BACKGROUND
Neonates, especially very low birth weight premature infants, are at an increased risk for infection after birth. Because of a relative deficiency in adaptive immune responses from lack of antigen exposure in utero, researchers have focused on innate immune responses with particular attention to neutrophils, since they are the first immune cells to respond to infection or inflammation. Numerous well-conducted studies have characterized global deficiencies in neonatal neutrophil function following birth. For example, impairments of transmigration through the vascular endothelium has been shown to result from (1) a reduced number of key membrane surface receptors, i.e. Mac-1 (CR-3, CD11b/CD18) and selectin [1–4,5,6–10], (2) impaired competency of signal transduction [5,11], (3) decreased mobilization of intracellular calcium [10], and (4) diminished concentrations of chemokines and cytokines from resident tissue macrophages and neutrophils [1]. Moreover, clinical stressors resulting from both infectious and non-infectious etiologies, such as premature birth and respiratory distress syndrome, have also been shown to further hinder neutrophil function [1–4,12,13].
The composition of the circulating neutrophil pool also differs significantly between neonates at birth and adults, but its impact on overall neutrophil performance remains mostly unexplored. Multiple studies have documented an increased number of the earliest developmental forms (including promyelocytes, myelocytes, and metamyelocytes), termed immature granulocytes, in neonates compared to adults but methods used for measurements have varied [14,15]. Using flow cytometric techniques to measure neutrophil composition, we have previously demonstrated that these early neutrophil precursors comprise 12% of circulating neutrophils in newborns, irrespective of labor exposure, but only 5% in healthy adults [15]. However, to date only a single study has investigated differences in neutrophil function based on cell maturity. In 1982, using microscopic evaluations of cell morphology to classify neutrophils as bands, bilobed, or multilobed, Boner and colleagues calculated that 30% of neonatal and 9% of adult neutrophils are immature and concluded that chemotactic ability was directly related to cell maturity with neonatal immature performing the least well [16].
Neutrophil development is reflected in the production of granule constituents [17–20]. Granulopoiesis, or the formation of granules within the maturing neutrophil, begins with azurophilic granules that form between the myoblast and promyelocyte stages of development and are rich in bactericidal proteins. These initial granules are followed by the formation of specific granules (myelocyte to metamyelocyte stage), gelatinase granules (band stage), and, finally, secretory granules that appear in mature, segmented neutrophils [18–21]. Secretory and gelatinase granules are important in the earliest stages of neutrophil-mediated inflammatory responses because they serve as the reservoirs for membrane-associated receptors [18]. Expulsion of granule substances, or granule exocytosis, occurs in reverse order with secretory granules being extruded first and azurophilic last. Thus, immature granulocytes have reduced concentrations of cell substrates necessary to mount a robust, effective response in the early stages of infection. Additionally, immature neutrophil numbers increase during times of stress or infection, which may explain variances in “healthy” and “ill” neonatal patients. For this study, we hypothesized that functional differences between neonatal and adult neutrophils, including chemotaxis and phagocytosis, would be directly related to differences in neutrophil composition and not generalized global deficiencies. We investigated differences in chemotaxis and phagocytosis for immature granulocytes (promyelocytes, myelocytes, and metamyelocytes) and mature neutrophils (bands and mature, segmented forms) in healthy newborns (≥37 weeks’ gestational age) born either by spontaneous vaginal delivery or by primary cesarean section without labor, and compared them to cells from healthy adult volunteers. Additionally, we evaluated a focused subset of pro-inflammatory cytokines and chemokines to determine if variations in their expression profiles would correlate to any identified functional differences.
METHODS
Neutrophil Isolation
Neutrophils were purified from the cord blood of healthy term infants, delivered vaginally (n=18) or by scheduled cesarean section without labor (n=18), and from the peripheral blood of healthy adults (n=16), using PolymorphPrep™ (Axis Shield, Oslo, Norway) according to the manufacturer's instructions. Informed consent was obtained from healthy, adult volunteers and mothers who were expected to deliver a healthy term infant at the Women and Newborn Pavilion of the Children’s Hospital at the University of Oklahoma (OU) Medical Center. Participants were enrolled in accordance with an approved protocol by the University of Oklahoma Health Sciences Center’s (OUHSC) Institutional Review Board (IRB). In brief, blood was collected into sterile tubes containing sodium citrate as an anticoagulant (Becton Dickinson, Franklin Lakes, NJ). Peripheral neutrophils were prepared from the anti-coagulated blood using gradient separation layering on PolymorphPrep™ in a one-to-one ratio. Adult samples were centrifuged at 450 × G for 35 minutes at 20°C in a swing-out rotor. PolymorphPrep™ was diluted to 90% with double distilled water for isolation of neonatal samples. The samples were then centrifuged at 400 × G for 30 minutes at 20°C in a swing-out rotor. Neutrophils were collected from the interphase, washed in Ca2+ and Mg2+-free Hanks's balanced salt solution (Life Technologies, Grand Island, NY), and collected by centrifugation at 400 × G for 12 minutes at 20°C. The supernatant was aspirated and contaminating red blood cells were lysed with the addition of 10 ml ice cold double distilled water via 15 second vortex, followed by the addition of 5 ml 3.6% NaCl and raising the volume to 50 ml with Ca2+ and Mg2+-free phosphate buffered saline (PBS; Life Technologies, Grand Island, NY).
Labeling for Flow Cytometry, Flow Cytometry, and Cell Sorting
Purified neutrophils were collected by centrifugation at 400 × G for 12 minutes at 20°C and resuspended in 100 µl of RPMI (Life Technologies, Grand Island, NY) with 10% native serum and gently vortexed for 3 seconds. Neutrophils were labeled with the addition of 15 µl of the following: CD16 (clone: NKP15) fluorescein isothiocyanate (FITC); CD11b (clone: D12) phycoerythrin (PE); and CD45 (clone: 2D1) (Becton Dickinson, Franklin Lakes, NJ). These were then incubated for 30 minutes in the dark at room temperature. After incubation, we added 400 µl of RPMI with 10% native serum and samples were placed at 4°C until analyzed by flow cytometry. No samples were stored for more than 12 hours.
