Abstract
Goals
To investigate the association of diabetes with risk of decompensated cirrhosis in patients with chronic hepatitis C (CHC).
Background
Direct-acting antivirals are highly effective in treating CHC but very expensive. CHC patients at high risk of progression to symptomatic liver disease may benefit most from early treatment.
Study
We conducted a retrospective cohort study using the 2006–2013 Truven Health Analytics MarketScan Commercial Claims and Encounters database including inpatient, outpatient and pharmacy claims from private insurers. CHC and cirrhosis were identified using ICD-9-CM diagnosis codes; baseline diabetes was identified by diagnosis codes or anti-diabetic medications. CHC patients were followed to identify decompensated cirrhosis. Multivariable Cox proportional hazards regression was used to model the risk of decompensated cirrhosis by baseline cirrhosis.
Results
There were 75,805 CHC patients with median 1.9 years follow-up. 10,317 (13.6%) of the CHC population had diabetes. The rates of decompensated cirrhosis per 1000 person-years were: 185.5 for persons with baseline cirrhosis and diabetes, 119.8 for persons with cirrhosis and no diabetes, 35.3 for persons with no cirrhosis and diabetes, and 17.1 for persons with no cirrhosis and no diabetes. Diabetes was associated with increased risk of decompensated cirrhosis in persons with baseline cirrhosis (adjusted hazard ratio (aHR) 1.4; 95% confidence interval [CI], 1.3–1.6) and in persons without baseline cirrhosis (aHR 1.9; 95% CI, 1.7–2.1).
Conclusions
In a privately insured US population with CHC, the adjusted risk of decompensated cirrhosis was higher in diabetic compared to non-diabetic patients. Diabetes status should be included in prioritization of antiviral treatment.
Keywords: chronic hepatitis C, decompensated cirrhosis, diabetes, retrospective cohort study, hepatic complication, cirrhosis
Introduction
In the US, an estimated 2.7–3.9 million people have chronic hepatitis C (CHC), making it the most common blood borne infection.[1] Roughly 75%-85% of acute hepatitis C infections persist as CHC.[2–5] The majority of infected people are unaware of their condition because most acute hepatitis C infections are asymptomatic or show non-specific mild symptoms,[6, 7] and symptomatic complications (e.g. cirrhosis) usually take more than 2 decades to develop.[8] The current incidence of acute hepatitis C ranges from 15,000 to 20,000 new infections per year, and recurrent infection rates after successful treatment range from 1–22%.[9] Infections peaked at an estimated 300,000 new infections per year in the late 1980s.[10] The prevalence of hepatitis C viremia in the population peaked in 1994, but given the long course of disease, the prevalence of decompensated cirrhosis has continued to rise. The age-adjusted mortality rate for hepatitis C increased between 1999 and 2007 to 4.58 deaths per 100,000 people, surpassing that of HIV in 2006.[11] Recent data from the Centers for Disease Control and Prevention (CDC) indicate that the age-adjusted CHC mortality rate has continued to increase to 4.65 deaths per 100,000 in 2010.[12]
Without treatment, morbidity and mortality caused by CHC are forecasted to peak in 2030 in the US with 24,300 newly diagnosed decompensated cirrhosis cases, 14,300 hepatocellular carcinoma cases, 3,100 liver transplants and 34,900 deaths.[13] The cost of treating advanced liver disease in the US was estimated at $6.5 billion in 2013, with an expected peak cost of $9.1 billion in 2024.[14] Recently available direct-acting antiviral drugs are highly effective in treating hepatitis C [15] and are usually well-tolerated in interferon-free regimens that spare patients the adverse effects and lower efficacy of interferon-based treatment. The high cost of these drugs has led some private health insurance plans and Medicaid programs to restrict treatment to certain high risk patients.[16] Universal treatment of all CHC patients should be the ultimate goal. However, in the setting of limited resources, proportionally more cases of decompensated cirrhosis can be prevented if treatment is prioritized to CHC patients at highest risk rather than treating all infected persons. Therefore, identifying patients with the highest risk of poor outcomes would allow more rational prioritization of treatment.
We hypothesized that prevalent diabetes mellitus is associated with increased risk of developing hepatic complications in CHC patients. To test this hypothesis, we studied the association of diabetes with the development of decompensated cirrhosis in a large population of CHC patients using a medical claims database of privately insured persons.
Materials and Methods
This was a retrospective cohort study using the 2006–2013 Truven Health Analytics MarketScan® Commercial Claims and Encounters database, including inpatient, outpatient and outpatient pharmacy claims from US employer-sponsored insurance plans covering employees and their dependents. Approximately 100 payers contributed to this dataset. An encrypted person identifier allows longitudinal follow-up. The Washington University Human Research Protection Office determined this study was exempt from Institutional Review Board oversight.
