Abstract
Objectives
C-reactive protein (CRP) is a well-established general marker of inflammation from both infectious and noninfectious exposures. Previous studies have shown that maternal CRP is associated with an increased risk of autism and schizophrenia. The aim of this study was to examine the association between early to mid-gestational serum CRP levels, prospectively assayed in maternal sera, and the risk of bipolar disorder (BPD).
Methods
This study is derived from the Finnish Prenatal Study of Bipolar Disorder (FIPS-B), based on a nested case-control study design. A total of 378 BPD cases and 378 controls, matched on date of birth and sex, with available maternal sera were identified from Finnish nationwide registers. Maternal CRP levels were assessed using a latex immunoassay from archived maternal serum specimens, collected primarily during the first and second trimesters of pregnancy.
Results
Increasing maternal CRP, examined as a continuous variable, was not associated with BPD (OR=0.92, 95% CI: 0.81–1.05, p=0.24). The result did not change appreciably following adjustment for potential confounders. There were no associations between CRP in the highest quintile or decile, compared with their respective reference groups, and BPD.
Limitations
The limitations of the study include: relative young age of the cohort and availability only of a single marker of inflammation.
Conclusions
In contrast to previous findings on schizophrenia and autism, gestational maternal serum CRP levels were not associated with an increased risk of BPD. It is likely that maternal inflammation may be an additional factor that differentiates schizophrenia from BPD.
Keywords: CRP, Bipolar disorder, prenatal inflammation, nationwide cohort, epidemiology
Introduction
Epidemiological studies have demonstrated that inflammation during pregnancy is associated with an increased risk of psychiatric disorders such as schizophrenia and autism spectrum disorders (ASD) (Brown et al., 2004; 2014; 2010; Canetta et al., 2014a; Buka et al., 2001). Maternal cytokines produced as a result of immune activation from infectious and other environmental insults have been suggested to increase risk of these outcomes by disrupting development of the fetal brain (Meyer et al., 2005; 2009; Watanbe et al., 2010; Kneeland et al., 2013). Birth cohort studies have demonstrated significantly elevated levels of proinflammatory cytokines, including interleukin-8 (IL-8) (Brown et al., 2004) and tumor necrosis factor-alpha (TNF-α) (Buka et al., 2001) among mothers of offspring with schizophrenia. More recently, elevated maternal serum C-reactive protein (CRP) was associated with an increased risk of schizophrenia (Canetta et al., 2014a) and ASD in the Finnish Prenatal Studies, described below (Brown et al., 2014). Maternal infection during pregnancy, which induces immune activation, has been associated with schizophrenia in offspring in many studies (Brown et al., 2010) and emerging research has also implicated prenatal infection in autism (Lee et a., 2015; Atladóttir et al., 2010).
Compared to schizophrenia and ASD, there is a relative paucity of studies examining prenatal inflammation and infection in relation to bipolar disorder (BPD), and the findings are inconsistent. Using prospectively documented diagnoses of maternal influenza during pregnancy, Parboosing et al. (2013) demonstrated a greater than 4-fold increased risk of BPD in offspring. These findings were confirmed in a study from the same birth cohort, which utilized serologically documented markers of prenatal infection. Specifically, Canetta et al. (2014b) assayed influenza antibody titers in maternal serum collected during pregnancy. Exposure to maternal influenza was associated with a 5-fold increased risk of BPD with psychotic features though no association was found for BPD without psychosis. Other studies, based on maternal interview and a documented influenza epidemic provided further supportive evidence for maternal infection and BPD (Sacker et al., 1995; Cannon et al., 1996; Machón et al., 1997). Studies of adult patients with BPD have also revealed elevations in peripheral CRP levels (Dargél et al., 2015). However, the role of maternal infection with other agents in BPD is unclear; for example, Mortensen et al. (2011), in a study of neonatal blood samples stored on filter paper, found no association between neonatal markers of prenatal infection (IgG and IgM antibodies for Toxoplasma gondii, herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and cytomegalovirus (CMV)) and BPD.
