Abstract
Background
Antidepressant response to a single subanesthetic dose infusion of the glutamatergic modulator ketamine is transient in most depressed patients; however, a minority continue to experience an extended response. This study examined depressive symptoms and potential clinical predictors of extended response to ketamine in subjects with mood disorders.
Methods
Subjects were diagnosed with either major depressive disorder (MDD) or bipolar depression. All subjects were treatment-resistant and experiencing a major depressive episode of at least moderate severity. MDD subjects were unmedicated and those with bipolar depression were receiving therapeutic-dose lithium or valproate. All subjects received a single 0.5 mg/kg ketamine infusion. Data were collected pre-infusion (baseline) and at days one, 14, and 28 post-infusion.
Results
Twelve of 93 (12.9%) participants continued to meet response criteria (50% reduction in Montgomery-Asberg Depression Rating Scale (MADRS) score) at two weeks. All depressive symptoms assessed by the MADRS were improved at two weeks in ketamine responders except for sleep duration/depth. A positive family history of alcohol use disorder in a first-degree relative (FHP) and greater dissociation during the infusion were associated with better antidepressant response at two weeks. Improved measures of apparent sadness, reported sadness, inability to feel, and difficulty concentrating at day 1 correlated most strongly with antidepressant effects at two weeks.
Limitations
Post-hoc design, small sample size, diagnostic heterogeneity. Conclusions: Static (FHP) and dynamic (improved depressive symptoms) factors may be clinically useful in predicting whether a patient will have an extended response to ketamine.
Keywords: major depressive disorder, bipolar depression, treatment-resistant depression, ketamine, NMDA receptor antagonist, antidepressant, glutamatergic modulator
Introduction
Worldwide, major depressive disorder (MDD) is associated with high rates of morbidity and disability. The disorder is thought to affect 4.3% of the world’s population (Vos et al., 2012), and lifetime prevalence is estimated at 14.6% (Bromet et al., 2011). Most currently available antidepressant treatments can take two to four weeks to elicit an initial response, and treatment often requires several months for a full response. Indeed, in the real-world effectiveness Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, only 36.8% of patients experienced a remission of their depressive symptoms in response to a standard course of the selective serotonin reuptake inhibitor citalopram (Rush et al., 2006). The same study found that one-third of patients did not achieve remission even after four different antidepressant treatment trials with agents of different classes. Taken together, the evidence underscores the significant need for more rapid-acting and effective antidepressants, presumably with alternative mechanisms of action.
Aberrant glutamatergic neurotransmission has been implicated in both preclinical models of depression and in MDD (Niciu et al., 2014b; Shors et al., 1989). As a result, glutamatergic agents have been investigated as novel antidepressants (Niciu et al., 2014a; Skolnick et al., 1996). Multiple clinical trials have demonstrated that subanesthetic dose ketamine has robust and rapid antidepressant efficacy in both treatment-refractory MDD and in bipolar depression (Coyle and Laws, 2015; Lee et al., 2015). However, the antidepressant effects of a single ketamine infusion are transient in most patients (Coyle and Laws, 2015); as an example, only 50% of ketamine responders continue to respond at two days post-infusion (Newport et al., 2015).
Multiple studies have attempted to extend antidepressant response to ketamine. For instance, the glutamatergic modulator riluzole was tested in two randomized, double-blind, placebo-controlled trials but the effect did not separate from placebo (Ibrahim et al., 2012; Mathew et al., 2010). D-cycloserine, a partial agonist at the glycine co-agonist site of the NMDA receptor, has also been studied in relapse prevention and was found to be effective in a small (n=12) proof-of-concept study in bipolar depression (Kantrowitz et al., 2015). Nevertheless, the most promising antidepressant maintenance strategy currently under investigation appears to be repeated-dose ketamine infusions; yet, to date, only a few such studies have been conducted (aan het Rot et al., 2010; Cusin et al., 2016; Diamond et al., 2014; Murrough et al., 2013; Rasmussen et al., 2013; Singh et al., 2016). These multiple infusion studies have observed extended time-to-relapse (Rasmussen et al., 2013) as well as an increased number of responders (Diamond et al., 2014; Murrough et al., 2013; Rasmussen et al., 2013). Early response to ketamine was also found to predict extended response; one study noted an average time to relapse of 18 days after the last infusion and approximately one-third of the 17 phase I responders maintained antidepressant response at the end of a naturalistic follow-up period (83 days) (Murrough et al., 2013). Given these observations, identifying clinical and/or sociodemographic correlates of extended response in single-infusion ketamine studies is critical as it may facilitate better research design and data interpretation in future multiple-infusion protocols.
