Abstract
Background
Primary hyperparathyroidism (PHPT) is increasing in adults but rarely reported in young patients where routine blood work is obtained more judiciously. We aim to determine how PHPT is currently being diagnosed in young patients and examine surgical outcomes.
Method
We retrospectively analyzed PHPT patients 24 years of age or less who underwent parathyroidectomy from 2001 to 2014. Patients were divided into 2 time periods: 2001 to 2007 (A) and 2008 to 2014 (B). Incidentally, diagnosed patients lacked objective symptoms of PHPT and had no family history.
Results
Forty young patients met inclusion criteria: 16 in group A and 24 in group B. Those in group A compared with group B had similar mean age, preoperative calcium, and parathyroid hormone (P > .05). Incidental diagnosis was more common in the contemporary group (42% vs 25%, P = .001).
Conclusions
Current diagnosis of PHPT in young patients is increasingly incidental. This trend may be attributed to the more liberal use of labs in younger patients.
Keywords: Primary hyperparathyroidism, Pediatric hyperparathyroidism, Adolescent hyperparathyroidism
Primary hyperparathyroidism (PHPT) is a rare finding in young patients. We examined patients <24 years with PHPT and how they came to be diagnosed. We found that more contemporary patients had increasingly incidental diagnosis of PHPT.
The incidence of primary hyperparathyroidism (PHPT) is increasing in the adult population, due in part to the implementation of routine calcium screening.1 Therefore, the clinical profile of adults with PHTP is also increasingly nonspecific and asymptomatic. PHPT, however, is rarely reported in children and young adults, where routine blood work is often obtained more judiciously. The incidence of PHTP in young patients is far less common than in adults, with 2 to 5 cases per 100,000 in young patients vs 1 in 1,000 in adults,2–4 which argues against routine screening. Younger patients tend to present with increased disease severity,5,6 and delay in evaluating young people for parathyroid disorders can lead to significant end-organ damage at presentation, such as nephrocalcinosis and pathologic fracturing.
As more is being understood about hyperparathyroidism in younger patients, there is increased awareness of sporadic PHPT in this population with its associated nonspecific symptoms. A recent literature review focusing on hyperparathyroidism in pediatric and adolescents report the incidence of PHPT may be underestimated, and to consider a lower threshold for ordering screening calcium levels to allow earlier diagnosis of parathyroid dysfunction.7 As these patients are all younger than 50 years, they meet current consensus guidelines for parathyroid surgery.8,9 In addition, the literature further supports that parathyroid surgery in young patients with PHPT is safe and effective.6,7,10–12 The aims of this study were to determine how PHPT is currently being diagnosed in young patients, and to assess characteristics and outcomes of parathyroid surgery in this population. We hypothesized that there has been a change over time in how young patients are being diagnosed with PHPT at our institution.
Methods
A retrospective analysis was performed on a surgical parathyroid database to include all patients who underwent parathyroidectomy for PHPT between January 1, 2001 and December 31, 2014. We defined surgical cure as normocalcemia at 6-months after surgery, and all the patients had a minimum of 6-months of follow-up. We chose age 24 years or less as a designated cut-off for young age based on the lowest 2 percentile groups in our database. Young patients were divided into 2 equal time periods for analysis: those who underwent surgery from 2001 to 2007 (A) and those who had surgery from 2008 to 2014 (B). Patients were divided into these groups to identify and compare any changes in our cohort over time. To determine which patients were incidentally diagnosed, we looked for those patients with a negative family history who lacked objective symptoms of PHPT such as kidney stones, bone pain, and hypercalcemic crisis. We examined parathyroid surgery outcomes in all patients, and then specifically looked at outcomes for patients with a positive family history. Recurrence was defined as hypercalcemia after 6 months after parathyroidectomy. Categorical variables were analyzed using chi-square test or Fisher's exact-test, and independent t tests were used to compare continuous variables. All statistical calculations were performed using SPSS (IBM SPSS Statistics for Windows, version 22.0. Armonk, NY: IBM Corp.)