CHC was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes. The ICD-9-CM diagnosis codes described by Kramer et al[17] were used to identify individuals with CHC, requiring at least one CHC code (ICD-9-CM, 070.44, 070.54 or V02.62) on an inpatient facility claim or 2 or more codes spaced >30 and ≤180 days apart in outpatient/provider claims, as described by Klabunde et al.[18] For people identified solely by outpatient/provider claims, the 1st indicator of Hepatitis C infection was expanded to include chronic, unspecified, or acute hepatitis C (ICD-9-CM, 070.41, 070.44, 070.51, 070.54, 070.70, 070.71 or V02.62), while the 2nd code was required to be specific for CHC. Diagnostic laboratory claims from outpatient providers (see Appendix for definition) were not used to identify CHC or other diagnoses since the conditions coded on these claims might be used for rule-out conditions rather than established diagnoses.
The study population included CHC patients 18 years and older having complete medical and prescription drug insurance coverage for a minimum of 6 months after meeting the definition of CHC (see above), in order to identify baseline conditions. Persons with decompensated cirrhosis (i.e., bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis or hepatorenal syndrome) or liver transplant identified during the baseline period were excluded.
Primary exposure and other baseline characteristics
Exposures were identified during the 6-month baseline period. Prevalent diabetes mellitus was defined by ≥1 inpatient facility claim(s) with an ICD-9-CM diagnosis code for diabetes (249.00–250.93 or 648.00–648.04) or ≥2 outpatient/provider claims coded for diabetes >30 days apart, or ≥1 paid prescription(s) for an oral hypoglycemic medication or insulin. Cirrhosis at baseline was identified using ≥1 ICD-9-CM diagnosis codes for cirrhosis, portal hypertension or non-bleeding esophageal varices to improve accuracy for identifying cirrhosis.[19] HBV and HIV infections were identified using ≥1 inpatient facility claim(s) or ≥2 outpatient/provider claims coded for the condition >30 days apart. Alcoholic liver disease/alcohol abuse and obesity were identified by ≥1 ICD-9-CM diagnosis code since these conditions do not require diagnostic evaluation (see Appendix). CHC treatment during the baseline period was defined as ≥1 paid pharmacy prescription(s) for interferon alpha, ribavirin, boceprevir or telaprevir. The number of healthcare encounters (hospitalizations and outpatient visits) was calculated in the 6 months preceding the date when the CHC definition was met in a subset of patients who had insurance coverage in that period.
Outcomes
Follow-up started 6 months after the date when the CHC definition was met. Diabetic and non-diabetic CHC patients were followed to identify new diagnosis of decompensated cirrhosis (the primary outcome), including any of: bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis or hepatorenal syndrome. Secondary outcomes included hepatocellular carcinoma and a composite outcome of death or liver transplant. Outcomes were identified using ICD-9-CM diagnosis and procedure codes (see Appendix) present on ≥1 claim. Follow-up continued until an outcome, end of insurance coverage or 12/31/2013; whichever came first. Figure 1 illustrates the study design.
Figure 1.
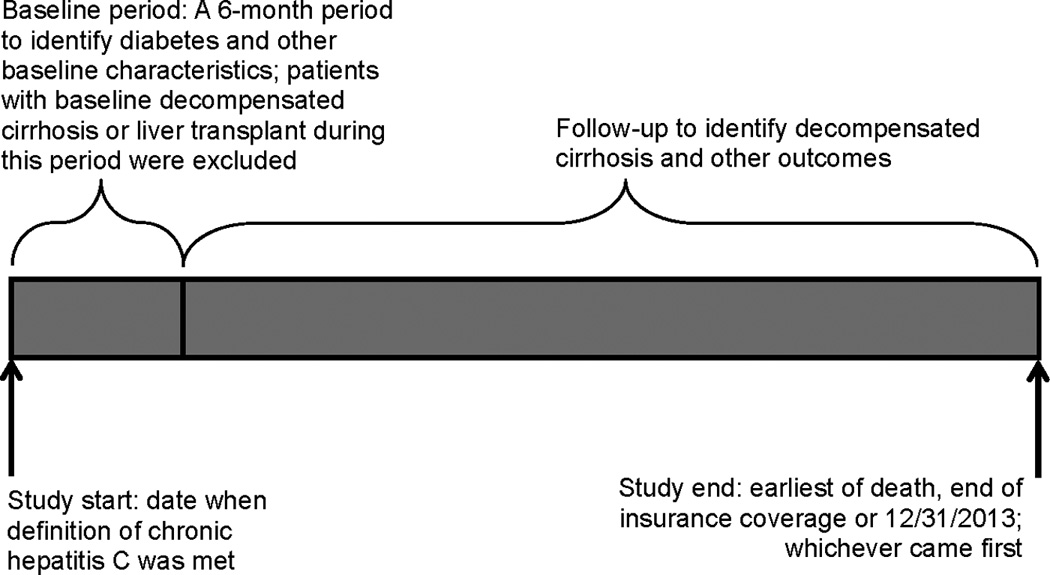
Illustration of Study Design
Chronic hepatitis C was defined by ICD-9-CM diagnosis codes: having ≥1 inpatient facility code for chronic hepatitis, or having ≥1 hepatitis C code (acute or chronic) followed by a chronic hepatitis C code >30 and ≤180 days apart on outpatient or provider claims.