Relatedly, efforts are underway to identify biomarkers of ketamine response (for a review, see (Iadarola et al., 2015)). Areas of investigation include BDNF (Haile et al., 2014; Laje et al., 2012; Machado-Vieira et al., 2009), Shank3 (Ortiz et al., 2015), D-serine (Moaddel et al., 2015), interleukin-6 (Yang et al., 2015), vascular endothelial growth factor receptor 1 (VEGF-1) (Permoda-Osip et al., 2014), and vitamin B12 (Lundin et al., 2014; Permoda-Osip et al., 2014). While these studies are promising, many of the findings need to be replicated.
With the exception of anhedonia (Lally et al., 2014), fatigue (Saligan et al., 2016), and suicidal ideation (DiazGranados et al., 2010b; Reinstatler and Youssef, 2015), the literature looking at specific depressive symptom improvement after ketamine has been limited. In MDD patients who remit with citalopram, residual depressive symptoms, most commonly sleep and appetite disturbance, persist (Nierenberg et al., 2010). Few baseline sociodemographic variables have been investigated with regard to their association with antidepressant response to ketamine past one week. Interestingly, studies have found that family history of an alcohol use disorder in a first-degree relative (FHP) correlated with increased antidepressant effects of ketamine in treatment-resistant individuals with either MDD (Niciu et al., 2014c; Niciu et al., 2014d; Phelps et al., 2009) or bipolar depression (Luckenbaugh et al., 2012; Permoda-Osip et al., 2014). In addition, patients with such a history had fewer depressive symptoms for up to four weeks post-infusion (Niciu et al., 2014d). Higher body mass index (BMI) was also found to correlate with greater improvement in depressive symptoms at 230 minutes and one day post-infusion, and no lifetime history of suicide attempt(s) correlated with antidepressant efficacy at one week post-infusion (Niciu et al., 2014c). Another study found that dimensional anxious depression (as defined as a Hamilton Depression Rating Scale (HAM-D) anxiety/somatization factor score ≥ 7) at baseline also correlated with improvement in depressive symptoms in individuals with treatment-resistant MDD (Ionescu et al., 2014). Finally, greater intra-infusion dissociation (as measured by the Clinician Administered Dissociative States Scale (CADSS)) was found to be correlated with increased antidepressant effects at 230 minutes and at one week post-infusion (Luckenbaugh et al., 2014).
Given the depressive symptoms noted above that have been associated with antidepressant response to ketamine at one week (accounting for significant amounts of the effect variance in prior studies at this time point), we hypothesized that FHP status, anxious depression, no lifetime history of suicide attempt(s), and greater dissociation would correlate with continued antidepressant effects at two weeks, and that all depressive symptoms assessed by the MADRS would be decreased two weeks after ketamine infusion in ketamine responders. We further sought to ascertain whether early symptom improvement would correlate with greater antidepressant effects at two weeks, hypothesizing that improvement in each individual MADRS symptom would correlate with overall antidepressant efficacy at two weeks.
Methods
Participant Selection
We examined data previously collected from four independent inpatient studies conducted by our group on the experimental use of ketamine in treatment-resistant MDD and bipolar I/II depression without psychotic features. The studies were conducted between October 2006 and June 2015. The primary results of three of the four studies have been previously published (Diazgranados et al., 2010a; DiazGranados et al., 2010b; Ibrahim et al., 2012; Zarate et al., 2012). Two papers reported the results of a double-blind, placebo-controlled study of a single subanesthetic dose of ketamine in current bipolar depression (“ketamine bipolar”; NCT#00088699, NIH Protocol #04-M-0222, substudy 2) (Diazgranados et al., 2010a; Zarate et al., 2012). One study examined the use of flexible-dose oral riluzole (100-200mg/day) versus placebo for 28 days following open-label ketamine infusion in treatment-resistant MDD (“ketamine riluzole”; NCT#00088699, NIH Protocol #04-M-0222, substudy 3) (Ibrahim et al., 2012); subjects from both the placebo and riluzole arms were included in this analysis. Results from an additional, ongoing study of ketamine in treatment-resistant depression are unpublished (NCT#00088699, NIH Protocol #04-M-0222, substudy 4).
