Abstract
Posttraumatic stress disorder (PTSD) is associated with executive functioning deficits, including disruptions in working memory (WM). Recent studies suggest that attention training reduces PTSD symptomatology, but the underlying neural mechanisms are unknown. We used high-density magnetoencephalography (MEG) to evaluate whether attention training modulates brain regions serving WM processing in PTSD. Fourteen veterans with PTSD completed a WM task during a 306-sensor MEG recording before and after 8 sessions of attention training treatment. A matched comparison sample of 12 combat-exposed veterans without PTSD completed the same WM task during a single MEG session. To identify the spatiotemporal dynamics, each group's data were transformed into the time-frequency domain, and significant oscillatory brain responses were imaged using a beamforming approach. All participants exhibited activity in left hemispheric language areas consistent with a verbal WM task. Additionally, veterans with PTSD and combat-exposed healthy controls each exhibited oscillatory responses in right hemispheric homologue regions (e.g., right Broca's area); however, these responses were in opposite directions. Group differences in oscillatory activity emerged in the theta band (4–8 Hz) during encoding and in the alpha band (9–12 Hz) during maintenance and were significant in right prefrontal and right supramarginal and inferior parietal regions. Importantly, following attention training, these significant group differences were reduced or eliminated. This study provides initial evidence that attention training improves aberrant neural activity in brain networks serving WM processing.
Keywords: Working memory, Prefrontal cortex, Attention, Cognitive control
Posttraumatic stress disorder (PTSD) involves reexperiencing, avoidance, and physiological arousal symptoms as well as negative alterations in mood and cognition (American Psychiatric Association, 2013). Innovative treatments targeting attention-level processes have recently emerged as promising interventions for PTSD, including attention bias modification treatment (ABMT), which trains attention away from threat (i.e., avoidance; Kuckertz et al., 2014), and attention control treatment (ACT), which trains participants to ignore threat-related contingencies and focus on the task at hand (Badura-Brack et al., 2015). Both treatments employ computer programs designed to normalize threat-related attention patterns by requiring participants to complete a neutral cognitive task in the face of threat-provoking stimuli (Beard, Sawyer, & Hofmann, 2012; Hakamata et al., 2010; Hallion & Ruscio, 2011; Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015). Attention is a logical target for PTSD treatment because patients with PTSD experience deficits in executive functioning including impaired working memory (WM) capacity and attention regulation (Aupperle, Melrose, Stein, & Paulus, 2012). In fact, a recent meta-analysis focusing on cognitive functioning reported that some of the strongest neurocognitive deficits associated with PTSD are in attention and WM (Scott et al., 2015), and meta-analyses of functional neuroimaging studies in PTSD have indicated disturbed neural activation patterns during performance of WM, executive control, and emotion regulation tasks (Hayes, Hayes, & Mikedis, 2013; Patel, Spreng, Shin, & Girard, 2012). In sum, WM is vital to everyday cognitive functioning, and the symptomatology of PTSD is strongly associated with deficits in WM processing (Koso & Hansen, 2005; Schweizer & Dalgleish, 2011).
Previous studies using electroencephalography (EEG) and magnetoencephalography (MEG) have characterized the neural dynamics of normative WM processing by focusing on neural oscillatory responses (Bonnefond & Jensen, 2012; Brookes et al., 2011; Heinrichs-Graham & Wilson, 2015; Jensen & Tesche, 2002; Jensen, Gelfand, Kounios, & Lisman, 2002; Jensen & Mazaheri, 2010; Palva, Monto, Kulashekhar, & Palva, 2010; Palva, Kulashekhar, Hamalainen, & Palva, 2011; Palva, Monto, & Palva, 2010; Tuladhar et al., 2007). Oscillatory analyses are ideal for studying neural responses that are extended in time, such as WM, which consists of three cognitive subprocesses: encoding, maintenance, and retrieval (Baddeley, 1992). Previous oscillatory studies of verbal WM in healthy participants have shown widespread alpha desynchronizations in left hemispheric language regions during encoding and maintenance, along with robust increases in parieto-occipital alpha (i.e., synchronization) during maintenance (Bonnefond & Jensen, 2012; Heinrichs-Graham & Wilson, 2015; Tuladhar et al., 2007). Several electrophysiological studies of WM have also found theta oscillations, although reports of theta activity are less common (Brookes et al., 2011; Jensen & Tesche, 2002). These studies have made important discoveries in normative samples. However, much remains to be elucidated in the context of PTSD.