Results
Patient selection and demographics
There were a total of 40 patients aged 24 years or less (range age 10 to 24) who underwent parathyroidectomy for PHPT between 2001 and 2014. This comprised 1.5% (40/2,601) of our total parathyroidectomy volume during this time period. The population demonstrates a slight female predominance (1.7:1). The PHPT patients were then divided into those undergoing surgery in 2 equal time periods, 16 individuals were in group A (2001 to 2007) and 24 in group B (2008 to 2014; Fig. 1). Patient characteristics remained comparable between the 2 time periods. There was no difference between patients in group A and B in terms of family history (P = .73), preoperative calcium (11.8 mg/dL vs 11.5 mg/dL, P = .50) and parathyroid hormone (118 pg/mL vs 187 pg/mL, P = .18; Table 1).
Figure 1.
Patient selection and groupings.
Table 1. Patient demographics.
| Variable | Group A, 2001–2007 (n = 16 patients) | Group B, 2008–2014 (n = 24 patients) | P value |
|---|---|---|---|
| Age, y (median [range]) | 19 [10–23] | 19 [10–24] | .79 |
| Sex | 1.00 | ||
| Male | 6 (37.5%) | 9 (37.5%) | |
| Female | 10 (62.5%) | 15 (62.5%) | |
| Positive family history* | 4 (25%) | 8 (33.3%) | .73 |
| Preoperative calcium (mg/dL; mean ± SEM) | 11.8 ± .3 | 11.5 ± .2 | .50 |
| Preoperative PTH (pg/mL; mean ± SEM) | 118 ± 21 | 187 ± 46 | .18 |
PTH = parathyroid hormone; SEM = standard error of the mean.
Family history includes multiple endocrine neoplasia type I and familial hyperparathyroidism.
Diagnosis of primary hyperparathyroidism
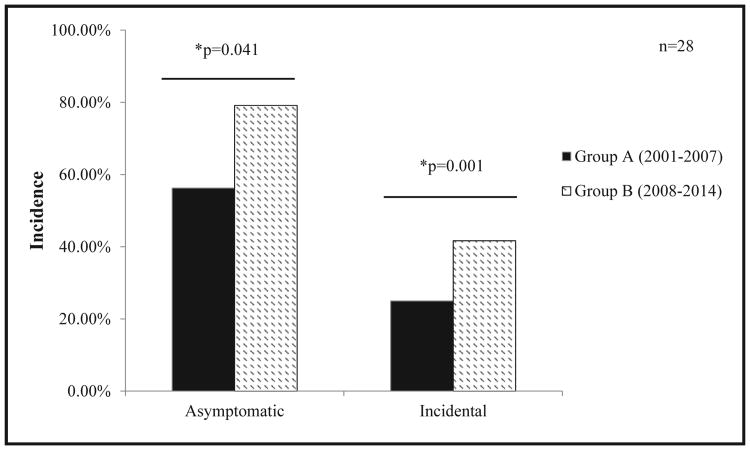
We examined medical records to determine how these young individuals were diagnosed with PHPT. Owing to the nonspecific nature of the symptoms associated with hyperparathyroidism, we considered PHPT as symptomatic only when specific symptoms of kidney stones, bone pain, or hypercalcemic crisis were present. Other nonspecific symptoms, such as abdominal pain, or the absence of symptoms at the time of presentation was considered incidental. In addition, we excluded patients with a positive family history when examining patients for incidental diagnosis, as this subset of patients have a higher suspicion of PHPT. Incidentally, diagnosed patients were therefore diagnosed during workup of nonspecific symptoms, most commonly abdominal pain, or based on labs drawn for suspicion of another disease process. (Table 2). We found that an asymptomatic presentation (group A, 56% vs group B, 79%, P = .041) and incidental diagnosis (group A, 25% vs group B, 42%, P = .001) were much more common in the later group (Fig. 2).