All participants were between the ages of 18 and 65 years old and admitted to the National Institute of Mental Health’s Mood and Anxiety Disorders research unit in Bethesda, Maryland. All participants provided written informed consent as approved by the National Institutes of Health Combined Central Nervous System Institutional Review Board. All neuropsychiatric diagnoses were based on the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision (DSM-IV-TR) criteria as confirmed by both a clinical interview performed by a licensed independent psychiatric practitioner and the Structured Clinical Interview for Axis I DSM-IV Disorders, Patient Version (SCID-I/P) (First et al., 2002); the DSM-5 was not used, given that three of the four studies included in this analysis were completed before the DSM-5 was released. All participants were experiencing a major depressive episode of at least moderate severity (defined as a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20 (Diazgranados et al., 2010a; Zarate et al., 2012) or ≥22 (Ibrahim et al., 2012)) at screening and prior to infusions. Subjects were also required to not have responded to at least one adequate antidepressant dose/duration trial, have no active substance use diagnosis for at least three months prior to inpatient admission, and have no unstable medical problems.
Study Design
After signing protocol-specific consent forms, all MDD subjects were tapered off their current psychotropic medication regimen and remained medication-free for at least two weeks (five weeks for fluoxetine) prior to infusion. Participants with bipolar I/II depression were tapered off all psychotropic medications except lithium or valproate; these subjects were then initiated and/or maintained on therapeutic levels of lithium or valproate for at least four weeks. All participants received a single subanesthetic (0.5mg/kg) infusion of ketamine hydrochloride over 40 minutes. In the “ketamine riluzole” open-label study, subjects received ketamine and were then randomized to either riluzole (100-200 mg/day) or placebo for 28 days. All studies administered rating scales at 60 minutes pre-infusion (baseline). The two “ketamine bipolar” studies and the treatment-resistant depression study (unpublished data) administered rating scales up to 14 days post-infusion, whereas the “ketamine riluzole” open-label study administered rating scales up to 28-days post infusion (Ibrahim et al., 2012).
Outcome Measures
The primary outcome measure for this analysis was the standard 10-item MADRS; it should be noted that this scale does not systematically examine atypical symptoms of depression (i.e., increased sleep, increased appetite, etc). Antidepressant response was defined as a ≥50% reduction in MADRS score from baseline at a given post-infusion time point, e.g. two weeks. Dissociative side effects were assessed using the CADSS (Bremner et al., 1998). Data from the HAM-D, Hamilton Anxiety Rating Scale (HAM-A), Beck Depression Inventory (BDI), and Young Mania Rating Scale (YMRS) were also assessed.
Demographic and clinical variables (reported in Table 1) were examined in relation to two-week antidepressant efficacy. These variables were chosen based on their association with antidepressant efficacy in longitudinal MDD and bipolar clinical trials but also included variables previously reported to be associated with ketamine’s antidepressant effects (Niciu et al., 2014c; Niciu et al., 2014d; Obrocea et al., 2002; Permoda-Osip et al., 2014; Trivedi et al., 2006). To determine whether specific residual symptoms post-infusion predicted antidepressant effect at two weeks post-infusion, individual symptoms at day one were also examined in relation to two-week antidepressant efficacy.
Table 1.
Demographic and Clinical Data of Subjects Receiving Ketamine, Separated by Antidepressant Response at Two Weeks
| Responders at Two Weeks (n=12) mean (SD) |
Non-Responders at Two Weeks (n=81) mean (SD) |
|
|---|---|---|
| Age, years | 43.8 (12.4) | 43.7 (12.8) |
| Body mass index | 32.7 (9.0) | 28.9 (6.3) |
| Age of Onset, years | 23.9 (11.9) | 17.6 (8.8) |
| Length of Illness, years | 19.9 (10.0) | 26.0 (12.7) |
| Length of Current Depressive Episode, months |
65.8 (111.4) | 50.5 (93.6) |
| Number of Previous Episodes | 29.5 (44.0) | 30.5 (40.4) |
| Total Lifetime Medication Trials | 5.7 (3.0) | 8.0 (4.3) |
| Clinical Ratings (at baseline) | ||
| MADRS | 31.6 (3.1) | 33.0 (5.0) |
| BDI | 25.8 (6.9) | 28.1 (8.7) |
| HAM-A | 19.7 (3.4) | 21.2 (5.2) |
| Percent change in MADRS score | ||
| 230 min | −60.4 (28.6) | −31.4 (34.7) |
| 1 day | −62.5 (30.9) | −28.6 (34.5) |
| 14 days | −66.1 (14.8) | −10.0 (19.8) |
| n (%) | n (%) | |
| Education | ||
| Not College Grad | 7 (58.3) | 33 (42.3) |
| College Grad | 5 (41.7) | 45 (57.7) |
| Sex, female | 5 (41.7) | 44 (54.3) |
| Race/ethnicity, white | 11 (91.7) | 72 (91.1) |
| Diagnosis (bipolar disorder) | 2 (20.0) | 21 (36.8) |
| Lifetime History of | ||
| Suicide Attempt | 6 (50.0) | 30 (38.5) |
| Anxiety Disorder | 5 (50.0) | 30 (54.5) |
| Alcohol Abuse | 2 (16.7) | 20 (28.6) |
| Substance Abuse (non-nicotine) | 6 (50.0) | 27 (36.5) |
| Abuse | ||
| Physical | 4 (40.0) | 14 (23.0) |
| Sexual | 4 (40.0) | 13 (21.3) |
| Family History Alcohol Use Disorder, 1st Degree Relative |
6 (50.0) | 28 (35.4) |
Abbreviations: MADRS, Montgomery-Åsberg Depression Rating Scale; BDI, Beck Depression Inventory; HAM-A, Hamilton Anxiety Rating Scale
Statistics
Baseline demographic and clinical characteristics were compared between two-week responder and non-responder groups using chi-square and independent samples t-tests.