Flow cytometric analysis and cell sorting was performed on an Influx cell sorter (Becton Dickinson, Franklin Lakes, NJ) located in the Flow and Image Cytometry Laboratory at OUHSC. Forward scatter (FSC), side scatter (SSC), and two-color fluorescence signals (FITC and PE at 531/40 and 572/21 nm, respectively) were collected and stored in list mode data files. A total of 10,000 neutrophil events were recorded for each sample, and a total of 50,000 cells were collected from each of the following groups: adult total neutrophils, adult mature neutrophils (bands and segmented forms), neonatal total neutrophils, neonatal mature neutrophils (bands and segmented forms), and neonatal immature granulocytes (promyelocytes, myelocytes, and metamyelocytes). The instrument settings were fixed for all data collection. Throughout the study, we performed quality control on the instrument before each measurement was taken using Flow Check beads (Beckman Coulter, Miami, FL). List mode data files were analyzed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Chemotaxis
One day before chemotaxis assays were performed, a Transwell 12-mm membrane containing 3-µm-pores (Corning Life Sciences, Tewksbury, MA) was coated with 2.5 µg/ml fibrinogen (Sigma-Aldrich Corp., St. Louis, MO) for each sample. To coat, 600 µl was added to the bottom of the Transwell and 200 µl was added to the top, and the membrane was incubated at 37°C for 1 hour. After incubation, the Transwells were washed twice with PBS. The PBS wash was aspirated off and the Transwells were dried overnight in a laminar flow hood. Purified neutrophils were counted and resuspended at a concentration of 4 × 105 cells in 200 µl Gey’s Buffer (Life Technologies, Grand Island, NY). Cells were added to the top of the Transwell. Chemoattractant, 1 × 10−7 M of N-formylmethionine-leucine-phenylalanine (fMLP; 600 µL; #F-3506, Sigma-Aldrich, St. Louis, MO) or buffer alone for control, was added to the bottom of the Transwell. Cells were allowed to migrate for 2 hours at 37°C and 5% CO2. Post incubation, 60 µl of 0.5 M EDTA was added to the bottom chamber and the plate was incubated for 15 minutes at 4°C. The number of cells was then quantified with an automated cell counter (Bio-Rad, Hercules, CA). Chemotaxed neutrophils were stained with 10 µl anti-human CD11b allophyocyanin (APC) and anti-Human CD16 phycoerythrin (PE) (eBioscience, San Diego, CA) and incubated 30 minutes at room temperature in the dark. Neutrophils were pelleted at 500 × G for five minutes and the media aspirated. Cells were resuspended in 500 µl 1-step Fix/Lyse Solution (eBioscience, San Diego, CA) for 30 minutes at room temperature in the dark. Cells were then pelleted at 500 × G for five minutes and the media aspirated and resuspended in 500 µl PBS for assessment of neutrophil composition by flow cytometry. Immature granulocytes included promyelocytes, myelocytes, and metamyelocytes, whereas mature neutrophils comprised band and segmented forms.
Phagocytosis
Whole blood was collected in sodium heparin tubes (Becton Dickinson, Franklin Lakes, NJ). Anticoagulated blood at a volume of 200 µl was transferred to a round bottom tube (Corning Life Sciences, Tewksbury, MA). To each tube 10 µl of eGFP Escherichia coli (1 × 108 E. coli/ml, K1-capsular strain RS218) was added and the control sample was transferred immediately to ice. Phagocytosis samples were transferred to a shaking incubator and incubated for 10 minutes at 37°C. Immediately after the incubation period samples were transferred to ice and stained with 10 µl anti-human CD11b APC and anti-human CD16 PE (eBioscience, San Diego, CA) and incubated 30 minutes on ice in the dark. Neutrophils were pelleted at 500 × G for five minutes and the media aspirated. Cells were resuspended in 500 µl 1-step Fix/Lyse Solution (eBioscience, San Diego, CA) for 30 minutes at room temperature in the dark. Cells were then pelleted at 500 × G for five minutes, and the media was aspirated and resuspended in 500 µl PBS for assessment of neutrophil composition by flow cytometry. To estimate GFP E.coli adherent to but not phagocytosed by the neutrophil, 1 ml of 3-mg/ml trypan blue (Invivogen, San Diego, CA) was added to the mixture of each sample to quench the fluorescence signal of extracellular bacteria after the initial analysis was completed. The residual fluorescence signal was measured again and the difference determined, which was minimal for all samples analyzed in this investigation [22]. Thus we conclude that adherent bacteria remaining on the neutrophil surface were rare. Immature granulocytes included promyelocytes, myelocytes, and metamyelocytes, while mature neutrophils comprised band and segmented forms.
Cytokine and Chemokine Gene Expression
Gene expression was quantified using a custom designed QuantiGene® 2.0 Plex Assay (Affymetrix, Santa Clara, CA) for each of the following groups: adult total neutrophils, adult mature neutrophils, neonatal total neutrophils, neonatal mature neutrophils, and neonatal immature granulocytes. The cells were pelleted by centrifugation at greater than 15,000 × G for 10 minutes. The supernatant was aspirated and 50 µl of RPMI with 10% native serum was added. We added 25 µl of working Lysis Mixture to each sample, mixing up and down 10–15 times. Samples were placed into a 55°C Vortemp for 30 minutes in order to lyse the cells. Samples were stored at −80°C until quantification. All samples were quantified according to the manufacturer’s protocol for the QuantiGene® 2.0 Plex Assay (part number 16659) using the Hand-Held Magnetic Plate Washer (Affymetrix, Santa Clara, CA).
Cytokine and Chemokine Protein Expression
Cytokine and Chemokine expression was quantified using a Procarta™ Human 7-plex Immunoassay (Affymetrix, Santa Clara, CA) for each of the following groups: adult total neutrophils, vaginal delivery cord blood neutrophils, cesarean section cord blood neutrophils, adult serum, vaginal delivery cord blood serum, and cesarean section cord blood serum. Serum was collected by centrifugation at 500 × G for 15 minutes at room temperature. Serum was transferred to a sterile tube and stored at −80°C until assayed. Purified neutrophils were pelleted and lysed with Procarta™ Cell Lysis Buffer (Affymetrix, Santa Clara, CA), debris was pelleted and supernatant was transferred to a sterile tube. Total protein was quantified with BCA assay (Pierce Biotechnology, Rockford, IL), and aliquoted for storage at −80°C. Neutrophil lysates (250 µg total protein) and 25 µl serum were quantified according to the manufacturer’s protocol for the Procarta™ Immunoassays (part number 17992-SPLB) using the Hand-Held Magnetic Plate Washer (Affymetrix, Santa Clara, CA).
Statistical Analysis
Study groups were classified into immature granulocytes (promyelocytes, myelocytes, and metamyelocytes) and mature neutrophils (bands and segmented), as well as by vaginal delivery (labor), cesarean delivery (no labor), and adult. Luminex QuantiGene® Gene Expression analysis and Luminex Procarta™ Immunoassays were completed in duplicate for all samples. Continuous variables were assessed for normality and comparisons among groups were made using ANOVA or Kruskal-Wallis tests. The student’s t-test and the Wilcoxon-Mann-Whitney test together with a Bonferroni corrected alpha level were used for post hoc comparisons of significant ANOVA and Kruskal-Wallis tests, respectively. Independent parametric and non-parametric variables were also analyzed with the student’s t-test and Wilcoxon-Mann-Whitney test, where appropriate. All analyses were carried out using either SAS 9.2 (SAS Institute, Cary NC) or Prism (GraphPad, San Diego, CA). A p-value ≤ 0.05 was considered statistically significant except for post hoc analyses in which p-values ≤ 0.05/24=0.002 (Bonferroni corrected) were considered statistically significant.