We hypothesize abnormalities in Hippocampus and gamma aminobutyric acid (GABA)ergic transmission as the possible biological models through which prenatal maternal immune activation (MIA) may influence risk of BPD. First, studies using mouse models of maternal exposure to the viral mimetic polyriboinosinic-polyribocytidilic acid [poly(I:C)], have demonstrated both presynaptic and postsynaptic hippocampal deficits as well as increased hippocampal pro inflammatory cytokine (IL-1β) levels (Giovanoli et al., 2016). Brain imaging studies have demonstrated reduction in sub regions of the hippocampus among patients with BPD (Bearden et al., 2008). In addition, impaired associative learning, which relies mainly on hippocampal neurons (Wirth et al., 2003) has been seen among BPD cases (Brambilla et al., 2011). Second, GABAergic transmission abnormalities have been hypothesized as being important in pathophysiology of BPD (Brambilla et al., 2003; Sanacora et al., 2000). A recent study using optogenetics and slice electrophysiology demonstrated reduced GABAergic transmission in the medial prefrontal cortex of adult MIA offspring (Canetta et al. 2016).
CRP, primarily synthesized by hepatocytes, is a sensitive general marker of systemic inflammation from both infectious and noninfectious exposures (Pepys and Baltz, 1983; Gabay and Kushner, 1999). The release of proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) stimulate the production of CRP. Factors associated with elevated CRP that might also alter fetal development include obesity, cigarette smoking, and hormone use (Pearson et al., 2003).
In this study we examined the association between CRP prospectively assayed in maternal sera and BPD in offspring. This study was based on the Finnish Prenatal Study of Bipolar Disorder (FIPS-B), a nested case-control study utilizing information from the Finnish nationwide registers. CRP has been considered in this study as an indicator of prenatal maternal inflammation resulting from both infectious and non-infectious insults. Based on previous findings from this same birth cohort, which demonstrated relationships between maternal CRP and both schizophrenia and autism, we hypothesized that elevated levels of maternal serum CRP during pregnancy would be associated with an increased odds of BPD in offspring.
Materials and methods
This study is derived from the Finnish Prenatal Study of Bipolar Disorder (FIPS-B), which is based on a nested case-control design. The study is a component of the Finnish Prenatal Studies, which derive from a national birth cohort with a large serum biobank and comprehensive psychiatric and other registries and which are aimed at examining prenatal factors and other major psychiatric outcomes including schizophrenia and autism. The sampling frame for the FiPS-B included all singleton live births in Finland between January 1st 1983 and December 31st 1998 (N= 1,009,846). The cases included BPD cases diagnosed in Finland, identified from the Finnish Hospital Discharge Register (FHDR) by December 31st 2008 (see “Identification of cases and controls” below). Further criteria used to define the subjects in the present study are elaborated below.
Description of the cohort and biobank
All offspring in the FiPS-B were derived from the Finnish Maternity Cohort (FMC). The FMC consists of virtually all pregnancies with archived prenatal serum specimens drawn beginning in 1983. The FMC serum biorepository contains blood samples from virtually all (≈99%) pregnant women in Finland; there are over 1 million serum samples, with an average of 60,000 new samples added each year. Following informed consent, blood samples were collected at Finnish maternity clinics for the purpose of screening for congenital infections (HIV, hepatitis B and syphilis). One maternal serum sample was obtained from each pregnancy. The median gestational age of serum collection for subjects in this study was 10 weeks (interquartile range: 8–12 weeks). After the screening, approximately 1–3 mls of serum from each pregnancy were stored at −25°C at the Prenatal Serology Laboratory of the National Institute of Health and Welfare in Oulu, Finland. All of the serum specimens can be linked with offspring by a unique personal identification code (PIC), which has been assigned to all residents of Finland since 1964 and has been computerized since 1971. The PIC is a unique code issued to all Finnish citizens and foreign residents registered in the population information system.
Description of other registers
The Finnish Population Register Center (PRC), established the Population Information System in 1969 which was subsequently computerized in 1971. It is a computerized national register containing basic information about Finnish citizens and foreign permanent residents. The personal data included in the system include: name, PIC, address, citizenship and native language, family relations and date of birth and death (if applicable). The Finnish Medical Birth Register (FMBR) was established in 1987 and includes comprehensive and standardized data on the perinatal period for all live births, and stillbirths of fetuses with birth weight of at least 500 g or gestational age of at least 22 weeks. Statistics Finland is the public authority established particularly for statistical services in Finland. The linkage of the information across all the registers is achieved through the PIC.