Recently, we conducted the first MEG study examining WM function in PTSD. This study found aberrant oscillatory alpha activity during WM encoding and maintenance in veterans with PTSD. These significant differences in oscillatory activity were found in the right inferior frontal gyrus (IFG), right supramarginal gyrus (SMG), and right inferior parietal areas (McDermott et al., 2015). In this study, we examined whether these neural abnormalities in WM processing could be ameliorated with attention training treatment by collecting pre- and posttreatment MEG recordings from a group of veterans who participated in a recent clinical trial of attention training for PTSD (Badura-Brack et al., 2015). Briefly, we compared pre- and posttreatment recordings from the veterans with PTSD to a control sample of psychologically healthy combat-exposed veterans who completed the identical WM task during a single MEG session. The pretreatment recordings of the veterans with PTSD and the data from the healthy combat-exposed veterans were both a subset of the data reported in McDermott and colleagues (2015), although that study did not examine treatment effects and included additional controls that had not been exposed to combat or any type of trauma. The current study accounted for combat exposure by using a control group comprised only of combat veterans without PTSD. Our hypothesis was that attention training would significantly normalize abnormal neural activity in the right IFG, right SMG, and right inferior parietal areas during WM processing in veterans with PTSD.
Material and method
Participants
Fourteen combat veterans with PTSD and 12 combat veterans without PTSD participated in this study. All 26 combat veterans in this study were participants in McDermott and colleagues (2015). We included the subset of participants with PTSD who completed a clinical trial of attention training for PTSD (Badura-Brack et al., 2015) and who also successfully completed pre- and posttreatment MEG sessions. Dropout rates for outcome studies of PTSD are typically quite high (Schottenbauer et al., 2008), and our study had a lower than average dropout rate. We included all 12 of the combat-exposed controls who participated in McDermott et al. (2015), and this group was closely matched to the PTSD group on age, sex, education, ethnicity, and handedness. All participants were male and right-handed. All participants were recruited from the community using television commercials, flyers, and/or social media, and all veterans had served in recent conflicts in Iraq or Afghanistan with their warzone service occurring between 2003 and 2014. Veterans in the PTSD group were diagnosed with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) in conjunction with the Life Events Checklist (Blake et al., 1995) and using the CAPS F1/I2 rule (Weathers, Ruscio, & Keane, 1999). Veterans with PTSD were further assessed using the Mini International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 2009) to rule out psychiatric diagnoses other than PTSD with or without concurrent anxiety and/or depression better explained by PTSD. No one in the PTSD group was receiving psychotherapy, and five (36 %) were on stable (no change for at least 6 months before beginning the study and no change during the study) doses of psychiatric medication (three on selective serotonin reuptake inhibitors [SSRIs] and two on mood stabilizers); the remaining 64 % were not taking psychiatric medication. Veterans in the control group were also assessed with the CAPS and M.I.N.I. to rule out PTSD and other psychiatric disorders. None of the veterans in the control group met diagnostic criteria for PTSD or any other psychiatric condition. Veterans were excluded from the study if they had another medical diagnosis likely to affect CNS function, brain neoplasm or lesion, history of significant head trauma, current substance dependence, or ferromagnetic implants such as shrapnel in the upper body. Written informed consent was obtained following the ethical guidelines of the Creighton University Institutional Review Board, who approved the study protocol. This study was carried out in accordance with the latest version of the Declaration of Helsinki.
Attention training treatment
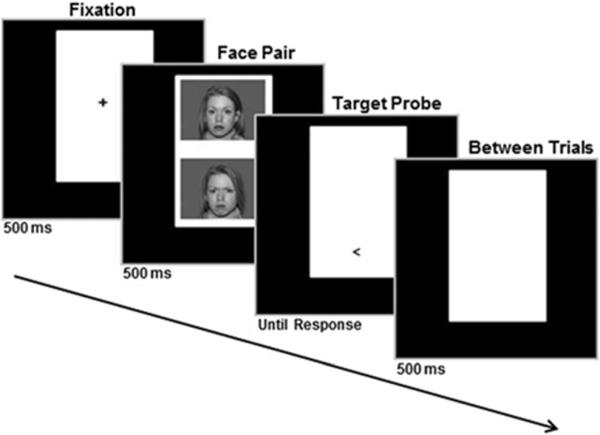
All veterans with PTSD completed two MEG sessions, before and after completing attention training using a faces-based variant of the dot probe task in which one angry face and one neutral face were displayed simultaneously, one above the other, on the computer screen (see Fig. 1). Each training trial began with a fixation cross (500 ms); followed by a face pair (500 ms) using a set of facial photographs (10 males and 10 females) from the NimStim stimulus set (Tottenham et al., 2009); then a target displayed (“<” or “>”) in the space vacated by either the angry or neutral face, which remained on-screen until patients responded; and, finally, there was a blank display for 500 ms between trials (see Fig. 1). Training consisted of eight 10–15 minute sessions over a 4-week period, during which veterans responded as quickly as possible to the direction of the probe (left or right) using a computer mouse (click either left or right). Participants were randomly assigned to ACT (eight participants) or ABMT (six participants) training conditions, which are described in the Tel Aviv University–National Institute of Mental Health ABMT protocol (http://people.socsci.tau.ac.il/mu/anxietytrauma/research/). In ABMT, the probe appears in the location of the neutral face in each of the 160 trials. Presenting the probe in the location of the neutral face implicitly trains participants to attend away from threat (Hakamata et al., 2010; Linetzky et al., 2015). High levels of anxiety are associated with high levels of attention to threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van Ijzendoorn, 2007; Cisler & Koster, 2010), so ABMT is designed to reduce threat bias, and this results in decreased anxious symptoms (Hakamata et al., 2010; Linetzky et al., 2015). On the other hand, in ACT, the probe appears in a fully counterbalanced manner, equally behind neutral and angry faces. This is thought to implicitly train participants to ignore threat related contingencies and focus on the task at hand. For a more detailed description of attention training (both ABMT and ACT), see Badura-Brack et al. (2015).
