Abstract
Studies have shown that many smokers begin using nicotine during adolescence, yet the influence of early nicotine use on the response to other drugs of abuse in adulthood is not fully understood. In the current study, nicotine was administered to adolescent and adult rats for seven days. Thirty days later, cocaine-induced locomotor activity and cocaine self-administration were examined when the rats pretreated as adolescents were adults. Rats exposed to nicotine during early adolescence were sensitized thirty days later to the locomotor-activating effects of cocaine and self-administered a greater number of cocaine infusions than adolescent rats pretreated with vehicle. As a result of this increased intake, the cocaine self-administration dose-response curve was shifted upward indicating an increase in cocaine reinforcement. Rats pretreated with nicotine as adults, however, did not show a difference in locomotor activity or cocaine self-administration thirty days later compared to adult rats pretreated with vehicle. These findings suggest that early exposure to nicotine has long-term consequences on cocaine use. These data further suggest that nicotine use may carry a greater risk during adolescence than adulthood and adolescents who smoke may be particularly vulnerable to stimulant use.
Keywords: adolescence, cocaine, locomotor activity, nicotine, self-administration
1. INTRODUCTION
Adolescence is the peak time of initiating smoking, with most users starting prior to age 18. Teens that start smoking prior to age 14 are 5.5 times more likely to smoke late in adolescence than do teens that do not smoke prior to age 14 (Korpi et al., 2015). In addition, early initiation leads to increased mortality from lung cancer (Funatogawa et al., 2012) and an increased risk of other disorders such as depression and anxiety (Breslau et al., 1991; Breslau, 1995). In adolescents, it also has been shown that there is a progression from licit drug use, such as smoking, to illicit drug use (Hornik, 2003). The most recent National Survey on Drug Use and Health showed that in 12 to 17 year olds, 53.9 percent of smokers also used an illicit drug compared with 6.1 percent of youths who did not smoke cigarettes (NSDUH, 2014). For all smokers aged 12 and up, there is a 5-fold increase in illicit drug use compared to non-smokers, thus this increase persists into adulthood. In addition, it has been shown that cigarette use before the age of 13 leads to a 3.3-fold increase in marijuana use compared to those who had never smoked (Merrill et al., 1999). Further, the earlier the age of onset of smoking the higher the probability of future hard drug use (Lewinsohn et al., 1999).
The adolescent rat can be used to model adolescent-onset drug use and evaluate the consequences of early nicotine exposure. In male rats, nicotine reward differs as a function of age with greater reward observed in adolescent than adult rats (Dannenhoffer and Spear, 2016; Lenoir et al., 2015; Torres et al., 2008). In addition, adolescent rats acquire nicotine self-administration more quickly and self-administer more nicotine than adult rats (Levin et al., 2007; Natividad et al., 2013). Nicotine administration during adolescence leads to increased nicotine self-administration in adults (Natividad et al., 2013), whereas self-administration is not altered by adult pre-exposure to nicotine. Exposure to nicotine during adolescence also can produce unique effects on the behavioral response to other drugs administered either immediately after nicotine treatment or at a later time point, compared to nicotine exposure during adulthood. In a previous study conducted in our laboratory, adolescent and adult male rats pretreated with nicotine were sensitized to cocaine on the day after pretreatment ended, with adolescent rats showing a higher degree of sensitization than adults (Collins and Izenwasser, 2004). In addition, nicotine-pretreated adolescent rats were sensitized to the locomotor-activating effects of amphetamine immediately after pretreatment ended and 30 days later, while adult rats pretreated with nicotine were not sensitized to amphetamine-stimulated locomotor activity at either time point (Collins et al., 2004a). It also has been shown that nicotine exposure prior to adolescence (starting at PND 22 during the post-weaning period) leads to increased cocaine-primed reinstatement (Anker and Carroll, 2011). These data suggest that nicotine may produce changes in the young brain that lead to unique behavioral responses to subsequent psychostimulant drug exposure within the adolescent period and at later time points past the adolescent period and into adulthood.
Nicotine treatment in adulthood can also alter cocaine-related behaviors. Daily nicotine injections given immediately before daily cocaine self-administration sessions over a 14-day period produced a significant increase in cocaine infusions by day 8 in adult rats (Bechtholt and Mark, 2002). Further, after eight days of nicotine treatment, adult rats had a more rapid acquisition of cocaine self-administration than after vehicle treatment and once acquired, they did not continue to take more cocaine over time (Horger et al., 1992). Together, these studies show that exposure to nicotine can alter the effects of stimulant drugs in adult rats during or soon after nicotine treatment, while less is known about the long-term effects of nicotine exposure during adolescence or in adults.
