Abstract
Introduction and Objective
Multiparametric MRI (mpMRI) and MR targeted biopsy have a growing role in the screening and evaluation of prostate cancer. We aim to evaluate the current knowledge, attitude, and practice patterns of urologists regarding this new technique.
Methods
An anonymous online questionnaire was designed to collect information on urologists’ beliefs and use of prostate mpMRI and MR targeted biopsy. The survey was sent to members of the Society of Urologic Oncology (SUO), the Endourological Society (ES), and European Association of Urology (EAU). Multivariate logistic regression analysis was performed to determine predictors for use of prostate MRI and MR targeted biopsy.
Results
A total of 302 responses were received [ES: 175, EAU: 23, SUO: 104]. The majority of respondents (83.6%) believe MR targeted biopsy to be moderately to extremely beneficial in the evaluation of prostate cancer. 85.7% of responders utilize prostate MR imaging in their practice, and 63.0% utilize MR targeted biopsy. The two most common settings for utilization of MR targeted biopsy include patients with history of prior negative biopsy (96.3%) and monitoring patients on active surveillance (72.5%). In those who do not utilize MR targeted biopsy, the principal reasons were lack of necessary infrastructure (64.1%) and prohibitive costs (48.1%). On multivariate logistic regression analysis, practice in an academic setting (1.86 [1.02–3.40], p = 0.043), and performing > 25 radical prostatectomies per year (2.32 [1.18–4.56], p = 0.015) remained independent predictors for utilizing MR targeted biopsy.
Conclusions
A majority of respondents to our survey look favorably upon use of prostate MRI and MR targeted biopsy in clinical practice. Over time, reduction in fixed costs and easier access to equipment may lead to further dissemination of this novel and potentially transformative technology.
Keywords: prostate cancer, multiparametric MRI, MR targeted biopsy, survey, trends
Introduction
One of the dilemmas currently faced in prostate cancer (PCa) screening and diagnosis is the over-detection of low-risk, indolent cancers that do not necessarily demand definitive treatment. This obstacle has led to a renaissance in the field, with the exploration of genomics, novel biomarkers, and imaging to bridge the deficiency (1–3). With respect to imaging, visualization of PCa has always been a challenging due to the deep location of the prostate within the pelvis, heterogenous complexity of PCa, and its multifocal nature (4). However, recent advances in multiparametric MRI (mpMRI) for prostate imaging coupled with the evolution of MR targeted biopsy have resulted in a paradigm shift in prostate cancer evaluation. MR targeted biopsy, performed either ‘in-bore’ or through cognitive or MRI-ultrasound fusion, allows for more precise sampling within the prostate, and, relative to systematic 12-core biopsy, has been repeatedly shown to identify more high-risk prostate cancer cases while avoiding detection of low-risk disease (5–8). This, in turn, has led to improved efforts in defining tumor burden and staging PCa prior to definitive treatment (9, 10). Futhermore, MR targeted biopsy has been shown to have proven utility in several different clinical scenarios, such as in those with prior negative transrectal ultrasound (TRUS) biopsy, lesions in areas within the prostate that are traditionally undersampled with TRUS biopsy (e.g., midline, anterior, distal apical), and monitoring of patients placed on active surveillance (11–15).
As the potential roles for mpMRI and MR targeted biopsy have expanded over time, the acceptance of these novel technologies within the urology community has been steadily increasing. Despite increasing use, there are still some opponents within the urology community and the acceptance has neither been uniform nor unanimous. To our knowledge, there is no study that has attempted to determine the current attitude of the urologic community towards mpMRI and MR targeted biopsy and reasons for acceptance or opposition of this novel technology. The aim of this present study is to gauge current knowledge, opinions, and practice patterns among urologists regarding use of mpMRI and MR targeted biopsy for screening and detection of prostate cancer.