Fig. 1.
Faces-based variant of the dot probe task. Each training trial began with a fixation cross (500 ms), followed by a face pair (500 ms), then a target displayed (“<” or “>”) in the space vacated by either the angry or neutral face, which remained on-screen until patients responded; finally, there was a blank display for 500 ms between trials
Previous studies of ACT and ABMT have found that both protocols significantly reduce symptoms of PTSD. A recent stand-alone clinical trial found a significantly stronger treatment effect for ACT over ABMT (Badura-Brack et al., 2015), whereas another recent study found a stronger effect for ABMT over ACT as an adjunctive treatment to psychotherapy for PTSD (Kuckertz et al., 2014). Importantly, both ACT and ABMT resulted in reduced PTSD severity in both trials. The only other clinical trial of ACT and ABMT for PTSD also found significant symptom improvement after both interventions, but no difference between treatments (Schoorl, Putman, & Van Der Does, 2013). Because both training protocols have been shown to reduce PTSD severity, and our goal was to examine whether PTSD symptom reduction was associated with alterations in the aberrant neural dynamics serving WM processing, we combined ACT and ABMT participants into an overarching attention training group for this study.
MEG experimental paradigm
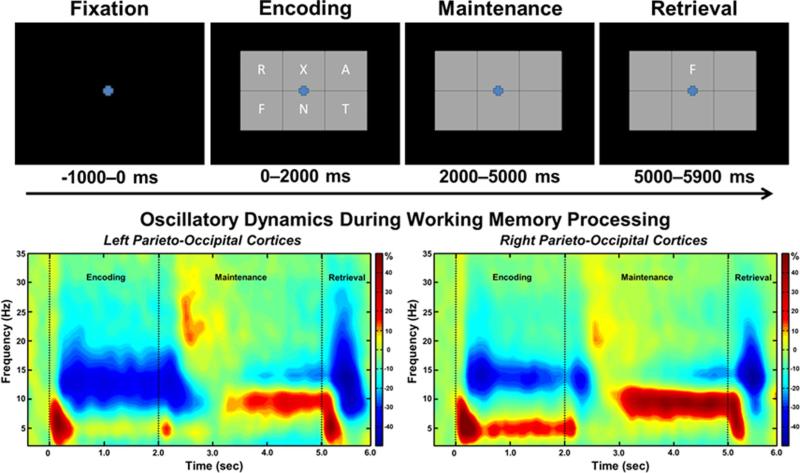
During the MEG recordings, participants completed a visual, verbal WM task while seated in the MEG chamber. First, participants were instructed to fixate on a centrally presented crosshair lasting 1,000 ms. A 19 × 13 cm grid (width-by-height) containing six letters was then presented for 2,000 ms. These letters then disappeared, and the empty grid remained on the screen for 3,000 ms, until a single “probe” letter appeared in the cell above the fixation for 900 ms (see Fig. 2). Participants indicated if the probe letter was one of the six previously presented during the encoding set using their index finger. Each trial lasted 6,900 ms, and each participant completed 128 trials; 50 % of trials were probe-positive (i.e., the probe appeared in the encoding set), and the remaining 50 % were probe-negative. After completing attention training, veterans with PTSD underwent a second, identical posttreatment MEG session. Note that equivalent task performance between patients and controls was expected on this WM paradigm, as a six-item memory load strongly burdens WM resources, but is not overly difficult for the vast majority of people. Task difficulty could have been increased with a higher WM load (e.g., seven or eight letters) or a more difficult WM task (e.g., four-back), but we specifically avoided this in the current study because accuracy differences between groups would have complicated the planned MEG analyses. Essentially, as described below, we focused on correct trials in this study, and a significant difference in the number of such trials between groups would have biased the signal-to-noise ratio of the MEG data in favor of the more accurate group.
Fig. 2.