Decompensated cirrhosis included any of: bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis or hepatorenal syndrome.
Statistical Analyses
The Chi-square test was used to test the association of diabetes with baseline conditions, and the non-parametric Wilcoxon-Mann-Whitney test was used to test the association of diabetes with duration of follow-up and the average number of healthcare encounters before the date of CHC definition, since both were not normally distributed. The incidence of study outcomes was calculated per 1000 person-years of observation and tested for statistical association with diabetes using the Chi-square test. Kaplan-Meier curves were generated to plot time to decompensated cirrhosis by baseline cirrhosis and diabetes. The risk of developing the study outcomes: decompensated cirrhosis, hepatocellular carcinoma and the composite outcome of liver transplant/death were modelled using separate Cox proportional hazard models stratified by baseline cirrhosis, controlling for gender, age group, alcoholic liver disease/alcohol abuse, obesity, HBV co-infection, HIV co-infection, recent interferon-based CHC treatment and diabetes. Hazard ratios (HR) and 95% confidence Wald intervals were reported. Separate multivariable Cox proportional hazards model tested the interaction of diabetes with baseline cirrhosis, the interaction of CHC treatment with baseline cirrhosis and the interaction of diabetes with CHC treatment. A sub-analysis including only persons with ≥3 years of follow-up was also performed (n= 16,750 persons, median follow-up=4.4 years [interquartile range, 3.6–5.6]). SAS Enterprise Guide version 7.1 (SAS Institute Inc., Cary, NC) was used for all data management and analyses.
Results
We identified 75,805 CHC patients with at least 6 months insurance coverage beginning on the date when the CHC definition was met who were enrolled in employer-sponsored health insurance plans in the US from 1/1/2006 through 6/30/2013. Figure 2 shows the algorithm with inclusion/exclusion criteria that resulted in the final study population. The median years of follow-up was 1.9 (range 0.5–8). The study population was predominantly male (61.8%) and had a median age of 53 years (range 18–64). Insured dependents of employees constituted 32.3% of the study population. Diabetes mellitus was identified in 10,317 (13.6%) of the CHC population at baseline. Among 10,317 diabetics, there were 2,840 persons identified only by diabetes medications. Of the 7,477 individuals having ICD-9-CM diagnosis codes for diabetes, there were 1,289 (17.2%) persons with at least one ICD-9-CM diagnosis code for type 1 diabetes and 79 (1.1%) persons with at least one code for secondary diabetes. Diabetic CHC patients were more likely to be male, older, obese, and have cirrhosis at baseline and were less likely to be coinfected with HIV and have received CHC treatment during the baseline period compared to non-diabetic CHC patients (Table 1). The median follow-up time for diabetic CHC patients (1.77 years, interquartile range [IQR] 0.96–3.09) was shorter than that of non-diabetic CHC patients (1.88 years, IQR 0.99–3.41, p<0.0001). Diabetic patients had more healthcare encounters in the 6 months prior to the date when the CHC definition was met (median 7 encounters, IQR 4–12) compared to non-diabetics (median 5, IQR 3–10, p<0.0001), in a subset of 44,657 patients with complete insurance coverage in the 6-month period before meeting the CHC definition.