Linear mixed models using restricted maximum likelihood estimation and a compound symmetry covariance structure were used to analyze the course of individual MADRS symptoms from baseline to two weeks post-infusion in the two-week responder vs. non-responder groups. Main effects for group and time and their interaction were included in the model, with the time points of 40, 80, 120, and 230 minutes as well as days one, two, three, seven, and 14. Baseline level was used as a covariate. In addition, for individual symptoms, subjects with a baseline of zero were removed from the analysis to minimize a floor effect. Bonferroni-corrected simple effects tests were used to compare groups at individual time points if the interaction between time and response was significant.
Pearson correlations were used to examine associations between percent change in MADRS score at two weeks post-ketamine infusion and the following: (1) the raw values of individual symptoms one day after infusion, and (2) baseline demographic and neuropsychiatric variables and total CADSS score at the end of the 40-minute infusion. Bonferroni corrections were used when correlations were run without an a priori hypothesis. Because FHP status, history of suicide attempt, dimensional anxious depression, and intra-infusion dissociation had all previously been found to correlate with ketamine’s antidepressant efficacy at one week, they were not corrected for multiplicity.
Results
Response Distribution and Correlates
Of the 122 participants in the four independent studies described above, 93 (76.2%) remained in their respective protocol for at least 14 days post-infusion, and 33 subjects completed four weeks of the open-label study (“ketamine riluzole”). Given the larger sample size, most analyses were conducted with data from the 14-day time point.
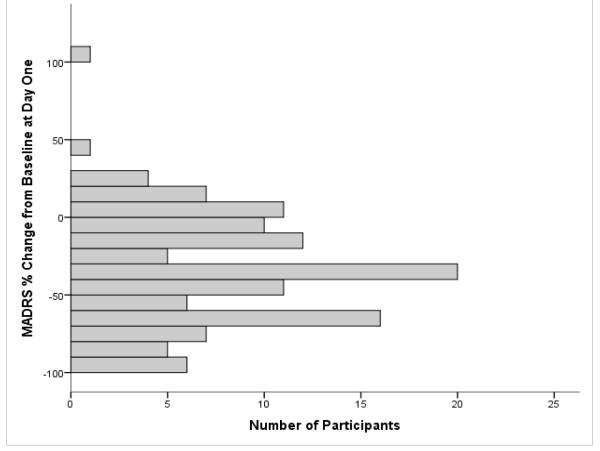
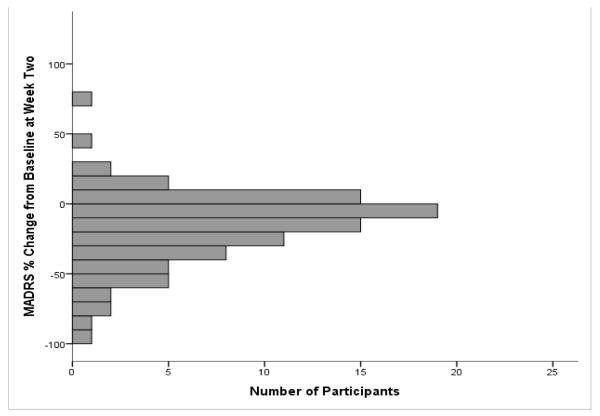
We first evaluated the distribution of antidepressant response to ketamine at one day and at two weeks post-infusion. Mean percent change in MADRS was −33.7% ± 35.2 at one day and −17.2% ± 26.9 at two weeks post-infusion. Forty-one of the 122 participants (32.5%) had an antidepressant response at 24 hours, and 12 of the 93 (12.9%) had a response at the two-week time point. The demographic and clinical features of the subjects who did (n=12) and did not (n=81) show an antidepressant response to ketamine at the two-week time point are presented in Table 1. Compared to non-responders at two weeks, the two-week responder group had greater percent change from baseline MADRS score at 230 minutes (p=.006), one day (p=.003), and two weeks (p<.001).
