Abstract
Rationale
Siglec-8 is a surface receptor predominantly expressed on human eosinophils where its ligation induces reactive oxygen species (ROS) formation and cell death. Since Siglec-8 has intracellular tyrosine-based motifs, we hypothesized that Src family kinases (SFKs) are involved in ROS formation and cell death induced by Siglec-8 engagement.
Methods
Human peripheral blood eosinophils were purified and incubated with anti-Siglec-8 monoclonal antibodies (mAb, agonist), IL-5, and SFK pharmacological inhibitors. We focused on Siglec-8-induced cell death in short-term IL-5-activated cells leading to a regulated necrosis-type cell death. ROS production was determined by dihydrorhodamine (DHR) 123 labeling and flow cytometry, or by chemiluminescence using Amplex red. Activation of SFK was determined using phospholuminex and Western blotting.
Results
In order to determine cellular localization of ROS production, we measured intra and extracellular ROS. While an ETosis stimulus (calcium ionophore A23187) led to extracellular ROS (ecROS) production, Siglec-8-engagement in short-term IL-5 activated cells led to intracellular ROS (icROS) accumulation. Consistently, inhibition of extracellular ROS by catalase inhibited ETosis, but not IL-5-primed Siglec-8-induced cell death. In order to determine signaling events for Siglec-8, we performed Western blotting and found SFK phosphorylation in lysates from eosinophils stimulated with anti-Siglec-8 mAb ± IL-5. In order to identify which SFKs were involved, we used the phospholuminex assay and found increased levels of phosphorylated Fgr in the cytoplasmic fraction of cells co-stimulated with anti-Siglec-8 and IL-5 for 3 hours compared with cells stimulated with IL-5 alone. To test the involvement of SFKs in ROS production and cell death, we used SFK inhibitors PP2 and dasatinib, both of which completely inhibited eosinophil ROS production and cell death induced by anti-Siglec-8 and IL-5 co-stimulation.
Conclusion
Siglec-8 engagement in short-term IL-5-activated eosinophils causes icROS production and SKF phosphorylation, and both are essential in mediating Siglec-8-induced cell death.
Introduction
Siglecs (sialic acid binding Ig-like lectin) are a family of I-type lectins that recognize sialic acid. Characterized by their preferential expression on hematopoietic and immune systems and their common structure in cytoplasmic domain bearing one or more immunoreceptor tyrosine-based inhibitory motifs (ITIMs), siglecs are assumed to have primarily inhibitory function in the immune system. [1,2] Except for a few instances such as Siglec-2 (CD22) mediated inhibition of BCR signaling in B cells, however, the roles and precise mechanisms of siglec function in immune responses are yet to be understood. [3]
Siglec-8 is predominantly expressed on human eosinophils, although it is also found on the surface of basophils and mast cells. [4,5] Interestingly, in vitro ligation of Siglec-8 induces apoptotic eosinophil cell death, and this function is paradoxically enhanced by co-stimulation or prior priming with IL-5, the latter being a prominent activation/survival factor for eosinophils. [6,7] Clinically, Siglec-8 has been implicated in asthma pathogenesis; a polymorphism of Siglec-8 was associated with asthma susceptibility in humans [8]. Mice bearing a genetic deficiency for Siglec-F (the functional paralog of Siglec-8) [9,10] or enzymes (e.g., ST3Gal-III) responsible for generation of its putative ligand (glycans on Muc5b) were shown to have exacerbated eosinophilic inflammation in models of type 2 responses. [11,12] Collectively, Siglec-8 and Siglec-F are suggested to have a role to prevent chronic lung inflammation of asthma by inducing cell death of activated eosinophils. [13]
It has been demonstrated that Siglec-8 ligation induces apoptosis in resting cells, and a form of regulated necrosis, involving reactive oxygen species (ROS) accumulation and prolonged extracellular signal-regulated kinase (ERK) phosphorylation in short-term IL-5 pretreated cells [14,15], consistent with the general notion that cells that receive a death signal but have apoptosis inhibited (e.g. by IL-5) activate backup cell death pathways such as regulated necrosis. [16] Recently, another form of ROS-dependent regulated necrosis was described in eosinophils, ETosis. [17] The authors demonstrated that extracellular ROS (ecROS) is produced in and required for the process of ETosis. On the other hand, previous results suggested intracellular ROS (icROS) is accumulated and promotes phosphorylation of nuclear ERK upon Siglec-8/IL-5 induced cell death. Whether the mechanism of regulated cell death induced by ETosis stimuli and Siglec-8/IL-5 are identical, i.e., whether ecROS and icROS play similar roles in Siglec-8/IL-5 induced cell death as in ETosis, is the first focus of the present study. Furthermore, the mechanism of ROS induction by an ITIM-bearing receptor is not known. In other siglec pathways, such as those involving CD22 on B cells or CD33 on neutrophils, Lyn, a member of the non-receptor tyrosine kinase Src family kinases (SFKs), is implicated in signaling, suggesting an affinity of SFKs for the intracellular membrane-proximal ITIM found in most siglecs. [18,19] In turn, SFKs are also implicated in chemoattractant-induced ROS production in neutrophils. [20] Therefore, to dissect the signaling pathways mediating eosinophil cell death following Siglec-8 engagement in short-term IL-5-primed eosinophils, we tested the hypothesis that SFKs are involved in ROS accumulation and cell death induced by Siglec-8.