Identification of cases and controls
Cases for the present study were identified from the FHDR. The FHDR contains computerized data on recorded diagnoses covering all somatic and psychiatric hospitals, as well as all inpatient wards of local health centers, military wards, prison hospitals, and private hospitals since 1969. Since 1998 the FHDR also includes outpatient care in public specialized hospital units. All Finnish citizens are entitled to Finland’s national health insurance, which is maintained by the state and financed through tax revenues. The FHDR contains the patient’s PIC (including date of birth and sex), area of residence, hospital ID, admission and discharge dates and primary and secondary diagnoses, according to the International classification of Disease (ICD). The cases in the FiPS-B consisted of individuals in the FMC with a diagnosis of BPD [International Statistical Classification of Diseases (ICD) diagnostic codes 2962A-G, 2963A-G, 2964A-G, 2967A (ICD-9) and F31.x (ICD-10)] using the most recent diagnosis who were followed up until December 31st, 2008. In the present study, the cases (and matched controls, see below) were drawn from all singleton offspring in the FMC born between January 1st 1988 and December 31st 1991 (N=257,608). The selection of cases and controls in this study was limited to these years due to the lack of availability of data from the Finnish Medical Birth Register (FMBR), which was established in 1987, the need to maintain greater uniformity of the serum specimens with regard to the time of sampling and storage, and to maximize the duration of follow-up (18–21 years) among the cohort given the age of onset of BPD. A diagnostic validity study of the FHDR for BPD-I found 92% specificity for a diagnosis of BPD-I (Kieseppä et al., 2000) as compared to diagnostic interview by DSM-III-R criteria (American Psychiatric Association, 1987), and 99.7% specificity for diagnoses of psychotic disorders including BPD-I (Perälä et al., 2007) compared to interview by DSM–IV criteria (American Psychiatric Association, 1994). For the present study, there were a total of 442 BPD cases identified. Among these cases, there were insufficient quantities of sera on 64 cases; thus, maternal CRP data were obtained on 378 case control pairs. These cases were matched 1:1 to controls drawn from the birth cohort who were without BPD, schizophrenia, or diagnoses related to these disorders (Supplemental material 1: list of diagnostic codes excluded from controls) on sex, date of birth (±30 days), and residence in Finland at the time of diagnosis of the matched case. A detailed description of the Finnish nationwide registers has been reported elsewhere and will only be briefly described here (Chudal et al., 2014).
Ethical approval for the study was obtained from the Hospital district of Southwest Finland and Institutional Review Board of the New York State Psychiatric Institute. The approval for utilizing the health register data and their linkage for scientific research was obtained from the Finnish data protection authority.
C-Reactive Protein Assay
The measurements of CRP were carried out blind to case/control status. CRP was measured on the clinical chemistry analyzer Architect c8200 (Abbott Laboratories, Abbott Park, IL, USA) using a latex immunoassay (Sentinel, Milan, Italy). The precision between series expressed as the coefficient of variation (mean ± SD) was 5.1% ± 2.3% and the systematic error (bias) (mean ± SD) was 2.7% ± 7.4 during the course of the study. Assay sensitivity is 0.10 mg/L.
Covariates
The covariates included maternal age, gestational week of the maternal blood draw, number of previous births, maternal smoking during pregnancy, maternal educational level, parental history of BPD, other parental psychiatric disorders (ICD codes used are shown in footnote, Table 1) and province of birth. Information on maternal age was obtained from the Population Register Center, gestational week of the maternal blood draw from the FMC; number of previous births and maternal smoking during pregnancy from the FMBR, maternal educational level from Statistics Finland; parental psychiatric history from the FHDR and PRC; and province of birth from the PRC and Statistics Finland. The covariates were classified as shown in Tables 1 and 2.
Table 1.
Relationship between covariates and maternal C-reactive protein levels (≥/< median) in control subjects.