(Top) Layout of working memory paradigm and (bottom) time-frequency spectrograms. (Top) Each trial consisted of four phases: (1) a fixation phase lasting 1,000 ms that included the baseline (−400 to 0 ms), (2) an encoding phase lasting 2,000 ms and consisting of six letters presented simultaneously within a grid, (3) a maintenance phase lasting 3,000 ms during which the six letters disappeared from the grid, and (4) a retrieval phase lasting 900 ms that required participants to identify whether the probe letter appeared in the original encoding set. Each participant completed 128 trials during each MEG session. (Bottom) Group mean time-frequency spectrograms averaged across all participants, with a sensor from the left parieto-occipital region shown on the left and the same for the right. In each spectrogram, time (sec) is shown on the x-axis and frequency (Hz) on the y-axis. Percentage of power change was computed by dividing the mean power of each time-frequency bin by the respective bin's baseline power and multiplying this value by 100. The color legend is displayed to the right. Strong theta synchronization and alpha desynchronization was observed shortly after the encoding grid was presented, and this evolved into a narrower alpha synchronization during maintenance (with theta oscillations dissipating)
MEG data acquisition and coregistration with structural MRI
With an acquisition bandwidth of 0.1–330.0 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (Version 2.2; Elekta), MEG data from each participant were individually corrected for head movement, coregistered with structural MRI, and subjected to noise reduction using the signal space separation method with a temporal extension (tSSS; Taulu & Simola, 2006; Taulu, Simola, & Kajola, 2005). Each participant's MEG data were coregistered with structural T1-weighted MRI data before source space analyses using BESA MRI (Version 2.0). Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. After beamformer analysis, each participant's functional images were also transformed into standardized space using the transform previously applied to the structural MRI volume and spatially resampled.
MEG time-frequency transformation and statistics
The MEG preprocessing and imaging analysis pipeline was closely based on the methods reported in our recent WM papers (Heinrichs-Graham & Wilson, 2015; McDermott et al., 2015; Proskovec, Heinrichs-Graham, & Wilson, 2016; Wiesman et al., 2016). The continuous magnetic time series was divided into epochs of 6,900 ms duration, with 0 ms being the onset of the encoding grid and the baseline being the −400–0 ms time bin (see Fig. 2). Cardio artifacts were removed from the data using signal-space projection, which was accounted for during source reconstruction (Uusitalo & Ilmoniemi, 1997). Epochs containing other artifacts that were not suppressed by tSSS (e.g., some eye blinks) were rejected using a fixed threshold method, supplemented with visual inspection. The final number of accepted epochs did not statistically differ between groups.
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation (resolution: 1.0 Hz, 50 ms; Papp & Ktonas, 1977), and the resulting data per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were then normalized by dividing the power value of each time-frequency bin by the respective bin's baseline power, which was calculated as the mean power during the −400–0 ms time period. To derive the time-frequency windows of interest for the source imaging analysis, we used a data-driven statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. This statistical analysis used a two-stage procedure to control for Type I error. Briefly, in stage one, one-sample t tests were conducted on each data point, and the output spectrogram of t values was thresholded at p < .05 to identify time-frequency bins containing potentially significant oscillatory deviations. In stage two, suprathreshold bins were clustered with neighboring bins that were also above the threshold, and a cluster value was derived by summing the t values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance of the observed clusters were tested directly using this distribution (Ernst, 2004; Maris & Oostenveld, 2007). Based on these analyses, time-frequency windows containing significant oscillatory events across all participants were selected for imaging. Further details of this statistical approach are available (Heinrichs-Graham & Wilson, 2015).
MEG source imaging and statistics
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Hillebrand, Singh, Holliday, Furlong, & Barnes, 2005; Van Veen, van Drongelen, Yuchtman, & Suzuki, 1997), which employs spatial filters in the frequency domain to calculate source power for the whole brain volume. The single images are derived from the cross-spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the power in these images was normalized per participant using a separately averaged prestimulus noise period of equal duration and bandwidth (Van Veen et al., 1997).
Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0-mm resolution. The resulting 3-D maps of functional brain activity were statistically evaluated using a mass univariate approach based on the general linear model. Briefly, the effect of group was examined using a random-effects analysis for the time-frequency bin and condition of interest (pre-/posttreatment). Statistical maps were displayed as a function of alpha level (p < .005) and adjusted for multiple comparisons using a spatial extent threshold (cluster restriction: 80 contiguous voxels) based on the theory of Gaussian random fields.
Results
Clinical measures and behavioral performance
Mean age was 33.4 years in the group with PTSD, and 30.8 years in the combat-exposed comparison sample; this difference was not significant, t(24) = .96, p = .39. All 26 participants were able to successfully complete the WM task. Participants correctly identified the probe in 82.73 % (SD = 6.23 %) of all trials, and only correct trials were included in MEG analyses. Task performance and reaction time did not differ between veterans with and without PTSD (all ps > .42). Equivalent task performance was expected based on the design of this WM task, which involved only a six-item load (i.e., not a more difficult seven- or eight-item load; see Method section). Likewise, task performance in participants with PTSD did not change following attention training, which was over 80 % correct before and after treatment, nor did it result in statistically significant change in reaction time. Importantly, after attention training treatment, CAPS scores significantly improved, t(13) = 5.07, p < .0005, from a pre-treatment average of 71.00 (SD = 16.31), indicating severe PTSD, to a posttreatment average of 45.21 (SD = 21.53), which is at or below most CAPS thresholds used for a diagnosis of PTSD (Weathers et al., 1999).