Materials and Methods
Eosinophil purification
Blood eosinophils were purified (to >95% purity) from healthy control subjects by using Percoll gradient separation and the CD16 magnetic bead negative selection system (Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described. Written informed consent was obtained from all blood donors, and the study was approved by the Institutional Review Board of Cincinnati Children’s Hospital.
Eosinophil culture and cell death measurement
Human eosinophils were cultured at 1×106/mL in RPMI 1640 containing 10% fetal bovine serum (FBS) (subsequently designated as “normal media”), or for some experiments, in RPMI 1640 containing 0.1% bovine serum albumin (BSA), in order to avoid the known ROS–scavenging effect of FBS [FBS(−) media]. To activate eosinophils, we added recombinant human IL-5 (PeproTech, Rocky Hills, NJ) (30 ng/mL), and anti–Siglec-8 or its isotype-matched control antibody (BioLegend, San Diego, CA) (2.5 μg/mL). Unless noted otherwise, IL-5 and anti-Siglec-8 were added simultaneously. Anti–Siglec-8 mAb 2C4 (mouse IgG1) was produced as previously described.5 In some experiments the clone 7C9 anti–Siglec-8 antibody (BioLegend) was used; results were identical regardless of which anti–Siglec-8 antibody clone used. ETosis was induced by exposure to the calcium ionophore A23187 (Sigma-Aldrich, MO) (2μM). The selective pharmacological inhibitor for SFKs (PP2) and its inactive control (PP3) were purchased from EMD-Millipore Chemicals (Merck, Darmstadt, Germany), and the pan-tyrosine kinase inhibitor dasatinib (DA) was obtained from Fisher Scientific (Pittsburgh, PA). Inhibitors or controls were added simultaneously with IL-5 and/or anti–Siglec-8, as specified in figure legends. When the ROS inhibitor diphenyleneiodonium (DPI; Sigma-Aldrich, St Louis, MO) was used, cells were preincubated with DPI for 5 minutes and then washed and resuspended with media prior to adding the indicated stimuli. Catalase (Sigma-Aldrich), which does not penetrate intact plasma membranes and thus works as an extracellular ROS scavenger, was added to the medium just prior to adding the indicated stimuli. Eosinophil apoptosis and death were assessed by means of flow cytometry with Annexin V and 7-aminoactinomycin D (7AAD) staining and a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). For detecting ETosis, cells were cultured in an 8 well chamber slide (Thermo Scientific) for 3 hours after stimulation, then stained with DAPI before determination of typical morphologic change of the nucleus by fluorescent microscopy.
Measurement of released eosinophil peroxidase (EPX) activity
EPX release was measured as described previously. [21] Briefly, following stimulation of eosinophils with indicated stimuli, the substrate O-phenylenediamine (OPD) was added directly to cell suspensions, reaction was stopped by H2SO4 and color intensity measured at 492-nm wavelength. Data are expressed as fold change, compared with level in supernatant of unstimulated cells.
