Abstract
The importance of patient satisfaction in modern healthcare is widely recognized, but research on satisfaction in the context of smoking cessation has not kept pace. The purpose of this study was to explore treatment satisfaction in a sample of smokers (N = 84) randomized to one of two smoking cessation treatment interventions (mHealth Reinforcement and mHealth Monitoring) that used cell phone-based procedures to monitor smoking status in individuals’ natural environments for 4 weeks. Starting on the target quit date, participants received usual care smoking cessation treatment consisting of 8 weeks of transdermal nicotine and 4 weeks of twice-weekly telephone counseling, and were also prompted 1 to 3 times daily (with exact number and timing not disclosed beforehand) to use a study cell phone and CO monitor to complete a CO self-test, video-record the process, and submit videos using multimedia messaging within 2 hours. mHealth Reinforcement participants could earn prizes for smoking-negative on-time CO tests. A treatment satisfaction survey was completed at the end of the 4-week monitoring/reinforcement phase. Results indicate that participants overwhelming endorsed high levels of overall satisfaction in both conditions. Treatment adherence did not differ between conditions, but was positively associated with endorsing the highest satisfaction with help quitting with the intervention (p < .01 to .03). mHealth Reinforcement was associated with increased longest duration of abstinence (p < .01). Controlling for relevant participant characteristics and treatment adherence, longest duration of abstinence robustly predicted highest satisfaction with help quitting and mediated the effect of treatment condition on that satisfaction. Further research on treatment satisfaction may aid the development of effective abstinence reinforcement and other smoking cessation interventions.
Keywords: contingency management, behavioral treatment, nicotine replacement therapy, tobacco, text-messaging, multi-media messaging
1. Introduction
Satisfaction can be defined as an individual’s experience compared with his or her expectations (Pascoe, 1983). The assessment of patient (or, consumer) satisfaction is now ubiquitous in health services sectors, and is consistent with patient-centered care, coordination of care, and other shared decision-making models of modern healthcare (The Institute of Medicine, 2001). Slower to occur has been the evaluation of patient satisfaction in randomized controlled trials (Kelley, Kraft-Todd, Schapira, Kossowsky, & Riess, 2014), especially in the field of smoking cessation or substance abuse treatment in general (Carroll & Rounsaville, 2003). Observational studies generally support a positive relation between patient satisfaction and substance use outcomes (Boden & Moos, 2009; Carlson & Gabriel, 2001; Crosier, Scott, & Steinfeld, 2012; Hawkins, Baer, & Kivlahan, 2008; Hser, Evans, Huang, & Anglin, 2004; Sanford, Donahue, & Cosden, 2014, c.f., McLellan & Hunkeler, 1998). For example, in a large national panel survey involving patients at 62 methadone, outpatient, and residential programs in the U.S., positive treatment satisfaction near discharge predicted improved drug use outcomes at 1-year, controlling for baseline patient characteristics, treatment duration, counseling intensity, and treatment adherence (Zhang, Gerstein, & Friedmann, 2008). A positive association between treatment satisfaction and long-term outcomes is also evident in other domains, such as treatment for comorbid psychiatric and substance use disorder treatment (Boden & Moos, 2009).
Feedback from patients has also been used to inform modifications to services that then lead to improved patient satisfaction (Crosier et al., 2012), with implications for improved treatment engagement and outcomes going forward. In the smoking cessation research literature, this is particularly evident in efforts to develop and evaluate technology-based interventions, including telemedicine (Richter et al., 2015), web-based (Shahab & McEwen, 2009) and text messaging-based (Kong, Ells, Camenga, & Krishnan-Sarin, 2014) programs. This is a relatively nascent but fast growing, area of research that often involves assessing the acceptability of interventions that differ in the frequency or intensity of technology-related aspects of treatment. For example, abstinence reinforcement (Contingency Management) is an efficacious behavioral treatment for reducing substance (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006), and recent work has examined technology-based methods to deliver this intervention remotely (i.e., in individuals’ natural environment, without intensive in-person demands). Reinforcement interventions use tangible incentives like vouchers for goods or services, or “prizes” like gift cards, to systematically reinforce objective evidence of abstinence. Dallery and colleagues have examined the acceptability of delivering reinforcement and other smoking treatment components via web-based procedures (Meredith & Dallery, 2013; Meredith, Grabinski, & Dallery, 2011; Raiff, Jarvis, Turturici, & Dallery, 2013; Reynolds et al., 2015). For example, in the first of a two-trial study (Raiff et al., 2013), smokers were randomized to a 7-week reinforcement condition with incentives of smoking-negative breath tests or a control condition without abstinence reinforcement. In both groups, participants conducted CO self-tests and submitted results via web cam, and received web-based counseling and feedback on CO results. In a second trial, participants were randomized to view a video description of the web-based reinforcement intervention or one that required users to deposit their own money and earn it back by testing smoking-negative on breath tests (as a potential means to off-set the cost of incentives). Overall, participants rated the internet-based treatment intervention acceptable.