RESULTS
Neutrophil chemotaxis varies based on cell maturation but not exposure to labor
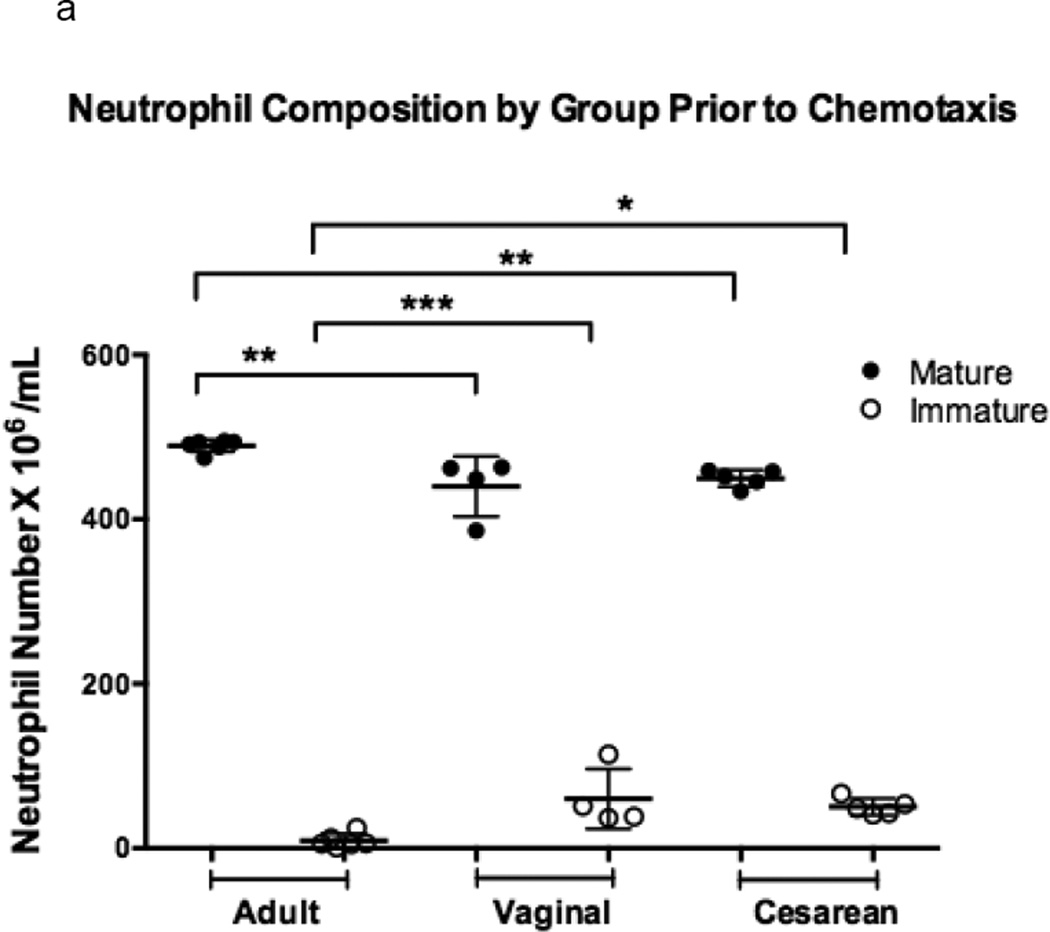
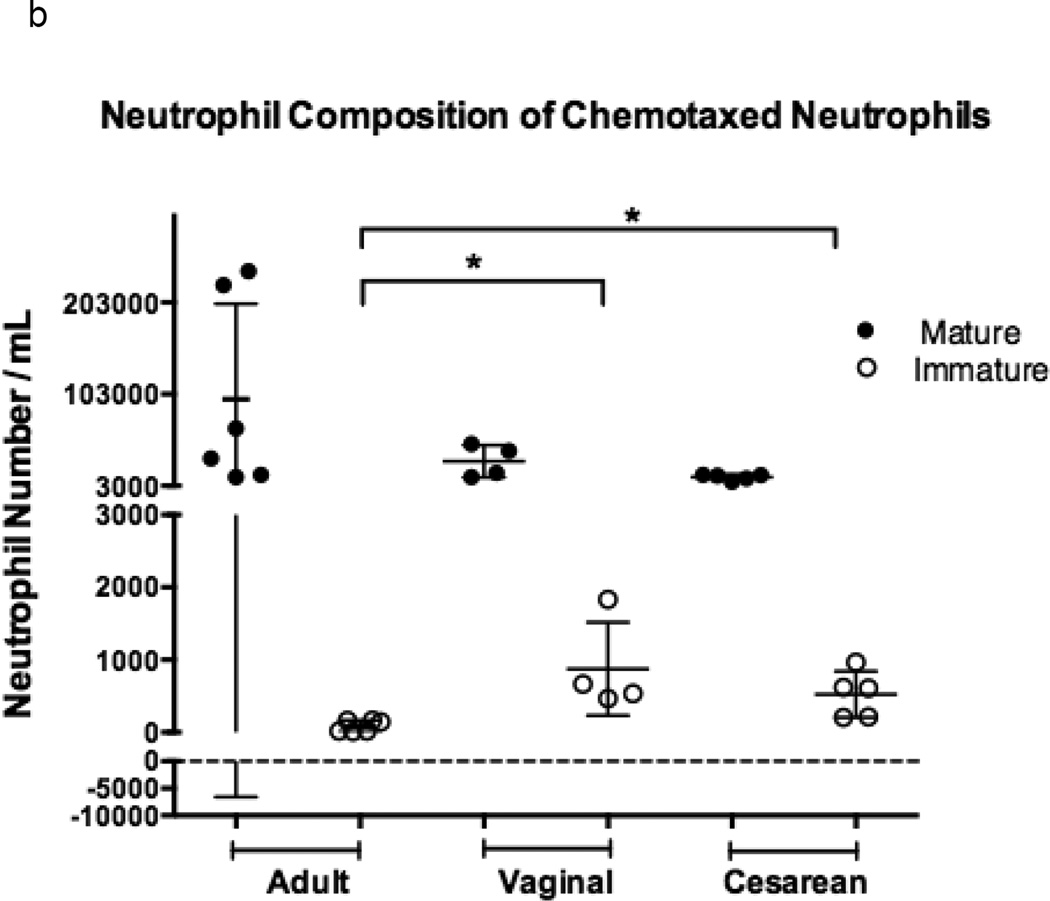
Neonatal neutrophil composition differed from that of adults prior to chemotaxis due the increased number of developmentally immature forms found in cord blood as compared to adult peripheral venous samples (p =0.001; Figure 1a) [14,15]. This relationship, though, was unchanged in the population of chemotaxed neutrophils for all experimental groups (p = 0.001; Figure 1b).