ROS production measurements
To measure icROS, after stimulation for the indicated times, cells were incubated in normal media with 1 mM dihydrorhodamine (DHR) 123 (Invitrogen, Waltham, MA) at 37 C for 30 minutes, fixed with 1% PFA, washed with PBS, and subsequently analyzed by flow cytometry. In some experiments, cells were incubated with DHR 123 for 15 minutes before the addition of stimuli; at the end of stimulation period, cells were fixed with 1% PFA. For measuring ecROS, the Amplex-Red® Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) was used according to manufacturer’s protocols. Briefly, isolated eosinophils were re-suspended in normal or FBS(−) media at 1×105/mL density, and following addition of stimuli, reagents and/or catalase, cells were transferred to a 96 well microplate. The microplate was kept at 37 °C in a plate reader and the fluorescence was measured every 10 minutes.
Immunochemical detection of phosphorylated signaling proteins
Phosphorylated signaling proteins were measured using a multiplex bead assay and Western blotting. For the multiplex bead assay, an antibody panel kit for 8 phosphoproteins related to SFK (Lck, Lyn, Src, Yes, Fgr, Fyn, Blk and Hck) was used (Milliplex; Millipore, Temecula, CA). Briefly, eosinophils (1×106/mL) were cultured with or without stimuli for the indicated times, washed with cold PBS, and lysed in the presence of a cocktail of protease/phosphatase inhibitors (Thermo Fisher Scientific, Uppsala, Sweden) using manufacturer-provided lysis buffer to generate lysates equivalent to 1×107 cells/mL, Lysates were then filtered with centrifugation in provided filter plates. Total protein concentrations of the lysates were measured and adjusted by diluting with lysis buffer. Lysates were mixed with a cocktail of antibody-specific beads overnight, treated with detection antibody and Streptavidin–PE, and analyzed with the Luminex system (Luminex, Austin, TX). In some experiments, cell lysates were fractionated with the NE-PER nuclear and cytoplasmic extraction kit (Thermo Fisher Scientific), according to the manufacturer’s protocols.
For Western blotting, protein lysates were electrophoresed through Bis-Tris 4–15% gradient NuPage gels (LifeScience-Invitrogen) and transferred to nitrocellulose membranes by using the iBlot dry blot system (LifeScience-Invitrogen), according to the manufacturer’s guidelines. Anti–phospho-Yes antibody, which recognizes phosphorylation of Tyr537 in multiple SFKs, was purchased from Thermo-Fisher. After blocking with 5% BSA in Tris-buffered saline–Tween, membranes were incubated overnight with primary antibodies (1:1000 in Tris-buffered saline–Tween/1% BSA). Specific binding to these antibodies was detected with HRP-conjugated secondary antibodies using the Luminata Forte chemiluminescent HRP detection kit (Millipore).
Statistical analysis
Prism® (GraphPad Software, CA) was used to determine statistical significance. Unless noted otherwise, data in figures represent mean values with error bars indicating standard deviation of replicates within an experiment. The number of independent experiments is denoted in figure legends. P value of less than 0.05 by t-test was regarded as significant and indicated with (*).