Figure 2.
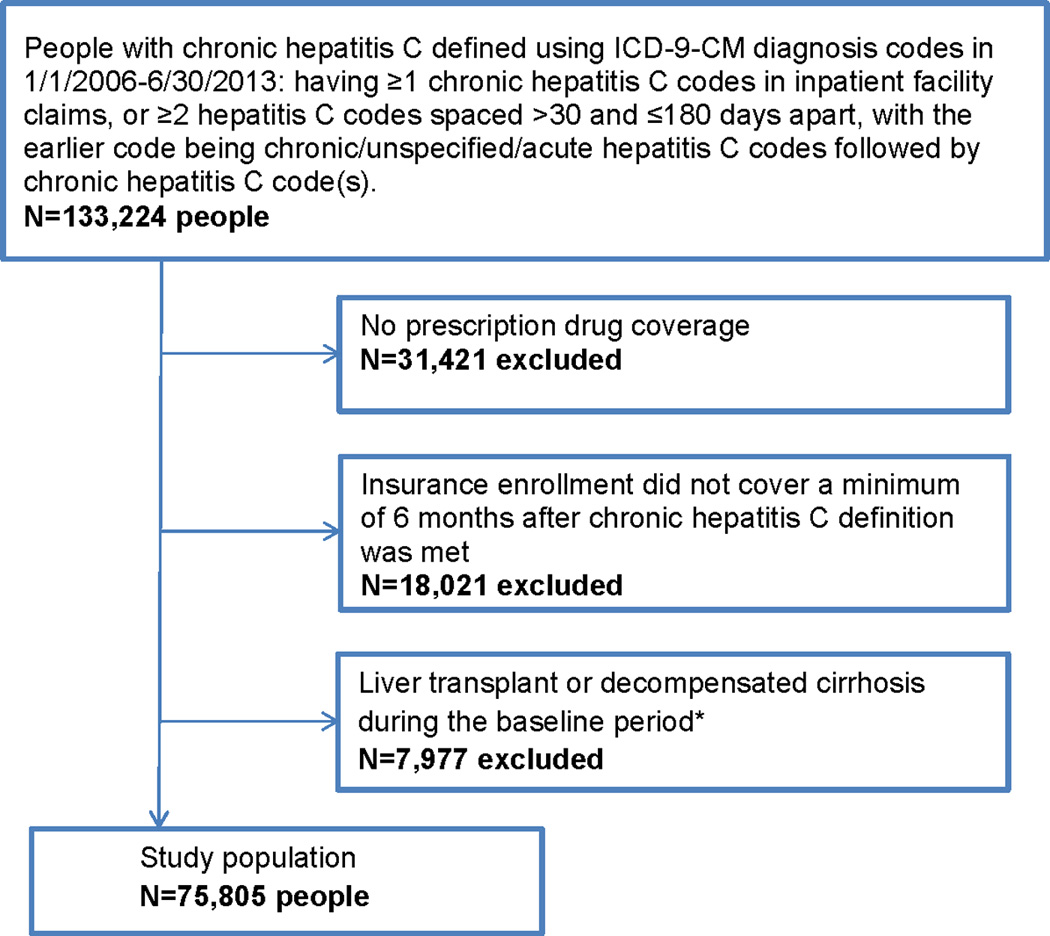
Algorithm with Inclusion/Exclusion Criteria to Identify Patients with Chronic Hepatitis C from Health Insurer Claims Data
*The baseline period was defined as the first 6 months beginning on the date when chronic hepatitis C definition was met
Table 1.
Baseline* Characteristics of Chronic Hepatitis C Patients
| Characteristic | All patients n=75,805 n (%) |
Diabetics n=10,317 n (%) |
Non diabetics n=65,488 n (%) |
p value |
|---|---|---|---|---|
| Male | 46,853 (61.81) | 7,184 (69.63) | 39,669 (60.57) | <.001 |
| Age 18–39 | 7,930 (10.46) | 316 (3.06) | 7,614 (11.63) | <.001 |
| 40–44 | 5,636 (7.43) | 498 (4.83) | 5,138 (7.85) | |
| 45–49 | 12,474 (16.46) | 1,423 (13.79) | 11,051 (16.87) | |
| 50–64 | 49,765 (65.65) | 8,080 (78.32) | 41,685 (63.65) | |
| Alcoholic liver disease/alcohol abuse | 2,563 (3.38) | 357 (3.46) | 2,206 (3.37) | 0.632 |
| Obesity | 3,257 (4.30) | 900 (8.72) | 2,357 (3.60) | <.001 |
| HBV | 617 (0.81) | 87 (0.84) | 530 (0.81) | 0.721 |
| HIV | 1,571 (2.07) | 153 (1.48) | 1,418 (2.17) | <.001 |
| Recent treatment | 16,597 (21.89) | 1,756 (17.02) | 14,841 (22.66) | <.001 |
| Cirrhosis | 11,827 (15.60) | 2,341 (22.69) | 9,486 (14.49) | <.001 |
The baseline period was defined as the first 6 months beginning on the date when the chronic hepatitis C definition was met
Diabetic CHC patients had higher incidence of all conditions comprising the diagnosis of decompensated cirrhosis (bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis and hepatorenal syndrome) as well as death, stratified by having cirrhosis at baseline (Table 2). People with both cirrhosis and diabetes at baseline had higher probability of progressing to decompensated cirrhosis than people with only cirrhosis, only diabetes or neither condition (Figure 3). In multivariable Cox proportional hazards models diabetes was associated with significantly increased risk of decompensated cirrhosis in persons coded for cirrhosis at baseline (adjusted HR 1.42, 95% CI 1.29–1.56), and in persons not coded for cirrhosis at baseline (adjusted HR 1.86, 95% CI 1.69–2.06, Table 3). Male gender and alcoholic liver disease/alcohol abuse were independently associated with higher risk of decompensated cirrhosis. Baseline CHC treatment was associated with significantly decreased risk of decompensated cirrhosis in both models, with the protective effect greater in patients with baseline cirrhosis (interaction p<0.001, Table 3). The interaction between diabetes and baseline cirrhosis was significantly associated with decompensated cirrhosis (p<0.001) in a separate multivariable Cox proportional hazards model.
Table 2.
