want EJ, Qin C, Trauger SA, Brandon TR, Custodio DE, Abagyan R, Siuzdak G. METLIN: a metabolite mass spectral database. Ther. Drug Monit. 2005;27:747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York: Springer; 2005. Limma: linear models for microarray data; pp. 397–420. [Google Scholar]
- Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015;101:1144–1154. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AM, Swiergiel AH, Lisowski P. Epigenetics of stress adaptations in the brain. Brain Res. Bull. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Cutler RG, Button C, Telljohann R, Mattson MP. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. J. Neurochem. 2011;118:611–615. doi: 10.1111/j.1471-4159.2011.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L, Le KA. Health effects of fructose and fructose-containing caloric sweeteners: where do we stand 10 years after the initial whistle blowings? Curr. Diab. Rep. 2015;15:54. doi: 10.1007/s11892-015-0627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekola-Ayele F, Adeyemo AA, Rotimi CN. Genetic epidemiology of type 2 diabetes and cardiovascular diseases in Africa. Prog. Cardiovasc. Dis. 2013;56:251–260. doi: 10.1016/j.pcad.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Takeshita K, Yamamoto K, Kikuchi R, Nakayama T, Nomura M, Cheng XW, Egashira K, Matsushita T, Nakamura H, Murohara T. Stress augments insulin resistance and prothrombotic state: role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes. 2012;61:1552–1561. doi: 10.2337/db11-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. XMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. doi: 10.1186/1471-2105-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Tietgens AJ, LoGrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J. Cell Sci. 2012;125:4902–4912. doi: 10.1242/jcs.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reedt Dortland AK, Vreeburg SA, Giltay EJ, Licht CM, Vogelzangs N, van Veen T, de Geus EJ, Penninx BW, Zitman FG. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology. 2013;38:209–218. doi: 10.1016/j.psyneuen.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Vicario M, Guilarte M, Alonso C, Yang P, Martinez C, Ramos L, Lobo B, Gonzalez A, Guila M, Pigrau M, Saperas E, Azpiroz F, Santos J. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav. Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J. Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wang R, Jiang A, Ding Y, Wu M, Ma X, Zhao Y, He J. Abdominal obesity and its association with health-related quality of life in adults: a population-based study in five Chinese cities. Health Qual. Life Outcomes. 2014;12:100. doi: 10.1186/1477-7525-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Li W, Teo K, Wang XY, Liu LS, Yusuf S, Investigators I-HC. Association of psychological risk factors and acute myocardial infarction in China: the INTER-HEART China study. Chin. Med. J. (Engl.) 2011;124:2083–2088. [PubMed] [Google Scholar]
- Ye Z, Wang S, Yang Z, He M, Zhang S, Zhang W, Wen J, Li Q, Huang Y, Wang X, Lu B, Zhang Z, Su Q, Hu R. Serum lipocalin-2, cathepsin S and chemerin levels and nonalcoholic fatty liver disease. Mol. Biol. Rep. 2014;41:1317–1323. doi: 10.1007/s11033-013-2977-5. [DOI] [PubMed] [Google Scholar]
- Yu T, Park Y, Li S, Jones DP. Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. J. Proteome Res. 2013;12:1419–1427. doi: 10.1021/pr301053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimomra ZR, Porterfield VM, Camp RM, Johnson JD. Time-dependent mediators of HPA axis activation following live Escherichia coli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1648–R1657. doi: 10.1152/ajpregu.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.