Abstract
Background
Conventionally, change in motor performance is quantified with discrete measures of behaviour taken pre- and post-practice. As a high degree of movement variability exists in motor performance after stroke, pre- and post-testing of motor skill may lack sensitivity to predict potential for motor recovery.
Objective
Evaluate the use of predictive models of motor learning based on individual performance curves and clinical characteristics of motor function in individuals with stroke.
Methods
Ten healthy and fourteen individuals with chronic stroke performed a continuous joystick-based tracking task over 6 days, and at a 24 hour delayed retention test, to assess implicit motor sequence learning.
Results
Individuals with chronic stroke demonstrated significantly slower rates of improvements in implicit sequence-specific motor performance compared to a healthy control (HC) group when root mean squared error (RMSE) performance data were fit to an exponential function. The HC group showed a positive relationship between a faster rate of change in implicit sequence-specific motor performance during practice and superior performance at the delayed retention test. The same relationship was shown for individuals with stroke only after accounting for overall motor function by including Wolf Motor Function Test (WMFT) rate in our model.
Conclusion
Nonlinear information extracted from multiple time points across practice, specifically the rate of motor skill acquisition during practice, relates strongly with changes in motor behaviour at the retention test following practice and could be used to predict optimal doses of practice on an individual basis.
Keywords: stroke, motor skills, learning curve, rehabilitation
Introduction
Motor outcomes after stroke rely on capacity for learning as individuals work to relearn old, and acquire new, motor skills.1 The amount, or “dose”, of practice required to stimulate learning after stroke is an important topic among clinicians and researchers alike.2 Motor learning refers to “relatively permanent changes in the capability for skilled behavior” and has conventionally been quantified with discrete measures of behaviour taken at post-practice time points.3,4–9 It is known that the acquisition of motor skills which result in behavioral change do not occur in a linear manner.9 Pre- and post-testing of motor skill may lack sensitivity to delineate nonlinear information regarding how quickly individuals improve as well as whether, and when, performance plateaus.9 In the early phase of motor skill acquisition individuals acquire basic movement patterns through trial and error movement strategies that are used to achieve the desired goal.10 If sufficient practice is provided, large improvements in motor performance typically occur in a non-linear fashion, followed by smaller rates of improvement when approaching a plateau in behaviour.1 While previous works have modeled motor behaviour using a curvilinear function in individuals with stroke,11–14 the possibility of exploiting the relationship between rate of motor skill acquisition and the number of trials until asymptote to determine whether this information can be used to enhance motor learning after stroke has not been studied.15 Undertaking this effort is important as it may enable prediction of the appropriate dose of practice on an individual basis, which could lead to optimized motor learning after stroke. Some work has used post-hoc analyses to quantify the dose of practice needed to induce clinically meaningful change,2 or dose escalation methods to identify the maximum tolerated dose.16 However, at this time no methodology exists that uses the rate of skill acquisition to predict retention performance and the dose of practice necessary to achieve performance plateau during motor skill acquisition.17 Additionally, this approach could enable the individualized prediction of the dose of practice required for rehabilitation of impaired motor skills after stroke. Understanding how skills are acquired on an individual level, beyond change scores, in the medical field has called into question the use of fixed numbers of procedures for credentialing and licensing18. Understanding the progression of learning through a proportional progress can help in the development of efficient practice paradigms in rehabilitation settings as the numbers of trials (i.e., dose) necessary for motor learning may vary from person to person.
Presently, motor outcomes are described with summary values, such as mean response time or percent of correct responses, taken at discrete points in time to produce pre-post change scores.4–7,15 However, behavioral changes can be characterized using models that encompass the evolution of performance changes over time. For example, curve fitting uses all data points, as opposed to small blocks, and as such captures the overall trend or the ‘evolution of performance of practice’. The most suitable method for quantifying how performance evolves is the learning curve, as learning does follow a robust pattern of change, and suggests that practice always helps improve performance with the most observable improvements occurring in the early phase of practice.19 In addition, with an adequate dose of practice individuals can achieve comparable levels of performance.20 This work has considered healthy adults,15,21–24 and detected differences in motor skill acquisition in healthy people and individuals with stroke.11 Yet, the application of these principles in clinical research has lagged. In addition, pre and post measurements have been viewed as limiting our understanding of the mechanisms actively involved in an intervention.25 In a recent review discussing the neural mechanisms supporting stroke rehabilitation, pre and post testing was posed as a hindrance to our understanding of how an intervention works, disallowing the isolation of the ‘active ingredient’ involved.25 The benefit of an approach that estimates parameters, is that it does not restrict nor define change solely to initial and final performance, but instead captures a broad scope of skill acquisition compared to a discrete change score calculated from the beginning to end of practice.26
Based on theoretical models of learning, in the current work we used an exponential function to generate a curve of improvement in implicit motor skill performance of a continuous tracking task (CTT) as a function of time.7,27 The use of a learning curve is advantageous in the investigation of the dynamic nature of performance as skill acquisition for the CTT involves known transitions throughout the learning process.28 Modeling mean scores as a continuous curve, so neighbouring blocks may have some influence on the succeeding block, accurately depicts the CTT learning process. Rather than calculating mean scores from each block individually, curve fitting incorporates more information to make a prediction; performance in the initial blocks sequentially influence performance in the latter blocks. Thus the purpose of the present study was two-fold. (1) To derive and compare practice parameters estimated from an exponential function between individuals with stroke and a healthy control group during implicit motor skill acquisition. (2) To determine the relationship between estimated practice parameters within groups and the retention of performance following practice. We hypothesized that exponential curve fitting of implicit sequence-specific motor performance across six days of skilled motor practice would result in group differences in the predictor values that would be associated with retention test performance. In addition, we hypothesized that a clinical measure of motor function, the Wolf Motor Function Test (WMFT), when added to the regression model, would help to predict the magnitude of change associated with implicit motor learning.