Materials and Methods
Survey instrument
After reviewing the literature on the subject, a 22-item questionnaire was designed to collect demographic data and information on urologist’s attitude and practice patterns regarding use of MRI and MR targeted biopsy for prostate cancer. The questionnaire was designed in a branching fashion such that respondents were taken to different follow-up questions based on how he/she responded to the previous question. Additionally, certain questions allowed respondents to “select all that apply” in response to the question. Information was obtained on the respondents’ age, practice type, years in practice, fellowship training in urologic oncology, AUA section, urological societies, number of prostate biopsies and radical prostatectomies performed, and use of prostate MRI and MR targeted biopsy in clinical practice. A shortened 16-item questionnaire was designed for Society of Urologic Oncology (SUO) members due to constraints on number of questions allowed by the society. Questions on AUA section, urological societies, approach for radical prostatectomy, and utilization of biomarkers were removed from the modified version.
Study design
A link to the survey was sent through email to the members of SUO and the Endourological Society (ES). The survey link was also posted on the twitter feed for the European Association of Urology (EAU) to target its members. Approximately 4,000 members of ES and 600 members of SUO received requests for the study. As all three societies have heterogeneous member populations (non-clinicians, mid-level providers, etc.), an unknown number of recipients qualified for the study, and therefore the response rate could not be accurately calculated. The responses were automatically collected in an Excel spreadsheet in an anonymous fashion. The study was determined to be exempt from review by an Institutional Review Board by the Office of Human Subjects Research Protection at the National Institutes of Health.
Statistics
Statistical analysis was perfomed using STATA version 13.0 (StataCorp LP, College Station, TX USA). Wilcoxon rank sum test was used to compare distribution of continuous variables. Pearson Chi square and Fisher’s exact test were used to compare proportions of categorical variables. Since there were separate links to the questionnaire sent to SUO and to the other two societies, we were able to ascertain the responses coming from SUO vs. ES+EAU. Univariate and multivariate logistic regression analysis was performed to identify predictors for utilization of MRI and MR targeted biopsy in practice and attitudes regarding the potential benefit of these techniques. Respondent’s age, practice type, oncology fellowship training, number of prostate biopsies/month, and number of radical prostatectomies/year were used in regression analysis. An additional variable based on whether the response was from the link sent to members of SUO vs. ES + EAU was also included in the multivariate analysis to evaluate if the preferences were different if the respondent was an SUO member. Statistical significance was defined as p < 0.05.
Results
A total of 302 responses were received, with 175 (58.0%) from ES, 23 (7.6%) from EAU, and 104 (34.4%) from SUO. Characteristics of the respondents are shown in Table 1. Mean age of the respondents was 48.6 (± 11.9) years, with 143 (48.2%) trained in urologic oncology and 208 (68.9%) practicing in an academic setting. Respondents practicing in an academic setting tended to be younger (46.85 (11.62) vs 52.27 (11.62) years, p < 0.001) and more often urologic oncology fellowship trained (54.1 vs 34.4%, p = 0.002) than those practicing in non-academic setting. Also, surgeons in an academic setting were more likely to do > 15 biopsies per month (30.7% vs. 19.8%, p = 0.029) and > 50 radical prostatectomies per year (37.3% vs. 14.1%, p = 0.001).
Table 1.