Incidence of Hepatic Complications and Death per 1000 Person-Years Stratified by Baseline Cirrhosis and Diabetes*
| Persons with baseline cirrhosis n=11,827 |
Persons without baseline cirrhosis n=63,978 |
|||||
|---|---|---|---|---|---|---|
| Diabetic*** n=2,341 persons |
Non diabetic*** n=9,486 persons |
p value | Diabetic*** n=7,976 persons |
Non diabetic*** n=56,002 persons |
p value | |
| Decompensated cirrhosis** | 185.5 (564/3,040) | 119.8 (1,726/14,403) |
<0.001 | 35.3 (504/14,264) | 17.1 (1,883/110,136) |
<0.001 |
| Bleeding esophageal varices | 41.6 (150/3,602) | 23.2 (377/16,255) | <0.001 | 4.6 (69/14,880) | 2.1 (237/112,561) | <0.001 |
| Ascites | 98.0 (329/3,358) | 64.5 (1,003/15,552) |
<0.001 | 16.9 (248/14,660) | 7.8 (877/111,855) | <0.001 |
| Hepatic encephalopathy | 89.3 (307/3,438) | 54.8 (863/15,761) | <0.001 | 14.3 (211/14,710) | 8.2 (919/111,682) | <0.001 |
| Hepatocellular carcinoma | 38.4 (139/3,616) | 27.1 (437/16,149) | <0.001 | 10.4 (154/14,778) | 4.8 (536/112,190) | <0.001 |
|
Spontaneous bacterial peritonitis |
14.7 (55/3,730) | 9.6 (160/16,589) | 0.007 | 2.0 (30/14,948) | 0.9 (100/112,861) | <0.001 |
| Hepatorenal syndrome | 14.4 (54/3,750) | 8.2 (137/16,641) | 0.001 | 1.9 (29/14,943) | 0.8 (85/112,904) | <0.001 |
| Liver transplant | 16.3 (60/3,690) | 13.4 (219/16,384) | 0.181 | 1.5 (23/14,926) | 1.2 (132/112,714) | 0.223 |
| Death | 24.0 (91/3,796) | 18.1 (303/16,769) | 0.019 | 11.0 (164/14,970) | 4.3 (487/112,953) | <0.001 |
The baseline period was defined as the first 6 months beginning on the date when the chronic hepatitis C definition was met
Decompensated cirrhosis was defined as any of: bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacteria peritonitis and hepatorenal syndrome.
Cells in these columns are rates/1000 person-years (number of persons with outcome/person-years of observation)
Figure 3.
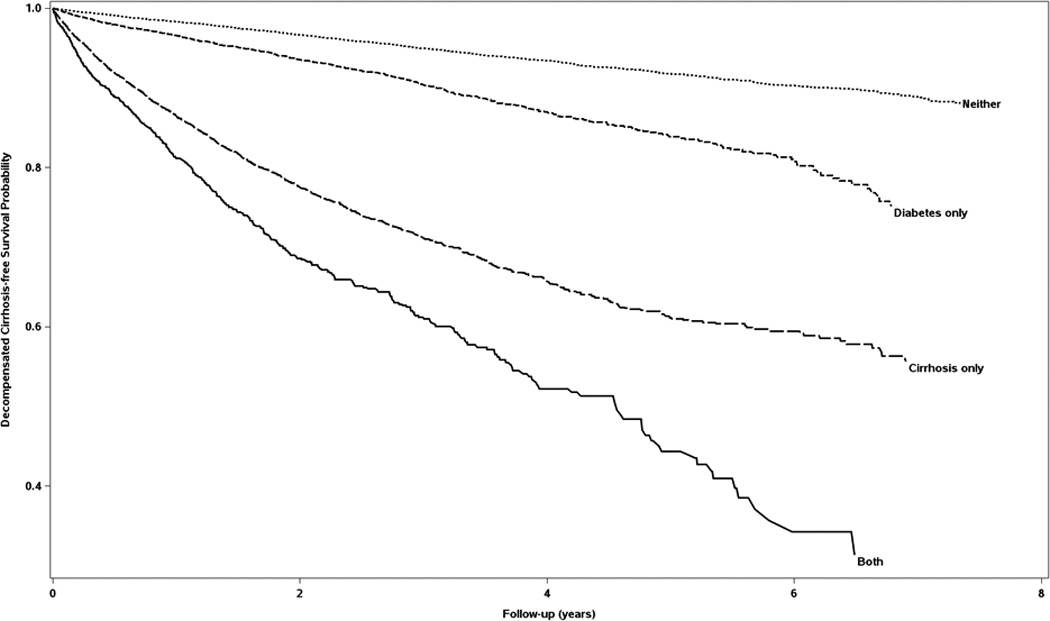
Kaplan-Meier Curves of Decompensated Cirrhosis-free Survival Stratified by Cirrhosis and Diabetes at Baseline
Decompensated cirrhosis included any of: bleeding esophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis or hepatorenal syndrome.
Neither= neither cirrhosis nor diabetes, both= both cirrhosis and diabetes
Table 3.