Methods
Participants
The stroke (ST) group included 14 individuals with chronic middle cerebral artery (MCA) stroke (Mean age = 64.7; SD = 7.24 years). Physical impairment level was assessed using the Fugl-Meyer (FM) upper extremity motor scale (Mean = 52.7; SD = 13.0; maximal score 66).29 Ten right-handed, older healthy individuals made up the control group (HC; Mean age = 64.8; SD = 8.5 years) (Table 1). Individuals were recruited from the university and local communities. The rights of all participants were protected by the ethical review board at the University of British Columbia; each individual signed an approved institutional informed consent form prior to enrollment.
Table 1.
Participant Characteristics.
| Group | n | Age (mean, SD) | Gender | MM | FM |
|---|---|---|---|---|---|
| HC | 10 | 64.8 (8.50) | 7 Female; 3 Male | 29.8 (0.422) | N/A |
| ST | 14 | 64.7 (7.24) | 4 Female; 10 Male | 29.2 (0.974) | 52.7 (13.0) |
Abbreviations: MM: Mini-Mental, FM: Fugl Meyer
Experimental Design
Individuals participated on seven separate days over a 2-week span, with no more than 5 days between practice sessions. On the first day, the ST group completed the Wolf Motor Function Test (WMFT) and both groups (ST, HC) performed four pre-test training blocks (30 trials/block) of the continuous tracking task (CTT). Days 2–6 involved CTT practice and on day 7, a 24-hour retention test consisting of four blocks was performed (30 trials/block). We selected this dose of practice for our study as our past work has shown that it is enough to facilitate motor learning26,30; however, it is unclear whether it is a sufficient dose to lead to asymptote in performance. On each practice day (i.e., days 2–6) participants practiced 100 trials (5 blocks; 20 trials/block); completion of each practice session took approximately 30 minutes (Figure 1A).
Figure 1.
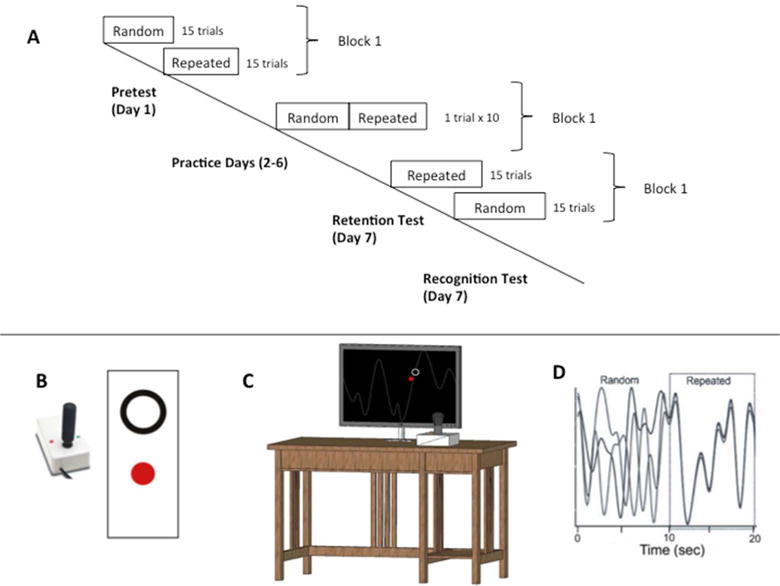
Continuous Tracking Task (CTT). (Panel A) During the pretest and posttest, the repeated and random waveforms segments were performed in separate blocks. During practice days (2–6) individuals tracked continuous 20 s waveform segments (multiple overlapping lines represent different trials) repeating sequences were flanked with random sequences in a single block (Panel D). (Panel B, C) Pictorial of the CTT apparatus used to perform the task during pre and post-testing and practice. Participants operated a joystick to move a closed red dot inside an open black circle on a computer screen.
Motor Function Assessment
On day 1, the WMFT of upper extremity function was assessed by a registered Physical Therapist. Movement time to complete 15 items of the WMFT with the paretic and non-paretic arms was determined. A task rate was calculated as 60 seconds/performance time(s) with a score of ‘0’ given if a participant was unable to perform the task.30 The WMFT mean rate has been shown to be a more sensitive method to characterize motor function of the upper extremity in individuals with stroke.30
Behavioral Task
The ST group used their paretic, left arm and the HC group used their non-dominant, left arm to track the vertical path of a target with wrist movements that controlled a joystick (Current Designs*). Participants’ movements were represented as a red filled circle and the target circle was outlined in white; both objects were visible on a black background (Figure 1B,C). See Wadden et al. 2014 and Supplementary Material for in-depth CTT details.31
Motor performance was evaluated using root mean squared error (RMSE), which is the average difference between the target pattern and participant movements, and reflects overall tracking errors in the kinematic patterni. RMSE was calculated separately for random and repeating segments on every trial. For the pre-and retention test, performance in the first block (15 trials of both random and repeated sequences) was evaluated. For each participant, a pre- to retention-test change score was calculated separately for random and repeated sequences (e.g., mean RMSE on Day 1, Block 1 minus mean RMSE from Block 1 at the retention test on day 7; Change ScoreDay1–7).