Results
Cellular localization of ROS
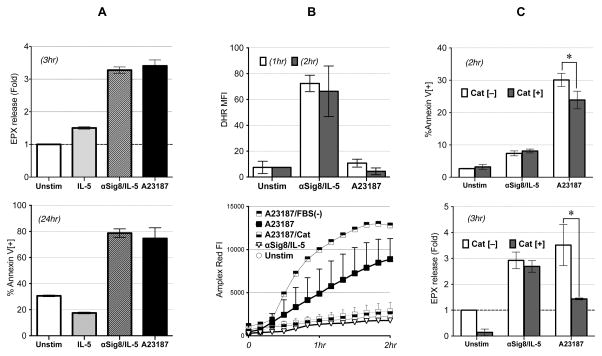
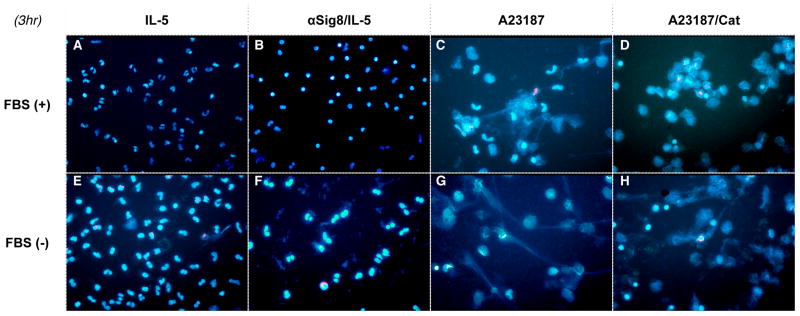
ROS is released to both intra- and extra-cellular compartments by NADPH-oxidase. [22] Intracellular ROS is also produced in various other intracellular compartments, most notably mitochondria, where they play roles in multiple functions, from host defense to regulation of cellular signaling. [23] In ETosis, extracellular ROS is produced and can cause eosinophil death.6 We have previously shown that intracellular ROS is produced and required for Siglec-8/IL-5 induced cell death7,15; however, the role of cellular localization of ROS, i.e., whether only intracellular or both intra and extracellular ROS are required for Siglec-8/IL-5-stimulated eosinophils, has not been determined. Thus, we compared production of intracellular and extracellular ROS in eosinophils in response to the calcium ionophore A23187, which induces ETosis, to ROS generated in response to anti-Siglec-8/IL-5. Both stimuli led to comparable degranulation and eosinophil death, as measured by extracellular EPX release and annexinV/7AAD flow cytometry (Figure 1A upper and lower panels, respectively), suggesting similar magnitudes on cell death. Interestingly, while A23187 led to extracellular ROS accumulation (as previously reported), anti-Siglec-8/IL-5 led to intracellular (icROS) accumulation but not extracellular ROS generation (Figure 1B upper and lower panels, respectively). Removal of FBS (a known ROS scavenger [24]) from the media (FBS(−)), enhanced ROS, while catalase completely eliminated the ROS produced in response to A23187; neither had any effect on anti-Siglec-8/IL-5-treated cells (data not shown). Consistent with A23187 leading to extracellular ROS, catalase completely scavenged ROS (Figure 1B lower panel) and prevented A23187-induced, but not anti-Siglec-8/IL-5-induced, cell death and EPX release (Figure 1C upper and lower panels, respectively). Finally, the expulsion of nuclear DNA (“net” formation, characteristic of ETosis) was not seen in anti-Siglec-8/IL-5 treated cells, even in FBS (−) (Figure 2). These finding suggest that while both stimuli led to eosinophil death, they do so employing different pathways and mechanisms.
Figure 1. Comparison of anti-Siglec-8/IL-5-induced ROS production and cell death with ETosis.
(A) Anti-Siglec-8/IL-5 co-stimulation and A23187 cause similar effects on eosinophils in terms of cell death and granule release. Granule release by eosinophils (evaluated by extracellular EPX assay at 3 hour time point) was evaluated with both Siglec-8/IL-5 and A23187, shown as fold increase from unstimulated cells (upper panel). Cell death was compared to no stimulation or IL-5 only stimulation at 24 hr (lower panel). Representative of 3 experiments.
(B) Anti-Siglec-8/IL-5 co-stimulation or A23187 evoke distinct patterns of intracellular/extracellular ROS production. icROS was detected by DHR flow cytometry (representative of 3 experiments) (upper panel). ecROS was detected by Amplex-Red fluorescence with or without catalase (Cat) (representative of 2 experiments) (lower panel). (C) Effect of catalase on cell death (upper panel) and EPX release (lower panel; shown as fold change from unstimulated, Cat[−] cells) induced by anti-Siglec-8/IL-5 or A23187 (*p<0.05). Representative of 2 experiments.
Figure 2. Morphological discrimination of anti-Siglec-8/IL-5-induced cell death and ETosis.
Cells were incubated with IL-5 (A, E), anti-Siglec-8/IL-5 (B, F), A23187 (C, G) or A23187 in the presence of catalase (D, H) in normal media (A–D) or FBS (−) media (E–D). Note that FBS(−) media, which should enhance ecROS, did not invoke ETosis after anti-Siglec-8/IL-5 stimulation, contrary to A23187 treated cells, suggesting irrelevance of ecROS to anti-Siglec-8/IL-5 induced cell death. IL-5 only or A23187/Cat treated cells are shown as controls (representative of 2 experiments).