As noted above, 33 subjects completed four weeks of the open-label “ketamine riluzole” study. For those subjects, mean MADRS percent change at four weeks was −20.1% ± 28.7. Of the 33 subjects, four (12.1%) continued to exhibit an antidepressant response at four weeks post-infusion. Notably, two of those responders were experiencing their first major depressive episode and all four had FHP status.
Specific Symptom Improvement Associated with Ketamine’s Extended Antidepressant Effect
We next investigated change in specific depressive symptoms at two weeks using individual MADRS items. Those who reported a zero at baseline for the symptom being examined were excluded. We hypothesized that there would be differences in depressive symptoms between responders and non-responders at two weeks and, indeed, significant group differences were observed for all symptoms at two weeks except for “Reduced Sleep” (MADRS item #4; F(1,697) = 1.64, p = .20). To further examine the lack of change in sleep symptoms, we performed linear mixed models on sleep-related items from other standardized rating scales. We were interested in the internal validity of the finding as well as in elucidating whether specific subtypes of insomnia were differentially affected. No significant difference was observed at two weeks in the YMRS sleep item, the HAM-D early insomnia item, the HAM-D middle insomnia item, or the HAM-D late insomnia item. Prior to multiplicity correction, a significant difference was only seen in the self-reported BDI sleep item (F(1, 227) = 7.55, p = .007).
Was Day One Antidepressant Symptom Improvement Related to Two-Week Antidepressant Efficacy?
To assess the degree by which residual symptoms could predict ketamine’s antidepressant efficacy, bivariate correlations examining the relationship between individual MADRS items at one day post-infusion and percent change in MADRS score at two weeks were conducted (Table 2). In general, more residual symptoms at day one was associated with decreased antidepressant efficacy at two weeks. Significant correlations with percent change in MADRS score at two weeks were found with reported sadness (r = −.34, p = .001), difficulty concentrating (r = −.32, p = .002), apparent sadness (r = −.30, p = .003), and inability to feel (r = −.31, p = .002) at day one. A stepwise linear regression showed that only reported sadness was an independent correlate.
Table 2.
Day 1 Symptom Severity Correlation with Antidepressant Response to Ketamine at Two Weeks (MADRS Percent Change from Baseline)
| Variable | r | r2 | p | padjusted* |
|---|---|---|---|---|
| Reported Sadness | −.34 | .116 | .001 | .01 |
| Difficulty Concentrating | −.32 | .102 | .002 | .02 |
| Inability to Feel | −.31 | .096 | .002 | .02 |
| Apparent Sadness | −.30 | .090 | .003 | .03 |
| Lassitude | −.26 | .068 | .012 | .12 |
| Pessimistic Thoughts | −.23 | .053 | .024 | .24 |
| Inner Tension | −.23 | .053 | .029 | .29 |
| Reduced Appetite | −.23 | .053 | .026 | .26 |
| Suicidal Thoughts | −.22 | .048 | .037 | .37 |
| Reduced Sleep | −.08 | .006 | .48 | 1.00 |
Bonferroni-corrected for multiple comparisons; bolded variables were significant.
Abbreviations: MADRS: Montgomery Åsberg Depression Rating Scale
Demographic and Clinical Correlates of Ketamine’s Extended Antidepressant Efficacy
The relationship between demographic and clinical variables and percent change in MADRS score from baseline to two weeks post-infusion were assessed with Pearson correlations. As mentioned previously, FHP status, no lifetime history of suicide attempt, dimensional anxious depression, and intra-infusion dissociation were previously found to correlate with ketamine’s antidepressant effects at one week and were thus treated as primary variables. Of the primary variables, only FHP status (r = −.23, p=.03) and dissociation at 40 minutes (r = −.29, p = .005) correlated with antidepressant efficacy.
Discussion
In this secondary analysis, we hypothesized that all individual depressive symptoms assessed by the MADRS would be lower in those who continued to manifest an antidepressant response to ketamine at two weeks post-infusion versus those who did not. We found that all MADRS depressive symptoms were improved at two weeks in ketamine responders except for sleep quality. In addition, a positive family history of alcohol use disorder in a first-degree relative (FHP) and greater dissociation during the infusion were associated with better antidepressant response at two weeks. Improved measures of apparent sadness, reported sadness, inability to feel, and difficulty concentrating at day one correlated most strongly with antidepressant efficacy at two weeks.