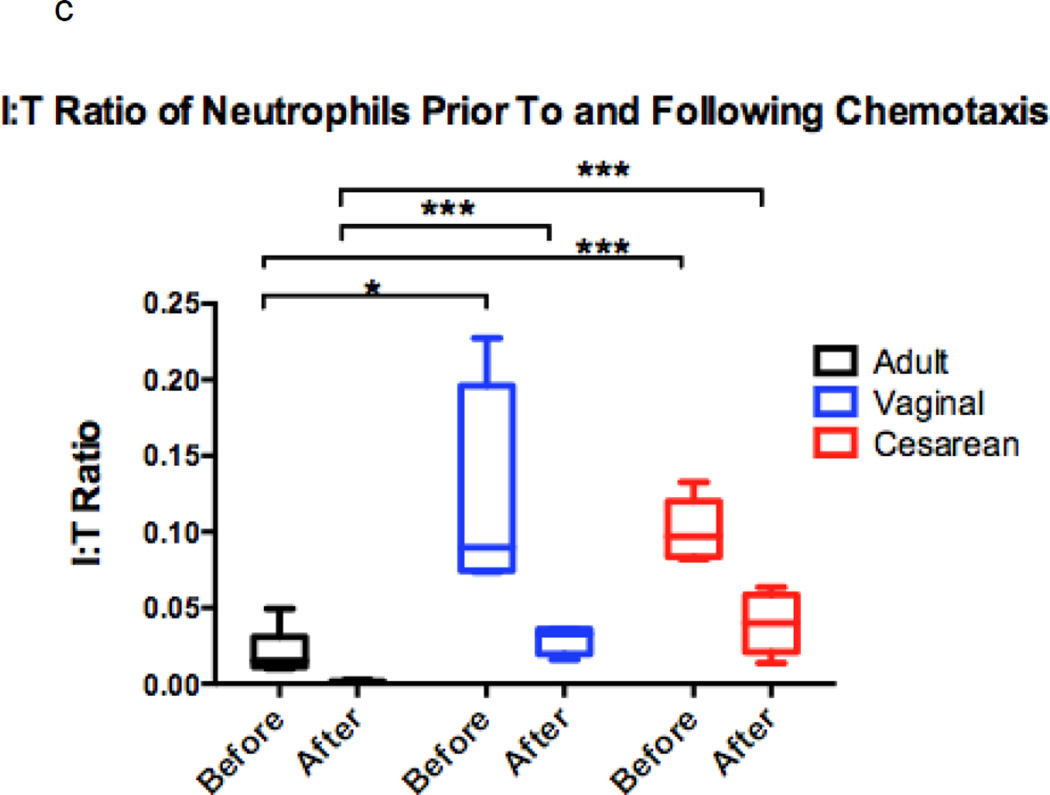
Figure 1. Comparison of chemotaxis based on neutrophil maturation between experimental groups.
Transwells containing a 12-mm diameter membrane (3 µm pores) coated with fibrinogen and using the chemoattractant, fMLP (1 × 10−7 M) or buffer alone, were used to measure chemotaxis in vaginal (n=6), cesarean (n=4,), and adult (n=5) neutrophil samples. Purified neutrophils were counted and resuspended to a concentration of 4 × 105 live cells in 200 µL Gey’s Buffer. Cells were allowed to migrate for 2 hours at 37°C and 5% CO2. Maturity of chemotaxed cells was determined by labeling of CD11b and CD16. Mature cells were defined by a population of cells high in CD11b (APC) and CD16 (PE), and consisted of segmented and banded neutrophils (closed circles). Immature neutrophils had lower levels of CD11b (APC) staining and varied levels of CD16 (PE). Metamyelocytes, promyelocytes, and myelocytes comprised the immature cell population (open circles). Neonates had a neutrophil composition consisting of a statistically higher number of immature neutrophil forms prior to (a) and (b) following chemotaxis. The total number of neutrophils completing chemotaxis was similar for all experimental groups (b). Comparison of immature to total neutrophils (I:T ratio) for each group is represented and demonstrates an increased number of immature granulocytes in neonates prior to and following chemotaxis (c). Neonates had similar findings irrespective of labor exposure (a–c). *p = 0.01, **p < 0.001, ***p< 0.0001.
Significantly more immature granulocytes were able to successfully complete chemotaxis in neonates as compared to adults. Neonates exhibited only a four-fold reduction in the number of immature neutrophils prior to and following chemotaxis (10% and 12% versus 3% and 4%; cesarean and vaginal, respectively), while adults experienced a 20-fold reduction (2% versus 0.1%). The total number of neutrophils that migrated between neonates and adults was found to be similar (p = 0.50) and neonatal groups did not differ as a result of labor exposure.
We further explored this relationship using the I:T neutrophil ratio, or the ratio of immature to total neutrophils, prior to and following chemotaxis (Figure 1c). Although neonates exhibited a higher I:T ratio compared to adults prior to and following chemotaxis, all groups experienced a reduced I:T ratio following chemotaxis, indicating that mature cells are superior to immature cells in their ability to migrate across the membrane in all experimental groups.
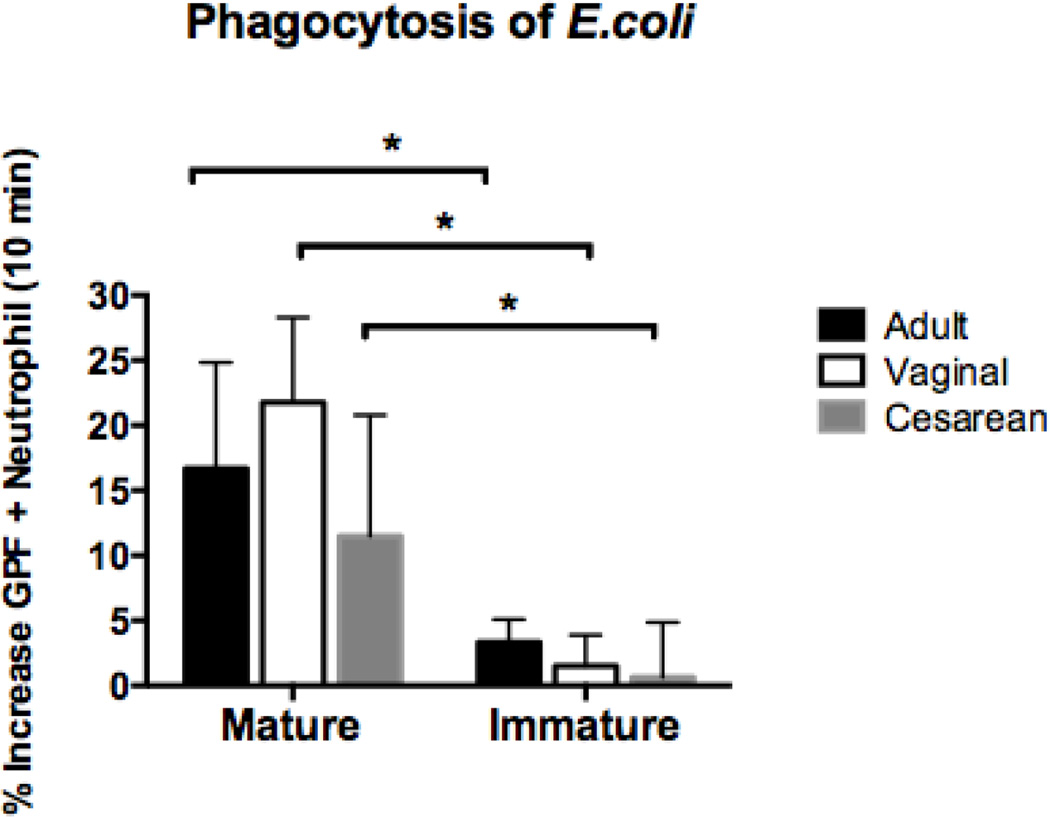
Mature but not immature neutrophils were able to phagocytose Escherichia coli. Neonatal neutrophils performed similarly, independent of labor exposure
We identified no differences in phagocytosis of this Gram-negative bacterial pathogen by neutrophils from healthy term newborns vs. adults (p = 0.65). However, considerable disparities were observed based on the maturation of the neutrophil (Figure 2). Whereas mature neutrophils from both newborns and adults were able to phagocytose E. coli equally well, immature forms performed poorly. Exposure to labor in neonates had no effect on E.coli phagocytosis.