Multivariable Cox Proportional Hazards Regression Models of Decompensated Cirrhosis for Persons with Cirrhosis and Persons without Cirrhosis at Baseline*
| Persons with cirrhosis n=11,827 |
Persons without cirrhosis n=63,978 |
||||||
|---|---|---|---|---|---|---|---|
| Adjusted HR |
95% Confidence Interval |
Adjusted HR |
95% Confidence Interval |
||||
| Male | 1.16 | 1.06 | 1.27 | 1.23 | 1.12 | 1.34 | |
| Age 18–39 | Reference | Reference | |||||
| 40–44 | 1.37 | 0.97 | 1.93 | 1.36 | 1.02 | 1.82 | |
| 45–49 | 1.68 | 1.24 | 2.26 | 2.27 | 1.78 | 2.88 | |
| 50–64 | 1.78 | 1.34 | 2.36 | 2.88 | 2.31 | 3.61 | |
| Alcoholic liver disease/alcohol abuse | 1.65 | 1.42 | 1.92 | 1.77 | 1.43 | 2.18 | |
| Obesity | 1.06 | 0.88 | 1.28 | 1.06 | 0.85 | 1.32 | |
| HBV | 1.14 | 0.76 | 1.71 | 1.45 | 0.96 | 2.18 | |
| HIV | 1.10 | 0.78 | 1.57 | 1.31 | 1.00 | 1.71 | |
| Recent treatment | 0.50 | 0.44 | 0.56 | 0.85 | 0.77 | 0.94 | |
| Diabetes | 1.42 | 1.29 | 1.56 | 1.86 | 1.69 | 2.06 | |
Interaction testing for association of diabetes and baseline cirrhosis with decompensated cirrhosis, p<0.001
Interaction testing for association of chronic hepatitis C treatment and baseline cirrhosis with decompensated cirrhosis, p<0.001
Interaction testing for association of chronic hepatitis C treatment and diabetes with decompensated cirrhosis, p=0.919 in model of persons with baseline cirrhosis and p=0.792 in model of persons without baseline cirrhosis
HR= Hazard ratio
The baseline period was defined as the first 6 months beginning on the date when the chronic hepatitis C definition was met
Secondary Outcomes and Sub-analysis
In a separate multivariable Cox proportional hazards model, diabetes was associated with increased risk of hepatocellular carcinoma in persons with baseline cirrhosis (adjusted HR 1.27, 95% CI 1.04–1.53) and in persons without baseline cirrhosis (adjusted HR 1.82, 95% CI 1.52–2.18). The risk of hepatocellular carcinoma associated with diabetes was significantly higher in persons with baseline cirrhosis compared to persons without baseline cirrhosis (interaction p=0.008). Diabetes was also associated with increased risk of the composite outcome of death/liver transplant (adjusted HR 1.26, 95% CL 1.04–1.52) in persons with baseline cirrhosis and significantly higher risk in persons without baseline cirrhosis (adjusted HR 2.01, 95% CI 1.70–2.38, p<0.001 for interaction term). In a subset analysis of the population with at least 3 years follow-up, diabetes was associated with increased risk of decompensated cirrhosis in both persons with baseline cirrhosis (adjusted HR 2.08, 95% CI 1.51–2.85) and without baseline cirrhosis (adjusted HR 2.15, 95% CI 1.74–2.66). The complete results of the secondary outcomes and sub-analysis are included in the Appendix.
Discussion
In this large population of CHC patients having employer-sponsored insurance in the US, diabetes mellitus was independently associated with increased risk of decompensated cirrhosis, hepatocellular carcinoma and liver transplant/death in patients both with and without baseline cirrhosis. This information may help guide decision-making regarding early treatment with the newly available direct-acting antivirals and appropriate management of diabetes
Prior research suggests an association of diabetes[20, 21], insulin resistance[20, 22–26] and serum glucose[27] with progression of hepatic fibrosis in CHC patients. Fewer studies looked at the association of diabetes with hepatocellular carcinoma[28–30] and other hepatic complications.[30, 31] These associations between diabetes and hepatic complications have not been firmly established since the studies were cross sectional,[20, 22–27, 32] had small sample sizes[21, 23] and/or included highly selected CHC populations.[21, 28, 29, 31] Results from one study[33] were difficult to interpret because a temporal relation between CHC and diabetes was not established.
Our results showed that older age was associated with higher risk to develop decompensated cirrhosis. This is not unexpected, since older age is likely a marker for longer duration of infection, and decompensated cirrhosis usually takes more than 2 decades to develop in CHC patients.[8] HIV and HBV co-infections were not significantly associated with decompensated cirrhosis. In agreement with prior research[21, 26, 34], we found that male gender was associated with increased risk of decompensated cirrhosis. Despite the fact that interferon-based CHC treatment was only identified in the baseline 6 months beginning on the date when the definition of CHC was met, treatment was independently associated with lower risk of decompensated cirrhosis. Baseline treatment had a larger protective effect in people with baseline cirrhosis than without baseline cirrhosis. The outcome of antiviral therapy cannot be assessed in our administrative data.
