As an emerging subdiscipline of pharmacology, one of the primary goals of systems pharmacology is to elucidate therapeutic mechanisms of combined pharmacological strategies used to treat diverse complex diseases in which multiple integrated cellular pathways are perturbed.
Mechanistic insights into disease pathogenesis are critical for optimal therapeutic development. With the exception of rare mono- or oligogenic disorders caused by clearly defined mutation(s), most common disorders such as neurological and heart diseases are caused by a combination of genetic, environmental, and unidentified factors making them highly complex in nature [1]. In addition to the multifactorial etiology that remains to be illuminated for most complex disorders, the molecular changes associated with a given disease state are increasingly recognized to be dynamic and multi-faceted. Crosstalk between multiple signaling and metabolic pathways is often initiated, and network changes occur as the disease progresses. In addition to perturbations in intracellular processes, changes in cell-cell interaction networks, which are extremely complicated even under normal physiological conditions, add another layer of complexity to delineating the etiology of a disease and ascertaining the most effective therapeutic approach [2]. Such intricacies generally necessitate multi-faceted treatment strategies. Drug combinations that therapeutically modulate different mechanistic nodes within a network may enable better outcomes than single drug treatments [3].
Developing a novel drug is notoriously time-consuming and requires enormous financial investment as well as large-scale research efforts. Thus, reducing the time and cost as well as improving success rates are of strategic importance in drug development. One practical avenue to achieve this goal is drug repurposing; a process which identifies novel applications for approved pharmacological agents.
Advances in “omics” have demonstrated that diseases often are better described not by symptoms or a single endpoint, but by the molecular environment of the whole cell, tissue or organism. Systems pharmacology identifies therapies or combinations of therapies that best normalize a perturbed cellular or tissue milieu (with minimal side effects) based on the breadth of molecular abnormalities associated with a particular disease. Thus, systems pharmacology could facilitate the development of new therapies based on existing drugs by seamlessly incorporating mechanistic findings of a given disease with known pharmacological profiles of drugs.
G protein-coupled receptors (GPCRs) have been identified as potential targets for treating retinal degenerative diseases [4,5]. Chen et al. recently reported the successful application of systems pharmacology involving the combination use of GPCR-modulating, FDA-approved drugs in protection of retinal photoreceptor cells against bright light-induced cell degeneration, a hallmark pathology leading to vision impairment or blindness in various retinal degenerative disorders [6]. This combination treatment consisted of bromocriptine, which is traditionally classified as a Gi-coupled D2 dopamine receptor agonist, the Gs-coupled β1-adrenergic receptor antagonist metoprolol and the Gq-coupled α1-adrenergic receptor selective antagonists tamsulosin or doxazosin. When administered individually, each of the drugs was effective at protecting the retinal photoreceptors against bright light-induced degeneration, but did not preserve the normal retinal transcriptome. Remarkably, when these drugs were administered in combination, each agent could be used at a dose that would be sub-therapeutic if given alone thereby reducing the possibility of dose-related adverse drug reactions. Functional assays demonstrated that such combined treatment resulted in stimulation of dopamine receptors D2R- and D4R-mediated Gi signaling as well as inhibition of D1R-mediated Gs signaling and α1A-adrenergic receptor-mediated Gq signaling. Transcriptome analyses further revealed superior preservation of normal retinal gene expression profiles by combined treatment compared to those observed after single drug treatment. Bromocriptine is mainly used in the treatment of hyperprolactinemia and acromegaly and was recently approved for adjunct treatment of type 2 diabetes. Metoprolol is a mainstay treatment for hypertension, angina and heart failure. Tamsulosin and doxazosin are both used to treat benign prostatic hyperplasia with the latter also approved as an antihypertensive agent.
Taken together, these findings support the concept that systems pharmacology can facilitate discovery of mechanisms of action that integrate multiple cellular signaling pathways. This approach, and future possible treatments, to chronic human illnesses could be expedited by the repurposing the drugs already in clinical use and targeted focusing on systems in which effective pharmacological modulation has not yet been achieved to enable the future development of combined treatment strategies for optimal management of complex human disorders (Fig. 1).