Practice Exponential Curve
Individual changes in implicit sequence-specific performance and motor control, as measured by the RMSE for repeated and random sequences, respectively, were fit to an exponential function with a least squares regression analysis.29 RMSE value for each trial across the pre-test (day 1) and 5 days of task performance (days 2–6) for each participant were parameterized using the following equation32:
E(RMSEN) is the expected value of RMSE on practice trial N; A is the expected value of RMSE after practice has been completed (asymptote parameter); B is the change in the expected value of RMSE from the beginning of practice to the end of practice (change score parameter); Alpha (α) is the exponential learning rate parameter (Figure 2 for HC single subject example).27
Figure 2.
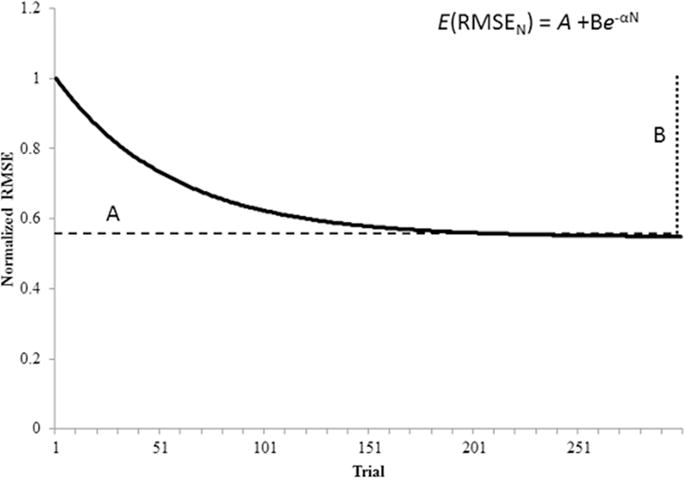
Skill acquisition follows an exponential decay as performance improves. Fitting RMSE data extracted from a motor skill practice to the function, E(RMSEN) = A + B−αN, produced practice parameters based on the nonlinear decay of performance during skill acquisition. E(RMSEN) is the expected value of RMSE on practice trial N; A is the expected value of RMSE after practice has been completed (asymptote parameter); B is the change in the expected value of RMSE from the beginning of practice to the end of practice (change score parameter); Alpha (α) is the exponential rate parameter.27 Data shown here were derived from a sample HC participant.
Retention Test Score
A retention test score (RTS) was calculated to determine the change in motor behaviour associated with learning. By accounting for early practice performance and the change score parameter, B, as predicted from the exponential function, a direct comparison between practice and retention was possible. The mean RMSE for block 1 for the pre-test (day 1) and retention test were calculated (RMSEPT and RMSERT, respectively).33,34 The RTS was determined as follows:
In this equation, a higher score indicates better retention (i.e., larger change in motor behaviour) of the motor skill. The RTS is interpreted as the increase or decrease in performance following the retention interval, and has been deemed the most informative measure of retention.35
Predicting dose of practice to asymptote
To evaluate the predictive capabilities of the exponential function to determine optimal dose of practice for motor learning, the exact trial was calculated that indicated individual performance was within 1% tolerance of the A value. The number of trials until asymptote was determined by the following equation:
Trialn is the expected trial N until asymptote; A is the expected values of RMSE after practice has been completed (asymptote parameter); B is the change in the expected value of RMSE from the beginning of practice to the end of practice (change score parameter); Alpha (α) is the exponential learning rate parameter (Figure 2).27
Statistical Analysis
Motor Performance
A between-group analysis of variance (ANOVA) was used to evaluate differences in motor performance between ST and HC groups on mean practice RMSEDays1 to 6 for each sequence separately (Repeated and Random).
Separate between-group multivariate analysis of variance (MANOVA) for each sequence (Repeated and Random) were used to evaluate differences in motor performance between ST and HC groups on the exponential practice parameter dependent measures (A, B, α) derived from the exponential function.
Motor Learning
Between-group ANOVAs were used to evaluate differences in motor learning for individuals in the ST and HC groups using mean retention RMSE and Change ScoreDay1–7 separately for each sequence as the dependent measures (Repeated and Random).
A between-group ANOVA was used to evaluate differences in motor performance for individuals in the ST and HC groups with the RTS ((RMSEPT − RMSERT)/B) for each sequence as the dependent measure (Repeated and Random).