The role of hepatitis C virus infection in the development of diabetes is controversial.[35–39] Liver cirrhosis can lead to diabetes (i.e. hepatogenous diabetes[40]). There were only 79 persons with ICD-9-CM diagnosis codes for secondary diabetes in our study population who possibly had hepatogenous diabetes or other types of secondary diabetes. Given the limitations of administrative databases, we cannot address the biological role of diabetes in liver disease progression. However, we were able to demonstrate that prevalent diabetes mellitus in CHC patients, regardless of whether it resulted from the hepatitis C infection, cirrhosis, or was due to other factors, was significantly associated with higher risk of liver disease progression. Because of the observational nature of this study, a causal association cannot be inferred between diabetes and decompensated cirrhosis. However, a diagnosis of diabetes can be used to assess the risk of progression to decompensated cirrhosis in CHC patients. Prior research has shown an association between glycemic control and metformin therapy with improved survival and lower risk of hepatic complications.[41] Patients with chronic liver disease and diabetes need appropriate diabetes management, and may also benefit from earlier antiviral treatment if they have CHC.
Limitations and Strengths
There were several limitations to this study. Using administrative data, we can only identify conditions that are diagnosed and coded on claims. Cirrhosis might be asymptomatic (therefore not coded on medical claims), which possibly resulted in misclassification. To minimize this bias, we used the first 6 months beginning on the date when the CHC definition was met to identify baseline conditions, including cirrhosis. With this baseline time period patients who underwent liver assessment soon after coding of CHC will be classified correctly, if the cirrhosis was diagnosed and coded. The time when individuals were infected with hepatitis C Virus cannot be determined from claims data (i.e., left censoring). Because CHC is usually asymptomatic and diabetics had higher number of healthcare encounters than non-diabetics, diabetics may be more likely to be screened and diagnosed with CHC earlier than non-diabetics, resulting in entry into our study population earlier in the course of their CHC. This would most likely result in a bias toward the null hypothesis, which would suggest that the association of diabetes and CHC with decompensated cirrhosis may be even stronger than we observed. Prior research suggests impaired glucose tolerance is associated with liver complications and lower survival in persons with cirrhosis[42, 43], including cirrhosis related to hepatitis C[44]. However, due to the administrative nature of our data, we cannot accurately identify persons with impaired glucose tolerance to study this factor. If persons with impaired glucose tolerance have higher risk of developing liver complications and we classified them as non-diabetic, this will lead to bias towards the null hypothesis and an underestimate of the impact of diabetes on our study outcomes.
The study population consisted of beneficiaries of employer-sponsored insurance plans, which is not completely representative of the US population. ICD-9-CM diagnosis codes are not perfectly accurate although the codes for CHC and cirrhosis were validated in VA administrative data with positive and negative predictive values of 93% and 92%, respectively, for chronic hepatitis C and 90% and 87%, respectively, for cirrhosis .[17] ICD-9-CM codes for diabetes mellitus have been validated in administrative data using both inpatient and outpatients records in elderly patients with cancer[45] (75.1% sensitivity 95.1% specificity) and in elderly people treated by primary care physicians [46] (62.2% sensitivity and 97.2% specificity). In addition to inpatient and outpatient records, we used paid prescriptions for anti-diabetic medications to identify diabetes; this likely improved the sensitivity to identify diabetes in our study.
The median follow-up in our study was 1.9 years, which might not be long enough to identify liver complications. To address this limitation, we performed a sub-analysis including only persons with at least 3 years of follow-up, which demonstrated approximately two-fold increased risk of decompensated cirrhosis associated with diabetes in persons with and without baseline cirrhosis. This is consistent with the Kaplan-Meier curves in Figure 3 in which the survival curve for diabetics diverges from the curve for non-diabetics with longer follow-up time.
The strengths of this study include the large study population including people from all regions in the US. Our results are generalizable to US adults with private insurance. We excluded persons with baseline decompensated cirrhosis to establish temporality of the association between diabetes and decompensated cirrhosis. In addition, our analyses showed that diabetes was consistently associated with not only decompensated cirrhosis, but also hepatocellular carcinoma and liver transplant/death in people with baseline cirrhosis and in people without baseline cirrhosis.
Conclusions
CHC patients with diabetes mellitus had increased risk of decompensated cirrhosis and other hepatic complications in a population of persons with employer-sponsored health insurance in the US. In the presence of limited fiscal resources and the inability to pay for treatment of all hepatitis C patients, persons with CHC and diabetes might benefit from prioritization and earlier treatment with one of the newer direct-acting antiviral regimens.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in part by the Washington University Institute of Clinical and Translational Sciences grant [UL1 TR000448] from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number [R24 HS19455] through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH).
The authors thank Dr. Kevin Korenblat at the division of Gastroenterology at Washington University in St. Louis for his help in reviewing this study.
Footnotes
Disclosures: MJS declares no conflict of interest. MAO has done consulting work for Merck, Pfizer and Sanofi-Pasteur. WGP has done consulting work for Merck and Abbvie. RMP declares no conflict of interest.