MEG sensor-level analysis
Sensor-level spectrograms were statistically examined using nonparametric permutation testing to derive the precise time-frequency bins for follow-up beamforming analyses. These analyses indicated a strong alpha desynchronization (9–16 Hz) that began shortly after onset of the encoding grid (~200 ms) and continued throughout the duration of the encoding phase and slightly into the maintenance period (p < .001, corrected). A theta synchronization (4–8 Hz) was also detected, and it began at the onset of the encoding grid (0 ms) and persisted until the end of encoding (p < .001, corrected). Finally, a narrower alpha band (9–12 Hz) synchronization was detected during the maintenance period, beginning about 2,200 ms after the onset of the encoding grid and continuing until the retrieval phase (p < .001, corrected). These three oscillatory responses can easily be discerned in the sensor-level spectrograms (see Fig. 2). To interrogate the temporal dynamics, the significant alpha oscillatory responses were divided into 12 nonoverlapping time windows of 400 ms duration (i.e., 200–600 ms, 600–1000 ms, 1000–1400 ms, 1400–1800 ms, 1800–2200 ms, 2200–2600 ms, 2600–3000 ms, 3000–3400 ms, 3400–3800 ms, 3800–4200 ms, 4200–4600 ms, 4600–5000 ms), and each window was imaged and statistically evaluated for group effects. The first five time windows (200–2200 ms) were imaged using the 9–16 Hz band, and the remaining were imaged from 9–12 Hz. The theta oscillatory response was also divided into nonoverlapping time windows of 400 ms duration (i.e., 0–400 ms, 400–800 ms, 800–1200 ms, 1200–1600 ms, 1600–2000 ms), and each window was imaged and statistically evaluated for group effects. These precise time windows were chosen because they correspond closely to the observed oscillatory dynamics (see Fig. 2).
MEG oscillatory analysis
Group-level statistical parametric maps (SPMs) were initially computed for the 14 veterans with PTSD (pretreatment) and the 12 combat veterans without PTSD. These maps showed strong alpha event-related desynchronization (ERD) in left hemispheric language areas of each group. In addition, combat-exposed veterans without PTSD had theta and alpha event-related synchronizations (ERS) in the right prefrontal cortices, which included the IFG, the dorsolateral prefrontal cortex (DLPFC), the right SMG, and right inferior parietal regions, whereas veterans with PTSD had theta and alpha ERD responses in these same brain regions.
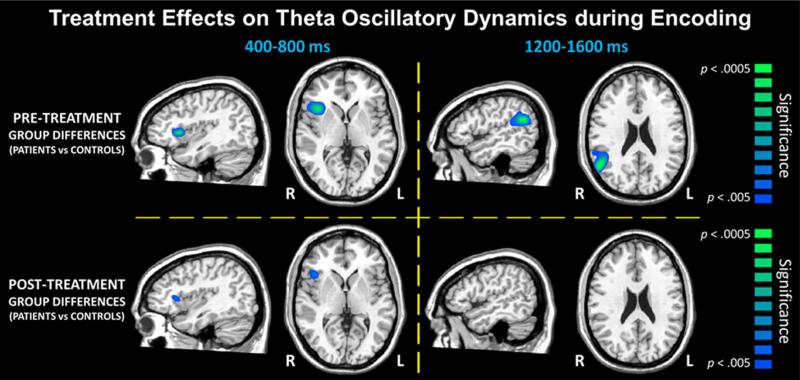
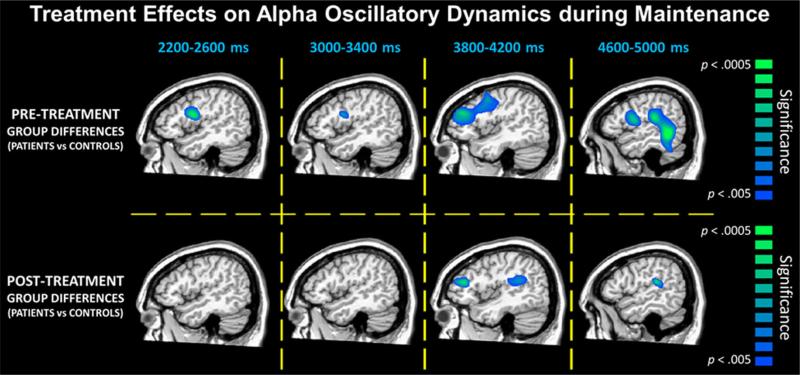
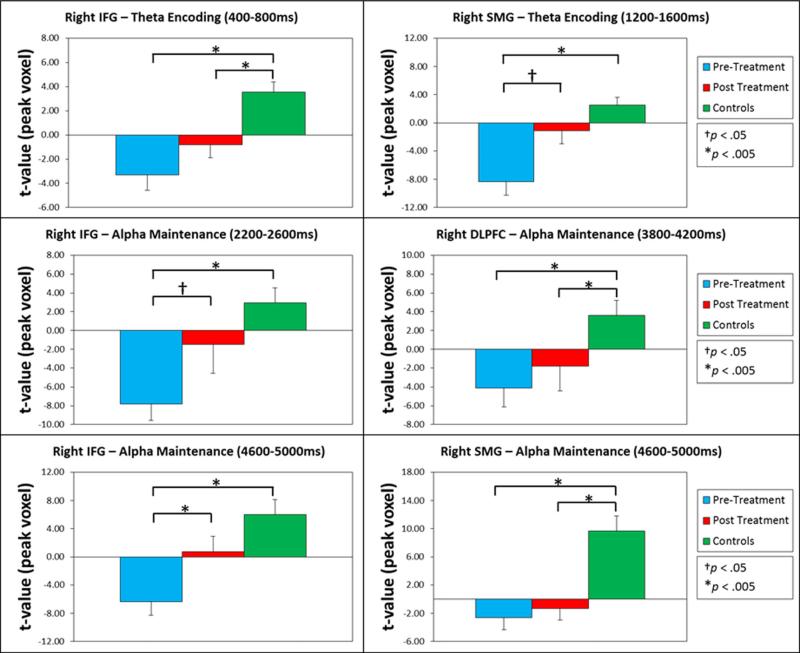
In regard to pretreatment group differences (pretreatment images compared to control images), we found significant effects during several time windows, but only in right hemispheric brain regions. For example, veterans with PTSD had significantly weaker theta activity (i.e., ERD) compared to those without PTSD in the right IFG in the 400–800 ms time window and in the right SMG during in the 1,200–1,600 ms time window (p < .005, corrected; see Fig. 3). No group differences in alpha activity were observed in the encoding period. During maintenance, veterans with PTSD had weaker alpha activity in the IFG (i.e., ERD), which first emerged in the 2,200–2,600 ms time window and then remerged in the 3000–3400 ms, 3800–4200 ms, and 4600–5000 ms time windows (p < .005, corrected; see Fig. 4). Similar differences in alpha were also found in the right DLPFC during the 3,800–4,200 ms time window and in the right SMG and inferior parietal areas during the 4,600–5,000 ms time window (p < .005, corrected; see Fig. 4).
