Abstract
Background
Gastrostomy tube (G-tube) placement is a common intervention for newborns with severe feeding difficulties. Infants with congenital diaphragmatic hernia (CDH) are at high risk for feeding problems. Prevalence of G-tube placement and consequent nutritional outcomes of infants with CDH and G-tubes has not been described.
Aims
Determine factors associated with G-tube placement and growth in infants with congenital diaphragmatic hernia.
Study design
Retrospective cohort study of infants with CDH to evaluate the association of G-tube placement with risk factors using logistic regression. We also assessed the association between growth velocity and G-tube placement and other risk factors using linear regression.
Subjects
The subjects of the study were infants with CDH treated at Duke University Medical Center from1997 to 2013.
Outcome measures
Weight gain in infants with CDH that had G-tube placement compared to those infants with CDH that did not.
Result
Of the 123 infants with CDH, 85 (69%) survived and G-tubes were placed in 25/85 (29%) survivors. On adjusted analysis, extracorporeal membrane oxygenation (OR=11.26 [95% CI: 1.92–65.89]; P=0.01) and proton pump inhibitor use (OR=17.29 [3.98–75.14], P ≤0.001) were associated with G-tube placement. Infants without G-tubes had a growth velocity of 6.5 g/day (95% CI: 2.5–10.4) more than infants with G-tubes.
Conclusion
Survivors with more complex inpatient courses were more likely to receive G-tubes. Further investigation is needed to identify optimal feeding practices for infants with CDH.
Keywords: Neonate, G-tube, Feeding
1. Introduction
Congenital diaphragmatic hernia is associated with 30–50% mortality despite ongoing efforts to improve outcomes by establishing management guidelines [1–4]. Surviving infants experience long term health complications that affect multiple organ systems, including the lungs, heart, and gastrointestinal tract. Gastrointestinal morbidity can be particularly severe and can manifest in oral aversion, gastroesophageal reflux disease (GERD), or malnutrition as evidenced by growth failure or delayed growth [2,5]. Clinical practices involved with addressing feeding issues vary across physicians and institutions, and one such practice includes placement of a G-tube to address severe feeding difficulties [1].
Risk factors that predict placement of G-tubes and guidelines which prompt the use of G-tubes, along with the consequent nutritional outcomes, in infants with CDH are not well established. Our current approach at Duke University Medical Center is to make a multidisciplinary decision among the primary medical provider, patient's family, occupational and speech therapists, and pediatric surgeons for the need for and timing of G-tube placement, with minimal objective standard approach. The primary aim of this study is to report the frequency of G-tube placement in infants with CDH at a single tertiary intensive care nursery over a 16 year period using this relatively subjective strategy, and identify factors that are associated with G-tube placement. The secondary aim is to assess how our current practice with use of G-tubes is associated with long term growth outcomes in infants with CDH.
2. Methods
2.1. Study design
This was a retrospective cohort study of infants with CDH cared for at the Duke University Medical Center ICN from 1997 to 2013. We included all surviving infants who: 1) received care at Duke University ICN, including transfers; 2) were diagnosed with right- or left-sided CDH prenatally via ultrasound or postnatally via clinical or imaging findings. Using electronic medical records, we collected demographic and clinical information including birth weight, gestational age, Apgar score at 5 min, chromosomal anomaly, extracorporeal membrane oxygenation (ECMO) requirement, ventilator support, type of repair, side of defect, inpatient medications (dexmedetomidine, sildenafil, nitrous oxide, proton pump inhibitor (PPI), H2 antagonist, metoclopramide, vasopressors, diuretics), discharge medications, G-tube placement in the first 12 months of life, and feeding information (including number of days before initiating feeds, TPN use, and type of feeds: mother's milk, donor human milk or formula). Outpatient data, focusing on weight and development, were also collected from the NICU follow-up clinic visit closest to one year of age. Growth percentiles were determined using WHO Child Growth Standards [6]. The Duke Institutional Review Board gave permission to conduct the study.