Phosphorylation of SFKs in IL-5/anti-Siglec-8 stimulated cells
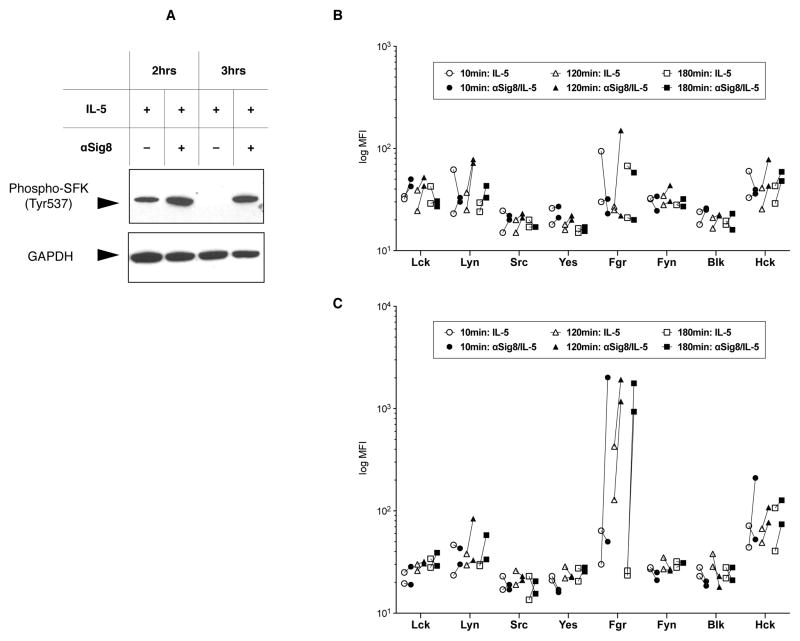
Since Siglec-8 has intracellular tyrosine-based signaling motifs, and its ITIM domain was necessary for signaling in mast cells [25], we hypothesized that Src family kinases (SFKs) are involved in this intracellular ROS formation and cell death induced by Siglec-8 engagement. Thus, we first performed Western blotting for phospho-SFK (using an antibody against the Y537 motif conserved in most SFK members) in anti-Siglec-8/IL-5 co-stimulated cells. As seen in Figure 3A, there was increased SFK phosphorylation in the cytoplasmic fraction of cells treated with anti-Siglec-8/IL-5 compared with IL-5 alone at 10–180 minutes. No phospho-SFK was seen in the nuclear fraction (data not shown). These results demonstrated that SFK was phosphorylated after Siglec-8 antibody engagement, this phosphorylation was sustained for at least 180 minutes, and was localized to the cytoplasmic cell fraction.
Figure 3. Detection of SFK phosphorylation by immunochemistry.
(A) Detection of phosphorylated Tyr537 of SFK (top) in cytoplasmic fraction lysates of eosinophils treated with IL-5 alone or anti-Siglec-8/IL-5 for the indicated time. Detection of GAPDH on the same membrane is used as a loading control (bottom). Representative of 2 experiments. (B & C) Results of the phospho-luminex assay detecting phosphorylation of eight SFKs in nuclear fractions (B) and cytoplasmic fractions (C) from human eosinophils stimulated with IL-5 ± anti-Siglec-8. Anti-Siglec-8 mAb was added simultaneously with IL-5. Data are shown as each line representing change in phosphorylation from cells treated with IL-5 alone to IL-5 with anti-Siglec-8 in an individual experiment.
Since this Western blot antibody recognized phosphorylated tyrosine in multiple SFKs, we used phospholuminex to screen more specifically for SFK phosphorylation in anti-Siglec-8/IL-5 co-stimulated cells. Based on the known spatial specificity of SFK function, we examined fractionated nuclear/cytoplasmic lysates, using cells stimulated by IL-5 only or anti-Siglec-8/IL-5. While no remarkable phosphorylation was found for each SFK among nuclear fractions (Figure 3B), phosphorylated-Fgr levels were uniquely increased upon Siglec-8 ligation in the cytoplasmic fractions (Figure 3C). These results supported our hypothesis that SFKs are involved in signaling events following engagement of Siglec-8.