| Covariates | CRP | CRP | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ≥ median | < median | |||||
|
| ||||||
| Mean | SD | Mean | SD | t | ||
| Maternal age (years) | 28.83 | 4.87 | 28.76 | 5.07 | −0.12 | 0.91 |
| Gestational week of blood draw | 11.57 | 3.55 | 9.91 | 3.48 | −4.54 | < 0.0001 |
| N | % | N | % | χ2 | ||
|
| ||||||
| Previous birthsa | 12.48 | 0.0004 | ||||
| 0 | 57 | 30.6 | 89 | 48.6 | ||
| ≥ 1 | 129 | 69.3 | 94 | 51.4 | ||
| Parental BPD historyb | 4.96 | 0.02 | ||||
| No | 184 | 97.3 | 185 | 100 | ||
| Yes | 5 | 2.7 | 0 | 0 | ||
| Parental psychiatric historyc | 0.32 | 0.57 | ||||
| No | 155 | 81.6 | 149 | 79.3 | ||
| Yes | 35 | 18.4 | 39 | 20.7 | ||
| Maternal smoking during pregnancyd | 0.002 | 0.96 | ||||
| No | 153 | 85 | 151 | 84.8 | ||
| Yes | 27 | 15 | 27 | 15.2 | ||
| Maternal education level | 7.83 | 0.02 | ||||
| University degree | 8 | 4.2 | 18 | 9.6 | ||
| High school graduate | 131 | 68.9 | 137 | 72.9 | ||
| Less than high school | 51 | 26.8 | 33 | 17.5 | ||
| Province of birth | 0.36 | 0.95 | ||||
| Southern Finland | 68 | 35.8 | 66 | 35.1 | ||
| Eastern Finland | 24 | 12.6 | 21 | 11.2 | ||
| Western Finland | 68 | 35.8 | 68 | 36.2 | ||
| Northern Finland | 30 | 15.8 | 33 | 17.5 | ||
Data missing for 9 controls
Data missing for 4 controls. Diagnoses defined according to the following ICD codes: ICD-10: F31.x; ICD-9: 2962A-G, 2963A-G, 2964A-G, 2967A; ICD-8: 296.10, 296.30.
Diagnoses defined according to the following ICD codes: ICD-10: F10-99, excluding F31.x, F70-79; ICD-9: 291-316, excluding 2962A-G, 2963A-G, 2964A-G, 2967A, 293-294; ICD-8: 291–308, excluding 296.10, 296.30, 292–294 except for 294.30
Data missing for 20 controls; CRP: C- reactive protein; SD: standard deviation; t: t-test value; χ2: Pearson’s chi squared test value.
Table 2.
Relationship between covariates and bipolar disorder in case and control subjects.
| Covariates | Controls | Cases | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | t | ||
| Maternal age (years) | 28.80 | 4.96 | 28.28 | 5.68 | 1.33 | 0.18 |
| Gestational week of blood draw | 10.75 | 3.61 | 10.85 | 3.83 | −0.37 | 0.71 |
| N | % | N | % | χ2 | ||
|
| ||||||
| Previous birthsa | 2.82 | 0.09 | ||||
| 0 | 146 | 39.6 | 169 | 45.7 | ||
| ≥ 1 | 223 | 60.4 | 201 | 54.3 | ||
| Parental BPD historyb | 31.63 | <0.0001 | ||||
| No | 369 | 98.7 | 327 | 88.6 | ||
| Yes | 5 | 1.3 | 42 | 11.4 | ||
| Parental psychiatric history | 62.18 | <0.0001 | ||||
| No | 304 | 80.4 | 202 | 53.4 | ||
| Yes | 74 | 19.6 | 176 | 46.6 | ||
| Maternal smoking during pregnancyc | 5.34 | 0.02 | ||||
| No | 304 | 84.9 | 288 | 78.3 | ||
| Yes | 54 | 15.1 | 80 | 21.7 | ||
| Maternal education level | 0.40 | 0.81 | ||||
| University degree | 26 | 6.9 | 24 | 6.3 | ||
| Upper secondary school | 268 | 70.9 | 263 | 69.6 | ||
| Less than high school | 84 | 22.2 | 91 | 24.1 | ||
| Province of birth | 12.44 | 0.006 | ||||
| Southern Finland | 134 | 35.4 | 168 | 44.4 | ||
| Eastern Finland | 45 | 11.9 | 52 | 13.8 | ||
| Western Finland | 136 | 36.0 | 93 | 24.6 | ||
| Northern Finland | 63 | 16.7 | 65 | 17.2 | ||
Data missing for 8 cases and 9 controls
Data missing for 9 cases and 4 controls
Data missing for 10 cases and 20 controls
SD: standard deviation; t: t-test value; χ2: Pearson’s chi squared test value
Statistical analyses
To assess the potential for confounding, we first utilized bivariate analyses to evaluate relationships between covariates and CRP in controls. Pearson’s chi squared test was used for dichotomous variables and Student’s t-test was employed for continuous variables. Subsequently, the covariates were examined for associations with case and control status. Covariates were considered for inclusion in the models based on associations with both CRP and BPD at p <0.1, in accord with standard texts (Rothman and Greenland, 1998).