Figure 2. Phagocytosis of E.coli based on neutrophil maturity in adults, neonates born vaginally, and neonates delivered by primary cesarean section.
GFP-labeled E.coli (RS218; 1 × 108 organisms/ml) were added to whole blood in sodium heparin tubes from vaginal (n=4), cesarean (n=5), and adult (n=7) samples. Duplicate samples were incubated at 37°C for 10 minutes. Immediately after the incubation period, the samples were transferred to ice and labeled with CD11b and CD16. Flow cytometry was used to measure mean fluorescence intensity of the neutrophils following ingestion of GFP- labeled E.coli. Mature cells were defined by a population of cells high in CD11b (APC) and CD16 (PE), and consisted of segmented and banded neutrophils. Immature granulocytes had lower levels of CD11b (APC) staining and varied levels of CD16 (PE). Metamyelocytes, promyelocytes, and myelocytes comprised the immature granulocyte population. Mature neutrophils in neonates and adults phagocytosed E.coli equally well, while immature cells performed poorly. **p < 0.001.
Variations in neutrophil gene expression and production of pro-inflammatory cytokines exist between neonates and adults
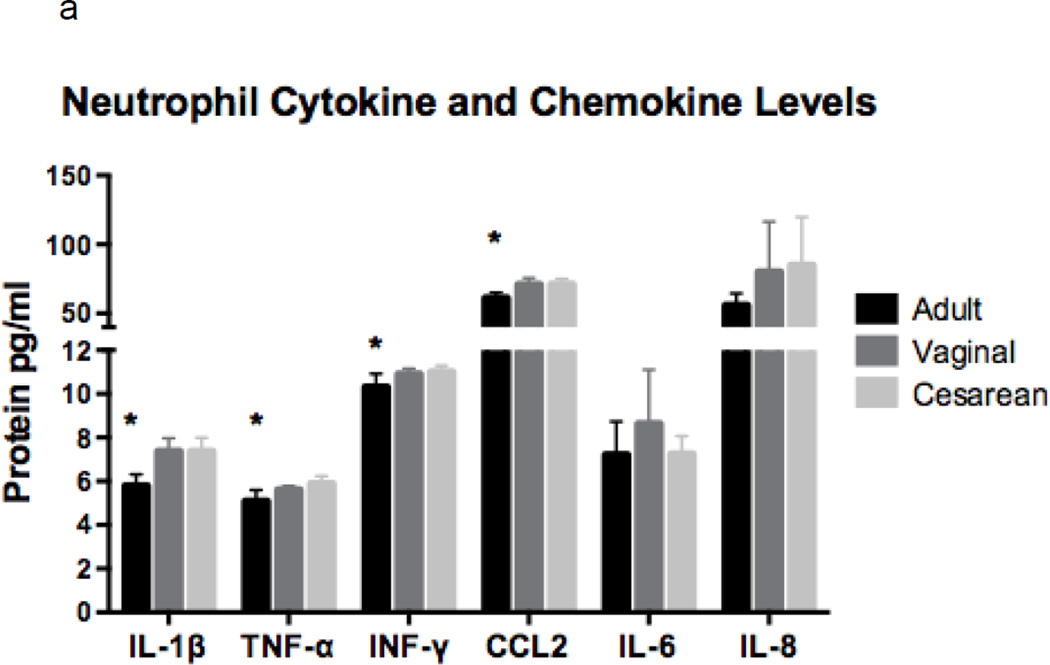
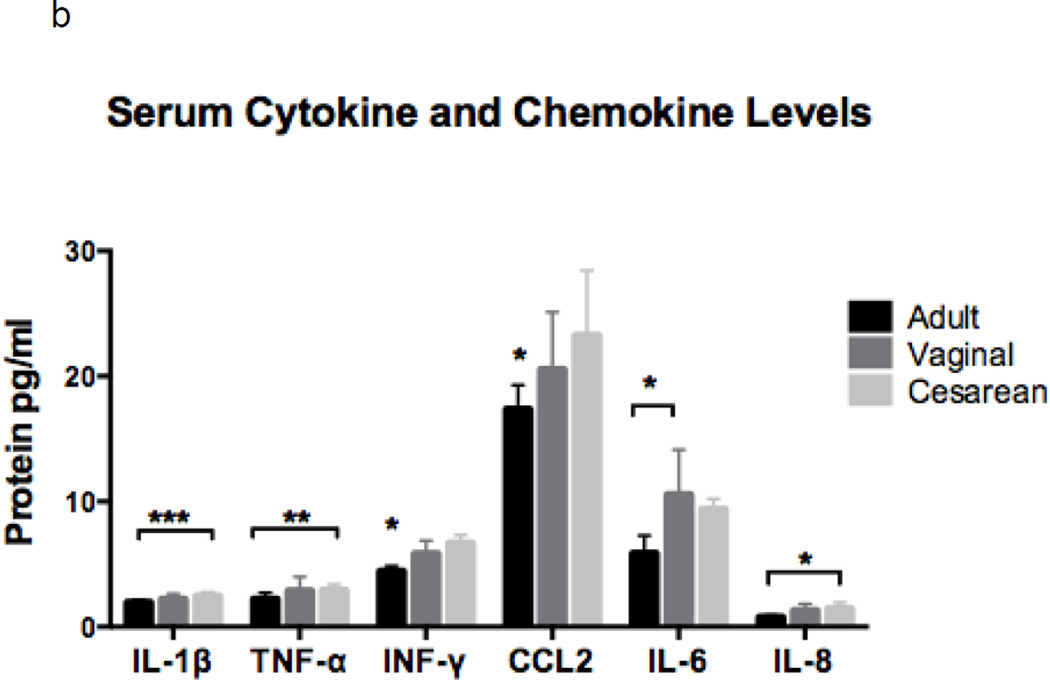
Serum concentrations of several pro-inflammatory cytokines and chemokines were assessed due to their potential release during neutrophil activation and/or processing. Additionally, these proteins can be produced and secreted by other immune cells thereby influencing neutrophil function. Newborns had similar neutrophil and serum concentrations of mediators tested whether or not they were exposed to labor (Figure 3a and b). However, when compared to adults, newborns had significantly higher neutrophil concentrations of IL-1β, TNF-α, IFN-γ, and CCL2, but equivalent levels of IL-8, IL-6, and resistin (not shown; Adult: 8663, Vaginal: 8773, and Cesarean: 9473 pg protein/mL; p=0.82).