In the present study, adolescent and adult rats were treated for seven days with nicotine or vehicle. Thirty days later, the effects of cocaine on locomotor activity or cocaine self-administration was measured in separate groups. The locomotor activity studies were conducted using a cumulative-dosing procedure so that an entire dose-response curve could be determined in one day. Likewise, for the self-administration studies, multiple doses of cocaine were used, so that a complete dose-response curve could be generated in one day. The purpose of these studies was to evaluate the long–term effects of nicotine on cocaine-related behaviors in adolescents compared to adults.
2. RESULTS
2.1 Experiment 1: Locomotor activity
Locomotor activity in response to cocaine was markedly influenced by nicotine pretreatment in adolescent male rats. There was a significant pretreatment drug x age interaction in response to cocaine on day 37 between the adolescent and adult rats pretreated with nicotine and vehicle (F[1,100] = 8.15, p = 0.0052; fig 1). Post-hoc tests showed that nicotine-pretreated adolescent rats had a greater locomotor response to cocaine than vehicle-pretreated adolescent rats overall (p ≤ 0.05; fig 1A) and that there were significant differences in response to 10 and 30 mg/kg cocaine. In contrast, the response to cocaine in adult rats did not differ significantly following nicotine and vehicle pretreatment (p ≥ 0.05; fig 1B). In an age-wise comparison, cocaine-induced locomotor activity was greater in nicotine-pretreated adolescent rats than nicotine-pretreated adult rats (p ≤ 0.05). The response to cocaine following vehicle pretreatment, however, did not significantly differ between the two age groups (p ≥ 0.05). Furthermore, the lack of a significant pretreatment drug x cocaine dose interaction, paired with a significant overall difference as an effect of pretreatment, suggests that the entire cocaine dose-response curve was displaced leftward in adolescent rats pretreated with nicotine relative to vehicle.
Figure 1.
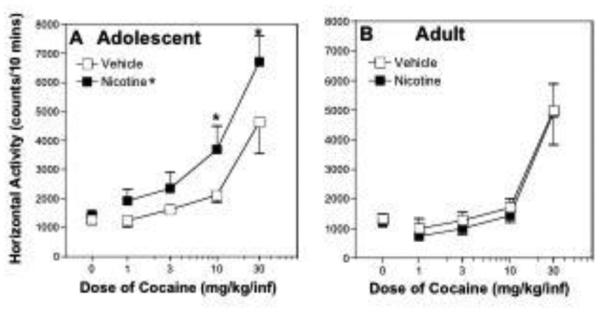
(A) Thirty days after the last injection of nicotine or vehicle, adolescent rats pretreated with nicotine were sensitized to the locomotor-activating effects of cocaine compared to adolescent rats pretreated with vehicle (PAM n=6/ group; p < 0.05). Overall, there was a significant effect of pretreatment and post hoc tests showed that nicotine significantly increased activity at the 10 and 30 mg/kg doses of cocaine compared to vehicle. (B) Adult rats pretreated with nicotine were not sensitized to the locomotor-stimulating effects of cocaine compared to adult rats pretreated with vehicle (n=5/group). *denotes a significant difference compared to vehicle controls (p < 0.05).
2.2 Experiment 2: Self-administration
As with locomotor activity, cocaine self-administration was markedly influenced by nicotine pretreatment in adolescent male rats. There was a significant pretreatment drug x age interaction between adolescent and adult rats pretreated with nicotine and vehicle (F[1,125] = 13.32, p = 0.0004; fig 2A). Post hoc tests showed that adolescent rats pretreated with nicotine self-administered a significantly greater number of cocaine infusions on day 37 than adolescent rats pretreated with vehicle (p ≤ 0.0001; fig 2A) and that there were significant increases in self-administration of 0.06, 0.125, and 0.25 mg/kg cocaine. In addition, the lack of a significant pretreatment drug x cocaine dose interaction effect shows that adolescent rats self-administered more cocaine after nicotine pretreatment than vehicle pretreatment regardless of cocaine dose, and that the entire cocaine dose-response curve was displaced upward in adolescent rats pretreated with nicotine relative to vehicle, while the shape of the curve was unchanged.