The analysis of the relationship between maternal CRP and BPD in offspring was based on a nested case-control design in which the control subjects for each case were selected from the population at risk and matched to cases on selected factors, as described in “Case and control identification.” Initially, maternal CRP was examined as a continuous variable in relation to BPD. The CRP variable was log transformed before analysis due to the skewed distribution. Maternal CRP was also analyzed as a categorical variable to explore whether there was a nonlinear relationship with BPD. As a CRP level ≥10 mg/L is considered clinically abnormal (Pepys and Baltz, 1983), maternal CRP levels above this threshold were examined in relation to risk of BPD, with levels < 10mg/L as the reference category. To further facilitate interpretation of the data, additional analyses of the association between CRP in quintiles (with the lowest quintile as the reference category) and in deciles (with the lowest decile as the reference category) and BPD were conducted.
Appropriate to the nested case-control design, point and interval estimates of odds ratios, unadjusted and adjusted for potential confounding factors, were obtained by fitting conditional logistic regression models for matched sets. A two-sided P-value of <0.05 was considered statistically significant. Statistical analyses were performed with SAS statistical software (SAS Version 9.4; SAS Institute Inc., Cary, NC).
Results
The mean age at diagnosis of the cases was 19.8 years (SD: 3.1, range: 4–25 years). As shown in Table 1, gestational week of blood draw, previous births, parental BPD history and maternal educational level were associated with maternal CRP in controls. Previous births, parental BPD, parental psychiatric history, maternal smoking during pregnancy and province of birth were associated with BPD (Table 2). Among the covariates, only previous births and parental history of BPD were associated with both CRP in controls and BPD; hence, we adjusted only for those covariates initially in the main analyses. For further reassurance we also adjusted for all covariates.
Figure 1 shows the distribution of maternal CRP levels among cases and controls. In the unadjusted analyses, increasing maternal CRP, defined as a continuous variable, was not associated with BPD in offspring (OR=0.92, 95% CI: 0.81–1.05, p=0.24). There association was not significant following adjustment for parental psychiatric history (OR=0.92, 95 % CI: 0.79–1.07, p=0.27), maternal age (OR=0.93, 95%CI: 0.81–1.06, p=0.26), and previous births (OR=0.91, 95% CI: 0.79–1.05, p=0.19). However, there were statistical trends following adjustment for parental BPD (OR= 0.88, 95% CI: 0.76–1.01, p=0.07), maternal smoking during pregnancy (OR=0.87, 95% CI: 0.76–1.01, p=0.06) and all available covariates (OR=0.87, 95% CI: 0.73–1.02, p=0.09). There were no differences in the proportions of cases (7.1%) and controls (7.1%) with maternal CRP levels ≥10 mg/L (OR=1.00, 95% CI: 0.56–1.78, p=1.00). The median maternal CRP level was 2.22 mg/L among cases and 2.80 mg/L among controls. Table 3 depicts the association between maternal CRP levels in quintiles and deciles and BPD. There was no increased odds of BPD for maternal CRP levels in the highest quintile (OR= 0.87, 95% CI: 0.54–1.41, p=0.57) and decile (OR=1.00, 95% CI: 0.52–1.94, p=0.99). The range of CRP values among controls in the highest quintiles and deciles were 5.96–63.34 mg/L and 8.80–63.34 mg/L, respectively. The corresponding reference values in the lowest quintiles and deciles were 0.11–0.53 mg/L and 0.11–0.90 mg/L.
Figure 1.