We recently completed a randomized controlled trial of a novel mHealth abstinence reinforcement intervention for treatment-seeking smokers (Alessi et al., submitted for publication). The World Health Organization defines mHealth as, for example, a component of eHealth involving the provision of health services and information via mobile technologies such as mobile phones, tablet computers and personal digital assistants (World Health Organization, 2015). To our knowledge, no studies have examined patient satisfaction in the context of mHealth-based abstinence reinforcement for smoking cessation. Information about participant experience is particularly valuable in the context of technology-based applications because of the rapid pace of change, and the related ability to potentially pivot based on feedback if needed. In addition, although our mHealth procedures reduce patient burden related to attending in-person appointments and provide services ecologically and with minimized delay, it also comes with higher demands in terms of patient-directed breath testing. Thus, assessment of acceptability is important. The purpose of the current study was to use data from our recent mHealth reinforcement trial to examine patient satisfaction across a number of areas, and to examine predictors and mediators of global patient satisfaction. Specifically, we expected high levels of satisfaction with treatment overall, and higher satisfaction among those in the mHealth Reinforcement condition. We also hypothesized that (1) treatment condition would predict treatment satisfaction, and that (2) adherence and (3) longest duration of abstinence (a primary outcome) may at least partially mediate the relation between treatment condition and satisfaction. We did not have specific hypotheses about relations between participant characteristics and treatment satisfaction.
2. Materials and Methods
2.1. Participants and Setting
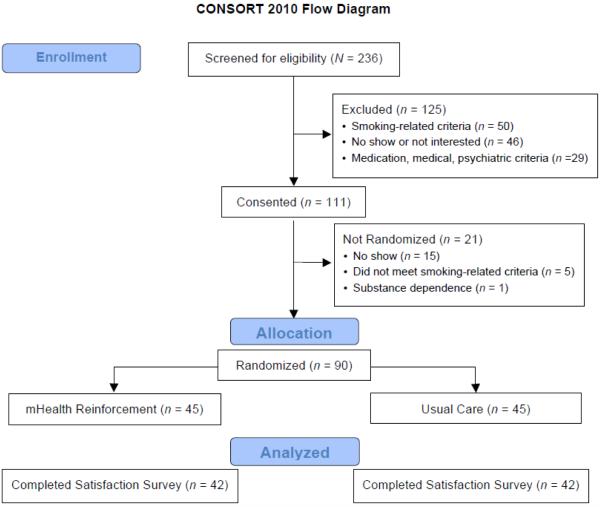
Data from participants in our mHealth abstinence reinforcement trial who completed a treatment satisfaction survey were examined for the purpose of this study (N = 84, 93.3% of the total sample). Participants were adults at least 18 years of age who (1) smoked a minimum of 10 cigarettes daily verified by a breath carbon monoxide (CO) test reading ≥ 8 parts per million (ppm), (2) had no past-year abstinence exceeding 3 months, (3) intended to quit within 3 weeks, and (4) had a valid photo I.D. and mailing address (for the purpose of loaning out study equipment). Individuals were excluded for (1) past month smoking cessation treatment, (2) serious and unstable psychiatric illness (e.g., schizophrenia, non-nicotine substance use disorder) or medical disease, (3) medication or other contraindications for transdermal nicotine, and (4) use of monoamine oxidase inhibitors, antipsychotics, mood stabilizers, bupropion, or naltrexone. Procedures were provided in English. A Masters-level licensed research therapist for telephone counseling sessions and a Baccalaureate-level research assistant completed remaining assessments. The in-person study visits (intake, follow-up) occurred at a university health center between January 2012 and December 2014. Participants provided written informed consent, and the Institutional Review Board approved study procedures. See Figure 1 for participant flow.
Figure 1.
Participant flow through the study.
2.2. Assessments
At intake, a patient form captured demographic and eligibility-related information. The Fagerström Test of Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and the Readiness to Change Questionnaire (Rollnick, Heather, Gold, & Hall, 1992) were completed. At intake, baseline, counseling sessions and the week 4 (end of behavioral treatment) interview, the Timeline Follow-back procedure (Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000; Sobell & Sobell, 1992) captured frequency and intensity of smoking in the past 30 days (intake) or since the last visit (remaining sessions). CO tests were conducted using a Micro Plus Smokerlyzer (Bedfont Scientific Ltd., Kent, England). Treatment satisfaction was assessed at the end of the mHealth phase (week 4) with a 5-item in-house form, to briefly evaluate satisfaction with the intervention overall, satisfaction with help quitting with the intervention, and satisfaction with specific aspects of the mHealth procedures.
2.3. Procedures
Procedures directly related to the current study are presented here and otherwise outlined; see the main study report for details (Alessi et al., submitted for publication). Briefly, starting on the target quit date, twice-weekly supportive telephone counseling was provided for 4 weeks, and a standard regimen of transdermal nicotine was provided for 8 weeks. Also on the quite date, participants were randomized to one of two treatment conditions: mHealth Reinforcement or mHealth Monitoring. All participants were instructed that an interactive voice response (IVR) system would send prompts to conduct CO self-tests up to 3 times daily between 7 a.m. and 10 p.m. for the next 4 weeks, with the exact number and timing not disclosed. When prompted, participants used the video-record function on their study cell phone (with a front-facing lens) to record the CO self-test process, and sent the date and time-stamped video to research staff using multimedia messaging. Participants also reported the CO results and number of cigarettes smoked using the IVR. Video test results were compared against IVR reports to confirm accuracy (confirmed in all but 2 instances). mHealth Reinforcement participants also earned chances for prizes contingent on on-time and smoking-negative breath tests (CO ≤ 6 parts per million (ppm)). Earnings were determined immediately via computer algorithm during IVR calls, and were available for redemption after IVR reports were confirmed against video clips. The mean (SD) amount earned for smoking-negative tests was $349.66 (184.12) out of an expected $502 maximum. Participants were compensated $25 for the intake interview, $35 for that at Week 4 and $50 for returning study equipment in good condition (100%). To promote adherence, all participants also received $1 per CO test and a $10 bonus each week for submitting all tests.