Figure 3. Neutrophil and serum concentrations of cytokines and chemokines by experimental group.
Cytokines and chemokines were measured in neutrophils (a) and in serum (b) in adults (n=6), neonates delivered vaginally (n=7), and in neonates delivered by primary cesarean section (n=7). Neonates had similar findings, irrespective of labor exposure. Neonatal neutrophils had higher concentrations of IL1-β, TNF-α, INF-γ and CCL2 as compared to adults, while IL-6 and IL-8 was equivalent (a). Serum levels showed higher levels of IL1-β and TNF-α in cesarean as compared to adult samples, while INF-γ and CCL2 and significantly decreased in adults as compared to neonates, regardless of labor. IL-8 concentrations were different among the groups (ANOVA, p = 0.03); but, failed to meet significance when evaluated by t-test analysis. Note: Resistin was not included in the charts, due to unexpectedly high concentrations in both neutrophils and adults. Neutrophil concentrations were Adult: 8663, Vaginal: 8773, and Cesarean: 9473 pg protein/mL; p=0.82; and serum levels were Adult: 387, Vaginal: 545, and Cesarean: 729 pg protein/mL; p=0.003. *p = 0.01, **p < 0.01, ***p < 0.001.
Serum levels of pro-inflammatory proteins also varied between neonates and adults (Figure 3b). Newborns delivered by cesarean had higher quantities of IL-1β and TNF-α compared to adults, while neonates born vaginally had increased levels of IL-6 compared to adults. CCL2 and IFN-γ levels were lower in adults, but similar in both groups of newborns. IL-8 and resistin differed among experimental groups with the highest levels in cesarean born neonates, followed by those delivered vaginally, and the lowest levels in adults (resistin = Adult: 387, Vaginal: 545, and Cesarean: 729 pg protein/mL; p=0.003).
Gene expression analysis was also completed for IL-1β, IL-8, TNF-α, and resistin. Again, results were generally similar between the two neonatal groups, irrespective of labor exposure, but varied significantly for neonatal compared to adult neutrophils (supplement). Notably, gene expression profiles did not necessarily correlate with protein concentrations.
DISCUSSION
The number and function of circulating neutrophils differs between neonates at birth and adults. Neonatal neutrophils have well documented impairments in function such as deficiencies in their ability to transmigrate through the vascular endothelium, resulting from reduced numbers of key membrane surface receptors [1–4,5,6–10], diminished levels of chemokine and cytokines from resident tissue macrophages and neutrophils [1], decreased competency of signal transduction [5,11], and delayed mobilization of intracellular calcium [10]. Due to unknown factors, neutrophil numbers surge in the first 24 hours of life to reach levels never again encountered during one’s lifetime while healthy [14]. This surge occurs despite limited bone marrow neutrophil storage pools, which are estimated to be only 10% that of adults [5,23]. After reaching their peak, the number of neutrophils will then steadily decline to achieve typical adult quantities by 72 hours of life [5,14,23], a time when neutrophil function, responsiveness, deformability, and levels of cell surface adhesion normalize to adult values [1,13,25–27].
During this three-day period, the composition of the circulating neutrophil pool also varies between neonates and adults due to the increased number of developmentally immature forms found in neonates [14,27]. Outside this initial newborn period, any rise in immature neutrophil levels is typically associated with illness and is generally considered the body’s attempt to get “all hands on deck” to fight the offending pathogen(s). These undeveloped neutrophils, however, lack vital early pro-inflammatory proteins and receptors due to absent or incomplete development of their gelatinase and/or secretory granules. While these deficiencies may leave the newborn vulnerable to infection, they can also provide essential protection against the development of an acute inflammatory response as the infant becomes colonized with their microbiome postpartum.
To date, only a single study by Boner and colleagues has reported on functional differences between neonatal and adult neutrophils based on cell maturity. Similar to our present study, these authors concluded that function was directly correlated with neutrophil development and maturity [16]. Unlike our study, they determined that immature granulocytes chemotaxed the least efficiently. Methods used to classify neutrophils could explain noted differences between our studies. Boner et al used microscopic evaluation of cell morphology to characterize neutrophils as bands, bilobed, or multilobed cells, while we used surface markers and flow cytometry. Additionally, we categorized bands as mature not only due to similarities in CD11b/CD16 surface receptors and flow processing, but also based on current recommendations from the College of American Hematology and the Clinical Microscopy Resource Committee. The latter committee advocates that bands be reported with segmented neutrophils to represent the total absolute neutrophil count, suggesting that the term “immature granulocytes” denote only cells that are less mature than the band neutrophil [28–30]. Hence, we found immature forms comprised 12% versus 5% of the total neutrophil population in neonates and adults, respectively, compared to 30% versus 9% as determined by Boner and colleagues.
In summary, our data verifies that chemotaxis is dependent upon the cell’s developmental stage and suggests that chemotaxis competency ranks in the following order: adult mature > neonatal mature > neonatal immature > adult immature neutrophils. Given that newborns at birth have an abundance of circulating immature granulocytes, while adults have very few, we conclude that differences in neutrophil composition contribute to known impairments of chemotaxis in neonates [31,32]. Although we found no difference in the total number of neutrophils able to complete chemotaxis between neonates and adults, this association has been well documented and we suggest that a low sample size might explain this variation. Our data, however, demonstrates statistically significant variations exist based on neutrophil maturation. Others, including Kraus and coworkers in 1989, have also contemplated the direct association of neutrophil maturation and function when they identified a “nonmotile” subgroup of neonatal and adult neutrophils. Because this nonmotile group was inundated with bands and immature granulocytes, these investigators concluded that developmentally immature neutrophils lacked the necessary cellular components to perform chemotaxis, although this notion was not directly tested [31]. More recently, Fox and associates proposed a similar hypothesis to explain variations of chemotaxis between neonates and adults using a dose-response curve for a variety of chemokines [32].
Although our results differ from previous investigations, variations in laboratory techniques and/or handling of blood specimens may also explain variances. Studies have shown that the concentration and type of chemoattractant used could influence neonatal and adult neutrophil function [32]. For example, historical chemotaxis experiments utilized the chemoattractant, zymosan-activated serum (ZAS), and methods employing agarose assays or Boyden (or modified Boyden) chambers with various sized filter pores (from 3–8µm) [11,24,30,33]. Boyden chambers, while effective for measuring chemotaxis, require skills that are difficult to master to ensure proper use. Additionally, neutrophils were commonly resuspended in bovine or adult serum, which could alter complement factors known to vary between neonates and adults [11,25,33,34]. In this study, Transwells containing 12-mm diameter membranes with 3µm filter pores coated with fibrinogen were used to measure chemotaxis and the bacterial-derived chemoattractant fMLP was employed. We also reconstituted the subject’s neutrophils in their own serum.