While most depressive symptoms were lower in responders, sleep quality was not significantly different from non-responders at two weeks. This finding was replicated in the YMRS sleep item as well as in the HAM-D early, middle, and late insomnia items, but not in the BDI sleep item. This difference may be due to variability in the range of possible scores (0-3 on the BDI versus 0-6 on the MADRS), wording differences across the instruments, or means of administration (BDI is a self-report questionnaire whereas the other scales are clinician-administered). In addition, the effects of ketamine on sleep in depressed patients are complex. Ketamine increased slow-wave sleep activity as well as total sleep time in MDD patients for two days post-ketamine infusion (Duncan et al., 2013), but had little effect on the sleep of bipolar patients taking concurrent mood stabilizers (Duncan and Zarate, 2013). Increased slow-wave sleep is considered a biomarker of enhanced synaptic plasticity (Vyazovskiy et al., 2008), which has been implicated in ketamine’s antidepressant mechanism of action (Bjorkholm and Monteggia, 2016; Duncan et al., 2013). Our findings suggest that, while earlier, more objective improvements in sleep patterns may occur, these changes may not be perceived by raters and may not persist in those with an overall protracted response to ketamine. These results also parallel previous findings that continued sleep problems are one of the most common residual symptoms after remission of depressive symptoms in response to citalopram (Nierenberg et al., 2010).
The present study also investigated what, if any, residual symptomatology at day one might predict antidepressant efficacy at two weeks, hypothesizing that fewer symptoms early in the course of treatment would predict continued antidepressant efficacy. We found that improvements in reported sadness, apparent sadness, inability to feel, and concentration at day one were most robustly correlated with percent change in MADRS score from baseline to two weeks. However, the individual correlations explained only a limited proportion of the variance in response. While this result may be clinically useful in predicting which subjects are more likely to have a protracted antidepressant response to ketamine, the limited amount of variance explained suggests the need for additional correlates to generate stronger predictors of response.
The need to identify demographic and clinical correlates of ketamine’s antidepressant effects is key to both clinical practice and the design of future studies, given that such correlates could generate spurious associations if not properly controlled or covaried. In this study, FHP status correlated with improved antidepressant response at two weeks post-infusion. FHP’s association with ketamine’s enhanced antidepressant efficacy is supported by previous publications from our group (Niciu et al., 2015; Phelps et al., 2009) and the finding was also noted in a Polish cohort with bipolar depression (Permoda-Osip et al., 2014). FHP is also associated with attenuation of ketamine’s adverse effects, e.g. acute dysphoria and positive symptoms (Petrakis et al., 2004) as well as with reward valence (Yoon et al., 2016). In addition, an emerging FHP neuroimaging literature that includes premorbid/at-risk FHP youth found differential subcortical and cerebellar brain volumes as well as differences in white matter microstructures (Cservenka, 2015). This suggests that increased antidepressant response to ketamine may be due to a variety of factors.
In this regard, one genetic factor that may play a role in the correlation with FHP is a polymorphism in the NR2A subunit of the NMDA receptor (Schumann et al., 2008). This polymorphism is associated with family history of alcoholism, early onset of alcohol use disorder, and risky drinking behavior. Epigenetic and/or neurophysiological factors may also play a role (e.g. individuals with FHP have been shown to have altered age-related differences in glutamatergic turnover (Cohen-Gilbert et al., 2015) as well as other structural and functional neuroimaging alterations (Cservenka, 2015)). Overall, however, little work has been done to date on the genetic or biomarker-based factors underlying this association with FHP.
The present study also found that greater dissociation during ketamine infusion correlated with increased antidepressant efficacy at two weeks post-infusion. This finding was also observed at earlier time points in an overlapping subsample of participants (Luckenbaugh et al., 2014). To our knowledge, however, no biomarkers to date have been linked to greater dissociation during ketamine infusions. It is possible that dissociation is related to ketamine’s ability to increase glutamate release, given that lamotrigine, a drug thought to inhibit glutamate release, attenuates ketamine’s dissociative effects (Anand et al., 2000). This increase in glutamate is thought to mediate ketamine’s antidepressant abilities through increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-to-NMDA receptor activation (Dwyer and Duman, 2013; Maeng et al., 2008). Ketamine’s antidepressant properties may also stem from the induction of profound experiences (Krupitsky and Grinenko, 1997), with dissociation potentially acting as a marker of such experiences. Another possibility is that experiencing significant dissociative symptoms unblinds participants as to whether they are receiving ketamine or placebo. However, an earlier study from our group found that dissociation—but not psychotomimetic symptoms—was associated with improved mood ratings, even though both could potentially unblind participants (Luckenbaugh et al., 2014). It should also be noted that a correlation between ketamine-induced dissociation and antidepressant efficacy was not observed in a smaller sample (n=11) (Valentine et al., 2011). Nevertheless, more research is needed to understand the mechanism(s) underlying this correlation.