Characteristics of the respondents
| Age (n = 284), mean (± SD), years | 48.55 (11.9) |
| Society (n = 302) | |
| Endourological Society, n (%) | 175 (58.0) |
| European Association of Urology, n (%) | 23 (7.6) |
| Society of Urologic Oncology, n (%) | 104 (34.4) |
| Practice Type (n = 302) | |
| Academic, n (%) | 208 (68.9) |
| Non-Academic, n (%) | 94 (31.3) |
| Years in Practice (n = 300) | |
| Trainee, n (%) | 28 (9.3) |
| 0–10, n (%) | 93 (31.0) |
| 11–20, n (%) | 73 (24.3) |
| >20, n (%) | 106 (35.3) |
| Fellowship trained in Urologic Oncology (n = 297) | |
| No, n (%) | 154 (51.9) |
| Yes, n (%) | 143 (48.2) |
| No. of prostate biopsies monthly (n = 296) | |
| 0–5, n (%) | 89 (30.1) |
| 6–10, n (%) | 87 (29.4) |
| 11–15, n (%) | 39 (13.2) |
| >15, n (%) | 81 (27.4) |
| No. of radical prostatectomies annually (n = 296) | |
| 0–25, n (%) | 144 (48.7) |
| 26–50, n (%) | 63 (21.3) |
| 51–75, n (%) | 30 (10.1) |
| >75, n (%) | 59 (20.0) |
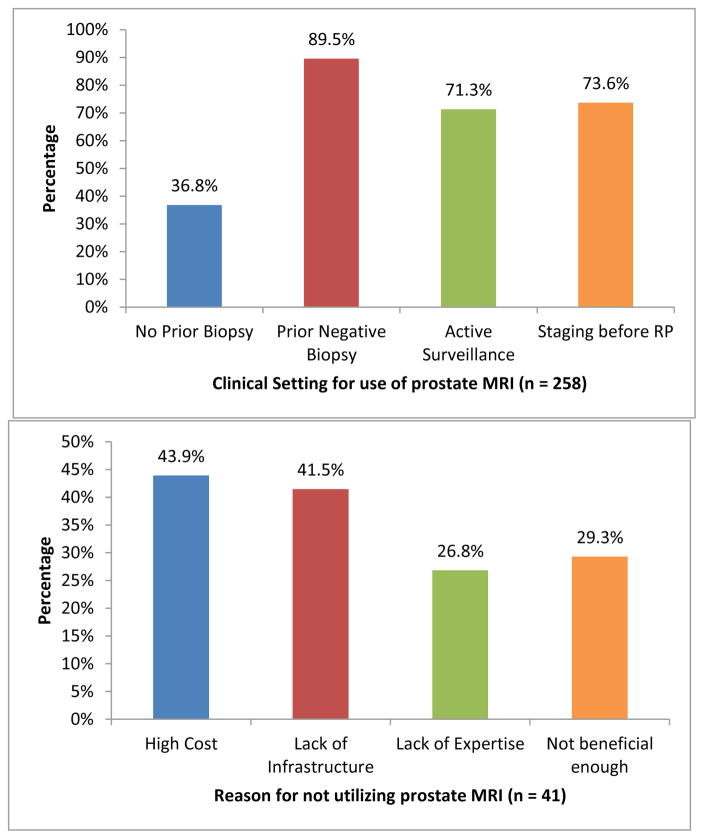
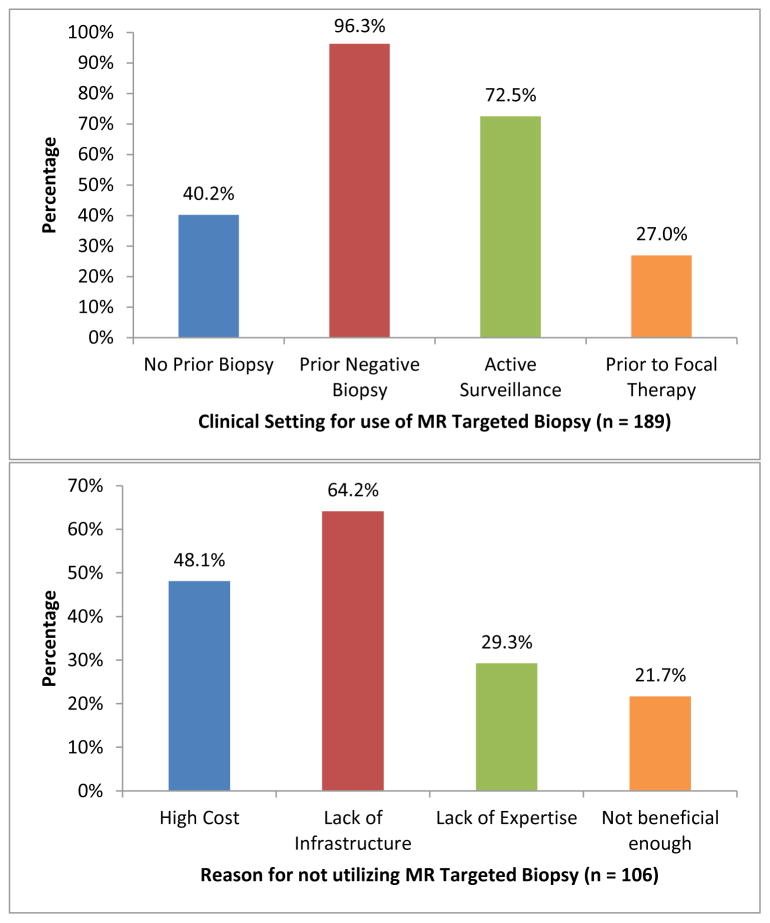
Participant’s responses to survey questions are presented in Table 2. The majority of respondents believed prostate mpMRI (87.6%) and MR targeted biopsy (83.6%) to be at least moderately to extremely beneficial for evaluation of prostate cancer. Overall, 85.7% of urologists responding to this survey stated they utilize prostate MRI in practice and 63.0% stated they utilize MR targeted biopsy, with MRI-ultrasound fusion biopsy (73.0%) being the most common method for targeted biopsy. Furthermore, a strong majority of respondents (65.7%) believed that MR targeted biopsy is more accurate than systematic biopsy in detecting clinically significant PCa. The top 2 settings for utilization of prostate MRI were in patients with prior negative biopsy (89.5%) and staging before radical prostatectomy (73.6%); [Figure 1], while the top 2 settings for utilization of MR targeted biopsy were in patients with prior negative biopsy (96.3%) and patients placed on active surveillance (72.5%) [Figure 2]. Prohibitive costs and lack of necessary infrastructure were the two most common reasons for not using prostate MRI and targeted biopsy in respondents’ clinical practice.
Table 2.
Participant’s responses to survey questions
| n (%) | |
|---|---|
| How beneficial is MRI for PCa? (n = 298) | |
| No benefit at all | 7 (2.4) |
| Slightly beneficial | 30 (10.1) |