To evaluate the relationship between exponential practice parameters and RTS in the HC group, simple linear regression analyses were conducted, where the rate of skill acquisition parameter, α, was regressed on the RTS separately for repeated and random sequences1. Because stroke-related movement deficits may interfere with motor behavior, we conducted hierarchical regression analyses in the stroke group designed to examine the relationship between α and RTS while accounting for individuals’ level of motor function. These hierarchical regression analyses were conducted separately for the Repeated and Random sequences; paretic WMFT rate was entered as a predictor in the first block, and the rate of skill acquisition parameter, α, was entered in the second block and these variables regressed on the RTSi. The Variance Inflation Factor (VIF) and tolerance statistics indicated minimal collinearity within the data as VIF value was under 2.0.36
Prediction of Optimal Dose
A between-group ANOVA was used to evaluate differences in the predicted numbers of trials until asymptote between individuals with chronic stroke and healthy individuals on the log transformed Predicted trialA_n by Group (ST, HC) for each sequence (Repeated and Random).
For all statistical tests, significance level was set to p < 0.05. SPSS 22.0 (IBM, New York, NY) statistical software was used for analyses. A Bonferroni correction was used on post-hoc analyses to correct for multiple comparisons.
Results
Differences in motor performance metrics between ST and HC groups
During practice, there was a near significant difference and significant difference between groups (ST, HC) in mean RMSE for repeated and random sequence (mean practice RMSEDays 1 to 6). Across practice days the ST group demonstrated worse performance than the HC group; mean RMSE for the repeated sequence for the ST group was 13.02 (SD = 3.75) and for the HC group mean RMSE was 9.7 (SD = 4.12), F(1,22) = 4.255, p = 0.051, η2ρ = 0.162. While for random sequence performance, the ST group demonstrated a mean RMSE of 12.2 (SD = 3.62) and the HC group a mean RMSE of 9.0 (SD = 3.41), F(1,22) = 4.648, p = 0.042, η2ρ = 0.174.
Using our exponential function to characterize change in motor performance across practice we discovered a significant main effect of group (ST, HC) for the exponential practice parameters derived from the repeated sequences (A, B, α), Wilks’ λ = 0.614, F(1,20) = 4.198, p = 0.019, η2ρ = 0.386. The post hoc univariate ANOVAs demonstrated that a significant difference for the exponential practice parameters was observed for the extracted α value, F(1,22) = 4.9, p = 0.0038, η2ρ = 0.182 (Figure 3). There were no significant differences between groups for the exponential practice parameters A or B for repeated sequence practice, F(1,22) ≤ 0.276, p ≥ 0.383. There was no significant main effect of group (ST, HC) on the practice parameters for random sequence detected by the MANOVA (A, B, α), Wilks’ λ = 0.693, F(1,20) = 2.960, p = 0.057, η2ρ = 0.307.
Figure 3.
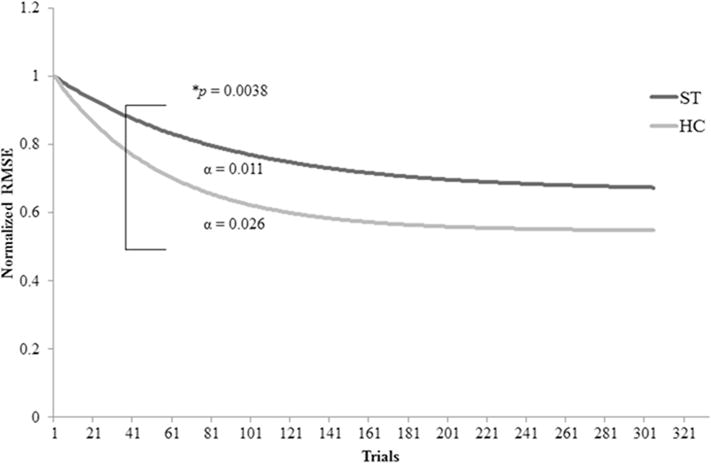
Repeated Sequence Practice Curves showing a significant difference between the HC and ST groups for exponential practice parameters for the extracted Alpha (α) value, F(1,22) = 4.9, p = 0.0038, η2ρ = 0.182.
Differences in motor learning metrics
At retention, there was significant main effect of group (ST, HC) for mean RMSE for repeated sequence and near significant difference for random sequence as detected by the ANOVAs. Similarly to practice, the ST group performed worse than the HC group at retention; mean RMSE for the repeated sequence was higher in the ST group (11.8; SD = 4.51) than the HC group (8.2; SD = 1.74) F(1,22) = 5.732, p = 0.026, η2ρ = 0.207. RMSE was also higher in the ST group for random sequences (11.6; SD = 4.17) as compared to the HC group (8.3; SD = 1.91) F(1,22) = 4.221, p = 0.052, η2ρ = 0.161. There was no significant main effect of group (ST, HC) on Change ScoreDay1–7 for repeated or random sequences as detected by the ANOVAs. ST repeated sequence Change ScoreDay1–7 was 4.1(SD = 3.22), HC repeated sequence Change ScoreDay1–7 was 7.7 (SD = 12.09) F(1,22) = 1.09, p = 0.307, η2ρ = 0.047. Similarly, ST random sequence Change ScoreDay1–7 was 5.3 (SD = 3.22) and HC random sequence Change ScoreDay1–7 was 4.7 (SD = 6.09) F(1,22) = 0.116, p = 0.736, η2ρ = 0.005.