2.2. Definitions
Presence of chromosomal anomaly, or clinical signs consistent with a chromosomal anomaly, was recorded based on prenatal ultrasound diagnosis or genetic testing conducted prenatally or postnatally. Infants with a structural cardiac abnormality on echocardiogram (including ventricular septal defect, atrial septal defect, hypoplastic left heart, and coarctation of the aorta) were classified as having congenital heart disease. Infants whose only structural cardiac defect(s) consisted of patent ductus arteriosus or patent foramen ovale were not classified as having congenital heart disease. We classified the source of feedings throughout hospitalization: mother's milk, donor human milk, cow's milk formula, or elemental formula. Type of repair included either patch repair or primary repair. The following comorbidities were defined by the types of medications that were prescribed: GERD (PPI, H2 antagonist, or metoclopramide); pulmonary hypertension (sildenafil or nitric oxide); hypotension (epinephrine); or chronic lung disease (inhaled budesonide, diuretics, supplemental oxygen). We also recorded the use of dexmedetomidine, which is often administered for sedation during and after ECMO at our center. Growth velocity was defined as the change in weight between date of discharge for the non G-tube group or G-tube placement and follow-up divided by the number of days between those time points. We determined growth to be “adequate” if an infant met at least 1 of 2 criteria: 1) weight percentile of >10% at approximately one year post-discharge follow-up; or 2) increased weight percentile at follow-up compared to time of G-tube placement or discharge from the hospital.
2.3. Statistical analysis
We divided surviving infants into 2 groups: those with G-tube placement and those without G-tube placement. Because our center instituted management guidelines for infants with CDH in 2002 [4], we evaluated the number of survivors and percentage of survivors with G-tubes over time. In order to evaluate the presence of long-term morbidities, we compared the prevalence of certain discharge medications (PPIs, ranitidine, metoclopramide, diuretics, and budesonide) between groups using Fisher's exact test. We also compared the presence of modifiable and non-modifiable risk factors for G-tube placement and growth velocity between these 2 groups using Fisher's exact test. Risk factors were divided into 3 categories: category 1) non-modifiable risk factors; category 2) modifiable risk factors that usually occur within the first week of life; and category 3) modifiable risk factors that usually occur after the first week of life.
We used multivariable logistic regression to identify significant risk factors associated with the decision to place a G-tube. We used multivariable linear regression to identify significant risk factors associated with growth velocity. In the first step of the logistic and linear regression analyses, Category 1 risk factors were entered to create a model (Model 1). Risk factors with P < 0.1 in this analysis were entered into a new model (Model 2) along with addition of Category 2 risk factors. Risk factors with P < 0.1 from Model 1 or Model 2 were entered into a new model (Model 3) with addition of risk factors from Category 3. The final model (Model 4) was created using all risk factors with P < 0.1 from Models 1, 2, or 3. P-values < 0.05 were considered significant. Preliminary data was collected using Microsoft Excel 2010, and statistical analyses were performed using Stata 13 (College Station, TX).