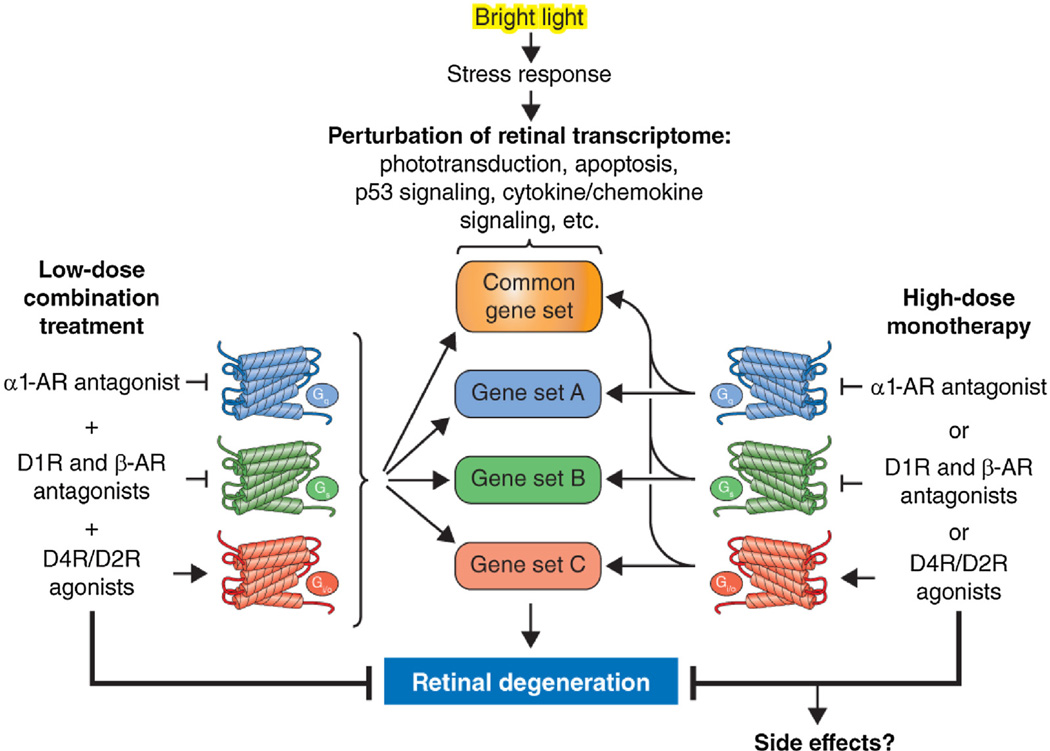
Fig. 1.
GPCR systems pharmacology protects against light-induced retinal degeneration.
Exposure of mice to bright light disrupts retinal transcription manifested as dysregulation of multiple gene sets, including but not limited to downregulation of the phototransduction pathway and upregulation of the apoptosis pathway, p53 signaling, cytokine-cytokine receptor interactions and chemokine signaling. Such disruption typically leads to retinal degeneration. Pretreatment with single drugs that act as antagonists at Gs-coupled GPCRs, agonists at Gi-coupled GPCRs or antagonists at Gq-coupled GPCRs at relatively high doses results in partial restoration of the transcriptional landscape and protection from phototoxicity, albeit with the potential for side effects. Administration of these drugs in combination at doses that would be sub-effective if given individually results in superior normalization of the retinal transcriptome and synergistic protection against light damage with a lower risk of adverse effects. This figure was adapted from Ref. [6] under the AAAS License to Publish Agreement.
Acknowledgments
This work was supported by funding from the National Institutes of HealthEY021126 and U01 EY025451 (KP) and R24 EY024864 (TSK), the National Natural Science Foundation of China81473732 (YC), and the Department of Veterans AffairsIK2BX002683 (PDK). The work also was supported by the Arnold and Mabel Beckman Foundation and the Canadian Institute for Advanced Research (CIFAR). K.P. is the John H. Hord Professor of Pharmacology.
Footnotes
Disclosure statements
K.P. is an inventor of US Patent No. 8722669 – “Compounds and Methods: of Treating Ocular Disorders” and US Patent No. 20080275134 – “Methods: for Treatment: of Retinal Degenerative Disease” issued to Case Western Reserve University (CWRU) whose values may be affected by this publication. CWRU may license this technology for commercial development. KP is a member of the scientific board of Polgenix, Inc. involved in developing systems pharmacology whose values may be affected by this publication.
References
- 1.Hunter DJ. Gene-environment interactions in human diseases. Nat. Rev. Genet. 2005;6(4):287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Palczewski K. Systems pharmacology links GPCRs with retinal degenerative disorders. Annu. Rev. Pharmacol. Toxicol. 2016;56:273–298. doi: 10.1146/annurev-pharmtox-010715-103033. http://dx.doi.org/10.1146/annurev-pharmtox-010715-103033, Epub 2015 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar R. Complex diseases require complex therapies. EMBO Rep. 2013;14(12):1039–1042. doi: 10.1038/embor.2013.177. http://dx.doi.org/10.1038/embor.2013.177, Epub 2013 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Okano K, Maeda T, Chauhan V, Golczak M, Maeda A, Palczewski K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012;287(7):5059–5069. doi: 10.1074/jbc.M111.315432. http://dx.doi.org/10.1074/jbc.M111.315432, Epub 2011 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Palczewska G, Mustafi D, Golczak M, Dong Z, Sawada O, Maeda T, Maeda A, Palczewski K. Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Invest. 2013;123(12):5119–5134. doi: 10.1172/JCI69076. http://dx.doi.org/10.1172/JCI69076, Epub 2013 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Palczewska G, Masuho I, Gao S, Jin H, Dong Z, Gieser L, Brooks MJ, Kiser PD, Kern TS, Martemyanov KA, Swaroop A, Palczewski K. Synergistically acting agonists and antagonists of G protein-coupled receptors prevent photoreceptor cell degeneration. Sci. Signal. 2016;9(438):ra74. doi: 10.1126/scisignal.aag0245. http://dx.doi.org/10.1126/scisignal.aag0245. [DOI] [PMC free article] [PubMed] [Google Scholar]