Conversely, our phagocytosis experiments were conducted using whole blood, instead of isolated neutrophils, and performed with live GFP-labeled E.coli. The results were obtained using sensitive flow cytometry measurements of labeled E.coli uptake into the neutrophils. Our conclusions were consistent with previous findings, including a recent study by Filias and colleagues, who demonstrated equivocal neutrophil phagocytosis of E.coli in whole blood between term newborns and healthy adults. These investigators, however, did not discriminate function based on cell maturity so further inference cannot be made [35].
Because labor is the end result of a substantial pro-inflammatory response in the mother, we also designed this study to investigate neonatal neutrophil function following labor exposure [36,37]. We speculated that neutrophils from neonates born vaginally (with labor exposure) would have improved function compared to those delivered by primary cesarean (without labor) due to cell stimulation and/or activation. However, we found no differences in chemotaxis or phagocytosis between neonatal groups due to labor exposure. Moreover, gene expression and protein concentration of pro-inflammatory cytokines and chemokines in both serum and cord blood neutrophils were largely similar.
When compared to adults, neonates were found to have significantly higher levels of these pro-inflammatory substances despite variations in gene expression profiles. Stress associated with delivery is an unlikely cause, given similar findings between neonatal groups, irrespective of labor exposure, and our obstetrical practice of immediately clamping the cord after birth. Protein levels were also measured on naïve cells without prior activation/stimulation by noxious stimuli and newborns involved in this study were healthy and delivered at term. Elevations in cytokine and chemokine levels are, therefore, most likely due to environmental influences when compared to adults. Normal fetal development occurs in oxygen levels of 1–5% [38], which correlates to an oxygen saturation of 25–40% in the inferior and superior vena cava [39]. Towards the end of pregnancy, however, fetal size and metabolic requirements begin to surpass placental capabilities leading to further declines in oxygen concentrations [40–42]. Under similar circumstances in adults, hypoxia inducible factor 1α (HIF-1α) would most likely be activated, thereby, increasing NF-κB levels and subsequently inducing the production of TNF-α and IL-1β [38,43,44]. Accordingly, TNF-α and IL-1β concentrations were found to be significantly higher in neonatal samples in this study, while increased amounts of NF-κB have been previously documented in neonatal neutrophils and correlated with elevations of IL-8 and IL-1β [45,46]. Moreover, HIF-1α influences neutrophil motility, bacterial phagocytosis and killing, and aggregation in adult cells, but companion studies in healthy neonates are lacking and should be encouraged [47,48].
Neonatal neutrophils are known to have qualitative impairments of chemotaxis, rolling adhesion, transmigration, and lamellipodia formation [49–52]. Our results confirm that a neutrophil’s ability to perform chemotaxis and phagocytosis is directly correlated with cell maturation and development, suggesting that variations in neutrophil composition at birth may explain previously described functional impairments rather than general global neutrophil dysfunction. Noted improvements in neutrophil function, responsiveness and deformability, as well as increased levels of cell surface adhesion molecules over the first three days of life supports this conclusion as these phenotypic changes are closely associated with normalization of neonatal neutrophil numbers and composition to adult values [1,13,24–26]. Clinical interventions that push neutrophil maturity may, therefore, enhance function and reduce the risk of sepsis. Alternatively, attenuation of inflammatory responses that have evolved and adapted over time may have undesired effects as the newborn becomes colonized with both synergistic and/or opportunistic organisms. Validation of these ideas, though, will require further investigation.
The importance of the present project is that it directly compares neonatal and adult neutrophil function based upon the developmental stage of the cells. It also utilizes current techniques to demonstrate this concept while accounting for possible influences of key pro-inflammatory cytokines and chemokines that could alter neutrophil function in neonates following labor exposure. Because this study was conducted in healthy, term newborns and adults, conclusions may not necessarily be generalized to premature or acutely ill neonates, which represents a limitation to this study. Furthermore, the paucity of circulating immature neutrophils in healthy adults restricted our ability to study these cells comprehensively. While we strive to replicate in vivo mechanisms in a laboratory setting, studying neutrophils in vitro is an imperfect science. Further investigations can use these lead points to further delineate neutrophil function in this vulnerable patient population.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000 Jul;110(1):18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hadithy H, Addison IE, Goldstone AH, Cawley JC, Shaw JC. Defective neutrophil function in low-birth-weight, premature infants. J Clin Pathol. 1981 Apr;34(4):366–370. doi: 10.1136/jcp.34.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr R. The role of colony stimulating factors and immunoglobulin in the prevention and treatment of neonatal infection. Arch Dis Chil Fetal Neonatal Ed. 2013 May;98(3):F192–F194. doi: 10.1136/archdischild-2011-301269. [DOI] [PubMed] [Google Scholar]
- 4.Klebanoff SJ, Waltersdorph AM. Prooxidant activity of transferrin and lactoferrin. J Exp Med. 1990 Nov 1;172(5):1293–1303. doi: 10.1084/jem.172.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urlichs F, Speer CP. Neutrophil function in preterm and term infants. Neoreviews. 2004 Oct;5(10):e417–e429. [Google Scholar]
- 6.Kim SK, Keeney SE, Alpard SK, Schmalstieg FC. Comparison of L-selectin and CD11b on neutrophils of adults and neonates during the first month of life. Pediatr Res. 2003 Jan;53(1):132–136. doi: 10.1203/00006450-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol. 2011 Jun;90(1):74–81. doi: 10.1016/j.jri.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy LT, Zakem-Cloud H, Tosi MF. Total cell content of CR3 (CD11b|CD18) and LFA-1(CD11a|CD18) in neonatal neutrophils: relationship to gestational age. Blood. 1996 May 1;87(9):3929–3933. [PubMed] [Google Scholar]
- 9.Speer CP, Johnston RB., Jr . Neutrophil function in newborn infants. In: Pollin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia, PA: WB Saunders; 1998. pp. 1954–1960. [Google Scholar]
- 10.Taniuchi S, Kinoshita Y, Yamamoto A, Fujiwara T, Hattori K, Hasui M, Kobayashi Y. Heterogeneity in F-actin polymerization of cord blood polymorphonuclear leukocytes stimulated with FMLP. Pediatr Internat. 1999 Feb;41(1):37–41. doi: 10.1046/j.1442-200x.1999.01017.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger B1, Laskin DL, Mariano TM, Sunil VR, DeCoste CJ, Heck DE, Gardner CR, Laskin JD. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J Leukoc Biol. 2001 Dec;70(6):969–976. [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenfeld L, Krause PJ, Herson V, Savidakis J, Bannon P, Maderazo E, Woronick C, Giuliano C, Banco L. Longitudinal study of neutrophil adherence and motility. J Pediatr. 1990 Dec;117(6):926–929. doi: 10.1016/s0022-3476(05)80139-8. [DOI] [PubMed] [Google Scholar]