3. Results
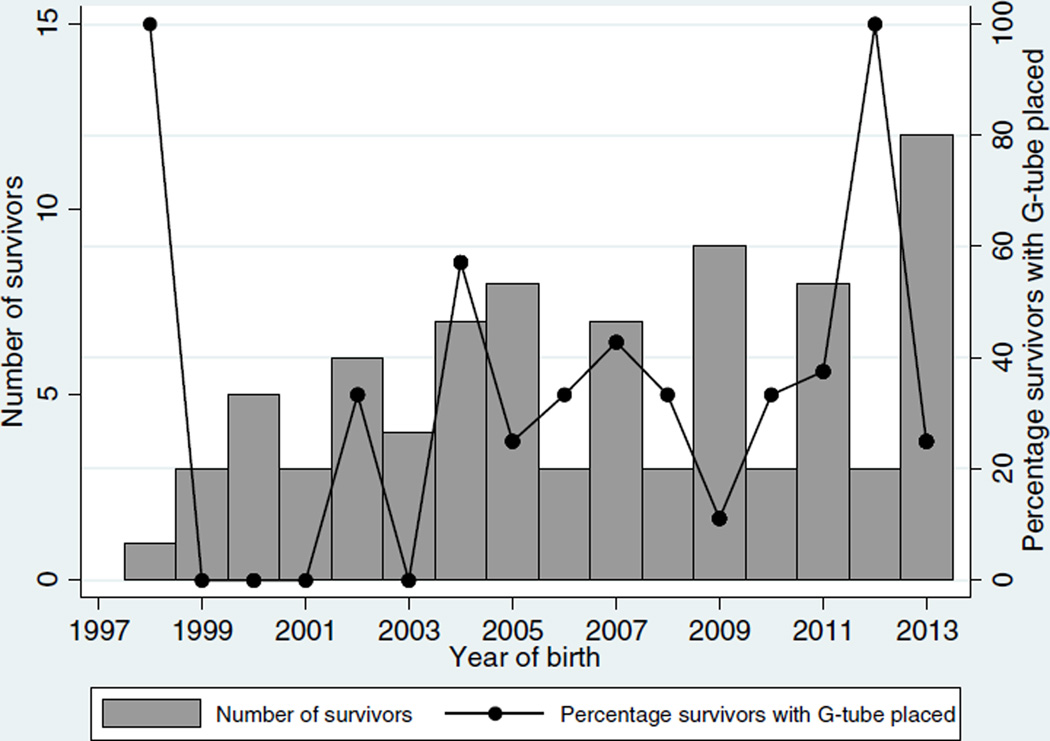
In the cohort of 123 infants with CDH, 85 (69%) survived. For these survivors, diaphragmatic hernia repair surgery was performed on median day of life 6 (25th–75th percentile: 3–11), and G-tubes were placed in 25/85 (29%). Of those with G-tube placement, 14/25 (56%) also had Nissen fundoplication, and 3/25 (12%) G-tubes were placed after discharge (26, 93, and 159 days after). The non-modifiable factors in the survivors were comparable between those with and without G-tube placement (Table 1). Those survivors who required G-tube placement had more intensive medical therapeutic interventions within the first week of life, including ECMO and epinephrine exposure and longer duration of hospitalization. The median length of initial hospitalization for infants with G-tube placement was 101 days (77–122), compared to 22 days (11−31) in those who did not have G-tube placement. Over the 16 year time span, there was variability in the frequency of G-tube placement and in the number of surviving infants with CDH without any apparent trends (Fig. 1). A greater proportion of survivors with G-tubes received GERD treatment during hospitalization (68% with PPI) compared to the non G-tube group (32% (P < 0.001)) (Table 1). Similarly, based on discharge medications, chronic lung disease was more common in those survivors who required G-tube placement (Table 2). Of the three factors included in the final model, only ECMO and PPI use were significantly associated with G-tube placement (Table 3).
Table 1.
Demographics.
| G-tube (n = 25) | No G-tube (n = 60) | P-value | |
|---|---|---|---|
| Category 1: non-modifiable risk factors | |||
| Birth weight ≥ 2500 g | 80% | 93% | 0.08 |
| Gestational age ≥ 36 weeks | 88% | 93% | 0.41 |
| Prenatal diagnosis | 72% | 47% | 0.06 |
| Female | 40% | 28% | 0.32 |
| Hispanic ethnicity | 20% | 23% | >0.99 |
| Apgar at 5 min ≥ 5 | 84% | 83% | 0.15 |
| Chromosomal anomaly | 4% | 5% | 0.79 |
| Congenital heart disease | 28% | 28% | >0.99 |
| Category 2: modifiable risk factors within first week | |||
| ECMO | 48% | 3% | <0.001 |
| Epinephrine | 76% | 42% | 0.01 |
| Nitric oxide | 84% | 40% | <0.001 |
| Repair by 1 week | 24% | 72% | <0.001 |
| Category 3: modifiable risk factors after first week | |||
| Proton pump inhibitor | 68% | 10% | <0.001 |
| H2 receptor antagonist | 76% | 37% | 0.002 |
| Sildenafil | 24% | 2% | 0.002 |
| Dexmedetomidine | 28% | 5% | 0.01 |
| Any maternal breast milk | 36% | 65% | 0.02 |
ECMO: extracorporeal membrane oxygenation; G-tube: gastrostomy tube.
Fig. 1.
Number of survivors and percentage of survivors with G-tube placement over time.
Table 2.
Discharge medicationsa.
| G-tube (n = 24) | No G-tube (n = 58) | P-value | |
|---|---|---|---|
| Proton pump inhibitor | 54% | 16% | 0.001 |
| Ranitidine | 21% | 26% | 0.78 |
| Metoclopramide | 29% | 24% | 0.78 |
| Diuretics | 54% | 7% | <0.001 |
| Budesonide | 54% | 3% | <0.001 |
G-tube: gastrostomy tube.
Discharge medications not available for 3 infants.
Table 3.
Predictors of gastrostomy tube (G-tube) placement and growth velocity.
| Predictors of G-tube placement | Odds ratio | P-value |
|---|---|---|
| Prenatal diagnosis | 4.50 (0.98, 20.59) | 0.05 |
| ECMO | 11.26 (1.92, 65.89) | 0.01 |
| Proton pump inhibitor | 17.29 (3.98, 75.14) | <0.001 |
| Predictors of growth velocity | Coefficient | P-value |
| ECMO | − 2.7 (− 7.3, 2.0) | 0.26 |
| G-tube | − 6.5 (− 10.4, − 2.5) | 0.002 |
ECMO: extracorporeal membrane oxygenation.