Figure 2.
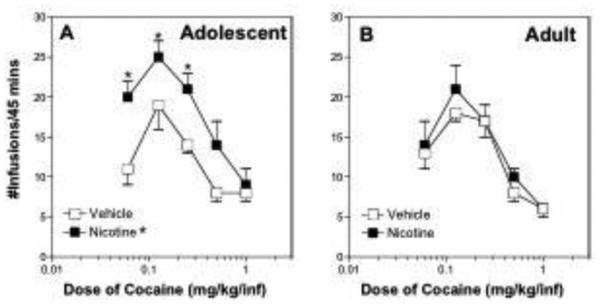
(A) Thirty days after the last injection of nicotine or vehicle, there was an overall significant increase in cocaine self-administration in adolescent rats pretreated with nicotine compared to adolescent rats pretreated with vehicle (n=6/group; p < 0.0001). Post hoc tests showed that there was a significantly greater number of infusions in the nicotine group at 0.06, 0.125, and 0.25 mg/kg/infusion of cocaine (p≤0.05). (B) There was no difference in the number of cocaine infusions taken by adult rats pretreated with nicotine compared to adult rats pretreated with vehicle (n=10/nicotine-pretreated group and n=7/vehicle-pretreated group). *denotes a significant difference compared to vehicle controls (p < 0.05).
There was no significant difference in the number of cocaine infusions self-administered between adult rats pretreated with nicotine and adult rats pretreated with vehicle (p ≥ 0.05; fig 2B). In an age-wise comparison, adolescent rats pretreated with nicotine self-administered significantly more cocaine infusions than adult rats pretreated with nicotine (p ≤ 0.0001), but there was no difference in cocaine self-administration between adolescent and adult rats pretreated with vehicle (p ≥ 0.05, (compare vehicle groups in Fig. 2A and 2B).
3. DISCUSSION
All of the rats used in these experiments were adults at the time of behavioral testing. Nonetheless, we continue to refer to the rats as “adolescents” and “adults” to reflect the age at which they were pretreated. It also should be noted that at the time of behavioral testing, the rats pretreated with nicotine as adults were 30 days older than the rats pretreated with nicotine as adolescents; thus, the two groups were possibly in different stages of adulthood when exposed to cocaine. Our data, however, show that there are no differences in either cocaine-stimulated locomotor activity or cocaine self-administration as a function of age in the vehicle-pretreated rats.
3.1 Locomotor activity
Thirty days after a seven-day pretreatment with nicotine, adolescent rats were sensitized to the locomotor-activating effects of cocaine, whereas the response of adult rats was not changed, compared to vehicle pretreatment. This is consistent with our previous studies showing that adolescent male rats pretreated with nicotine for seven days were sensitized to the locomotor-activating effects of cocaine and amphetamine one day after the pretreatment period, on day 8 (Collins and Izenwasser, 2004; Collins et al., 2004a) and to amphetamine 30 days later, on day 37 (Collins et al., 2004a). Thus, sensitization to the effects of nicotine is not evident only immediately after exposure to nicotine, but rather persists into adulthood.
In the current study, adult rats pretreated with nicotine were not sensitized to the locomotor-stimulant effects of cocaine 30 days later, compared to adult rats pretreated with vehicle, in contrast to the adolescent rats. In our previous study, we found that adult male rats were significantly less sensitized to the locomotor-activating effects of cocaine than adolescent male rats (Collins and Izenwasser, 2004) and did not sensitize to the effects of amphetamine (Collins et al., 2004a) one day after nicotine pretreatment. Similarly, a previous study showed that nicotine treatment for nine days in adult male rats did not produce sensitization to cocaine-stimulated locomotor activity on the next day (Schenk et al., 1991). Adult male rats also were not sensitized to amphetamine-induced locomotor activity thirty days after nicotine pretreatment (Collins et al., 2004a), similar to our current results with cocaine. Thus, unlike the adolescent rats, in which nicotine-induced sensitization to stimulants persists 30 days later, the adult rats show no evidence of long-term sensitization to stimulants following nicotine exposure.