Table 2. Presenting complaint of incidentally diagnosed young patients with negative family history.
| Group | Age (y), sex | Presenting symptom |
|---|---|---|
| A | 21, male | Cloudy urine |
| A | 19, male | Abdominal pain |
| A | 21, male | Hypertension |
| A | 21, female | Abdominal pain |
| B | 18, male | Asymptomatic |
| B | 23, male | Abdominal pain |
| B | 22, female | Polyuria |
| B | 10, male | Abdominal pain, fatigue |
| B | 23, female | Dysphagia |
| B | 20, female | Asymptomatic |
| B | 17, male | Polyuria, patient with developmental delay |
| B | 24, male | Asymptomatic |
| B | 23, male | Asymptomatic |
| B | 15, female | Asymptomatic |
Figure 2.
Differences in patient characteristics at diagnosis of primary hyperparathyroidism by group.
Parathyroid surgery outcomes
Single adenoma accounted for 67.5% (27/40) of all young patients undergoing parathyroidectomy, with this figure increasing to 82.1% in the absence of family history. We did not find a significant difference in transient or permanent complication rates between time groups. Only 1 patient (2.5%) developed a permanent complication, which was permanent hypocalcemia, after undergoing a total parathyroidectomy with forearm implantation for multiple endocrine neoplasia (MEN)–associated PHTP. In addition, all 40 patients achieved cure, defined as normal calcium, 6 months after their operation (Table 3). The recurrence rate, defined as hypercalcemia more than 6 months after surgery, was 7.5%. The time to recurrence ranged from 9 to 59 months after surgery, and recurrence only occurred in patients with a positive family history. Overall, 12/40 (30%) patients had a positive family history, including MEN and non-MEN familial hyperparathyroidism. Not surprisingly, young patients with a positive family history had a higher incidence of multigland disease and were more likely to undergo an open bilateral operation (P = .01). A positive family history was also associated with a higher complication (P = .02), and recurrence rates (P = .005). (Table 4).
Table 3. Parathyroid surgery details outcomes by group.
| Parameter | Group A, 2001–2007 (n = 16 patients) | Group B, 2008–2014 (n = 24 patients) | P value |
|---|---|---|---|
| Operation performed | .74 | ||
| MIP | 11 (69%) | 14 (58.3%) | |
| Bilateral exploration | 5 (31%) | 10 (41.7%) | |
| Etiology | .59 | ||
| Hyperplasia | 5 (31.3%) | 8 (33.3%) | |
| Adenoma | 11 (68.8%) | 16 (66.7%) | |
| Transient complications | .67 | ||
| Hoarseness | 0 (0%) | 0 (0%) | |
| Hypocalcemia | 3 (18.8%) | 3 (12.5%) | |
| Permanent complications | 1.00 | ||
| Hoarseness | 0 (0%) | 0 (0%) | |
| Hypocalcemia | 0 (0%) | 1 (4.3%) | |
| Wound complications | 1.00 | ||
| Hematoma | 0 (0%) | 0 (0%) | |
| Infection | 0 (0%) | 0 (0%) | |
| Cure at 6-month follow-up | 16 (100%) | 24 (100%) | 1.00 |
| Recurrence | 2 (12.5%) | 1 (4.2%) | .55 |
| Mean follow-up time (mo) | 28.4 ± 7.1 | 8.3 ± 1.7 | - |
Results represented as n (%).