Inhibition of SFKs prevents icROS accumulation and cell death in eosinophils following Siglec-8 engagement
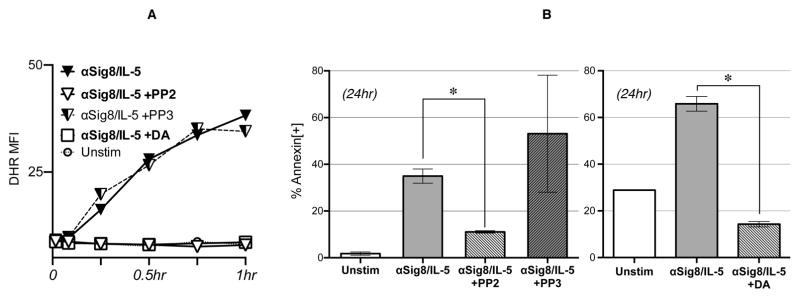
To test if activation of SFKs is required for the downstream outcomes of anti-Siglec-8–initiated signaling, namely ROS production and cell death, we examined the effect of pharmacological SFK inhibitors. As shown in Figure 4A, the pan-tyrosine kinase inhibitor dasatinib (DA; 10 nM) and the the SFK inhibitor PP2 (7.5 μM), but not its inactive control PP3, effectively inhibited anti-Siglec-8/IL-5 mediated intracellular ROS production, detected by DHR123 flow cytometry. When evaluating their effect on cell death, co-incubation with PP2 or DA also significantly prevented eosinophil cell death following anti-Siglec-8 ± IL-5 stimulation as measured by Annexin-V/7AAD flow cytometry (Figure 4B left and right panels, respectively). Together, these results suggest that activation of SFKs is required for intracellular ROS production and cell death following Siglec-8 stimulation.
Figure 4. SFK inhibitors prevent ROS production and cell death of anti-Siglec-8/IL-5 stimulated eosinophils.
(A) DHR123 flow cytometry was used for detection of icROS production. Cells were incubated with or without anti-Siglec-8/IL-5 for 1 hour in the presence of the selective SFK inhibitor PP2 (7.5 μM) or its inactive control PP3 (7.5 μM), or the pan-tyrosine kinase inhibitor dasatinib (DA; 10 nM). (B) Annexin V flow cytometry for detection of cell death. Cells were stimulated with anti-Siglec-8/IL-5 overnight in the presence or absence of PP2 and PP3 (7.5 μM, left) or DA (10 nM, right). Representative of 5 (A), 5 (B left) and 6 (B right) experiments. (*p<0.05)
Discussion
In this study, we elucidate the signaling pathway of cell death that occurs in IL-5/anti-Siglec-8 co-stimulated eosinophils. Specifically, our data support a model wherein engagement of Siglec-8 in short-term IL-5 activated eosinophils leads to SFK-mediated intracellular ROS accumulation and cell death that is distinct from the recently described process of ETosis.
We demonstrate that there are at least two distinct modes of ROS production and requirement for eosinophil cell death. Unequivocally, ETosis (induced experimentally by calcium ionophore A23187) and anti-Siglec-8/IL-5-induced cell death showed distinct modes of ROS production and usage, as the former was shown to be exclusively ecROS dependent while the latter is essentially icROS–predominant. The source of ecROS is presumably NADPH oxidase located on the plasma membrane or membrane of released granules, but definitive proof of this will require further experimentation. The exact source of icROS induced by Siglec-8 engagement is not known, but may include diverse sources such as NADPH oxidase on intracellular granules, or oxidative phosphorylation in mitochondria. In addition to mechanistic insight, this difference in ROS generation may have implications on consequences of the two types of eosinophil cell death. While both ETosis and IL-5/anti-Siglec-8-induced cell death led to some release of eosinophil granule proteins, ecROS may further contribute to tissue injury in ETosis and not IL-5/anti-Siglec-8-induced cell death. On the other hand, another study demonstrated that ecROS can deactivate damage associated molecular patterns (DAMPs) to abrogate further tissue damage. [26] Regardless, the presence or absence of ecROS may affect the extent of tissue damage caused by different mechanisms of eosinophil cytolysis, and studies, preferably using physiological stimuli, are warranted.