Fig. 3.
Significant group differences (p < .005, corrected) in theta oscillatory activity during the encoding phase. Data representing the veterans with PTSD before treatment compared to the veterans without PTSD are shown on the top row, whereas the bottom row depicts comparison of the posttreatment images of veterans with PTSD to the veterans without PTSD. Note that data from the veterans without PTSD are the same in both panels, as these participants completed only one MEG session. Before treatment, veterans with PTSD had significantly reduced theta activity (i.e., strong ERD) during several time bins in the right inferior frontal and supramarginal gyri (p < .005, corrected) compared to veterans without PTSD (top row). Following treatment, these differences in theta activity were strongly diminished and/or fully suppressed (bottom row)
Fig. 4.
Significant group differences (p < .005, corrected) in alpha oscillations during the maintenance phase. Similar to Fig. 3, data representing veterans with PTSD before treatment compared to veterans without PTSD are shown on the top row, while the bottom row depicts comparison of the posttreatment veterans with PTSD to the veterans without PTSD. Note that data from the veterans without PTSD are the same in both panels, as these participants completed only one MEG session. In most time windows, veterans with PTSD had significantly weaker alpha activity (i.e., ERD responses) in right prefrontal and supramarginal areas before attention training treatment compared to veterans without PTSD (p < .005, corrected; top row). Following attention training, differences in most time bins were no longer present, and the posttreatment veterans generally showed no significant differences relative to those without PTSD (bottom row)
In regard to our primary focus on posttreatment group differences (posttreatment images compared to control images), we observed either a reduction or normalization of the pre-treatment group differences during both theta encoding and alpha maintenance. Note that SPMs were displayed as a function of alpha level (p < .005) such that differences above this threshold were considered nonsignificant. For theta encoding, the group difference in the right IFG during the 400–800 ms time window was still present (p < .005, corrected; see Fig. 3), although markedly reduced, and the group difference in the right SMG during the 1,200–1,600 ms time window was no longer present (p > .005, corrected; see Figs. 3 and 5). For alpha maintenance, the group differences in the right IFG for the 2200–2600, 3000–3400, and 4600–5000 ms time windows were no longer present (p > .005, corrected; Figs. 4 and 5). The group differences in the right DLPFC for the 3,800–4,200 ms time window and in the right SMG and inferior parietal areas for the 4,600–5,000 ms time window were still present posttreatment (p < .005, corrected; see Fig. 4) and were relatively unaffected by treatment (see Fig. 5). Finally, there was a significant treatment effect in the right IFG in the 4,600–5,000 ms time window (p < .005, corrected; see Fig. 5), and marginal treatment effects in brain areas where group differences were no longer observed posttreatment (Fig. 5).