- 13.Carr R, Pumford D, Davies JM. Neutrophil chemotaxis and adhesion in preterm babies. Arch Dis Arch. 1992 Jul;67(7 Spec No):813–817. doi: 10.1136/adc.67.7_spec_no.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol. 2008 Apr;28(4):275–281. doi: 10.1038/sj.jp.7211916. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence SM, Eckert J, Makoni M, Pereira HA. Is the Use of Complete Blood Counts with Manual Differentials for Determining Neutrophil Composition in Newborns Antiquated?". ACLS. 2015;45(4):403–413. [PubMed] [Google Scholar]
- 16.Boner A, Zeligs BJ, Bellanti JA. Chemotactic responses of various differential stages of neutrophils from human cord and adult blood. Infect Immun. 1982 Mar;35(3):921–928. doi: 10.1128/iai.35.3.921-928.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham TN. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006 Jul;6(7):541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 18.Gullberg U, Bengtsson N, Bülow E, Garwicz D, Lindmark A, Olsson I. Processing and targeting of granule proteins in human neutrophils. J Immunol Methods. 1999 Dec 17;232(1–2):201–210. doi: 10.1016/s0022-1759(99)00177-5. [DOI] [PubMed] [Google Scholar]
- 19.Gullberg U, Andersson E, Garwicz D, Lindmark A, Olsson I. Biosynthesis, processing and sorting of neutrophil proteins: insight into neutrophil granule development. Eur J Haematol. 1997;58:137–153. doi: 10.1111/j.1600-0609.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 20.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate proteins. Trends Immunol. 2007 Aug;28(8):340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Boström EA1, Tarkowski A, Bokarewa M. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochem Biophys Acta. 2009 Dec;1793(12):1894–1900. doi: 10.1016/j.bbamcr.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JP, Carter WO, Narayanan P. Functional Assays by Flow Cytometry. In: Rose NR, de Marcario E, Folds JD, Lane HC, Nakurmura R, editors. Manual of Clinical and Laboratory Immunology. Washington, D.C., USA: American Society of Microbiology Press; 1997. pp. 245–254. [Google Scholar]
- 23.Edwards SW. Biochemistry and Physiology of the Neutrophil. first. New York: Cambridge University Press Publishing; 1994. [Google Scholar]
- 24.Fox SE, Lu W, Maheshwari A, Christensen RD, Calhoun DA. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine. 2005 Feb 7;29(3):135–140. doi: 10.1016/j.cyto.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Sacchi F, Rondini G, Mingrat G, Stronati M, Gancia GP, Marseglia GL, Siccardi AG. Different maturation of neutrophil chemotaxis in term and preterm newborn infants. J Pediatr. 1982 Aug;101(2):273–274. doi: 10.1016/s0022-3476(82)80139-x. [DOI] [PubMed] [Google Scholar]
- 26.Hilmo A, Howard TH. F-actin content of neonate and adult neutrophils. Blood. 1987 Mar;69(3):945–949. [PubMed] [Google Scholar]
- 27.Abughali N, Berger M, Tosi MF. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood. 1994 Feb;83(4):1086–1092. [PubMed] [Google Scholar]
- 28.Ferguson E, Etzell JE. To and fro on band count reporting and clinical utility. Q&A, CAP Today. 2010 Nov [Google Scholar]
- 29.Clinical and Laboratory Standards Institute (CLSI) Reference leukocyte (WBC) differential count (proportional) and evaluation of instrumental methods: approved standard. (2nd) 2007 [Google Scholar]
- 30.Cornbleet PJ, Novak RW. Classifying segmented and band neutrophils: Interlaboratory variability leads to a combining of the proficiency testing categories. CAP Today. 1994 May;:38–41. [PubMed] [Google Scholar]
- 31.Krause PJ, Kreutzer DL, Eisenfeld L, Herson VC, Weisman S, Bannon P, Greca N. Characterization of nonmotile neutrophil subpopulations in neonates and adults. Pediatr Res. 1989 May;25(5):519–524. doi: 10.1203/00006450-198905000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Yegin O. Chemotaxis in Childhood. Pediatr Res. 1983;17(3):183–187. doi: 10.1203/00006450-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Krause PJ, Herson VC, Boutin-Lebowitz J, Eisenfeld L, Block C, LoBello T, Maderazo EG. Polymorphonuclear leukocyte adherence and chemotaxis in stressed and healthy neonates. Pediatr Res. 1986 Apr;20(4):296–300. doi: 10.1203/00006450-198604000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Repo H, Jokipii AM, Leirisalo M, Kosunen TU. Leucocyte motility in the newborn: determination of spontaneous movement is essential in the in vitro assessment of neutrophil chemotaxis. Clin Exp Immunol. 1980 Jun;40(3):620–626. [PMC free article] [PubMed] [Google Scholar]
- 35.Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M. BMC Pediatr. 2011 Apr 14;11:29. doi: 10.1186/1471-2431-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dossett JH, Williams RC, Jr, Quie PG. Studies on interaction of bacteria, serum factors and polymorphonuclear leukocytes in mothers and newborns. Pediatrics. 1969 Jul;44(1):49–57. [PubMed] [Google Scholar]
- 37.Mendelson CR. Minireview Fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009 Jul;23(7):947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imanirad P, Dzierzak E. Hypoxia and HIFs in regulating the development of the hematopoietic system. Blood Cells Mol Dis. 2013 Dec;51(4):256–263. doi: 10.1016/j.bcmd.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy PJ. The fetal circulation. CEACCP. 2005 Nov;5(4):107–112. [Google Scholar]
- 40.Vorherr H. Placental insufficiency in relation to postterm pregnancy and fetal postmaturity. Evaluation of fetoplacental function; management of the postterm gravida. Am J Obstet Gynecol. 1975 Sep 1;123(1):67–103. doi: 10.1016/0002-9378(75)90951-5. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed AI, Versi E. Prolonged pregnancy. Curr Opin Obstet Gynecol. 1993 Oct;5(5):669–674. [PubMed] [Google Scholar]
- 42.Freeman RK, LaGrew DC. Prolonged Pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 2nd. New York: Churchill Livingstone; 1991. pp. 945–956. [Google Scholar]
- 43.Bhandari T, Nizet V. Hypoxia-Inducible Factor (HIF) as a Pharmacological Target for Prevention and Treatment of Infectious Diseases. Infect Dis Ther. 2014 Jun 24; doi: 10.1007/s40121-014-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009 Jul 1;315(11):1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Vancurova I, Bellani P, Davidson D. Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res. 2001 Feb;49(2):257–262. doi: 10.1203/00006450-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 46.McDonald PP, Bald A, Cassatella MA. Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood. 1997 May 1;89(9):3421–3433. [PubMed] [Google Scholar]
- 47.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1a is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005 Feb;4(2):256–258. [PubMed] [Google Scholar]
- 49.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007 May;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 50.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000 Jul;110(1):18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 51.Urlichs F, Speer CP. Neutrophil function in preterm and term infants. Neoreviews. 2004;5:e417–e430. [Google Scholar]
- 52.Fox SE, Lu W, Maheshwari A, Christensen RD, Calhoun DA. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine. 2005 Feb 7;29(3):135–140. doi: 10.1016/j.cyto.2004.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.