REFERENCES
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Shakil AO, Conry-Cantilena C, Alter HJ, et al. Volunteer blood donors with antibody to hepatitis C virus: clinical biochemical, virologic, histologic features. The Hepatitis C Study Group. Ann Intern Med. 1995;123:330–337. doi: 10.7326/0003-4819-123-5-199509010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 4.Esteban JI, Lopez-Talavera JC, Genesca J, et al. High rate of infectivity and liver disease in blood donors with antibodies to hepatitis C virus. Ann Intern Med. 1991;115:443–449. doi: 10.7326/0003-4819-115-6-443. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LB, Buskell-Bales Z, Wright EC, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 6.Aach RD, Stevens CE, Hollinger FB, et al. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991;325:1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- 7.Koretz RL, Abbey H, Coleman E, et al. Non-A, non-B post-transfusion hepatitis. Looking back in the second decade. Ann Intern Med. 1993;119:110–115. doi: 10.7326/0003-4819-119-2-199307150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 9.Bryony Hill AS, Saleem Jawaad, Cooke Graham. Five–Year Risk of Late Relapse or Reinfection With Hepatitis C After Sustained Virological Response: Meta-analysis of 49 Studies in 8534 Patients. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington, USA. 2015. [Google Scholar]
- 10.Ward JW. The hidden epidemic of hepatitis C virus infection in the United States: occult transmission and burden of disease. Top Antivir Med. 2013;21:15–19. [PMC free article] [PubMed] [Google Scholar]
- 11.Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 12.Viral Hepatitis Surveillance -United State. Centers for Disease Control and Prevention. 2011 [Google Scholar]
- 13.Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack A. Hepatitis C Treatment Wins Approval, but Price Relief May Be Limited. The New York Times; 2014. [Google Scholar]
- 17.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petta S, Camma C, Di Marco V, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136–1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 21.Kita Y, Mizukoshi E, Takamura T, et al. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metabolism. 2007;56:1682–1688. doi: 10.1016/j.metabol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 24.Hickman IJ, Powell EE, Prins JB, et al. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 25.Muzzi A, Leandro G, Rubbia-Brandt L, et al. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41–46. doi: 10.1016/j.jhep.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Cua IH, Hui JM, Kench JG, et al. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48:723–731. doi: 10.1002/hep.22392. [DOI] [PubMed] [Google Scholar]
- 27.Ratziu V, Munteanu M, Charlotte F, et al. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39:1049–1055. doi: 10.1016/s0168-8278(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 28.Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 29.Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 30.Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823–831. doi: 10.1002/hep.27228. [DOI] [PubMed] [Google Scholar]
- 31.Everhart JE, Lok AS, Kim HY, et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz V, Berenguer M, Rayon JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 33.Huang YW, Yang SS, Fu SC, et al. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study. Hepatology. 2014;60:807–814. doi: 10.1002/hep.27212. [DOI] [PubMed] [Google Scholar]
- 34.Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology. 2011;54:1127–1134. doi: 10.1002/hep.24503. [DOI] [PubMed] [Google Scholar]
- 35.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naing C, Mak JW, Ahmed SI, et al. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montenegro L, De Michina A, Misciagna G, et al. Virus C hepatitis and type 2 diabetes: a cohort study in southern Italy. Am J Gastroenterol. 2013;108:1108–1111. doi: 10.1038/ajg.2013.90. [DOI] [PubMed] [Google Scholar]
- 38.Ruhl CE, Menke A, Cowie CC, et al. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60:1139–1149. doi: 10.1002/hep.27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusi K. The relationship between hepatitis C virus infection and diabetes: time for a divorce? Hepatology. 2014;60:1121–1123. doi: 10.1002/hep.27252. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Compean D, Gonzalez-Gonzalez JA, Lavalle-Gonzalez FJ, et al. Current Concepts in Diabetes Mellitus and Chronic Liver Disease: Clinical Outcomes, Hepatitis C Virus Association, and Therapy. Dig Dis Sci. 2015 doi: 10.1007/s10620-015-3907-2. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Compean D, Gonzalez-Gonzalez JA, Lavalle-Gonzalez FJ, et al. The treatment of diabetes mellitus of patients with chronic liver disease. Ann Hepatol. 2015;14:780–788. doi: 10.5604/16652681.1171746. [DOI] [PubMed] [Google Scholar]
- 42.Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70–75. doi: 10.1111/j.1572-0241.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Compean D, Jaquez-Quintana JO, Lavalle-Gonzalez FJ, et al. Subclinical abnormal glucose tolerance is a predictor of death in liver cirrhosis. World J Gastroenterol. 2014;20:7011–7018. doi: 10.3748/wjg.v20.i22.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calzadilla-Bertot L, Vilar-Gomez E, Torres-Gonzalez A, et al. Impaired glucose metabolism increases risk of hepatic decompensation and death in patients with compensated hepatitis C virus-related cirrhosis. Dig Liver Dis. 2016;48:283–290. doi: 10.1016/j.dld.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 46.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