The median day of G tube placement was 82 days (54–114), and the median day of discharge in the infants without G-tube placement was 22 days (11–30.5). However, the number of days between G-tube placement or discharge and follow-up in both groups respectively was not significantly different (284 days (184–351) for the G-tube group and 221 days (120–291) for the non-G-tube group; P = 0.10). Median growth velocity of survivors without G-tubes was 20 g/day (18–28) at median age of follow up of 8 months (4–10) compared to 14 g/day (11–18) in survivors with G-tubes at median age of follow up of 11 months (9–13). On adjusted analysis, the factors that were included in the final model to predict growth velocity were ECMO and G-tube placement. Infants without G-tubes had 6.5 g/day (95% CI: 2.5–10.4) more weight gain than infants with G-tubes (Table 3).
4. Discussion
Our cohort of 123 infants represents one of the largest studies to date from a single institution analyzing infants with CDH and their nutritional outcomes. Of the 85 surviving infants, we found that 25 (29%) had a G-tube placed. We chose to examine predictors of G-tube placement that were indicative of either 1) the infant's medical complexity, which may lead to increased metabolic load and successive requirement for G-tube feedings, or 2) interruption or impairment of oral feeding because of neurologic injury or gastrointestinal pathophysiology.
Surprisingly, the incidence of congenital heart disease was not significantly different between infants with and without G-tubes. This finding may have been due to several reasons such as a broad definition of congenital heart disease or a low number of infants with congenital heart defects in our sample population (n = 37, with 24 survivors). In our cohort, we found that predictors of G-tube placement included prenatal diagnosis, ECMO, and PPI use. A prenatal diagnosis is suggestive of a larger size of defect as it is detected during the second trimester anatomy ultrasound. The larger defects found earlier in pregnancy are likely to lead to more difficult to manage lung pathology such as pulmonary dysmaturity, lung hypoplasia, and persistent pulmonary hypertension compared to infants with lesions that do not appear this early in pregnancy [3,7]. In a multi-center analysis of infants with CDH in Western Australia, a larger percentage of infants who were prenatally diagnosed compared with postnatally diagnosed infants were born at lower birth weights and earlier gestational ages [8]. Regardless of timing of diagnosis, CDH has a high incidence of pulmonary comorbidities and interventions. These included ECMO use, mechanical ventilation, and treatment for pulmonary arterial hypertension, which have been shown in other studies of various neonatal intensive care unit populations to be associated with likelihood of G-tube placement [9,10].
While our study shows that GERD, as indicated by PPI use, is associated with G-tube placement, our cohort of infants with CDH did not show improved growth with this intervention. On the contrary, G-tube placement and ECMO exposure were negative predictors of growth. Our findings are in agreement with a cohort study of 116 infants with CDH, which found that feeding tube placement was not associated with improved growth outcomes in childhood. The authors of this study attributed this finding to the increased metabolic load, based on REE, of the patients with feeding tube placement, which leads to their subsequent increased caloric needs. They found that the measured REE was actually greater, ranging from 83% to 137%, than the predicted value which is estimated by formulas based on metabolically active tissue, which is reduced in patients with failure to thrive. They postulate that contributing factors leading to the increased REE in this population include increased respiratory effort due to inflammation and increased work of breathing, but analysis of pulmonary function tests (PFTs) were not conducted in this study to be able to validate this hypothesis [11]. Our findings of G-tube placement and ECMO as negative growth predictors are in line with this previous study's findings because both are invasive interventions which identify infants who have a greater degree of disease (and likely chronic respiratory impairments), which may lend to increased REE.
While G-tube placement was not associated with improved growth in our cohort, in another small cohort study, infants with CDH or CDH with omphalocele (n = 24) had improved weight gain after G-tube placement [12]. This different finding could be accredited to several reasons: the study had a small sample size, greater variability in the follow-up weight, ranging from 52 days to 56 months, and the diagnosis of omphalocele in the CDH patients could be a confounding variable that had an impact on the degree of weight gain with G-tube placement.