| Moderately beneficial | 86 (28.9) |
| Very beneficial | 112 (37.6) |
| Extremely beneficial | 63 (21.1) |
| How beneficial is MR Targeted Biopsy for PCa? (n = 298) | |
| No benefit at all | 9 (3.0) |
| Slightly beneficial | 40 (13.4) |
| Moderately beneficial | 91 (30.5) |
| Very beneficial | 97 (32.6) |
| Extremely beneficial | 61 (20.5) |
| Believe that MR Targeted Biopsy is more accurate than systematic biopsy in detecting CS PCa?* (n = 198) | |
| Yes | 130 (65.7) |
| No | 10 (5.1) |
| Sometimes | 54 (27.3) |
| Do not know | 4 (2.0) |
| Use MRI for PCa? (n = 301) | |
| No | 43 (14.3) |
| Yes | 258 (85.7) |
| If yes, reasons for using prostate MRI** (n = 258) | |
| Patients with no prior biopsy | 95 (36.8) |
| Patients with prior negative biopsy | 231 (89.5) |
| Patients on active surveillance | 184 (71.3) |
| Staging before radical prostatectomy | 190 (73.6) |
| If no, reasons for not using prostate MRI** (n = 41) | |
| Cost | 18 (43.9) |
| Lack of infrastructure | 17 (41.5) |
| Lack of expertise | 11 (26.8) |
| Not beneficial enough | 12 (29.3) |
| Use MR Targeted Biopsy for PCa? (n = 300) | |
| No | 111 (37.0) |
| Yes | 189 (63.0) |
| If yes, what technique? (n = 189) | |
| Cognitive Fusion | 43 (22.8) |
| MRI-MRI fusion (“in-bore” biopsy) | 8 (4.2) |
| MRI-ultrasound Fusion Biopsy | 138 (73.0) |
| Reasons for using MR Targeted Biopsy for PCa** (n = 189) | |
| Patients with no prior biopsy | 76 (40.2) |
| Patients with prior negative biopsy | 182 (96.3) |
| Patients on active surveillance | 137 (72.5) |
| Patients prior to focal therapy | 51 (27.0) |
| If no, reasons for not using MR Targeted Biopsy for PCa** (n = 106) | |
| Cost | 51 (48.1) |
| Lack of infrastructure | 68 (64.2) |
| Lack of expertise | 31 (29.3) |
| Not beneficial enough | 23 (21.7) |
| How difficult to learn MR Targeted Biopsy Technique? (n = 297) | |
| Not difficult at all | 85 (28.6) |
| Slightly Difficult | 111 (37.4) |
| Moderately Difficult | 85 (28.6) |
| Very Difficult | 15 (5.1) |
| Extremely Difficult | 1 (0.3) |
| Utilize biomarkers (Oncotype DX, Prolaris) for PCa?* (n = 197) | |
| Yes | 52 (26.4) |
| No | 145 (73.6) |
| Believe MRI/MR Targeted Biopsy can decrease need for biomarker tests?* (n = 197) | |
| Yes | 51 (25.9) |
| No | 54 (27.4) |
| Maybe | 92 (46.7) |
Question asked only to members of ES and EAU, CS = clinically significant
Select all that apply question
Figure 1.