It is interesting that nicotine produces cross-sensitization to cocaine and amphetamine in adolescents. Our prior studies showed that while sensitization developed to the locomotor-stimulant effects of nicotine in adults, this was not evident in adolescent rats (Collins et al., 2004a; Collins et al., 2004b). These findings correlated with changes in nicotine receptors in that daily injections of nicotine increased receptor density in the caudate putamen and nucleus accumbens of adult, but not adolescent rats (Collins et al., 2004b). Thus, changes in nicotine receptor densities in brain regions that mediate locomotor activity and/or drug reward do not appear to be correlated with changes in the response to other stimulants.
In vehicle-pretreated animals, there were no differences in cocaine-stimulated locomotor activity regardless of age. This is not entirely surprising since all of the animals were adults at the time of cocaine testing even though the pretreatment period occurred during either adolescence or adulthood. This information is interesting on its own, in that it shows that cocaine-stimulated locomotor activity is constant during early to mid-adulthood.
3.2 Self-administration
In separate groups of rats, cocaine self-administration was examined 30 days after pretreatment with nicotine or vehicle. Similar to the results of the locomotor activity studies, there were no significant differences in cocaine self-administration between rats pretreated with vehicle either during adolescence or as adults. Again, this is not surprising, because all of the rats were adults at the time of testing even though the adult pretreated rats were older than the adolescent pretreated rats. This further shows that cocaine self-administration is consistent across adulthood in rats.
Rats exposed to nicotine during early adolescence self-administered a greater number of cocaine infusions as adults than did rats that were exposed to vehicle as adolescents or to vehicle or nicotine as adults. Increases were seen across the full range of cocaine doses, with effects observed on both the ascending and descending limbs of the curve. These data are consistent with the recent findings that that there were increases in self-administration of doses on the descending limb of the cocaine self-administration dose-response curve (i.e. in that study only doses on the descending limb were used) in mice subsequent to long-term (28 days) continuous infusion of nicotine during adolescence (Dickson et al., 2014). It also has been shown that adolescent rats acquire cocaine self-administration behavior more rapidly after very low doses of nicotine (0.03 or 0.1 mg/kg) than after vehicle injections (McQuown et al., 2007). Thus, it appears that these findings cross species, and that sensitization to the reinforcing effects of cocaine occurs after a much shorter period of exposure to nicotine during adolescence (7 days in the current study). In contrast to the self-administration studies, mice pretreated with nicotine through adolescence showed a reduction in cocaine-induced conditioned place preference and cocaine drug discrimination in adulthood, however since only one dose of cocaine was tested, it is difficult to know in which direction the curve was shifted (Kelley and Middaugh, 1999). A study in rats showed that cocaine CPP was increased in adults exposed to nicotine for 10 days during adolescence (McMillen et al., 2005). In contrast to the results in adolescence, a previous study in adult rats showed that nicotine increased the rate at which subsequent cocaine self-administration was acquired one day after the pretreatment ended, but that the increased response rate did not continue past the acquisition stage (Horger et al., 1992). Our dose-effect curves confirm and extend these findings that there is no long-term increase in cocaine self-administration subsequent to adult-onset nicotine administration.
The present data suggest that there are changes occurring in the adolescent brain during nicotine exposure that may cause different behavioral responses at distinct time points past the adolescent period and into adulthood. This is supported by a number of studies that have shown neurochemical changes subsequent to adolescent exposure to nicotine. Since adolescence into young adulthood is a really important period of brain maturation (Spear, 2013 for review) and nicotine has been shown to produce changes in neuroplasticity in adulthood, one might conclude that the effects of nicotine would be even greater, or more detrimental, during the maturational period. Korpi and colleagues have elegantly discussed the effects of nicotine use on brain development, including during adolescence, in a recent review paper (Korpi et al., 2015). For example, it has been shown that adolescent but not adult exposure to nicotine alters serotonin receptors in the cerebral cortex (Slotkin and Seidler, 2009), decreases striatal serotonin activity and content (Slotkin and Seidler, 2007) increases dopamine transporter densities and decreases serotonin transporter densities in the striatum (Collins et al., 2004b), and can lead to increased dopamine D2 receptor function (Dao et al., 2011; McQuown et al., 2007). Nicotine-induced increases in dopaminergic function and decreases in serotonergic function all can contribute to increased rewarding properties of cocaine. Together, these data show that the effects of nicotine on cocaine during early adolescence are not behavior-specific, since both cocaine-stimulated locomotor activity and cocaine self-administration are increased. This suggests the possibility that these two behaviors may be mediated via the same mechanisms.