G-tubes have been shown to be advantageous in other medical conditions-ultra-short gut, Pierre Robin, and cystic fibrosis that predispose to poor growth and nutrition. In patients with ultra-short gut, G-tube placement allows for the introduction of feeds to increase bowel adaptation and growth [13]. Infants and children with Pierre Robin sequence cannot adequately feed by mouth due to their mandibular anatomy. In a study of 37 children with Pierre Robin sequence, 32% had a weight increase after G-tube placement while another 43% maintained their weight [14]. A cohort of pediatric cystic fibrosis patients showed improved weight gain one and two years after G-tube placement, especially in those individuals with G-tube feedings for >6 months and mild CF. In comparison, those patients with CF without G-tubes actually lost mean weight at those two time points [15]. The increased metabolic load in infants with CDH may be even more pronounced than other conditions due to the embryological defect that leads to pulmonary hypoplasia and pulmonary hypertension. Because of this, studies like ours are informative to help develop management guidelines to address the nutritional morbidities that this population faces.
Because our center's neonatal intensive care unit, like many other centers, includes speech and occupational therapists in the care team for infants with complex problems and clinical courses due to their skill for assessing coordination of swallowing and detecting oral hypersensitivity, we noted their involvement in the care for our CDH infants [16]. Of those who had a speech consult, 40% received a G-tube, and of those who had an OT consult, 38% received a G-tube. These findings stress the importance of incorporating other disciplines in the care of this population.
One of the greatest strengths of our study is the large available cohort of infants with CDH cared for at our single center. Having a large population at one center had the advantages of reduced variability in providers who made decisions on nutritional management and surgeons involved with the diaphragmatic repair and G-tube placement. The results from the study have the potential to assist clinicians in predicting earlier which infants may be at risk for feeding difficulties and may ultimately have a greater need for G-tube placement. At our center, we have developed an approach inclusive of introduction of feeding therapists early in the hospital course, and have included interventions such as early antacid and diuretic use that we believe may reduce comorbidities like GERD and CLD in our CDH management guidelines.
Our study was limited by its retrospective nature. Infants were not followed up at a uniform duration of time and differences in growth outcomes may have been influenced by length of time between discharge and follow-up visits when post-G-tube weights were obtained. While there was a difference in the day of life that G-tube placement occurred compared to the day of life that infants without G-tube placement were discharged, the total number of days evaluated for growth were comparable between the groups. Data regarding daily caloric intake or type of feedings after discharge were not available. Additionally, while changes in practice, including the management guidelines established in 2002 [4], may have impacted outcome across the time span studied, we did not observe a change in the proportion of survivors with G-tube placement. This finding is consistent with the fact that these management guidelines provide target goals for oxygen saturation, ventilatory parameters, and laboratory values, but do not specifically address feeding strategies. We were also unable to evaluate neurodevelopmental outcomes, since most infants did not have formal neurodevelopmental testing. Thus, we were unable to explore a possible association between G-tube placement and neurodevelopment impairment. Additionally, it is likely that infants who underwent G-tube placement were at a higher risk for poor growth as they were identified early in life due to their comorbidities, most evident being CLD. We did not evaluate whether infants underwent G-tube placement after the time of the last documented follow-up visit, nor do we know if families and physicians were able to remove G-tubes after the last documented follow-up visit. As a result of these limitations, while our data indicates that those infants who did not have G-tube placement showed better growth, whether this difference is clinically significant cannot be inferred. Finally, although our center follows a general feeding advancement protocol, there is no feeding protocol specific to infants with CDH. Thus, there may have been provider variability in the choice of type of formula, additives, and feeding advancements.
In summary, infants with CDH have significant long term nutritional morbidities, including decreased growth potential. While G-tube placement may be the clinically appropriate intervention for the comorbidities present in this patient cohort, further studies need to be conducted to develop and then test the effectiveness of management guidelines for optimizing feeding and growth outcomes in this population.
Acknowledgments
Dr. Greenberg received support from NIH training grants (5T32HD043728-10 and 5T32HD043029-13). Dr. Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01). Dr. Cotten received support from the NIH (5U10 HD040492-10).
Abbreviations
- CDH
congenital diaphragmatic hernia
- CLD
chronic lung disease
- ECMO
extracorporeal membrane oxygenation
- GERD
gastroesophageal reflux disease
- G-tube
gastrostomy tube
- ICN
intensive care nursery
- OT
occupational therapy
- PFT
pulmonary function tests
- PPI
proton pump inhibitor
- REE
resting energy expenditure.
Footnotes
Conflicts of interest
None declared.
Contributor Information
Sharmistha Rudra, Email: rudras@email.chop.edu.
Obinna O. Adibe, Email: obinna.adibe@dm.duke.edu.
William F. Malcolm, Email: william.malcolm@duke.edu.