2.4. Data Analysis
Demographic and baseline data were examined for differences between treatment conditions, as well as for differences between those who did and did not endorse the highest satisfaction with help quitting with the intervention, using chi-square, Mann-Whitney U, and analysis of variance (ANOVA) depending on the underlying distribution. Response patterns on all treatment satisfaction survey items were examined visually, and differences between treatment conditions tested using Mann-Whitney U and chi-square as needed. Demographic and baseline variables that differed between conditions p ≤ .20 or were correlated with condition r = 0.20 or higher were included as predictors in subsequent regression analyses predicting treatment satisfaction (i.e., highest grade completed, lives with a smoker, longest quit attempts in months, number of past quit attempts lasting at least 24 hours). Variables that similarly differed between or were correlated with endorsing the highest satisfaction with help quitting were also included as predictors in regression analyses (i.e., intake CO, Readiness to Change score, past 30-day cigarettes per day, prior use of nicotine replacement therapy, Readiness to Change score), as were variables that were theoretically or empirically indicated (age, gender, nicotine dependence severity (FTND score). Ethnicity and submitting at least 1 smoking-negative CO during baseline (pre-quit date) met these covariate criteria but were excluded because the data were extremely skewed.
Longest duration of prolonged abstinence (LDA; measured in days) was examined as a potential mediator of the significant relation between study condition and highest satisfaction with help quitting with the intervention. LDA was defined as the greatest number of consecutive days of no smoking on self-report and all CO tests reading negative. Differences between treatment conditions were examined with ANOVA (Step 2 of mediation test, below). Mediation tests of the two remaining primary smoking outcomes (percentage of smoking-negative CO tests and objectively-verified point-prevalence abstinence) showed similar patterns (not presented; available from first author).
We used a 4-step process to test for mediation effects (Baron & Kenny, 1986; Kenney, 2013; Kenny, 2015). Mediation test Step 1 tests for an association between the independent variable (treatment condition) and outcome (highest satisfaction with help quitting), and was examined in logistic regression model 1, along with demographic and baseline characteristics that were significantly correlated with highest satisfaction and therefore were other potential predictors. Logistic regression model 2 added effects of two measures of treatment adherence (the number of days of transdermal nicotine use (not limited to the 4-week monitoring/reinforcement period) and the percentage of counseling sessions completed. Mediation test Step 2 assesses for an association between the treatment condition and presumed mediator (LDA). Mediation test Step 3 evaluates if increases in the presumed mediator accounts for differential changes in outcome (highest satisfaction with help quitting), and was examined in logistic regression model 3. Also in regression model 3 was assessment of mediation test Step 4, which establishes whether differences between conditions on highest satisfaction remain significant after LDA is added to the analysis. Finding that the relation between treatment condition and highest satisfaction is no longer significant would fulfill the criteria for full mediation (steps 1-4 are met); if effects of study condition on highest satisfaction remain significant but the absolute size of the relation is reduced, the criteria for partial mediation are met (steps 1-3, or at least 2-3, are met). Statistical significance was evaluated using two-tailed tests at alpha < .05. Analyses were conducted with IBM® SPSS® Statistics version 21.
3. Results
3.1. Demographic and Baseline Participant Characteristics
Participant characteristics by treatment condition (mHealth Reinforcement versus mHealth Monitoring) and by highest satisfaction with help quitting with the intervention (yes versus no) are presented in Table 1. mHealth Reinforcement participants were more likely to live with another smoker, p = .05. Mean cigarettes per day and intake CO were lower in participants who endorsed the highest satisfaction. No other differences were significant.
Table 1.
Participant Characteristics, Treatment Adherence, and Smoking Outcomes