Practice patterns for prostate MRI
Figure 2.
Practice patterns for MR targeted biopsy
On univariate logistic regression analysis, fellowship training in urologic oncology (p = 0.029), performing > 5 prostate biopsies per month (p = 0.027), and performing > 25 radical prostatectomies per year (p = 0.047) were associated with utilizing prostate MRI in practice [Table 3]. However, on multivariate logistic regression, no independent predictors for utilizing prostate MRI were found. On multivariate logistic regression analysis for the use of MR targeted biopsy, practice in an academic setting (1.86 [1.02–3.40], p = 0.043), performing 26–75 (2.32 [1.18–4.56], p = 0.015) radical prostatectomies per year, and performing greater than 75 radical prostatectomies per year (2.99 [1.27–7.02], p = 0.012) remained independent predictors for utilizing MR targeted biopsy in practice.
Table 3.
Univariate analysis for utilization of prostate MRI and MR Targeted Biopsy. OR, odds ratio; CI, 95% confidence interval
| Variable | Use of MRI | Use of MR Targeted Biopsy | ||
|---|---|---|---|---|
| Univariate OR [CI] | P value | Univariate OR [CI] | P value | |
| Age | 0.99 [.96 – 1.02] | 0.521 | 0.99 [.98–1.02] | 0.712 |
| Practice Type | ||||
| Non-academic (reference) | 1 | - | 1 | - |
| Academic | 1.09 [.55–2.18] | 0.799 | 2.06 [1.25–3.40] | 0.005 |
| Society response | ||||
| ES + EAU (reference) | 1 | - | 1 | - |
| SUO | 1.26 [.63–2.53] | 0.521 | 1.86 [1.11–3.11] | 0.018 |
| Fellowship trained | 2.14 [1.08–4.23] | 0.029 | 2.04 [1.26–3.31] | 0.004 |
| No. Prostate Biopsies per month | ||||
| 0–5 (reference) | 1 | - | 1 | - |
| 6–15 | 2.32 [1.10–4.89] | 0.027 | 1.97 [1.13–3.43] | 0.016 |
| >15 | 3.62 [1.37–9.55] | 0.009 | 4.12 [2.09–8.13] | <0.001 |
| No. of RARPs per year | ||||
| 0–25 (reference) | 1 | - | 1 | - |
| 26–75 | 2.25 [1.01–5.02] | 0.047 | 3.08 [1.75–5.44] | <0.001 |
| >75 | 3.32 [1.11–9.93] | 0.032 | 5.26 [2.47–11.18] | <0.001 |
When asked on difficulty in learning to perform the MR targeted biopsy technique, the majority of respondents (66%) stated the technique is either not difficult at all or only slightly difficult Only 16 (5.4%) respondents stated that the technique is very or extremely difficult. Having fellowship training in urologic oncology was associated with perceived lower difficulty in learning how to perform MR targeted biopsy (p = 0.047). No difference was found between responses from SUO compared to other two societies (p = 0.341).
Lastly, a majority of respondents (73.6%) indicated that they do not utilize biomarkers such as Oncotype DX and Prolaris in their practice, and most (46.7%) responded with “maybe” when queried on their belief on if MRI/MR targeted biopsy can decrease the need for such biomarker tests.