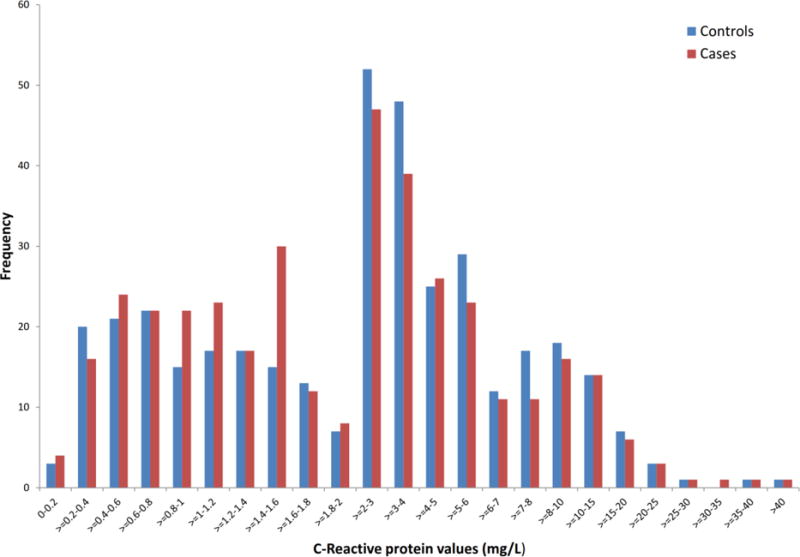
Distribution of maternal C-reactive protein levels in case and control subjects
Table 3.
Maternal CRP levels in quintiles and deciles in BPD case and control subjects.
| CRP by quintile (%) (mg/L)a | Cases | Controls | Odds Ratio | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| ≤ 20 (0.11–0.90) | 75 | 19.6 | 74 | 19.8 | Reference | Reference | |
| 21–40 (0.91–2.09) | 106 | 20.4 | 77 | 28.0 | 1.34 | 0.89–2.03 | 0.16 |
| 41–60 (2.10–3.51) | 60 | 19.8 | 75 | 15.9 | 0.79 | 0.50–1.23 | 0.29 |
| 61–80 (3.52-5.95) | 69 | 20.1 | 76 | 18.2 | 0.89 | 0.57–1.39 | 0.61 |
| >80 (5.96–63.34) | 68 | 20.1 | 76 | 18.0 | 0.87 | 0.54–1.41 | 0.57 |
| CRP by decile (%) (mg/L)b | Cases | Controls | Odds Ratio | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| ≤ 10 (0.11–0.53) | 35 | 9.8 | 37 | 9.2 | Reference | Reference | |
| 11–20 (0.54–0.90) | 40 | 9.8 | 37 | 10.6 | 1.08 | 0.56–2.08 | 0.81 |
| 21–30 (0.91–1.35) | 49 | 10.3 | 39 | 13.0 | 1.29 | 0.72–2.33 | 0.39 |
| 31–40 (1.36–2.09) | 57 | 10.0 | 38 | 15.1 | 1.51 | 0.83–2.74 | 0.17 |
| 41–50 (2.10–2.80) | 38 | 9.8 | 37 | 10.0 | 1.06 | 0.56–1.99 | 0.87 |
| 51–60 (2.81–3.51) | 22 | 10.0 | 38 | 5.8 | 0.58 | 0.29–1.18 | 0.14 |
| 61–70 (3.52–4.57) | 38 | 10.0 | 38 | 10.0 | 1.03 | 0.53–2.00 | 0.93 |
| 71–80 (4.58–5.95) | 31 | 10.0 | 38 | 8.2 | 0.86 | 0.45–1.65 | 0.66 |
| 81–90 (5.96–8.79) | 31 | 10.0 | 38 | 8.2 | 0.81 | 0.41–1.60 | 0.54 |
| 91–100 (8.80-63.34) | 37 | 10.0 | 38 | 9.8 | 1.00 | 0.52–1.94 | 0.99 |
CRP values among controls in each quintile
CRP values among controls in each decile
Discussion
This is the first study of the association between prenatal inflammation documented by maternal serum CRP levels and the risk of BPD in offspring. No significant associations were observed regardless of whether maternal CRP was defined as a continuous, dichotomous or categorical variable. The findings of this study are in contrast to previous studies, in this same birth cohort which documented associations between elevated maternal CRP levels and both childhood autism (Brown et al., 2014) and schizophrenia (Canetta et al., 2014a). It should be noted that the methods used in the studies on schizophrenia and BPD were identical in terms of the source population, identification of cases and controls, timing of maternal serum collection and CRP assay.