P. Brian Smith, Email: brian.smith@duke.edu.
C. Michael Cotten, Email: michael.cotten@duke.edu.
Rachel G. Greenberg, Email: rachel.greenberg@duke.edu.
References
- 1.van den Hout L, Sluiter I, Gischler S, De Klein A, Rottier R, Ijsselstijn H, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr. Surg. Int. 2009;25:733–743. doi: 10.1007/s00383-009-2425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawahara H, Okuyama H, Nose K, Nakai H, Yoneda A, Kubota A, et al. Physiological and clinical characteristics of gastroesophageal reflux after congenital diaphragmatic hernia repair. J. Pediatr. Surg. 2010;45:2346–2350. doi: 10.1016/j.jpedsurg.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Malowitz JR, Hornik CP, Laughon MM, Testoni D, Cotten CM, Clark RH, et al. Management practice and mortality for infants with congenital diaphragmatic hernia. Am. J. Perinatol. 2015;32:887–894. doi: 10.1055/s-0035-1544949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracy ET, Mears SE, Smith PB, Danko ME, Diesen DL, Fisher KA, et al. Protocolized approach to the management of congenital diaphragmatic hernia: benefits of reducing variability in care. J. Pediatr. Surg. 2010;45:1343–1348. doi: 10.1016/j.jpedsurg.2010.02.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaillard SM, Pierrat V, Dubois A, Truffert P, Lequien P, Wurtz AJ, et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann. Thorac. Surg. 2003;75:250–256. doi: 10.1016/s0003-4975(02)04278-9. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Child growth standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 7.Mesas Burgos C, Hammarqvist-Vejde J, Frenckner B, Conner P. Differences in outcomes in prenatally diagnosed congenital diaphragmatic hernia compared to postnatal detection: a single-center experience. Fetal Diagn. Ther. 2015 doi: 10.1159/000439303. [DOI] [PubMed] [Google Scholar]
- 8.Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–e363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- 9.Leeuwen L, Walker K, Halliday R, Karpelowsky J, Fitzgerald DA. Growth in children with congenital diaphragmatic hernia during the first year of life. J. Pediatr. Surg. 2014;49:1363–1366. doi: 10.1016/j.jpedsurg.2014.02.081. [DOI] [PubMed] [Google Scholar]
- 10.Pierog A, Aspelund G, Farkouh-Karoleski C, Wu M, Kriger J, Wynn J, et al. Predictors of low weight and tube feedings in children with congenital diaphragmatic hernia at 1 year of age. J. Pediatr. Gastroenterol. Nutr. 2014;59:527–530. doi: 10.1097/MPG.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 11.Haliburton B, Mouzaki M, Chiang M, Scaini V, Marcon M, Moraes TJ, et al. Long-term nutritional morbidity for congenital diaphragmatic hernia survivors: failure to thrive extends well into childhood and adolescence. J. Pediatr. Surg. 2015;50:734–738. doi: 10.1016/j.jpedsurg.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg J, Amaral JG, Sklar CM, Connolly BL, Temple MJ, John P, et al. Gastrostomy and gastrojejunostomy tube placements: outcomes in children with gastroschisis, omphalocele, and congenital diaphragmatic hernia. Radiology. 2008;248:247–253. doi: 10.1148/radiol.2481061193. [DOI] [PubMed] [Google Scholar]
- 13.Wales PW, Jancelewicz T, Romao RL, Piper HG, de Silva NT, Avitzur Y. Delayed primary serial transverse enteroplasty as a novel management strategy for infants with congenital ultra-short bowel syndrome. J. Pediatr. Surg. 2013;48:993–999. doi: 10.1016/j.jpedsurg.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Al-Attar H, Shergill AK, Brown NE, Guernsey C, Fisher D, Temple M, et al. Percutaneous gastrostomy tubes in children with Pierre Robin sequence: efficacy, maintenance and complications. Pediatr. Radiol. 2012;42:566–573. doi: 10.1007/s00247-011-2301-2. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh SI, Ryan-Wenger NA, McCoy KS. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr. Pulmonol. 2013;48:556–562. doi: 10.1002/ppul.22630. [DOI] [PubMed] [Google Scholar]
- 16.Guimber D, Michaud L, Storme L, Deschildre A, Turck D, Gottrand F. Gastrostomy in infants with neonatal pulmonary disease. J. Pediatr. Gastroenterol. Nutr. 2003;36:459–463. doi: 10.1097/00005176-200304000-00007. [DOI] [PubMed] [Google Scholar]