Discussion
The field of urology has continuously embraced novel technologies (e.g. robotics, laparoscopy, shockwave lithotripsy, lasers) that enhance patient outcomes, reduce patient morbidity, and improve efficiency and accuracy of therapy. As with all innovation, new technology must first endure a process of initial skepticism followed by rigorous clinical evaluation and proper validation. One such example is the introduction and adoption of robotic surgery within the field of urology (16, 17). Robotic technology was initially met with heavy uncertainty due to several factors, such as exorbitant cost, high degree of technical training, required assistant personnel, and necessity of dedicated operating rooms with bulky equipment (18). Therefore, it was believed that use of robotics would be limited to high-level academic centers, yet now robotic technology and the field of urology are fully integrated with ubiquitous use even amongst small community hospitals
MR targeted biopsy has recently emerged for more precise and smarter detection of prostate cancer. MR targeted biopsy, in relation to the current standard of care (systematic 12-core biopsy), has been shown to detect more high-risk cases, while simultaneously decreasing the detection of low-risk, indolent cancers (5, 6). However, the main question that is currently faced with respect to MR targeted biopsy is how generalizable the technology is. The MR targeted biopsy technique, similar to challenges initially faced with robotic surgery, requires up-front financial investment, specialized equipment, and expert personnel and support staff working in concert to accurately interpret prostate MRI scans and perform the MR targeted biopsy (19). While many large academic centers have adopted this technology with good results (20), there is no report on acceptance and enthusiasm about this technology in the urology community in general.
Therefore, we sought to assess current opinions and practice patterns among urologists regarding prostate MRI and MR targeted biopsy. Surveys have proven to be useful in gauging perceptions, beliefs, and practice patterns of urologists regarding a wide variety of topics (21–23). In our study, respondents seemed to look favorably upon use of prostate MRI and MR targeted biopsy, as 88% and 84% of respondents believed these two to be moderately to extremely beneficial, respectively. Furthermore, a large proportion of respondents actually utilize prostate MRI (86%) and MR targeted biopsy (63%) in their practice. The top setting for use of both prostate MRI and MR targeted biopsy was in patients with prior negative biopsy; this makes intuitive sense as the literature has consistently established MR targeted biopsy to have proven benefit in this specific patient cohort (24–26). Despite a lack of overwhelming evidence establishing utility in a biopsy naïve cohort, a high proportion of respondents (40%) indicated that they utilize MR targeted biopsy in patients with no prior biopsy; this is an interesting finding that illustrates increased adoption of this technique and potential expansion of the clinical scenarios in which MR targeted biopsy can be useful. Interestingly, the most common reasons for not utilizing prostate MRI and MR targeted biopsy included high costs and lack of necessary infrastructure, as opposed to the belief that these technologies are not beneficial enough to justify their use. These results suggest that reduction of cost and easier access to the necessary equipment may further increase the usage of this technology.
MR targeted biopsy is a challenging multi-step procedure that requires proficiency in spatial reasoning, hand-eye coordination, and basic knowledge of image-guidance to ensure accuracy; defining the learning curve for this technique is currently an area under investigation (27). In our study, however, a majority of respondents (66%) believed it is either not difficult at all or only slightly difficult. Despite being a relative recent adoption by the urology community, perceived ease in learning to perform MR targeted biopsy might suggest that it has a short learning curve. Amongst our respondents, urologists with fellowship training in urologic oncology, relative to those without fellowship training, more often pursed a career in academics. Furthermore, those in academics tended to perform more biopsies and radical prostatectomies, and more often utilized MR targeted biopsy in their practice. The regression analysis further delineated that practicing in academic setting and performing greater than 25 radical prostatectomies a year predicted use of MR targeted biopsy. This is an interesting trend which may be due to several plausible factors. Urologists practicing in an academic setting may be more inclined to embrace novel technologies, may have the financial backing (e.g. grants) to pursue these investments, and/or may have patient populations that demand these more advanced cutting-edge technologies. The principal limitation of this present study is the survey design implying reporting bias and lack of information on non-respondents. The survey might have appealed more to urologists who are more cognizant of this technology and/or utilize the technology in their practice. In addition, due to the electronic design of the survey, an additional component of reporting bias may be present in that urologists who accessed the survey may be more inclined to accept novel technologies in general. Therefore, this bias may limit the generalizability of these results to the overall urology community represented by these societies. Lastly, the results of this survey represent a snapshot in time, as opinions and practice patterns are constantly shifting. Despite these limitations, we believe this study provides valuable information regarding the beliefs and practice trends of urologists regarding prostate MRI and MR targeted biopsy. It will be interesting to see how these opinions and practice patterns evolve over time in the coming years as the indications for MRI and MR targeted biopsy continue to expand.