Nicotine exposure during early adolescence alters the effects of subsequent stimulant drug administration. In the current study, adolescent rats pretreated with nicotine showed sensitization to the locomotor-activating effects of cocaine and an increase in the number of self-administered cocaine infusions 30 days after nicotine treatment, once the rats were adults. Neither of these effects was observed in rats exposed to nicotine as adults. Thus, both the stimulant and reinforcing effects of cocaine are elevated in rats exposed to nicotine during adolescence. Even though all groups were adults at the time of testing, it appears that the critical factor was the time of initial exposure to nicotine. These data suggest that adolescent males who smoke cigarettes may be at an increased risk for subsequent stimulant use disorders, compared to adult-onset cigarette smokers.
4. EXPERIMENTAL PROCEDURE
4.1 Chemicals
Drugs were obtained from the following sources: (−)-Nicotine hydrogen tartrate salt from Sigma Chemical Co. (St. Louis, MO); cocaine hydrochloride from the National Institute on Drug Abuse (Rockville, MD).
4.2 Subjects
Sprague-Dawley rats (Charles River, Wilmington, MA) were used. Adolescent rats weighing an average of 88 ± 1 g (corresponding to approximately postnatal day 30 at the start of the experiment) and adult rats weighing 284 ± 1 g (approximately postnatal day 60 at the start of the experiment) were housed in individual cages in a temperature and humidity-controlled environment under a 12 h light/dark cycle. All of the adolescent rats were pre-pubertal at the start of the experiments. Between experimental sessions, the rats had continuous access to water in their home cages. Food was available ad libitum for rats in the locomotor activity experiment. For rats in the self-administration experiment, food was available ad libitum during the nicotine exposure period. During food shaping and cocaine self-administration sessions, food was restricted to approximately 16 g per day.
4.3 Pretreatment
All rats were injected once daily for seven days with 0.4 mg/kg nicotine/day (dose was based on weight of the base; i.p.) or vehicle (saline). Our prior studies showed that this dose of nicotine produced similar rewarding effects in adolescent and adult male rats (Lenoir et al., 2015). This dose has been shown to produce blood levels similar to those following smoking in humans. In addition, doses at or near 0.4 mg/kg nicotine are commonly used doses for preclinical behavioral studies of nicotine (Ahsan et al., 2014; Buffalari et al., 2016; Casarrubea et al., 2015).
4.4 Experiment 1: Locomotor Activity
4.4.1 Locomotor Activity Apparatus
Rats were placed in clear acrylic chambers (16 × 16 inches) inside Digiscan activity monitors (Omnitech Electronics, Columbus, OH) that were equipped with infrared light sensitive detectors mounted 2.5 cm apart along two perpendicular walls. Mounted along the opposing walls were infrared light beams that were directed at the detectors. One count of horizontal activity was registered each time the subject interrupted a beam. Animals were maintained on a 12 h light/dark schedule with lights on at 7 a.m. and off at 7 p.m. All behavioral testing was completed during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour each day and the groups randomized over the course of the day.
4.4.2 Locomotor Activity Testing
Thirty days after the last injection of nicotine or vehicle, all rats were injected with vehicle (saline), followed by 1.0, 3.0, 10.0 and 30.0 mg/kg cocaine (i.p.) in a cumulative dosing regimen (actual injections of 1.0, 2.0, 7.0 and 20.0 mg/kg cocaine) as described previously (Collins and Izenwasser, 2004). Following vehicle injections and each cumulative dose of cocaine, locomotor activity was measured for a 10-min period. An earlier study by Terry (Terry, 1992) showed that this time course produced the same results as testing individual doses on different days. Since this study is focused on the effects of cocaine during development, the ability to test all of the doses at once eliminated the potential variability of changes in development while testing multiple doses of a drug. Timeline: Days 1-7 pretreatment, day 37 locomotor activity.
4.4.3 Locomotor Activity Data analysis
Locomotor activity data were analyzed using a three-way (cocaine dose × pretreatment drug × age) Analysis of Variance (ANOVA) with repeated measures. Significant interactions were followed by tests for simple treatment (drug) effects and Fisher's Protected Least Significant Difference (PLSD) was used for post hoc analysis when warranted. P values less than 0.05 were considered significant for all tests.