Fig. 5.
Bar graphs showing peak voxel values in brain regions where significant group differences were observed. In each panel, the group mean for veterans with PTSD before treatment (aqua), veterans with PTSD after treatment (red), and healthy controls (green) is shown for the peak voxel in a specific time window and brain area where group differences were observed. The legend appears on the right, and error bars reflect one standard error of the mean. These graphs illustrate the widespread normalization of theta and alpha oscillatory responses following attention training in veterans with PTSD
Discussion
We evaluated the effects of attention training on the neural dynamics serving WM encoding and maintenance in combat veterans with PTSD using MEG. We found that PTSD was associated with altered dynamics during WM processing in the right IFG, right DLPFC, right SMG, and inferior parietal regions when compared to a combat-exposed control sample. Importantly, following attention training, we found that these altered neural dynamics were either reduced or eliminated.
Normative studies of WM processing have emphasized the importance of oscillatory activity in left-hemispheric language areas during encoding and maintenance (Brookes et al., 2011; Jensen et al., 2002) consistent with the presumed functioning of Baddeley's phonological loop (Baddeley, 2000). We observed expected activity in these language regions in both groups; however, differences emerged between veterans with PTSD (pretreatment) and veterans without PTSD in theta and alpha activity in the right prefrontal (mainly, inferior frontal gyrus) and supramarginal regions stretching into inferior parietal during some time windows. These findings reiterate the WM group differences in alpha activity identified between the larger sample (McDermott et al., 2015), but in this study we also examined differences in the theta oscillatory range, broadening our perspective on oscillatory aberrations in PTSD.
Our most important finding was that veterans with PTSD who completed an attention training protocol, which had been shown to successfully reduce PTSD symptoms (Badura-Brack et al., 2015), also displayed partially normalized neural activity during WM processing after training. A recent meta-analysis by Scott and colleagues (2015) reported that attention and WM deficits are among the strongest neurocognitive deficits in PTSD and are closely associated with the symptomatology, so combining these lines of research and discovering that attention training treatment improves aberrant neural dynamics during WM processing in veterans with PTSD is note-worthy. Although attention allocation and WM capacity are not necessarily equivalent constructs, there is considerable overlap between them as components of executive function (Bleckley, Durso, Crutchfield, Engle, & Khanna, 2003; McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010). This overlap may help explain how attention training normalized aberrant oscillatory activity during performance of a WM task, reducing the need to engage in compensatory recruitment. In a similar vein, we recently showed that attention training resulted in improved performance on an emotional Stroop task in veterans with PTSD (Khanna et al., 2015), supporting the assertion that attention training may exert part of its action through improved executive functioning.
Our current findings suggest that simple cognitive training geared to enhance inhibitory control modulates the brain network(s) serving WM processing, with neural responses in the prefrontal cortices and inferior parietal region becoming more like those of non-PTSD controls following therapy. Before closing, it is important to recognize several limitations of this study. First, the lack of a second MEG session in the healthy controls is probably the most significant limitation of the study. Such a repeated session would have provided a powerful control for natural variability over time in WM responses. We did not collect a second MEG session in controls because of the cost of MEG, and because there are many publications that have shown high test–retest reliability for MEG metrics (Ahonen, Huotilainen, & Brattico, 2016; Becker et al., 2012; Edgar et al., 2015; Martín-Buro, Garcés, & Maestú, 2016; Tan, Gross, & Uhlhaas, 2015). Importantly, Ahonen and colleagues (2016) found that neurophysiological responses during an N-back WM task were highly reliable. Nonetheless, a second MEG session in controls would have improved the study. Second, the relatively small sample size, lack of a placebo-treatment group, and that one third of the patients were receiving medication during the study are also limitations. Future studies should use larger and medication-free samples, and implement an attention training treatment protocol that includes a placebo group. Finally, our study also focused on men and combat-related trauma, as well as verbal WM (i.e., not spatial). Future studies should evaluate women, different types of trauma, and other WM and executive control tasks. Furthermore, the goal of this study was to identify whether attention training in general was associated with neurophysiological changes in PTSD. Future work should directly compare various forms of attention training (ACT, ABMT) and explore the use of attention training on other psychiatric conditions associated with executive function deficits. Nonetheless, our findings provide initial evidence that attention training improves aberrant neural activity in WM executive control networks in patients with PTSD.