Conclusion
A majority of respondents to our survey seem to look favorably upon use of prostate mpMRI and MR targeted biopsy in clinical practice. Both modalities are most commonly used in the settings of patients with prior negative biopsy and patients placed on active surveillance. Practice in an academic setting and higher surgical volume predicted utilization of MR targeted biopsy by the urologists. Reduction of cost and easier access to the necessary equipment may further increase the usage of this technology in the urologic community.
Highlights.
Respondents to our survey, in general, look favorably upon use of prostate MRI and MR targeted biopsy for prostate cancer evaluation
MR targeted biopsy most often utilized in setting of patients with prior negative biopsy Reduction in cost and easier access to equipment may further increase use of MR targeted biopsy
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen H, Liu X, Brendler CB, Ankerst DP, Leach RJ, Goodman PJ, et al. Adding genetic risk score to family history identifies twice as many high-risk men for prostate cancer: Results from the prostate cancer prevention trial. The Prostate. 2016 doi: 10.1002/pros.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudreau PO, Stagg J, Soulieres D, Saad F. The Present and Future of Biomarkers in Prostate Cancer: Proteomics, Genomics, and Immunology Advancements. Biomarkers in cancer. 2016;8(Suppl 2):15–33. doi: 10.4137/BIC.S31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George AK, Pinto PA, Rais-Bahrami S. Multiparametric MRI in the PSA screening era. BioMed research international. 2014;2014:465816. doi: 10.1155/2014/465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George AK, Turkbey B, Valayil SG, Muthigi A, Mertan F, Kongnyuy M, et al. A urologist’s perspective on prostate cancer imaging: past, present, and future. Abdominal radiology (New York) 2016;41(5):805–16. doi: 10.1007/s00261-016-0751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. European urology. 2015;68(1):8–19. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Rastinehad AR, Turkbey B, Salami SS, Yaskiv O, George AK, Fakhoury M, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. The Journal of urology. 2014;191(6):1749–54. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MM, George AK, Rubin R, Rais-Bahrami S, Parnes HL, Merino MJ, et al. Efficiency of Prostate Cancer Diagnosis by MR/Ultrasound Fusion-Guided Biopsy vs Standard Extended-Sextant Biopsy for MR-Visible Lesions. Journal of the National Cancer Institute. 2016;108(9) doi: 10.1093/jnci/djw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raskolnikov D, George AK, Rais-Bahrami S, Turkbey B, Shakir NA, Okoro C, et al. Multiparametric magnetic resonance imaging and image-guided biopsy to detect seminal vesicle invasion by prostate cancer. Journal of endourology / Endourological Society. 2014;28(11):1283–9. doi: 10.1089/end.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255(1):89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fascelli M, George AK, Frye T, Turkbey B, Choyke PL, Pinto PA. The role of MRI in active surveillance for prostate cancer. Current urology reports. 2015;16(6):42. doi: 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kongnyuy M, George AK, Rastinehad AR, Pinto PA. Magnetic Resonance Imaging-Ultrasound Fusion-Guided Prostate Biopsy: Review of Technology, Techniques, and Outcomes. Current urology reports. 2016;17(4):32. doi: 10.1007/s11934-016-0589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthigi A, Sidana A, George AK, Kongnyuy M, Shakir N, Kadakia M, et al. Midline lesions of the prostate: role of MRI/TRUS fusion biopsy and implications in Gleason risk stratification. International urology and nephrology. 