2.5 Experiment 2: Self-administration
4.5.1 Self-administration Apparatus
Experimental chambers for rats (Med Associates, Model ENV-008CT, East Fairfield, Vt.) equipped with two response levers, a pellet dispenser, which emits an audible click during delivery of 45-mg food pellets (Noyes, Traditional Formula, Lancaster, N.H.); and a white stimulus light, which is mounted 7 cm above the active lever, were used. Pressing the inactive lever had no scheduled consequences. Each chamber was outfitted with a single channel fluid swivel and spring leash assembly, which was connected to a counterbalanced arm assembly (Med Associates). Each chamber was enclosed within a sound-attenuating cubicle (Med Associates, Model ENV-018 M) equipped with an overhead light to provide general illumination and a fan to provide ventilation and mask extraneous sounds. Cocaine was delivered to rats via 3.33 r.p.m. syringe pumps (Med Associates). A 486 AT-compatible computer programmed in Medstate Notation and connected to an interface (Med Associates) that controls experimental events was used.
4.5.2 Surgery
Ten days after the end of the pretreatment period, rats were anesthetized with i.p. injections of 90 mg/kg ketamine plus 10 mg/kg xylazine. Incisions were made to expose the right jugular vein and skull, and a catheter made from silicon tubing (I.D. 0.51 mm, O.D. 0.94 mm) was implanted as described previously (Collins and Kantak, 2002; Kantak et al., 2000). The catheters were maintained by flushing them daily (Sunday through Saturday) with 0.1 ml 0.9% saline solution containing 0.3 IU heparin (LymphoMed, Inc., Rosemont, Ill.) and 6.7 mg timentin (SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.). Catheters were checked daily for leaks and as needed for function by infusing 0.1 ml of a solution containing 1.0 mg methohexital sodium (Brevital, Eli Lilly and Co., Indianapolis, Ind.) and noting the presence or absence of sedation. The catheters in two adult male rats became non-functional before self-administration training began and new catheters were implanted into the right femoral vein.
4.5.3 Self-administration training and testing
Three days after the end of the pretreatment period, rats were trained to press a lever for the delivery of food pellets over a 7-day period of food training. This was done to try to minimize the variability in acquiring self-administration. Because it was important to try to have all of the animals doing the self-administration part of the experiment in close temporal proximity (due to the developmental factors involved in studying adolescence and to try to minimize variability in the amount of time between nicotine pretreatment and self-administration testing), and there is variability in learning to lever press for a reinforcer, food training minimized the differences across animal during the cocaine self-administration studies. After the food training period was completed, jugular vein catheters were implanted, followed by a 10-day recovery period before daily 45 min cocaine self-administration sessions began. Rats were trained over a 10-day self-administration training period to self-administer 0.25 mg/kg cocaine on a fixed ratio 1 (FR1) schedule of operant responding where every lever press (FR1) resulted in a cocaine infusion. A 20 sec timeout period, during which lever presses had no scheduled consequences, followed each presentation of a reinforcer. Responding was considered stable when there was less than 20% variability across three consecutive days. Sessions were conducted Monday through Friday during the light phase.
Once response rates were stable, a dose of 0.25 mg/kg cocaine was available daily for 45 min until testing began on day 37 of the study, 30 days after the nicotine pretreatment ended. At that time, each rat was exposed to a full cocaine dose–response curve. Cocaine was delivered at a rate of 1.8 ml/min and concentrations of all cocaine doses were adjusted to ensure that drug delivery times remained constant for each dose. The doses, tested in a descending series, were 1.0, 0.5, 0.25, 0.125, 0.06 mg/kg. Each dose was available for 45 min. There were 45 min intervals between doses, during which rats were removed from the box, catheters were flushed with 0.1 ml 0.9% saline solution containing 0.3 IU heparin, and returned to their home cages. Timeline: Days 1-7 pretreatment, days 10-16 food training, day 17 surgery, day 27 self-administration training began, day 37 testing.
4.5.4 Self-administration data analysis
Data were analyzed by a three-way (cocaine dose × pretreatment drug × age) Analysis of Variance (ANOVA) with repeated measures. Significant interactions were followed by tests for simple treatment effects and Fisher's Protected Least Significant Difference (PLSD) was used for post hoc analysis when warranted. P values less than 0.05 were considered significant for all tests.
Highlights.