We attempted to define the intracellular molecular events that link Siglec-8 activation to icROS production, and identified an indispensable role for the SFKs. Western blot data show phosphorylation of the C-terminal SFK tyrosine, Y537. Regulation of SFK activity is complex, with two tyrosines, and four possible phosphorylation states (neither, both, or each tyrosine single phosphorylation state). While the phosphorylation of Y416 is associated with an active status, phosphorylation at Y537 can be associated with an active status (if Y416 is also phosphorylated) or an inactive status of the enzyme (if only Y537 is phosphorylated). [27] Thus, we used pharmacologic inhibitors to assess if function of SFK is required for downstream effects. As can be seen in Figure 4, using two independent inhibitors we show that SFK activity is required for IL-5/anti-Siglec-8 co-stimulated ROS accumulation and cell death.
Furthermore, the motif surrounding the C-terminal tyrosine is conserved among the Src family kinase members, and thus the antibody does not distinguish between family members. Thus, we turned to phospholuminex, where beads are coated with a panel of SFK-family member specific capture antibodies, and phosphorylation is detected with a pan-phosphotyrosine antibody. This approach enabled us to identify Fgr as one SFK (among those tested) that is phosphorylated following Siglec-8 engagement. Interestingly, the change in phosphorylation status was evident only following subcellular fractionation (data with whole cell lysates not shown), and specifically in the cytoplasmic fraction. Src family kinases traffic to different intracellular compartments to perform different functions, and thus focusing on a specific fraction increases the signal-to-noise ratio enabling differences to be seen which were not seen when whole cell lysates were used.
Previously, SFKs have been implicated in ROS production in neutrophils. Specifically, neutrophils require SFKs (Lyn, Fgr or Hck) to generate ROS production in an immediate response to chemoattractants (such as fMLP) or in a late response to integrin–mediated adhesion.5 Our finding that SFK activity is required for ROS production and eosinophil cell death in IL-5/anti-Siglec-8 co-stimulated cells may appear unexpected given that ITIM-bearing receptors, like those found in siglecs, are traditionally believed to signal via tyrosine phosphatases [28], and that SFKs are traditionally associated with activation and survival pathways. However, multiple studies have demonstrated that same mediators, such as SFKs, can be responsible for triggering both the activation and inhibitory pathways, thus allowing temporally regulated control of signaling. [29,30]
Our study has some limitations. For instance, we used antibodies to crosslink Siglec-8 and calcium ionophore to induce ETosis. These are commonly used surrogates for their specific cell death types, although previous studies have shown their equivalence to physiological ligands.17,31] Furthermore, many questions remain, including which tyrosine motif on the Siglec molecule is involved (ITIM or ITSM), what is the source of intracellular ROS (NADPH oxidases or mitochondria), and whether SFKs and ROS are involved in Siglec-8 signaling in mast cells. Furthermore, our studies with inhibitors of SFK suggest that an SFK is upstream of ROS production. However, this inhibitor is not specific for Fgr and thus does not unequivocally place Fgr upstream of ROS production. Furthermore, the possibility that Fgr can work both up- and down-stream of ROS [32] has not been fully excluded. These and other questions will be the subject of future studies.
In summary, our study identifies a novel signaling pathway leading to regulated eosinophil cell death, which involves Src family kinase activity leading to intracellular ROS accumulation and consequent lytic cell death.
Acknowledgments
This work was funded in part by R21 AI03853 (to NZ), R01 AI 072265 (to BSB) and P30 DK078392 (Cincinnati Digestive Diseases Research Core Center).