Table 4. Parathyroid surgery outcomes in patients with positive family history.
| Variable | Family history (n = 12 patients) | No family history (n = 28 patients) | P value |
|---|---|---|---|
| Operation performed | .01 | ||
| MIP | 4 (33.3%) | 22 (78.6%) | |
| Bilateral exploration | 8 (66.7%) | 6 (21.4%) | |
| Overall complication rate* | 5 (41.7%) | 2 (7.1%) | .02 |
| Permanent complication | 1 (8.3%) | 0 (0%) | .30 |
| Etiology | .008 | ||
| Hyperplasia | 8 (66.7%) | 5 (17.9%) | |
| Adenoma | 4 (33.3%) | 23 (82.1%) | |
| Cure at 6 months | 12 (100%) | 28 (100%) | 1.00 |
| Recurrence† | 4 (33.3%) | 0 (0%) | .005 |
Bold denotes statistical significance.
Results represented as n (%).
MIP = minimally invasive parathyroidectomy.
Complication rate includes transient and permanent complications.
Recurrence defined as development of hypercalcemia after 6 months.
Comments
We have demonstrated that young patients from 2008 to 2014 are increasingly diagnosed with PHPT in the absence of specific symptoms compared with their counterparts from 2001 to 2007. It has only been during the last several decades that more attention has been focused on examining PHPT in younger patients. The reported incidence of symptoms on presentation of PHTP in young patients varies throughout the literature from 67% to 94%.5,6,11,13 Many of these reports, however, include common nonspecific symptoms such as abdominal pain, fatigue, nausea, and weakness. These symptoms can be attributed to any number of physiologic or behavioral disorders in children. Therefore, we excluded these vague symptoms associated with PHPT and limited our definitions of overtly symptomatic disease to nephrolithiasis, bone pain, or crisis. With this focused definition, we found an increasing frequency of incidental PHTP diagnosis in young patients in the contemporary group. This supports the supposition that serum laboratory tests, especially calcium, are being obtained more liberally in younger patients. Guidelines on when to use screening calcium and parathyroid hormone (PTH) in young patients would be helpful, though beyond the scope of this study.
Increased suspicion of PHPT in younger patients may also be in part because of the high incidence of end-organ dysfunction reported with later PHPT diagnosis.5,6,13 A delay in diagnosis of PHPT in young patients can range anywhere from 7.2 months14 in patients with adenomas to between 2 and 4.7 years.4,11 Our findings seem to contrast prior studies that report higher incidence of severe symptoms at the time of presentation, as we report here an increase in incidentally diagnosed PHPT over time. It may be that we are seeing an earlier diagnosis of disease, and over time, we may find that the true incidence of PHPT in young patients is higher than previously reported.
Our cohort demonstrated a female predominance of 1.7:1 (total 25 females and 15 males), which approaches that of adults. The literature for pediatric PHPT is mixed, with several studies reporting male predominance, especially in younger age groups, as a difference between pediatric and adult presentations of the disease.4,10,12 A pooled review of available literature in 2012 of youth and adolescent PHPT reports an overall slight female predominance, with an increase in the number of female patients starting in the 21 to 30 age range.7 There were a total of 14 patients (35%) over the age of 21 in our cohort, and the slight female dominance remained in our population when stratified by age.
As several studies have reported differences in the presentation of PHPT in young patients compared with adults, there has also been discussion of different treatments.5,13 Despite concerns for higher complication rates in smaller patients, parathyroid surgery remains the treatment of choice.7 The low incidence of PHPT in young patients leads to limited data regarding the optimal conditions for surgery in this population. A sampling of the Healthcare Cost and Utilization Projection National Inpatient Sample examining pediatric endocrine surgery outcomes showed that high-volume endocrine surgeons had improved outcomes; however, only 8% of this sample comprised patients undergoing parathyroidectomy.15 Previous reports of complications after parathyroidectomy in pediatric patients have been as high as 56% with a 17% reoperation rate,11 but no effective medical treatment exists. We report a permanent complication rate of 2.5% in our young cohort of patients, which is comparable to the overall complication rates in our high-volume practice of mostly adult patients.