Using our exponential function to characterize change in motor learning from practice to retention, there was no significant main effect of group (ST, HC) on the RTS for repeated and random sequence as detected by the ANOVAs. The RTS for the repeated sequence for the ST group was 0.55 (SD = 0.385), and for the HC group was 0.63 (SD = 0.248), F(1,22) = 0.286, p = 0.598, η2ρ = 0.013, while for random sequence performance the ST group demonstrated a RTS of 0.62 (SD = 0.259) and the HC group was 0.456 (SD = 0.321), F(1,22) = 2.003, p = 0.171, η2ρ = 0.083.
Relationship between α and retention score for HC group
Simple linear regressions were used to evaluate the relationships between α and the RTS ((RMSEPT − RMSERT)/B) for both repeated and random sequence performance for the HC group. The practice parameter, α, accounted for significant variance in repeated sequence RTSs (R2 = 0.465, F(1,8) = 6.952, p = 0.03) (Figure 4a), indicating a significant relationship between rate of skill acquisition in practice and motor behavior at the retention test; however, this was not observed in the analysis of random sequence performance at the retention test (R2 =0.094, F(1,8) = 0.827, p = 0.39, respectively).
Figure 4.
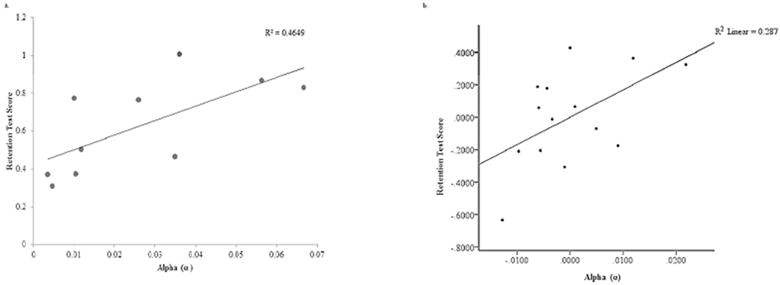
a and b: a. Relationships of RTS and Alpha (α) during repeated sequence performance in healthy individuals (HC Group) (p < 0.05). b. Partial plot generated by hierarchical regression analysis for Repeated Sequence Performance. The relationship between RTS and Alpha (α) was significant after accounting for WMFT rate for the hemiparetic limb in individuals with chronic stroke (ST Group) (p < 0.05).
Relationship between α, WMFT, and RTS for ST group
In the hierarchical regression models, paretic WMFT rate accounted for significant variance in RTS for both repeated and random sequences (R2 =0.424, F(1,12) = 8.825, p = 0.012; R2 =0.410, F(1,12) = 8.335, p = 0.014, respectively). Addition of α in the second block significantly improved the repeated sequence model (Δ R2 = 0.165, F(1,11) = 4.425, p = 0.05) (Figure 4b; table 2), indicating a significant relationship between α and repeated sequence RTS when accounting for level of motor function. In contrast, addition of α did not significantly improve the random sequence model (Δ R2 = 0.059, F(1,11) =1.229, p = 0.291). The VIF was 1.074.
Table 2.
Summary of Regression Modeling for Repeated Sequence (ST group)
| Predictors | R2 | F Statistic | Sig. | ΔR2 | Sig. (ΔR2) | βWMFT_AFF | |
|---|---|---|---|---|---|---|---|
| Model 1 | WMFTAFF | 0.424 | 8.83 | 0.012 | 0.424 | 0.012 | −0.651 |
| Model 2 | WMFTAFF, α | 0.589 | 7.885 | 0.008 | 0.165 | 0.05 |
Abbreviations: WMFTAFF: Paretic Limb Wolf Motor Function, α = Rate of Motor Skill Acquisition, ST = stroke.
Significant at p < 0.05.
Predicted dose of practice between ST and HC groups
There was not a significant main effect of group (ST, HC) for repeated and random sequence in predicted number of trials to asymptote (Predicted trialA_n) by the ANOVAs. Predicted trialA_n for repeated sequence performance was greater for the ST than the HC groups for the repeated sequences (ST predicted trials= 1755.2 (SD = 3504.68); HC predicted trials = 414.3 (SD = 436.66), F(1,22) = 2.968, p = 0.099, η2ρ = 0.119. This group difference was not present for random sequences (ST predicted trials = 328.3 (SD = 399.15); HC predicted trials = 520.4 (SD = 802.02), F(1,22) = 0.008, p = 0.928, η2ρ = 0.000.
Testing of Explicit Knowledge
Participants could only recognize sequences at a chance level, and failed to gain explicit knowledge of the repeated sequence. The ST group correctly identified 53.4% of sequences; the HC group accurately recognized 53.3%.
Discussion
The present study demonstrates that individual implicit sequence-specific motor learning can be successfully modeled by an exponential function and this shows significant relationships with the retention of the newly learned motor skill. During motor sequence acquisition individuals with chronic stroke demonstrated significantly slower improvements in skilled motor performance compared to a HC group. Inferior motor sequence performance and retention by individuals with stroke as compared to matched controls has been previously reported.22,32 The current study expands on these previous findings;11–14 the rate of motor skill acquisition both differed between individuals with stroke and healthy controls and was predictive of changes in motor learning when combined with clinical measures of motor function. The HC group showed a positive relationship between a faster rate of change in motor performance during practice and superior performance at the delayed retention test for the repeated sequence. Interestingly the same relationship was shown for individuals with stroke after we accounted for overall motor function by including WMFT rate in our model. Thus when controlling for level of clinical motor function, the rate of motor skill acquisition during practice related strongly with individuals’ ability to retain previously acquired motor skills.