Adolescent, but not adult, rats exposed to nicotine exhibit an increased response to cocaine as adults
Adolescent, but not adult, rats exposed to nicotine self-administer more cocaine as adults
Nicotine use may carry a greater risk during adolescence than adulthood
Adolescents that smoke may be particularly vulnerable to stimulant use as adults
ACKNOWLEDGMENTS
We would like to thank Dr. Kathleen Kantak for her helpful comments on a previous version of this manuscript. The animals used in this study were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1985. This work was supported by NIDA grants DA 13936, DA 15119, and DA 15947.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahsan HM, de la Pena JB, Botanas CJ, Kim HJ, Yu GY, Cheong JH. Conditioned place preference and self-administration induced by nicotine in adolescent and adult rats. Biomol Ther (Seoul) 2014;22:460–6. doi: 10.4062/biomolther.2014.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Adolescent nicotine exposure sensitizes cue-induced reinstatement of cocaine seeking in rats bred for high and low saccharin intake. Drug Alcohol Depend. 2011;118:68–72. doi: 10.1016/j.drugalcdep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology. 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–74. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Mollica JK, Smith TT, Schassburger RL, Rinaman L, Thiels E, Donny EC, Sved AF. Nicotine Enhances Footshock- and Lithium Chloride-Conditioned Place Avoidance in Male Rats. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarrubea M, Davies C, Faulisi F, Pierucci M, Colangeli R, Partridge L, Chambers S, Cassar D, Valentino M, Muscat R, Benigno A, Crescimanno G, Di Giovanni G. Acute nicotine induces anxiety and disrupts temporal pattern organization of rat exploratory behavior in hole-board: a potential role for the lateral habenula. Front Cell Neurosci. 2015;9:197. doi: 10.3389/fncel.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Kantak KM. Neuronal nitric oxide synthase inhibition decreases cocaine self-administration behavior in rats. Psychopharmacology. 2002;159:361–369. doi: 10.1007/s00213-001-0935-8. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Developmental Brain Research. 2004a;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. European Journal of Pharmacology. 2004b;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Dannenhoffer CA, Spear LP. Age differences in conditioned place preferences and taste aversions to nicotine. Dev Psychobiol. 2016;58:660–6. doi: 10.1002/dev.21400. [DOI] [PubMed] [Google Scholar]
- Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology. 2011;36:1319–31. doi: 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Miller MM, Rogers TD, Blaha CD, Mittleman G. Effects of adolescent nicotine exposure and withdrawal on intravenous cocaine self-administration during adulthood in male C57BL/6J mice. Addict Biol. 2014;19:37–48. doi: 10.1111/j.1369-1600.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatogawa I, Funatogawa T, Yano E. Impacts of early smoking initiation: long-term trends of lung cancer mortality and smoking initiation from repeated cross-sectional surveys in Great Britain. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hornik R. Alcohol, tobacco, and marijuana use among youth: same-time and lagged and simultaneous-change associations in a nationally representative sample of 9- to 18-year-olds. In: Romer D, editor. Reducing adolescent risk: toward an integrated approach. Sage Publications; Thousand Oaks, CA.: 2003. pp. 335–343. [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. Journal of Addictive Diseases. 1999;18:27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, Hyytia P, Dawe GS. Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. Pharmacol Rev. 2015;67:872–1004. doi: 10.1124/pr.115.010967. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B, Izenwasser S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–65. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rhode P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94:913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Davis BJ, Williams HL, Soderstrom K. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. European Journal of Pharmacology. 2005;509:161–164. doi: 10.1016/j.ejphar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JC, Kleber HD, Shwartz M, Liu H, Lewis SR. Cigarettes, alcohol, marijuana, other risk behaviors, and American youth. Drug and Alcohol Dependence. 1999;56:205–212. doi: 10.1016/s0376-8716(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O'Dell LE. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav Brain Res. 2013;257:275–85. doi: 10.1016/j.bbr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSDUH . Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- Schenk S, Snow S, Horger BA. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology. 1991;103:62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. A unique role for striatal serotonergic systems in the withdrawal from adolescent nicotine administration. Neurotoxicol Teratol. 2007;29:10–6. doi: 10.1016/j.ntt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Nicotine exposure in adolescence alters the response of serotonin systems to nicotine administered subsequently in adulthood. Dev Neurosci. 2009;31:58–70. doi: 10.1159/000207494. [DOI] [PubMed] [Google Scholar]
- Terry P. Differential effects of injection regimen on behavioral responses to cocaine. Pharmacology Biochemistry and Behavior. 1992;41:365–369. doi: 10.1016/0091-3057(92)90112-s. [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