| Variables | mHealth Reinforcement (n = 42) |
Usual Care (n = 42) |
Highest Satisfaction (n = 60) |
Not Highest Satisfaction (n = 24) |
|---|---|---|---|---|
| Age | 44.7 (10.3) | 45.2 (11.6) | 44.2 (10.7) | 47.0 (11.3) |
| Female (n) | 52.4% (22) | 60.0% (27) | 55.0% (33) | 66.7% (16) |
| Ethnicity (n) | ||||
| Not Hispanic | 92.9% (39) | 92.2% (39) | 91.7% (55) | 95.8% (23) |
| Hispanic | 2.4% (1) | 7.1% (3) | 6.7% (4) | 0% (0) |
| Not reported | 4.8% (2) | 0.0% (0) | 1.7% (1) | 4.2% (1) |
| Race (n) | ||||
| European American | 73.8% (31) | 81.0% (34) | 75% (45) | 83.3% (20) |
| African American | 14.3% (6) | 9.5% (4) | 13.3% (8) | 8.3% (2) |
| Asian/More than one/not reported | 11.9% (5) | 11.9% (5) | 11.7% (7) | 8.3% (2) |
| Highest grade (n) | ||||
| Grade 12, GED, or less | 66.7% (28) | 47.6% (20)† | 46.7% (28) | 33.3% (8) |
| 1-3 years college or more | 33.3% (14) | 52.4% (22) | 53.3% (32) | 66.7% (16) |
| Cigarette smoking characteristics | ||||
| Age first smoked | 16.7 (3.9) | 16.8 (4.3) | 17.0 (4.2) | 15.9 (3.3) |
| Past 30 days cigarettes per day | 17.7 (9.7) | 19.8 (8.0) | 17.4 (7.8) | 22.0 (10.6)* |
| Fagerström score | 4.0 (1.7) | 3.9 (1.6) | 4.0 (1.7) | 3.9 (1.7) |
| 1st cigarette within 5 minutes of waking (n) | 37.8% (16) | 35.6% (15) | 33.3% (20) | 45.8% (11) |
| Intake CO value | 16.0 (8.3) | 16.5 (7.3) | 15.1 (6.6) | 18.9 (9.8)† |
| Lives with smoker(s) (n) | 54.8% (23) | 33.3% (14)† | 43.4% (26) | 45.8% (11) |
| At least 1 baseline CO ≤ 6 ppm (n) | 7.1% (3) | 4.8% (2) | 8.3% (5) | 0.0% (0) |
| Prior quit history | ||||
| # ≥ 24-hour voluntary quit attempts | 7.2 (12.0) | 7.2 (21.1) | 7.0 (16.0) | 7.6 (20.2) |
| Longest quit attempt (months)a | 3.6 (8.8) | 1.6 (9.0) | 2.0 (8.5) | 2.3 (9.6) |
| Previous use of nicotine replacement (n) | 47.6% (20) | 54.8% (23) | 46.7% (28) | 62.5% (15) |
| Previous use non-nicotine medication (n) | 26.2% (11) | 33.3% (14) | 30.0% (18) | 29.2% (7) |
| Readiness to change | 6.0 (1.8) | 6.2 (1.9) | 5.8 (1.8) | 6.7 (5.8)† |
| Self-efficacy | 47.6 (7.3) | 46.1 (8.1) | 47.0 (7.9) | 46.6 (7.4) |
| Relapse Preparedness (WIP) | 13.8 (4.4) | 14.6 (4.2) | 14.0 (4.3) | 14.6 (3.7) |
| Craving – Negative expectancies (F1) | 5.1 (1.3) | 5.3 (1.0) | 5.2 (1.1) | 5.2 (1.1) |
| Craving – Positive expectancies (F2) | 3.2 (1.3) | 2.9 (1.3) | 3.1 (1.3) | 2.8 (1.2) |
| Treatment Adherence | ||||
| Days of transdermal nicotine use | 34.5 (13.1) | 30.8 (15.5) | 35.4 (12.6) | 25.8 (16.4)** |
| Percent of counseling sessions completed | 82.7% (30.2%) | 72.6% (32.2%) | 82.3% (27.6%) | 66.1% (33.7%)* |
| Longest duration of abstinence (days) a | 27.5 (10.3) | 17.0 (25.3)** | 28.0 (10.8) | 7.0 (21.8)** |
Note: Values are mean (SD) unless otherwise noted.
Median (interquartile range).
p < .10;
p < .05;
p < .01; all other relations, p ≥ .13.
3.2. Treatment Condition and Treatment Satisfaction
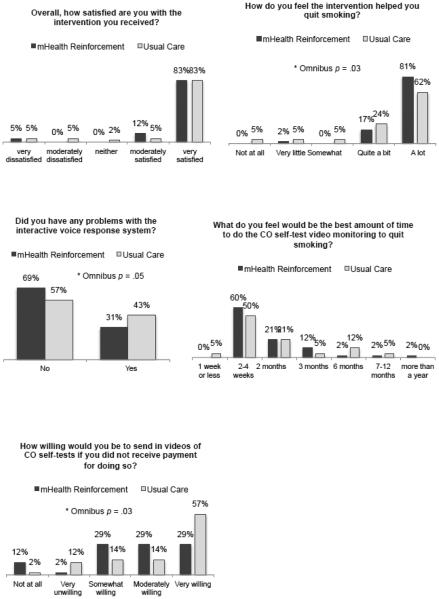
The percentage of participants who endorsed each response option on each treatment satisfaction survey item is depicted in Figure 2. The mHealth Reinforcement and mHealth Monitoring conditions differed significantly on pattern of responding on satisfaction with help quitting with the intervention (Panel 2), U = 698.0, p = 0.03, with the percentage who endorsed the highest level of satisfaction being greater in the mHealth Reinforcement condition compared to mHealth Monitoring, χ2 (d.f. = 1, N = 84) = 3.73, p = 0.05, phi = 0.21. Conditions also differed on willingness to submit CO tests without payment (Panel 5), U = 654.0, p = .03. Remaining differences by treatment condition were not significant, p-values 0.59 to 1.00, including on the overall satisfaction item (Panel 1).
Figure 2.
Treatment satisfaction survey response patterns by treatment condition.
3.3. Treatment Adherence
Differences between conditions were not significant for days of transdermal nicotine use, F (1, 82) = 1.44, p = .23, and percent of counseling sessions completed, F (1, 82) = 2.40, p = .13. Those who endorsed the highest satisfaction with help quitting had more days of transdermal nicotine use on average compared to those who endorsed less satisfaction, F (1, 82) = 8.39, p < .01, and completed a higher percentage of counseling sessions, F (1, 82) = 5.15, p = .03.
3.4. Smoking Behavior
Longest duration of abstinence was increased in mHealth Reinforcement compared to Monitoring, F (1, 82) = 8.42, p < .01, d = 0.63, and in those who endorsed highest satisfaction with help quitting with the intervention, F (1, 82) = 20.76, p < .01, d = 1.00. See Table 1 for descriptive statistics.