Footnotes
Disclosure statement: Dr. Bochner has current or recent consulting or scientific advisory board arrangements with, or has received honoraria from, Sanofi-Aventis, Pfizer, Biogen Idec, TEVA, AstraZeneca and Allakos; and owns stock in Allakos and Glycomimetics. He receives publication-related royalty payments from Elsevier and UpToDate™, and is a co-inventor on existing and pending Siglec-8-related patents and thus is entitled to a share of future royalties received by Johns Hopkins University on the potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. Journal of Allergy and Clinical Immunology. 2015 doi: 10.1016/j.jaci.2014.11.031. http://doi.org/10.1016/j.jaci.2014.11.031. [DOI] [PMC free article] [PubMed]
- 2.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellusova J, Nitschke L. Regulation of B Cell Functions by the Sialic Acid-Binding Receptors Siglec-G and CD22. Frontiers in Immunology. 2012:2. doi: 10.3389/fimmu.2011.00096. http://doi.org/10.3389/fimmu.2011.00096. [DOI] [PMC free article] [PubMed]
- 4.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. The Journal of Biological Chemistry. 2000;275(2):861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 5.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. The Journal of Allergy and Clinical Immunology. 2000;105(6 Pt 1):1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 6.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–5020. doi: 10.1182/blood-2002-10-3058. http://doi.org/10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 7.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. 2010;18(6):713–719. doi: 10.1038/ejhg.2009.239. http://doi.org/10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, et al. Mice deficient in the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. The Journal of Allergy and Clinical Immunology. 2014;133(1):240–7. e1–3. doi: 10.1016/j.jaci.2013.05.018. http://doi.org/10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–40. e1–9. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–36. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochemical and Biophysical Research Communications. 2005;336(3):918–924. doi: 10.1016/j.bbrc.2005.08.202. http://doi.org/10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 15.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. The Journal of Allergy and Clinical Immunology. 2013;132(2):437–445. doi: 10.1016/j.jaci.2013.03.024. http://doi.org/10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death and Differentiation. 2011;18(4):656–665. doi: 10.1038/cdd.2010.138. http://doi.org/10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121(11):2074–2083. doi: 10.1182/blood-2012-05-432088. http://doi.org/10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. The Journal of Biological Chemistry. 2001;276(47):44315–44322. doi: 10.1074/jbc.M105446200. http://doi.org/10.1074/jbc.M105446200. [DOI] [PubMed] [Google Scholar]
- 19.Paul SP, Taylor LS, Stansbury EK, McVicar DW. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96(2):483–490. [PubMed] [Google Scholar]
- 20.Fumagalli L, Campa CC, Germena G, Lowell CA, Hirsch E, Berton G. Class I phosphoinositide-3-kinases and SRC kinases play a nonredundant role in regulation of adhesion-independent and -dependent neutrophil reactive oxygen species generation. The Journal of Immunology. 2013;190(7):3648–3660. doi: 10.4049/jimmunol.1201951. http://doi.org/10.4049/jimmunol.1201951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101–8. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Bylund J, Brown KL, Movitz C, Dahlgren C, Karlsson A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? Free Radical Biology & Medicine. 2010;49(12):1834–1845. doi: 10.1016/j.freeradbiomed.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiological Reviews. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 25.Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, et al. Inhibition of Fc ε RI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Lotfi R, Herzog GI, DeMarco RA, et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. The Journal of Immunology. 2009;183(8):5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 27.Sun G, Sharma AK, Budde RJ. Autophosphorylation of Src and Yes blocks their inactivation by Csk phosphorylation. Oncogene. 1998;17(12):1587–1595. doi: 10.1038/sj.onc.1202076. http://doi.org/10.1038/sj.onc.1202076. [DOI] [PubMed] [Google Scholar]
- 28.Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Annual Review of Immunology. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- 29.Tourdot BE, Brenner MK, Keough KC, Holyst T, Newman PJ, Newman DK. Immunoreceptor Tyrosine-Based Inhibitory Motif (ITIM)-Mediated Inhibitory Signaling Is Regulated by Sequential Phosphorylation Mediated by Distinct Nonreceptor Tyrosine Kinases: A Case Study Involving PECAM-1. Biochemistry. 2013;52(15):2597–2608. doi: 10.1021/bi301461t. http://doi.org/10.1021/bi301461t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scapini P, Pereira S, Zhang H, Lowell CA. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol Rev. 2009;228(1):23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. Journal of Pharmacology and Experimental Therapeutics. 2009;330(2):608–612. doi: 10.1124/jpet.109.152439. http://doi.org/10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acín-Pérez R, Carrascoso I, Baixauli F, et al. ROS-Triggered Phosphorylation of Complex II by Fgr Kinase Regulates Cellular Adaptation to Fuel Use. Cell Metabolism. 2014;19(6):1020–1033. doi: 10.1016/j.cmet.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]