A pre- to post-testing approach ignores large amounts of information that characterizes individual capacity for change, rate of improvement and time to asymptote. Within-individual variability has been reported to be between 37 to 53% of between-individual variability, which challenges the idea that motor performance on one day represents a person’s characteristic performance.37 Further, when motor performance scores, such as clinical outcome measures, are used to prognosticate and determine whether therapeutic resources should be deployed, a measurement at a single point in time may not adequately reflect the full potential of the individual. Findings from the present study in combination with previous motor learning literature,38 demonstrate that performance across multiple practice sessions may be useful in predicting the retention of motor skills. Here we extend this finding and showed that for individuals with stroke, incorporating clinical assessment measures of motor function (WMFT), enhanced our ability to predict capacity to learn a new motor skill. RTS was calculated from ratio of the exponential derived parameter ‘B’ (similar to the change score from the beginning to the end of practice) and the change in performance from the beginning to retention. This is a unique approach as a change in practice performance (y values) across their entire practice (x values) is based on their expected asymptotic value ‘A’. Parameterizing practice and learning to an individual’s predicted performance values may offer more insight into her/his potential capacity for change beyond constraints of the limited number of trials.
The asymptotic value we derived in the present work describes the estimated value of an individual’s performance when an apparent plateau is achieved. Some individuals may achieve their asymptote relatively quickly, while others may take substantially more trials to do so, which will significantly impact the ‘α’ value. We did not observe a significant difference in the predicted trial to asymptote between individuals with stroke and healthy controls. This finding may result from our testing of individuals with relatively mild levels of stroke related motor impairment (average upper extremity Fugl-Meyer motor score 52 of 66). However, calculating predicted time to asymptote (A) immediately following practice has the potential to guide decisions regarding the correct dose of practice required for optimal improvement in motor behaviour and functional outcomes. Optimal dose of practice is an attractive concept in the field of rehabilitation following stroke as the dose required for neuroplastic change to occur is extremely high.2 The optimal dose of practice is an individualized number of repetitions a patient would need in order to maximize retention of the desired task. Due to the exponential nature of learning, if dose prescription could be calculated based on performance data from individual learning curves and therapists could quantify an amount of practice necessary for the retention of motor skills, then the somewhat abstract concept of dose could become a tangible notion. In addition, this predictive methodological approach may not only specify if the individual may require more practice trials, but also provide an indication of the challenge level of the practice session. If the practice or rehabilitation session was too easy, the predicted rate of skill acquisition during practice will be high and the number of trials to asymptote low; this may translate into lower long-term retention of the motor skill performance.39,40 Thus, the present study contains a roadmap for future rehabilitation research that employs predictive models of motor learning based on individual performance curves and clinical characteristics of motor function.
Limitations & Future Directions
There are limitations to the present study. We studied a relatively small (n = 14) group of mildly impaired individuals with stroke. This limits our ability to form robust association between practice parameters, clinical measures and motor learning. To increase the ecological validity between clinical research and rehabilitation settings, where tests are administered by researchers and treatments are delivered by clinicians, respectively, a deeper understanding of the performance and learning relationship must be achieved in larger patient populations and across a variety of motor tasks. We do not claim that our curve fitting approach is better than other methods; instead we present it as an alternative method that captures different data that are useful in understanding patterns of change and doses of practice associated with motor learning. Additionally, we present the idea that information about patterns of change may be more helpful in the rehabilitation setting than is pre to post characterization of behavioural change. Performance in each block of practice are connected, meaning performing a block will make the participant better at the task, and thus perform better in the next block.41 Rather than narrowing our focus on the performance score of one block of data in sequential order, functional forms (i.e., exponential function) fit a curve through the middle of all the data. Neighbouring blocks have an influence on performance score the participant is predicted to obtain in each block, which is more ecological valid and representative of the learning process. Better characterization of skill acquisition measures could enable data driven manipulation of motor practice (to ensure adequate dose of practice is delivered) and task (to optimize rates of change). Together, these shifts should enhance the impact of practice or rehabilitation sessions to optimize motor learning.
Conclusions
While rates of skill acquisition have been used to quantify performance following stroke, no study has investigated the association with retention performance. Further, the use of exponential functions to estimate the number of trials until plateau in motor behavior has not been considered. Therefore, we propose an innovative use for these curvilinear measures. Individualized dose of practice is an important step in the field of rehabilitation following stroke, not only to attain economic efficiency, but also to work towards optimizing personalized treatment plans. Currently, the most documented use of learning curves in a practical setting is the evaluation of skill acquisition for healthcare professionals.42–44 In a rehabilitation setting, where individuals with stroke are receiving interventions to relearn motor skills, learning curves could be used to determine an individualized set number of trials that predict when performance will reach a plateau. In addition, the rate of skill acquisition between different interventions could be compared to determine practice conditions (i.e., feedback, contextual interference) that yield a more productive method of learning. For example, if the physiotherapist understands the normal or excepted and rate of skill acquisition then s/he can determine suitable practice conditions to positively affect the length of time and difficulty level to achieve an intended outcome. If the rate of skill acquisition is abnormally slow, the physiotherapist could decrease the level of difficulty, i.e., increase the target size, shorten the distance of a reaching task, etc. If performance is only assessed by the final level of behaviour then large amounts of valuable information are lost that could have been used to update how interventions were being administered. Prescribing rehabilitative exercises based on predictive values could help to construct an idealized practice paradigm on a subject wise basis.