The optimal surgical approach in pediatric patients remains controversial. There is a higher suspicion of familial syndromes in younger patients, which is associated with multiglandular disease and a bilateral neck exploration. Our overall incidence of multiglandular disease was 32.5% (13/40), which doubles to 66.7% (8/12) when a family history is present. Owing to the underlying genetic defect affecting all parathyroid tissue, parathyroidectomy outcomes for MEN1, the most common form of familial PHPT, vary widely but are generally worse than in sporadic hyperparathyroidism.16 Our data support this finding, with positive family history patients having higher rates of overall complications compared with sporadic PHPT. We report an overall recurrence rate of 7.5% (3/40), which is greater than reported in the literature for the general population undergoing parathyroidectomy for PHPT. Our cohort, however, not only focuses on the youngest PHPT patients but also comprises 30% of the patients with positive family history of parathyroid disease. All cases of recurrence were in those with a positive family history and hyperplastic disease, which has been found to be associated with higher rates of recurrence in accordance with our findings.17
Sporadic PHPT, however, behaves quite similarly across age groups in the absence of family history.12 A literature review of hyperparathyroidism in young patients has found the incidence of single gland adenomas in children and young patients to be 80%.7 We report an 82.1% incidence of adenoma when excluding those in our cohort with a positive family history. In addition, minimally invasive parathyroidectomy can be safely performed with appropriate preoperative localization and intraoperative parathyroid hormone levels in these patients.18 Several centers adopt the radio-guided approach to parathyroidectomy.19,20 Examining this method specifically in pediatric patients revealed that a radio-guided approach could be effective and useful in pediatric PHPT patients.21
This study has several limitations. First, our study has a small sample size, which is prohibitive for multivariate modeling. However, the typical sample size of young patients with PHPT from a single institution ranges anywhere from 4 patients to 110 patients.7,12 The terminologies “young” and “pediatric” are also not standardized throughout the literature and vary widely from age 4 to 40 in some studies.11,12 Although our cohort is not adequately powered to perform predictive modeling, we have shown for the first time that young patients are increasingly being incidentally diagnosed with PHPT. We hypothesized that this association may be due to more liberal use of screening calcium and PTH in all patients, but formal prospective evaluation in specifically young patients would be needed to support this. We included bone pain in our definition of PHPT symptomology. Growing pains can be common in a pediatric population; however, given our older cohort with a median age of 19 years, we used this as a surrogate for end-organ damage specific to hyperparathyroidism. Finally, this study was performed in a retrospective manner and limited to patients who were referred to our surgery clinic, which may have introduced selection bias over the extended time period of the study. Notwithstanding these limitations, we were able to make comparisons between 2 groups of similarly aged patients at different time periods, rather than comparing young patients to a larger matched adult cohort.
In conclusion, we have found that PHPT in young patients is increasingly being diagnosed during the workup of nonspecific symptoms or in asymptomatic patients. This trend may be in part a reflection of the increasing use of screening calcium and PTH in younger patients. Parathyroid surgery is safe and effective with a low-permanent complication rate and achieves a cure rate of 100% at 6 months at our institution.