3.5. Treatment Satisfaction
Outcomes of the logistic regression analyses of endorsing highest satisfaction with help quitting smoking are presented in Table 2. Model 1 (with treatment condition and identified demographic and baseline variables entered) was significant, χ2 (d.f. = 11) = 26.86, p < .01. Significant predictors were (1) treatment condition, ß (SE) = 1.51 (0.66), Wald (d.f. = 1) = 5.14, p = .02, (2) highest grade completed, ß (SE) = −1.51 (0.72), Wald (d.f. = 1) = 4.37, p = .04, (3) cigarettes per day in the past 30 days at intake, ß (SE) = −0.09 (0.04), Wald (d.f. = 1) = 4.59, p = .03, and (4) intake Readiness to Change score, ß (SE) = −0.39 (0.20), Wald (d.f. = 1) = 3.92, p = .05. Model 2 (with treatment adherence variables added) was significant, χ2 (d.f. = 13) = 32.31, p < .01, with the same predictors significant and nonsignificant as above. Specifically, significant predictors were: (1) treatment condition, ß (SE) = 1.53 (0.71), Wald (d.f. = 1) = 4.68, p = .03, (2) highest grade completed, ß (SE) = −1.54 (0.76), Wald (d.f. = 1) = 4.12, p = .04, (3) cigarettes per day in the past 30 days, ß (SE) = −0.09 (0.04), Wald (d.f. = 1) = 4.36, p = .04, and (4) Readiness to Change score, ß (SE) = −0.43 (0.22), Wald (d.f. = 1) = 3.78, p = .05. Model 3 (with LDA added) was also significant, χ2 (d.f. = 14) = 41.75, p < .01. Significant predictors were (1) highest grade completed, ß (SE) = −2.39 (0.93), Wald (d.f. = 1) = 6.27, p = .01, cigarettes per day in the past 30 days, ß (SE) = −0.09 (0.04), Wald (d.f. = 1) = 4.39, p = .04, and (3) LDA, ß (SE) = 0.11 (0.04), Wald (d.f. = 1) = 7.40, p < .01; the effect of treatment condition was no longer significant.
Table 2.
Regression Analyses Predicting Highest Satisfaction with Help Quitting with the Treatment Intervention (Odds Ratios, 95%CI)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Treatment Condition | 4.51 (1.23, 16.60)* | 4.63 (1.16, 18.58)* | 3.05 (0.68, 13.64) |
| Age | 0.97 (0.92, 1.03) | 0.97 (0.91, 1.03) | 0.95 (0.88, 1.03) |
| Gender | 0.44 (0.11, 1.71) | 0.59 (0.15, 2.42) | 0.93 (0.19, 4.67) |
| Highest grade | 0.22 (0.05, 0.91)* | 0.21 (0.05, 0.95)* | 0.09 (0.01, 0.60)** |
| Longest previous quit attempt (months) | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) |
| Lives with another smoker | 0.47 (0.13, 1.76) | 0.44 (0.11, 1.81) | 0.32 (0.06, 1.70) |
| Fagerström score | 0.96 (0.67, 1.39) | 0.97 (0.66, 1.41) | 1.04 (0.69, 1.60) |
| Cigarettes per day in past 30 days | 0.92 (0.84, 0.99)* | 0.91 (0.84, 1.00)* | 0.92 (0.85, 0.99)* |
| Intake CO value | 0.93 (0.86, 1.01) | 0.92 (0.85, 1.00) | 0.96 (0.88, 1.04) |
| Prior use of nicotine replacement | 0.50 (0.14, 1.79) | 0.50 (0.13, 1.94) | 0.34 (0.07, 1.58) |
| Readiness to change | 0.68 (0.46, 1.00)* | 0.65 (0.42, 1.00)* | 0.70 (0.44, 1.12) |
| Days of transdermal nicotine use | 1.05 (0.99, 1.11) | 1.04 (0.97, 1.11) | |
| % of counseling sessions completed | 1.01 (0.98, 1.03) | 0.99 (0.97, 1.03) | |
| Longest duration of abstinence | 1.12 (1.03, 1.21)** |
Note. Variable (reference code): Intervention Condition (Usual Care), Gender (Male), Highest grade (less than any college), Lives with another smoker (no), Prior use of nicotine replacement (no).
p < .05;
p < .01; all other relations, p > .05.
4. Discussion
The current study is the first to examine patient satisfaction with mHealth-based abstinence reinforcement, and one of few to examine satisfaction in the context of technology-based abstinence reinforcement interventions. In this study, participants in both conditions endorsed high levels of overall satisfaction. This suggests in part that individuals’ expectation about transdermal nicotine and smoking cessation counseling were generally met. mHealth reinforcement participants were more likely to endorse the highest satisfaction with help quitting than mHealth monitoring participants, and the association remained significant after controlling for important participant characteristics, smoking history and motivation to quit, as well as treatment adherence. In research, adherence is often used as a proxy for acceptability or satisfaction with procedures. In this study, results of bivariate analyses demonstrating a positive association between treatment adherence and satisfaction suggest that there is overlap between adherence and satisfaction. However, the lack of a significant association in multivariate analyses indicates that the relation is more complex. Ultimately, smoking outcome exerted the strongest effect on the association between treatment condition and treatment satisfaction association, as a mediator. These results suggest that the positive relation between treatment outcome and satisfaction in the substance abuse treatment literature (discussed above) generalizes to the smoking cessation intervention examined in this study.
The finding of high treatment satisfaction in the mHealth reinforcement condition as well as overall in both conditions is consistent with research on internet-based smoking abstinence reinforcement (Meredith & Dallery, 2013; Meredith et al., 2011; Raiff et al., 2013; Reynolds et al., 2015). In one study, for example, smoking abstinence rates were higher in the internet reinforcement condition compared to control condition (Raiff et al., 2013). In that study, reported willingness to use the treatment again was also higher in the reinforcement compared to control condition, and willingness to use it again was correlated with abstinence in the reinforcement condition but not in the control condition. In the current study, abstinence was similarly greater in the reinforcement compared to control condition, but abstinence mediated the relation between treatment condition and satisfaction regardless of treatment condition. At least half of participants also reported that 2 to 4 weeks would be the best amount of time to complete CO self-tests to quit smoking, and a majority of nonreinforcement participants indicated that they would be very willing to send in CO test videos without any payment for doing do. Somewhat surprising was the extent to which participants suggested that a longer mHealth CO testing period would be “best” (endorsed by 41.7% of participants). These results indicate that individuals in both conditions would be willing to use the intervention again and that they valued the CO tracking component, consistent with prior research on internet-based reinforcement (Meredith & Dallery, 2013; Meredith et al., 2011; Raiff et al., 2013).