Supplementary Material
Footnotes
Current Designs Inc, 3950 Haverford Ave. Philadelphia PA 19104
There was no significant relationship between the exponential function parameters alpha (α) and B within the ST and HC groups (p=0.425). Therefore, the parameter B, used to calculate the retention score and did not confound the correlational analysis between practice parameter alpha (α) and the retention score.
References
- 1.Muratori LM, Lamberg EM, Quinn L, Duff SV. Applying principles of motor learning and control to upper extremity rehabilitation. Journal of hand therapy: official journal of the American Society of Hand Therapists. 2013;26(2):94–102. doi: 10.1016/j.jht.2012.12.007. quiz 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt RA, L TD. Motor control and learning: A behavioural emphasis. 4th. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 4.Boyd L, Winstein C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. Journal of Neurologic Physical Therapy. 2006;30(2):46–57. doi: 10.1097/01.npt.0000282566.48050.9b. [DOI] [PubMed] [Google Scholar]
- 5.Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol Learn Mem. 2009;92(1):35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Boyd LA, Winstein CJ. Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neurosci Lett. 2001;298(1):65–69. doi: 10.1016/s0304-3940(00)01734-1. [DOI] [PubMed] [Google Scholar]
- 7.Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Memory. 2004;11(4):388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidoni ED, Boyd LA. Motor sequence learning occurs despite disrupted visual and proprioceptive feedback. Behav Brain Funct. 2008;4 doi: 10.1186/1744-9081-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitall J. Stroke rehabilitation research: time to answer more specific questions? Neurorehabil Neural Repair. 2004;18(1):3–8. doi: 10.1177/0888439003262876. author reply 9–11. [DOI] [PubMed] [Google Scholar]
- 10.Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural brain research. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Deuschl G, Toro C, Zeffiro T, Massaquoi S, Hallett M. Adaptation motor learning of arm movements in patients with cerebellar disease. Journal of neurology, neurosurgery, and psychiatry. 1996;60(5):515–519. doi: 10.1136/jnnp.60.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioffe ME, Ustinova KI, Chernikova LA, Kulikov MA. Supervised learning of postural tasks in patients with poststroke hemiparesis, Parkinson’s disease or cerebellar ataxia. Experimental brain research. 2006;168(3):384–394. doi: 10.1007/s00221-005-0096-9. [DOI] [PubMed] [Google Scholar]
- 13.Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. Journal of neurophysiology. 1999;82(5):2108–2119. doi: 10.1152/jn.1999.82.5.2108. [DOI] [PubMed] [Google Scholar]
- 14.Lang CE, Bastian AJ. Additional somatosensory information does not improve cerebellar adaptation during catching. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2001;112(5):895–907. doi: 10.1016/s1388-2457(01)00518-1. [DOI] [PubMed] [Google Scholar]
- 15.Cousineau D, Helie S, Lefebvre C. Testing curvatures of learning functions on individual trial and block average data. Behavior research methods, instruments, & computers: a journal of the Psychonomic Society, Inc. 2003;35(4):493–503. doi: 10.3758/bf03195528. [DOI] [PubMed] [Google Scholar]
- 16.Dite W, Langford ZN, Cumming TB, Churilov L, Blennerhassett JM, Bernhardt J. A Phase 1 exercise dose escalation study for stroke survivors with impaired walking. International journal of stroke: official journal of the International Stroke Society. 2015 doi: 10.1111/ijs.12548. [DOI] [PubMed] [Google Scholar]
- 17.Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95(3):861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman LS, Cao J, Andalib A, Fraser S, Fried GM. A method to characterize the learning curve for performance of a fundamental laparoscopic simulator task: defining “learning plateau” and “learning rate”. Surgery. 2009;146(2):381–386. doi: 10.1016/j.surg.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Cousineau D, Lacroix GL. Getting parameters from learning data. Tutorials in quantitative methods in psychology. 2006;2:77–83. [Google Scholar]
- 20.Ritter F, Schooler L. The learning curve in International encyclopaedia of the social and behavioural sciences. Amsterdam: Pergamon; 2002. pp. 8602–8605. http://www/.iesbs.com. [Google Scholar]
- 21.Newell KM. Motor skill acquisition. Annual review of psychology. 1991;42:213–237. doi: 10.1146/annurev.ps.42.020191.001241. [DOI] [PubMed] [Google Scholar]