We also hypothesized that dimensional anxious depression at baseline would correlate with ketamine’s extended antidepressant effects (Ionescu et al., 2014), but this was not found in our sample at this time point. In addition, while BMI and lack of prior suicide attempt had previously been found to correlate with antidepressant efficacy at earlier time points (Luckenbaugh et al., 2014; Niciu et al., 2014c), these correlations were not replicated in our larger sample at two weeks.
Our study has many strengths as well as a number of limitations. Strengths include the combined subject-level data from several independent ketamine depression trials, which resulted in a relatively large sample of two-week responders. All participants were well-characterized inpatients studied at the same site by the same research group with high inter-rater reliability for the studied outcome measures. Potential limitations include the secondary/post-hoc design, the pooling of MDD and bipolar depressed subjects, the differences in initial medication status between subjects (unmedicated (MDD) vs medicated (lithium or valproate-maintained bipolar) subjects), the differences in study design (double-blind, placebo-controlled versus open-label), and the fact that a subset of subjects in the “ketamine riluzole” study received medication (riluzole) during the post-infusion period. Of those included in this analysis, 24/78 received adjunctive riluzole, and five of 24 responders received riluzole in addition to ketamine. While this may confound results, it should be noted that adjunctive riluzole did not significantly alter changes in depressive symptoms and did not delay time to relapse (Niciu et al., 2015). The significant subject heterogeneity reinforces the need for independent researchers to replicate these findings in more uniform samples. Another limitation is the lack of physiological sleep measures at two weeks—for instance, sleep polysomnography (PSG) or 24-hour actigraphy—to confirm poor sleep quality as reported on the clinician-administered scales.
By generating data that could be used to predict which subjects are likely to have an extended response to a single ketamine infusion, these findings may be useful in designing future studies. Interestingly, Murrough and colleagues (2013) reported a sensitivity and specificity of 0.88 and 0.71, respectively, for 24-hour antidepressant response and at the end of their open-label, six-infusion, 12-day protocol (Murrough et al., 2013). This suggests that the predictive ability of early response to a single infusion may also be useful in predicting response to multiple infusions. In addition, as ketamine continues to be developed as a treatment for depression, the finding that significant response to ketamine at day one may correlate with antidepressant effects two weeks later may help determine how often a patient will need repeated dosing; that is, those who are significantly improved after a single infusion—an effect apparent as early as 24 hours post-infusion—may not require repeat administration until much later, thereby minimizing potential risks associated with repeated exposure.
Taken together, our findings in two-week ketamine responders addresses previously unanswered questions and provides future avenues of research. Notably, while many patients experience a remarkable initial response to ketamine treatment, only 12.9% of our sample had an antidepressant response at two weeks. Though it is impressive that a single dose of ketamine can have such a profound effect on these extended responders, this result also highlights the fact that most subjects with treatment-resistant depression may not experience a lasting benefit from ketamine or, to sustain that benefit, will require regular ketamine maintenance therapy. Thus, it may be clinically advantageous to identify those who respond robustly early on in order to discuss maintenance therapies including, but not limited to, repeated-dose ketamine.
The correlation between improvements in specific depression symptoms (reported sadness, apparent sadness, inability to feel, and difficulty concentrating) at day one with overall two-week antidepressant efficacy may provide a clinically useful way to gauge the likelihood of extended antidepressant response to a single ketamine administration in individual subjects. Unchanged sleep symptoms at two weeks suggests that sleep may be one of the more refractory symptoms in both effective monoaminergic and glutamatergic antidepressant trials. In addition, the findings that FHP and increased intra-infusion dissociation correlated with antidepressant effects at two weeks are also key to understanding and maximizing ketamine’s effects. We hope these findings lead to further discoveries into glutamate-based antidepressant mechanisms as well as assist clinicians in predicting which subjects are more likely to experience longer antidepressant effects in response to ketamine.
Figure 1.
Percent Change from Baseline MADRS at (A) Day One, mean = −33.7, SD = 35.2 (n = 122) (B) Week Two, mean = −17.2, SD = 26.9 (n = 93).
Figure 2.
Residual Depressive Symptoms in Ketamine Responders (n=12) and Non-Responders (n=81) at 2 weeks Post-Infusion (controlled for baseline differences).