The current study adds to that literature by extending findings of acceptability to another technology-based abstinence reinforcement modality (mHealth), and demonstrating the overall acceptability of frequent breath testing. Further, results support the acceptability of testing when the exact frequency and timing is not disclosed to participants beforehand, consistent with our prior study on mHealth reinforcement for alcohol breath tests (Alessi & Petry, 2013). These results are perhaps particularly noteworthy given that participants used study-provided phones, and that being prepared to complete CO tests when prompted required keeping this phone (likely in addition to one’s personal cell) as well as a CO monitor close at hand throughout the day. New technology that permits, for example, breath testing via a smartphone adaptor should increase feasibility and acceptability, as well as present new opportunities for research and smoking intervention.
The robustness of the association between smoking outcome and treatment satisfaction in this study is especially compelling, with significant effects of LDA as well as the other two primary outcomes of the main trial (noted above; not presented to avoid redundancy). In each case, adding participant characteristics and treatment adherence to the model had minimal consequences. In addition, few participant characteristics were associated with treatment satisfaction. Nicotine dependence severity (Fiore et al., 2008) is associated with worse smoking treatment outcomes, which might be expected to affect treatment satisfaction. We found that a higher rate of baseline smoking (cigarettes per day, intake CO value) was associated with decreased satisfaction with help quitting, even after controlling for treatment adherence. Higher readiness to change smoking behavior at baseline was also associated with decreased treatment satisfaction. It may be that individuals who reported high readiness to quit had disproportionately high outcome expectancies (Cropsey et al., 2014), resulting in decreased satisfaction when those expectations were not met. Another consideration is that readiness or motivation to quit is not necessary to realize benefits of treatment (Jardin et al., 2014). In the current study, the association between readiness to quit and treatment satisfaction was no longer significant after controlling for smoking outcome.
A guiding principle behind efforts to improve patient adherence is that doing so will improve treatment outcomes and satisfaction, and mobile technology is increasingly a means by which adherence might be targeted. For example, an estimated 85% of people worldwide own a cell phone and over 80% of adults in the United States text (Duggan, 2013), and Millennials typically average more than 100 text messages daily (Smith, 2011). In a 2014 literature review of studies since 2003 that used text message-based interventions to target medication and other treatment adherence, 77% of studies reported improved treatment outcomes (Kannisto, Koivunen, & Välimäki, 2014). There is mounting evidence of increased smoking quit rates with mobile phone-based interventions, most of which to date have been centered around text messaging (Hartmann-Boyce, Stead, Cahill, & Lancaster, 2013). In the current study, the mHealth procedures required more time and effort than, for example, automated text message reminders or on-demand motivational messages, and intensive mHealth interventions may not be appropriate or preferred by all smokers trying to quit (e.g., Bock, Heron, Jennings, Magee, & Morrow, 2013). However, the positive association between smoking abstinence and treatment satisfaction in both conditions suggests there are circumstances in which relatively intensive mHealth intervention like that examined here are acceptable.
Limitations of this study to consider include that we took a relatively broad view approach to assessing patient satisfaction. Single-item assessments of satisfaction have been found useful. For example, a 1-item assessment of satisfaction was found predictive of improved substance use outcomes 1 year later (controlling for relevant participant characteristics and process variables like adherence) in a U.S. national panel survey of patients in diverse treatment settings (Zhang et al., 2008). Nevertheless, future efforts may benefit from a more in-depth exploration of the complex and multidimensional nature of the construct of patient satisfaction (Pascoe, 1983). It is also important to note that it is not possible to determine the causal direction of effects between adherence, abstinence, and satisfaction definitively from this study given that these are ongoing processes. Future studies might incorporate procedures to more closely examine the temporal relations between these variables (e.g., ecological momentary assessment). For example, it is possible that people who were more satisfied with and ongoing process measurements would shed light on these potential relations. Another consideration is that participants in the current study predominantly endorsed high satisfaction, overall and with regard to how the intervention helped with quitting smoking. While this reflects positively on the current procedures, circumstances likely to present challenges to satisfaction and acceptability are not difficult to imagine (although also not necessarily insurmountable). We also allowed a somewhat generous 2-hour acceptable time window between CO test prompt and submission. The finding that more than 98% of requested tests were submitted on time may suggest the possibility of tightening that window in future work, but it would be important to monitor the effect of this change on satisfaction. Finally, we focused on end-user treatment satisfaction; ultimately, the dissemination of incentive-based interventions will also require buy-in by healthcare providers, the public, and others with vested interests (Lynagh, Bonevski, Symonds, & Sanson-Fisher, 2011; Lynagh, Sanson-Fisher, & Bonevski, 2013; Park, Mitra, & Asch, 2012; Promberger, Brown, Ashcroft, & Marteau, 2011; Raiff et al., 2013; Rash et al., 2012).