- 22.Sampaio-Baptista C, Filippini N, Stagg CJ, Near J, Scholz J, Johansen-Berg H. Changes in functional connectivity and GABA levels with long-term motor learning. Neuroimage. 2015;106:15–20. doi: 10.1016/j.neuroimage.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampaio-Baptista C, Khrapitchev AA, Foxley S, et al. Motor skill learning induces changes in white matter microstructure and myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(50):19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampaio-Baptista C, Scholz J, Jenkinson M, et al. Gray matter volume is associated with rate of subsequent skill learning after a long term training intervention. Neuroimage. 2014;96:158–166. doi: 10.1016/j.neuroimage.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward NS. Does neuroimaging help to deliver better recovery of movement after stroke? Curr Opin Neurol. 2015;28(4):323–329. doi: 10.1097/WCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 26.Neva JL, Henriques DY. Visuomotor adaptation and generalization with repeated and varied training. Experimental brain research. 2013;226(3):363–372. doi: 10.1007/s00221-013-3444-1. [DOI] [PubMed] [Google Scholar]
- 27.Heathcote A, Brown S, Mewhort D. The power law repealed: The case for an exponential law of practice. Psychonomic Bulletin & Review. 2000;7(2):185–207. doi: 10.3758/bf03212979. [DOI] [PubMed] [Google Scholar]
- 28.Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit Sequence-Specific Motor Learning After Subcortical Stroke is Associated with Increased Prefrontal Brain Activations: An fMRI Study. Hum Brain Mapp. 2011;32(2):290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 30.Hodics TM, Nakatsuka K, Upreti B, Alex A, Smith PS, Pezzullo JC. Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Arch Phys Med Rehabil. 2012;93(11):1963–1967. doi: 10.1016/j.apmr.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadden K, Brown K, Maletsky R, Boyd LA. Correlations between brain activity and components of motor learning in middle-aged adults: an fMRI study. Front Hum Neurosci. 2013;7:169. doi: 10.3389/fnhum.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown S, Heathcote A. Averaging learning curves across and within participants. Behavior Research Methods, Instruments, & Computers. 2003;35(1):11–21. doi: 10.3758/bf03195493. [DOI] [PubMed] [Google Scholar]
- 33.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(23):8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modabber M, Neva J, Gill M, Budge I, Henriques D. Learning and retaining visuomotor adaptation across time. Journal of Vision. 2008;8(6):610–610. [Google Scholar]
- 35.Haibach P, Reid G, Collier D. Motor learning and development. Human Kinetics; 2011. [Google Scholar]
- 36.Field A. Discovering statistics using SPSS. Sage publications; 2009. [Google Scholar]
- 37.Nesselroade JR, Salthouse TA. Methodological and theoretical implications of intraindividual variability in perceptual-motor performance. The journals of gerontology. Series B, Psychological sciences and social sciences. 2004;59(2):49–55. doi: 10.1093/geronb/59.2.p49. [DOI] [PubMed] [Google Scholar]
- 38.Lee TDS, D A. Contextual interference. In: Williams MA, Hodges NJ, editors. SKill acquisition in sport: Research, theory and practice. London: Routledge; 2004. pp. 29–44. [Google Scholar]
- 39.Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. Journal of motor behavior. 2004;36(2):212–224. doi: 10.3200/JMBR.36.2.212-224. [DOI] [PubMed] [Google Scholar]
- 40.Wright D, Verwey W, Buchanen J, Chen J, Rhee J, Immink M. Consolidating behavioral and neurophysiologic findings to explain the influence of contextual interference during motor sequence learning. Psychon Bull Rev. 2015 doi: 10.3758/s13423-015-0887-3. [DOI] [PubMed] [Google Scholar]
- 41.Haith AM, Krakauer JW. Motor Learning: The Great Rate Debate. Current Biology. 2014;24(10):R386–R388. doi: 10.1016/j.cub.2014.03.077. [DOI] [PubMed] [Google Scholar]
- 42.Eversbusch A, Grantcharov T. Learning curves and impact of psychomotor training on performance in simulated colonoscopy: a randomized trial using a virtual reality endoscopy trainer. Surgical Endoscopy And Other Interventional Techniques. 2004;18(10):1514–1518. doi: 10.1007/s00464-003-9264-9. [DOI] [PubMed] [Google Scholar]
- 43.Flamme C, Stukenborg-Colsman C, Wirth C. Evaluation of the learning curves associated with uncemented primary total hip arthroplasty depending on the experience of the surgeon. Hip international: the journal of clinical and experimental research on hip pathology and therapy. 2005;16(3):191–197. doi: 10.1177/112070000601600302. [DOI] [PubMed] [Google Scholar]
- 44.Hernandez J, Bann S, Munz Y, et al. Qualitative and quantitative analysis of the learning curve of a simulated surgical task on the da Vinci system. Surgical Endoscopy And Other Interventional Techniques. 2004;18(3):372–378. doi: 10.1007/s00464-003-9047-3. [DOI] [PubMed] [Google Scholar]
- 45.Lundy-Ekman L. Neuroscience: fundamentals for rehabilitation. Philadelphia: WB Saunders; 1998. [Google Scholar]
- 46.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 47.Wulf G, Schmidt RA. Variability of practice and implicit motor learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23(4):987. [Google Scholar]
- 48.Wadden KP, Woodward TS, Metzak PD, et al. Compensatory motor network connectivity is associated with motor sequence learning after subcortical stroke. Behavioural brain research. 2015;286:136–145. doi: 10.1016/j.bbr.2015.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