Acknowledgments
Irene Lou is currently receiving grant support from NIH T32 CA090217-15. For the remaining authors, none are declared.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med. 2011;365:2389–97. doi: 10.1056/NEJMcp1106636. [DOI] [PubMed] [Google Scholar]
- 2.Mannix H. Primary hyperparathyroidism in children. Am J Surg. 1975;129:528–31. doi: 10.1016/0002-9610(75)90311-6. [DOI] [PubMed] [Google Scholar]
- 3.Allo M, Thompson NW, Harness JK, et al. Primary hyperparathyroidism in children, adolescents, and young adults. World J Surg. 1982;6:771–6. doi: 10.1007/BF01655371. [DOI] [PubMed] [Google Scholar]
- 4.Rapaport D, Ziv Y, Rubin M, et al. Primary hyperparathyroidism in children. J Pediatr Surg. 1986;21:395–7. doi: 10.1016/s0022-3468(86)80505-x. [DOI] [PubMed] [Google Scholar]
- 5.Pashtan I, Grogan RH, Kaplan SP, et al. Primary hyperparathyroidism in adolescents: the same but different. Pediatr Surg Int. 2013;29:275–9. doi: 10.1007/s00383-012-3222-3. [DOI] [PubMed] [Google Scholar]
- 6.Harman CR, van Heerden JA, Farley DR, et al. Sporadic primary hyperparathyroidism in young patients: a separate disease entity? Arch Surg. 1999;134:651–5. doi: 10.1001/archsurg.134.6.651. discussion 655–6. [DOI] [PubMed] [Google Scholar]
- 7.Belcher R, Metrailer AM, Bodenner DL, et al. Characterization of hyperparathyroidism in youth and adolescents: a literature review. Int J Pediatr Otorhinolaryngol. 2013;77:318–22. doi: 10.1016/j.ijporl.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Consensus development conference statement. J Bone Miner Res. 1991;6(Suppl 2):S9–13. doi: 10.1002/jbmr.5650061406. [DOI] [PubMed] [Google Scholar]
- 9.Bilezikian JP, Potts JT, Fuleihan GH, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–61. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]
- 10.Loh KC, Duh QY, Shoback D, et al. Clinical profile of primary hyperparathyroidism in adolescents and young adults. Clin Endocrinol (Oxf) 1998;48:435–43. doi: 10.1046/j.1365-2265.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 11.Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115:974–80. doi: 10.1542/peds.2004-0804. [DOI] [PubMed] [Google Scholar]
- 12.Sneider MS, Solorzano CC, Montano RE, et al. Sporadic primary hyperparathyroidism in young individuals: different disease and treatment? J Surg Res. 2009;155:100–3. doi: 10.1016/j.jss.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Hsu SC, Levine MA. Primary hyperparathyroidism in children and adolescents: the Johns Hopkins Children's Center experience 1984-2001. J Bone Miner Res. 2002;17(Suppl 2):N44–50. [PubMed] [Google Scholar]
- 14.Lawson ML, Miller SF, Ellis G, et al. Primary hyperparathyroidism in a paediatric hospital. QJM. 1996;89:921–32. doi: 10.1093/qjmed/89.12.921. [DOI] [PubMed] [Google Scholar]
- 15.Tuggle CT, Roman SA, Wang TS, et al. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008;144:869–77. doi: 10.1016/j.surg.2008.08.033. discussion 877. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli F, Marcucci T, Fratini G, et al. Is total parathyroidectomy the treatment of choice for hyperparathyroidism in multiple endocrine neoplasia type 1? Ann Surg. 2007;246:1075–82. doi: 10.1097/SLA.0b013e31811f4467. [DOI] [PubMed] [Google Scholar]
- 17.Dotzenrath C, Cupisti K, Goretzki PE, et al. Long-term biochemical results after operative treatment of primary hyperparathyroidism associated with multiple endocrine neoplasia types I and IIa: is a more or less extended operation essential? Eur J Surg. 2001;167:173–8. doi: 10.1080/110241501750099294. [DOI] [PubMed] [Google Scholar]
- 18.Durkin ET, Nichol PF, Lund DP, et al. What is the optimal treatment for children with primary hyperparathyroidism? J Pediatr Surg. 2010;45:1142–6. doi: 10.1016/j.jpedsurg.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 19.Norman J, Chheda H. Minimally invasive parathyroidectomy facilitated by intraoperative nuclear mapping. Surgery. 1997;122:998–1003. doi: 10.1016/s0039-6060(97)90201-4. discussion 1003–4. [DOI] [PubMed] [Google Scholar]
- 20.Chen H. Radioguided parathyroid surgery. Adv Surg. 2004;38:377–92. [PubMed] [Google Scholar]
- 21.Burke JF, Jacobson K, Gosain A, et al. Radioguided parathyroidectomy effective in pediatric patients. J Surg Res. 2013;184:312–7. doi: 10.1016/j.jss.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]