Despite these limitations, this study demonstrates that relations between smoking outcome and smoking treatment satisfaction in the context of the mHealth-based smoking intervention are robust, and contributes to the literature on the acceptability of technology-based reinforcement and other smoking treatment in end-users. Research and theory suggests that treatment adherence affects outcomes, and outcomes are related to treatment satisfaction, which is turn can affect future engagement with treatment and health-related behaviors. To the extent that treatment adherence, outcomes, and satisfaction are ongoing processes, there are temporal opportunities for these variables to interact and for intervention to potentially occur, and mHealth procedures present intriguing opportunities. The prevalence and use of cell phones and health-oriented applications (“apps”) and other mobile technology is growing at an extremely rapid rate (Nielsen, 2014). The acceptability of the mHealth procedures in this study may in part reflect that trend, and the acceptability and utility of mHealth generally is expected to grow. Applying such technology to improve understanding of relations between such contextual reinforcement and other smoking cessation interventions.
Highlights.
The importance of patient satisfaction in modern healthcare is widely recognized.
Patient satisfaction in the context of mHealth-based contingency management is unknown.
Participants received transdermal nicotine and telephone counseling with mHealth monitoring only or mHealth monitoring and abstinence-contingent incentives.
Longest duration of abstinence mediated effects of study condition on satisfaction with help quitting with the intervention, controlling for other relevant variables.
Results indicate the acceptability of procedures in end-users.
Acknowledgements
Thank you to the research participants, without whom this study would not have been possible. Sarah Coughlin, Karen Gilliam, Amy Novotny, and Sean Sierra contributed to data collection and oversight for the randomized clinical trial on which this report is based. Ellen Ciesielski assisted with IRB and other regulatory communications, and Laura LaBlanc and Wendy Soneson provided administrative support.
Funding. This research was supported by National Institutes of Health grants R21-DA029215, R01-DA01344, R21-DA031897, P50 DA092410, R01-AA021446, R01-HD075630, and P60 AA03510
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier: NCT01484717
References
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108(5):900–909. doi: 10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bock BC, Heron KE, Jennings EG, Magee JC, Morrow KM. User preferences for a text message–based smoking cessation intervention. Health Education & Behavior. 2013;40(2):152–159. doi: 10.1177/1090198112463020. [DOI] [PubMed] [Google Scholar]
- Boden MT, Moos R. Dually diagnosed patients’ responses to substance use disorder treatment. Journal of Substance Abuse Treatment. 2009;37(4):335–345. doi: 10.1016/j.jsat.2009.03.012. http://doi.org/10.1016/j.jsat.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MJ, Gabriel RM. Patient Satisfaction, Use of Services, and One-Year Outcomes in Publicly Funded Substance Abuse Treatment. Psychiatric Services. 2001;52(9):1230–1236. doi: 10.1176/appi.ps.52.9.1230. http://doi.org/10.1176/appi.ps.52.9.1230. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. Bridging the Gap: A Hybrid Model to Link Efficacy and Effectiveness Research in Substance Abuse Treatment. Psychiatric Services. 2003;54(3):333–339. doi: 10.1176/appi.ps.54.3.333. http://doi.org/10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Leventhal AM, Stevens EN, Trent LR, Clark CB, Lahti AC, Hendricks PS. Expectancies for the Effectiveness of Different Tobacco Interventions Account for Racial and Gender Differences in Motivation to Quit and Abstinence Self-Efficacy. Nicotine & Tobacco Research. 2014;16(9):1174–1182. doi: 10.1093/ntr/ntu048. http://doi.org/10.1093/ntr/ntu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosier M, Scott J, Steinfeld B. Improving Satisfaction in Patients Receiving Mental Health Care: A Case Study. The Journal of Behavioral Health Services & Research. 2012;39(1):42–54. doi: 10.1007/s11414-011-9252-0. http://doi.org/10.1007/s11414-011-9252-0. [DOI] [PubMed] [Google Scholar]
- Duggan M. Cell Phone Activities. 20132013:18. Retrieved from http://pewinternet.org/~;/media//Files/Reports/2013/PIP_Cell Phone Activities May 2013.pdf\nhttp://www.pewinternet.org/files/old-media//Files/Reports/2013/PIP_Cell Phone Activities May 2013.pdf.
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. http://doi.org/10.1037/0022-006X.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Bennet G, Benowitz NL, Hasselblad V. A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update. A U.S. Public Health Service Report. American Journal of Preventive Medicine. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. http://doi.org/10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction. 2013;108(10):1711–1721. doi: 10.1111/add.12291. http://doi.org/10.1111/add.12291. [DOI] [PubMed] [Google Scholar]
- Hawkins EJ, Baer JS, Kivlahan DR. Concurrent monitoring of psychological distress and satisfaction measures as predictors of addiction treatment retention. Journal of Substance Abuse Treatment. 2008;35(2):207–216. doi: 10.1016/j.jsat.2007.10.001. http://doi.org/10.1016/j.jsat.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. http://doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Evans E, Huang D, Anglin DM. Relationship Between Drug Treatment Services, Retention, and Outcomes. Psychiatric Services. 2004;55(7):767–774. doi: 10.1176/appi.ps.55.7.767. http://doi.org/10.1176/appi.ps.55.7.767. [DOI] [PubMed] [Google Scholar]
- Jardin BF, Cropsey KL, Wahlquist AE, Gray KM, Silvestri GA, Cummings KM, Carpenter MJ. Evaluating the Effect of Access to Free Medication to Quit Smoking: A Clinical Trial Testing the Role of Motivation. Nicotine & Tobacco Research. 2014;16(7):992–999. doi: 10.1093/ntr/ntu025. http://doi.org/10.1093/ntr/ntu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannisto KA, Koivunen MH, Välimäki MA. Use of Mobile Phone Text Message Reminders in Health Care Services: A Narrative Literature Review. Journal of Medical Internet Research. 2014;16(10):e222. doi: 10.2196/jmir.3442. http://doi.org/10.2196/jmir.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PloS One. 2014;94:e94207. doi: 10.1371/journal.pone.0094207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney DA. Mediation with dichotomous outcomes. 2013 Retrieved from http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Mediation+with+Dichotom ous+Outcomes#0.