Schizophrenia and BPD share several clinical features and epidemiological characteristics. Studies suggest that one reason for these similarities is an overlap between genetic factors in these two disorders (Lichtenstein et al., 2009; Mortensen et al., 2010; Lencz et al., 2013). There are, of course, notable differences in the clinical manifestations of these disorders, originally elaborated by Kraepelin (Kendler and Jablensky, 2011). Recently, Brown has suggested that the identification of maternal risk factors including prenatal infection may offer one strategy to parse the phenotypes of schizophrenia and BPD, in addition to clinical characteristics and neurobiological findings (Brown. 2015). While there have not been a sufficient number of studies of maternal infection and BPD, particularly using prenatal specimens, the evidence to date suggests that, apart from influenza (Canetta et al., 2014a; Parboosing et al., 2013), some infections associated with schizophrenia do not appear to be strong risk factors for BPD (Mortensen et al., 2011). Although one study indicated that maternal exposure to the type I strain of T. gondii was related to an increased risk of affective psychosis, a separate analysis specifically for BPD was not conducted (Xiao et al., 2009). Hence, while further work on maternal inflammation and infection in relation to BPD is necessary, our study provides one piece of evidence that maternal inflammation, at least as indicated by CRP levels, may be an additional factor that differentiates schizophrenia from BPD. This finding can be reconciled with our previously observed association between maternal influenza and BPD (Parboosing et al., 2013) if the effect of influenza is mediated by mechanisms other than inflammation or other molecules that mediate the maternal immune response.
The strengths of the study include: 1) availability of CRP obtained from maternal serum specimens, collected during early to mid-pregnancy; 2) selection of cases and matched controls from nationwide registers, which provides a representative sample and minimizes selection bias. 3) prospectively collected data from medical records which minimizes recall bias and 4) the availability of information on several potential confounders.
Limitations
The limitations of the study are as follows: 1) the study sample was smaller (n=756) than the previous studies on schizophrenia (N=1554) and ASD (N=1354) in this birth cohort. Therefore this study with a two sided significance level of 0.05, assuming a common standard deviation of 0.5, had a power of 69% to detect a difference in the log (CRP) means of 0.8 in cases and 0.88 in controls. This is relatively low in comparison to 90% power in the previous study on schizophrenia. However, on conducting a simulation run using the present study unadjusted odds ratio (OR=0.92), mean CRP values and standard deviation (SD) and imputing the sample sizes of previous studies on Schizophrenia, the associations remained insignificant [(p=0.09 (N= 1554) and p=0.11 (N= 1354)]”; 2) the mean age of the BPD cases was 19.8 years and the oldest subjects were 25 years of age. Due to the predefined sampling frame, the duration of follow-up for this study is limited and not enough to identify all cases that will develop BPD in the cohort. Thus, the lack of association may only be applicable to BPD cases with an earlier age of onset. 3) as noted above, the cases were identified from nationwide registers including inpatient and outpatient diagnoses, which do not accurately differentiate cases with or without psychotic features. Hence, were we were not able to test for an association between maternal CRP and BPD with psychotic features; 4) the availability of several inflammatory markers would have enabled examining the complex interactions among different markers of inflammation and acute phase reactant proteins in terms of immune mediated risk of BPD in offspring. However, due to the study protocol and financial restrictions of serological analyses only one marker of maternal inflammation i.e. CRP was used in this study; 5) maternal blood sample used for CRP analysis was collected at only one time point during pregnancy. Therefore, it is possible that our sample would not accurately measure the elevated CRP levels resulting from an exposure long before collection of maternal blood sample; 6) lastly, we did not have information on important confounders e.g. maternal Body Mass Index (BMI) and maternal drug use during pregnancy, which are associated with CRP levels during pregnancy. In addition, we had no information on maternal infections during pregnancy and thus cannot separately examine maternal CRP levels due to infectious versus non- infectious causes.
In conclusion, we have examined, for the first time, the relationship between maternal CRP and BPD. Our data suggest that maternal inflammation, at least as indicated by CRP, was not related to odds of BPD, in contrast to our findings on schizophrenia and autism in this same birth cohort. Future studies are essential to attempt to replicate these findings and to assess the relationship between maternal CRP and BPD with psychotic features.
Supplementary Material
Highlights.
Inflammation in pregnancy is associated with increased risk of psychiatric disorders.
CRP is marker of inflammation from both infectious and noninfectious exposures.
Elevated maternal CRP is associated with an increased risk of schizophrenia and ASD.
No significant associations were seen with elevated maternal CRP and offspring BPD.
Maternal inflammation may be a factor that differentiates schizophrenia from BPD.