2016 doi: 10.1007/s11255-016-1336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nix JW, Turkbey B, Hoang A, Volkin D, Yerram N, Chua C, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU international. 2012;110(11 Pt B):E694–700. doi: 10.1111/j.1464-410X.2012.11503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radtke JP, Boxler S, Kuru TH, Wolf MB, Alt CD, Popeneciu IV, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate cancer and prostatic diseases. 2015;18(3):288–96. doi: 10.1038/pcan.2015.29. [DOI] [PubMed] [Google Scholar]
- 16.Gettman MT, Blute ML, Peschel R, Bartsch G. Current status of robotics in urologic laparoscopy. European urology. 2003;43(2):106–12. doi: 10.1016/s0302-2838(02)00579-1. [DOI] [PubMed] [Google Scholar]
- 17.Thiel DD, Winfield HN. Robotics in urology: past, present, and future. Journal of endourology / Endourological Society. 2008;22(4):825–30. doi: 10.1089/end.2007.9830. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Gautam G. Robotics in urologic oncology. Journal of minimal access surgery. 2015;11(1):40–4. doi: 10.4103/0972-9941.147687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay KJ, Gupta RT, Rastinehad AR, Tsivian E, Freedland SJ, Moul JW, et al. Navigating MRI-TRUS fusion biopsy: optimizing the process and avoiding technical pitfalls. Expert review of anticancer therapy. 2016;16(3):303–11. doi: 10.1586/14737140.2016.1131155. [DOI] [PubMed] [Google Scholar]
- 20.Rastinehad AR, Abboud SF, George AK, Frye TP, Ho R, Chelluri R, et al. Reproducibility of Multiparametric Magnetic Resonance Imaging and Fusion Guided Prostate Biopsy: Multi-Institutional External Validation by a Propensity Score Matched Cohort. The Journal of urology. 2016;195(6):1737–43. doi: 10.1016/j.juro.2015.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto BJ, Osterberg EC, Salgado S, Scherr DS, Shariat SF. Prostate cancer risk estimation tool use by members of the American Urological Association: a survey based study. The Journal of urology. 2015;193(6):1933–7. doi: 10.1016/j.juro.2014.12.090. [DOI] [PubMed] [Google Scholar]
- 22.Sidana A, Donovan JF, Gaitonde K. Surgeons’ preferences and practice patterns regarding intraoperative frozen section during partial nephrectomy. Urologic oncology. 2014;32(6):864–8. doi: 10.1016/j.urolonc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Ficarra V, Wiklund PN, Rochat CH, Dasgupta P, Challacombe BJ, Sooriakumaran P, et al. The European Association of Urology Robotic Urology Section (ERUS) survey of robot-assisted radical prostatectomy (RARP) BJU international. 2013;111(4):596–603. doi: 10.1111/bju.12100. [DOI] [PubMed] [Google Scholar]
- 24.Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. The Journal of urology. 2012;188(6):2152–7. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendhiratta N, Meng X, Rosenkrantz AB, Wysock JS, Fenstermaker M, Huang R, et al. Prebiopsy MRI and MRI-ultrasound Fusion-targeted Prostate Biopsy in Men With Previous Negative Biopsies: Impact on Repeat Biopsy Strategies. Urology. 2015;86(6):1192–8. doi: 10.1016/j.urology.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidana A, Kadakia M, Maruf M, George A, Kongnyuy M, Muthigi A, et al. MP53-17 CANCER DETECTION ON MRI FUSION BIOPSY IS INDEPENDENT OF PRIOR NEGATIVE BIOPSY HISTORY: A MULTI-INSTITUTIONAL ANALYSIS. The Journal of urology. 2016;195(4):e704. [Google Scholar]
- 27.Muthigi A, George A, Su D, Yan P, Kruecker J, Narayanan H, et al. MP20-16 TRAINING AND SKILLS ASSESSMENT FOR FUSION-GUIDED PROSTATE BIOPSY: DEFINING THE LEARNING CURVE. The Journal of urology. 2016;195(4):e219–e20. [Google Scholar]