- Kenny D. a. Mediation. 2015 Retrieved from http://davidakenny.net/cm/mediate.htm.
- Kong G, Ells DM, Camenga DR, Krishnan-Sarin S. Text messaging-based smoking cessation intervention: A narrative review. Addictive Behaviors. 2014;39(5):907–917. doi: 10.1016/j.addbeh.2013.11.024. http://doi.org/10.1016/j.addbeh.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon J. a., Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. http://doi.org/10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Lynagh M, Bonevski B, Symonds I, Sanson-Fisher RW. Paying Women to Quit Smoking During Pregnancy? Acceptability Among Pregnant Women. Nicotine & Tobacco Research. 2011;13(11):1029–1036. doi: 10.1093/ntr/ntr108. http://doi.org/10.1093/ntr/ntr108. [DOI] [PubMed] [Google Scholar]
- Lynagh M, Sanson-Fisher R, Bonevski B. What’s Good for the Goose is Good for the Gander. Guiding Principles for the Use of Financial Incentives in Health Behaviour Change. International Journal of Behavioral Medicine. 2013;20(1):114–120. doi: 10.1007/s12529-011-9202-5. http://doi.org/10.1007/s12529-011-9202-5. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Hunkeler E. Alcohol & Drug Abuse: Patient Satisfaction and Outcomes in Alcohol and Drug Abuse Treatment. Psychiatric Services. 1998;49(5):573–575. doi: 10.1176/ps.49.5.573. http://doi.org/10.1176/ps.49.5.573. [DOI] [PubMed] [Google Scholar]
- Meredith SE, Dallery J. Investigating group contingencies to promote brief abstinence from cigarette smoking. Experimental and Clinical Psychopharmacology. 2013;21(2):144–154. doi: 10.1037/a0031707. http://doi.org/10.1037/a0031707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence. 2011;118(1):23–30. doi: 10.1016/j.drugalcdep.2011.02.012. http://doi.org/10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen Hacking health: How consumers use smartphones and wearable tech to track their health. 2014 [Google Scholar]
- Park JD, Mitra N, Asch DA. Public opinion about financial incentives for smoking cessation. Preventive Medicine. 2012;55(SUPPL.):S41–S45. doi: 10.1016/j.ypmed.2012.06.013. http://doi.org/10.1016/j.ypmed.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Pascoe GC. Patient satisfaction in primary health care: A literature review and analysis. Evaluation and Program Planning. 1983;6(3-4):185–210. doi: 10.1016/0149-7189(83)90002-2. http://doi.org/10.1016/0149-7189(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. http://doi.org/10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Promberger M, Brown RCH, Ashcroft RE, Marteau TM. Acceptability of financial incentives to improve health outcomes in UK and US samples. Journal of Medical Ethics. 2011;37(11):682–687. doi: 10.1136/jme.2010.039347. http://doi.org/10.1136/jme.2010.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Jarvis BP, Turturici M, Dallery J. Acceptability of an internet-based contingency management intervention for smoking cessation: Views of smokers, nonsmokers, and healthcare professionals. Experimental and Clinical Psychopharmacology. 2013;21(3):204–213. doi: 10.1037/a0032451. http://doi.org/10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, Stitzer ML. Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug and Alcohol Dependence. 2012;121(3):205–212. doi: 10.1016/j.drugalcdep.2011.08.027. http://doi.org/10.1016/j.drugalcdep.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, Lewis R. Experimental and Clinical Psychopharmacology A Feasibility Study of Home-Based Contingency Appalachia A Feasibility Study of Home-Based Contingency Management With Adolescent Smokers of Rural Appalachia. 2015 doi: 10.1037/pha0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Cox LS, Preacher KJ, Hunt JJ. Comparative and Cost Effectiveness of Telemedicine Versus Telephone Counseling for Smoking Cessation. Journal of Medical Internet Research. 2015;17(5):e113. doi: 10.2196/jmir.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Heather N, Gold R, Hall W. Development of a short “readiness to change” questionnaire for use in brief, opportunistic interventions among excessive drinkers. British Journal of Addiction. 1992;87(5):743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. http://doi.org/10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- Sanford A, Donahue M, Cosden M. Consumer perceptions of trauma assessment and intervention in substance abuse treatment. Journal of Substance Abuse Treatment. 2014;47(3):233–238. doi: 10.1016/j.jsat.2014.05.011. http://doi.org/10.1016/j.jsat.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction. 2009;104(11):1792–1804. doi: 10.1111/j.1360-0443.2009.02710.x. http://doi.org/10.1111/j.1360-0443.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- Smith A. Americans and text messaging. Pew Research Center; Washington, DC: 2011. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. In: Allen RZLJP, editor. Humana Press; Totowa, NJ, US: 1992. pp. 41–72. http://doi.org/10.1007/978-1-4612-0357-5_3. [Google Scholar]
- The Institute of Medicine . Crossing the quality chasm: a new health system for the 21st century. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- World Health Organization World Health Organization. Digital health for the end of TB strategy: An agenda for action. 2015;1 Retrieved from http://www.journals.cambridge.org/abstract_S1935789300000094. [Google Scholar]
- Zhang Z, Gerstein DR, Friedmann PD. Patient Satisfaction and Sustained Outcomes of Drug Abuse Treatment. Journal of Health Psychology. 2008;13(3):388–400. doi: 10.1177/1359105307088142. http://doi.org/10.1177/1359105307088142. [DOI] [PMC free article] [PubMed] [Google Scholar]