Acknowledgments
This work was supported by grants from University of Turku Graduate school (UTUGS) (R.C), Orion pharma foundation (RC), Yrjö Jahnsson Foundation (R.C), Jalmari and Rauha Ahokkaan Foundation (R.C), NARSAD Independent Investigator Award, USA (A.S), the Sigrid Juselius Foundation, Finland (A.S), 5K02-MH65422 (A.S.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Revised. 3rd. Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 1994. [Google Scholar]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Soares JC, Klunder AD, Nicoletti M, Dierschke N, Hayashi KM, Narr KL, Brambilla P, Sassi RB, Axelson D, Ryan N, Birmaher B, Thompson PM. Three-dimensional mapping of hippocampal anatomy in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:515–525. doi: 10.1097/CHI.0b013e31816765ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Cerruti S, Bellani M, Perlini C, Ferro A, Marinelli V, Giusto D, Tomelleri L, Rambaldelli G, Tansella M, Diwadkar VA. Shared impairment in associative learning in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1093–1099. doi: 10.1016/j.pnpbp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–37. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Brown AS. The Kraepelinian Dichotomy From the Perspective of Prenatal Infectious and Immunologic Insults. Schizophr Bull. 2015;41:786–91. doi: 10.1093/schbul/sbv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19:259–64. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15:411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, Kellendonk C. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014a;171:960–8. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, Terajima M, Shen L, Kellendonk C, Schaefer CA, Brown AS. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry. 2014b;171:557–63. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Cotter D, Coffey VP, Sham PC, Takei N, Larkin C, et al. Prenatal exposure to the 1957 influenza epidemic and adult schizophrenia: a follow-up study. Br J Psychiatry. 1996;168:368–71. doi: 10.1192/bjp.168.3.368. [DOI] [PubMed] [Google Scholar]
- Chudal R, Sucksdorff D, Suominen A, Lehti V, Hinkka-Yli-Salomäki S, Huttunen J, et al. Finnish Prenatal Study of Bipolar Disorders (FIPS-B): overview, design and description of the sample. Nord J Psychiatry. 2014;68:169–79. doi: 10.3109/08039488.2013.789073. [DOI] [PubMed] [Google Scholar]
- Dargél AA, Godin O, Kapczinski F, Kupfer DJ, Leboyer M. C-reactive protein alterations in bipolar disorder: a meta-analysis. J Clin Psychiatry. 2015;76:142–50. doi: 10.4088/JCP.14r09007. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav Immun. 2016;55:25–38. doi: 10.1016/j.bbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jablensky A. Kraepelin’s concept of psychiatric illness. Psychol Med. 2011;41:1119–26. doi: 10.1017/S0033291710001509. [DOI] [PubMed] [Google Scholar]
- Kieseppä T, Partonen T, Kaprio J, Lönnqvist J. Accuracy of register and record-based bipolar I disorder diagnoses in Finland- a study of twins. Acta Neuropsychiatr. 2000;12:106–109. doi: 10.1017/S0924270800035535. [DOI] [PubMed] [Google Scholar]
- Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:35–48. doi: 10.1016/j.pnpbp.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–5. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Guha S, Liu C, Rosenfeld J, Mukherjee S, DeRosse P, et al. Genome-wide association study implicates NDST3 in schizophrenia and bipolar disorder. Nat Commun. 2013;4:2739. doi: 10.1038/ncomms3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–39. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machón RA, Mednick SA, Huttunen MO. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1997;54:322–8. doi: 10.1001/archpsyc.1997.01830160040006. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–47. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–72. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, McGrath JJ, Hougaard DM, Nørgaard-Petersen B, Mors O, et al. Neonatal antibodies to infectious agents and risk of bipolar disorder: a population-based case-control study. Bipolar Disord. 2011;13:624–9. doi: 10.1111/j.1399-5618.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen MG, Pedersen CB. Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol Med. 2010;40:201–10. doi: 10.1017/S0033291709990419. [DOI] [PubMed] [Google Scholar]
- Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–85. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for health-care professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- Sacker A, Done DJ, Crow TJ, Golding J. Antecedents of schizophrenia and affective illness. Obstetric complications. Br J Psychiatry. 1995;166:734–41. doi: 10.1192/bjp.166.6.734. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit Rev Neurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–30. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Xiao J, Buka SL, Cannon TD, Suzuki Y, Viscidi RP, Torrey EF, et al. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11:1011–8. doi: 10.1016/j.micinf.2009.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