Highlights.
Some subjects display antidepressant response to ketamine two weeks post-infusion
Two-week post-infusion data from multiple ketamine trials (MDD & BD) were combined
Dissociation & family history of alcohol use were linked to response at two weeks
Less sadness, anhedonia & trouble concentrating at day 1 predicted greater response
Sleep quality was not significantly improved in responders at two weeks
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Bjorkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the clinicianadministered dissociative states scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSMIV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Sneider JT, Crowley DJ, Rosso IM, Jensen JE, Silveri MM. Impact of family history of alcoholism on glutamine/glutamate ratio in anterior cingulate cortex in substance-naive adolescents. Dev Cogn Neurosci. 2015;16:147–154. doi: 10.1016/j.dcn.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152–163. doi: 10.1002/hup.2475. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, Eikermann M, Durham K, Swee MB, Chang T, Dording C, Soskin D, Kelley J, Mischoulon D, Brown EN, Fava M. Ketamine augmentation for outpatients with treatmentresistant depression: Preliminary evidence for two-step dose escalation. Aust N Z J Psychiatry. 2016 Feb 18; doi: 10.1177/0004867416631828. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28:536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr. A randomized add-on trial of an N-methyl-Daspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatmentresistant major depressive disorder. J Clin Psychiatry. 2010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Jr., Zarate CA., Jr. Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep. 2013;15:394. doi: 10.1007/s11920-013-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73:1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders Research Version, Patient Edition with Psychotic Screen (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney J.J.r., De La Garza R.n., Charney DS, Newton TF, Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA. Ketamine and other Nmethyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Vande Voort JL, Brutsche NE, Zarate CA., Jr. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. 2014;75:e932–938. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily D-Cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry. 2015;76:737–738. doi: 10.4088/JCP.14l09527. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997;29:165–183. doi: 10.1080/02791072.1997.10400185. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche NE, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate CAJ. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EE, Della Selva MP, Liu A, Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37:178–184. doi: 10.1016/j.genhosppsych.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, Cassarly C, Zarate CA., Jr. Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin NB, Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Brutsche NE, Machado-Vieira R, Zarate CAJ. Baseline vitamin B12 and folate levels do not predict improvement in depression after a single infusion of ketamine. Pharmacopsychiatry. 2014;47:141–144. doi: 10.1055/s-0034-1377042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA., Jr. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatmentresistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Luckenbaugh DA, Xie Y, Villasenor A, Brutsche NE, Machado-Vieira R, Ramamoorthy A, Lorenzo MP, Garcia A, Bernier M, Torjman MC, Barbas C, Zarate CA, Jr., Wainer IW. D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology (Berl) 2015;232:399–409. doi: 10.1007/s00213-014-3669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr., Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014a;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014b;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA., Jr. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014c;75:e417–423. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, Brutsche NE, Furey ML, Zarate CA., Jr. Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol. 2014d:18. doi: 10.1093/ijnp/pyu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, Brutsche NE, Furey ML, Zarate CA., Jr. Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrocea GV, Dunn RM, Frye MA, Ketter TA, Luckenbaugh DA, Leverich GS, Speer AM, Osuch EA, Jajodia K, Post RM. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biol Psychiatry. 2002;51:253–260. doi: 10.1016/s0006-3223(01)01206-9. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Niciu MJ, Lukkahati N, Saligan LN, Nugent AC, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr. Shank3 as a potential biomarker of antidepressant response to ketamine and its neural correlates in bipolar depression. J Affect Disord. 2015;172:307–311. doi: 10.1016/j.jad.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permoda-Osip A, Skibinska M, Bartkowska-Sniatkowska A, Kliwicki S, Chlopocka-Wozniak M, Rybakowski JK. [Factors connected with efficacy of single ketamine infusion in bipolar depression] Psychiatr Pol. 2014;48:35–47. [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15:37–43. doi: 10.1007/s40268-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA., Jr. An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, crossover study in bipolar disorder. J Affect Disord. 2016;194:115–119. doi: 10.1016/j.jad.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji HK, Drevets WC, Van Nueten L. Intravenous esketamine in adult treatment-resistant depression: a double-blind, doublerandomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine's antidepressant effect in treatmentresistant patients with major depression. Biol Psychiatry. 2015;77:e19–20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Yoon G, Pittman B, Limoncelli D, Krystal JH, Petrakis IL. Familial alcoholism risk and the ratio of stimulant to sedative effects of ketamine. Biol Psychiatry. 2016;79:e69–70. doi: 10.1016/j.biopsych.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]