Acknowledgments
This research was supported by a grant from the nonprofit organization At Ease, USA (ABB), by a Creighton University College of Arts and Science Summer Undergraduate Research Fellowship (TJM), and Grants R01-MH103220 from the National Institutes of Health (TWW) and #1539067 from the National Science Foundation (TWW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Compliance with ethical standards All research was conducted in compliance with ethical standards and was approved by the Creighton University IRB. All authors participated in the research and writing of this article.
Disclosures Mr. McDermott, Dr. Badura-Brack, Ms. Ryan, Ms. Becker, Dr. Bar-Haim, Dr. Pine, Dr. Khanna, Dr. Heinrichs-Graham, and Dr. Wilson report no competing financial or other interests.
References
- Ahonen L, Huotilainen M, Brattico E. Within- and between-session replicability of cognitive brain processes: An MEG study with an N-back task. Physiology & Behavior. 2016;158:43–53. doi: 10.1016/j.physbeh.2016.02.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Aupperle R, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, Bar-Haim Y. Effect of attention training on attention bias variability and PTSD symptoms: Randomized controlled trials in Israeli and U.S. combat veterans. American Journal of Psychiatry. 2015;172(12):1233–1241. doi: 10.1176/appi.ajp.2015.14121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43:724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Fabrizio M, Sudre G, Haridis A, Ambrose T, Aizenstein HJ, Bagic A. Potential utility of resting-state magnetoencephalography as a biomarker of CNS abnormality in HIV disease. Journal of Neuroscience Methods. 2012;206(2):176–182. doi: 10.1016/j.jneumeth.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current Biology. 2012;22:1969–1974. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Brookes M, Wood J, Stevenson C, Zumer J, White T, Liddle P, Morris P. Changes in brain network activity during working memory tasks: A magnetoencephalography study. NeuroImage. 2011;55:1804–1815. doi: 10.1016/j.neuroimage.2010.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Murray R, Kuschner ES, Pratt K, Paulson DN, Dell J, Roberts TP. The maturation of auditory responses in infants and young children: A cross-sectional study from 6 to 59 months. Frontiers in Neuroanatomy. 2015;9:131. doi: 10.3389/fnana.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: A basis for exact inference. Statistical Science. 2004;19:676–685. [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis towards the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood Anxiety Disorders. 2013;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex. 2015;69:121–130. doi: 10.1016/j.cortex.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. The European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Khanna MM, Badura-Brack AS, McDermott TJ, Shepherd A, Heinrichs-Graham E, Pine DS, Wilson TW. Attention training normalises combat-related post-traumatic stress disorder effects on emotional Stroop performance using lexically matched word lists. Cognition & Emotion. 2015 doi: 10.1080/02699931.2015.1076769. doi:10.1080/02699931.2015.1076769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koso M, Hansen S. Executive function and memory in post-traumatic stress disorder: A study of Bosnian war veterans. European Psychiatry. 2005;21:167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kuckertz JM, Amir N, Boffa JW, Warren CK, Rindt SE, Norman S, McLay R. The effectiveness of an attention bias modification program as an adjunctive treatment for post-traumatic stress disorder. Behaviour Research and Therapy. 2014;63:25–35. doi: 10.1016/j.brat.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. 2015;32:383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martín-Buro MC, Garcés P, Maestú F. Test-retest reliability of resting-state magnetoencephalography power in sensor and source space. Human Brain Mapping. 2016;37(1):179–190. doi: 10.1002/hbm.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology. 2010;23:222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: A MEG study. Journal of Psychiatry and Neuroscience. 2015;41(4):251–260. doi: 10.1503/jpn.150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Kulashekhar S, Hamalainen M, Palva JM. Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. Journal of Neuroscience. 2011;31:5013–5025. doi: 10.1523/JNEUROSCI.5592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proceedings of the National Academy of Sciences. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. NeuroImage. 2010;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation. 1977;13:135–145. [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Human Brain Mapping. 2016;37(6):2348–2361. doi: 10.1002/hbm.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoorl M, Putman P, Van Der Does W. Attentional bias modification in posttraumatic stress disorder: A randomized controlled trial. Psychotherapy and Psychosomatics. 2013;82:99–105. doi: 10.1159/000341920. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH. Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry. 2008;71(2):134–168. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Dalgleish T. Emotional working memory capacity in posttraumatic stress disorder (PTSD). Behaviour Research and Therapy. 2011;49:498–504. doi: 10.1016/j.brat.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Schweinsburg BC. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin. 2015;141:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Harnett-Sheehan K, Sheehan M, Gray C, Lecrubier Y, Even C. Mini international neuropsychiatric interview (Version 6.0.0) University of South Florida College of Medicine; Centre Hospitalier Sainte-Anne; Tampa, USA: Paris, France: 2009. [Google Scholar]
- Tan HR, Gross J, Uhlhaas PJ. MEG-measured auditory steady-state oscillations show high test-retest reliability: A sensor and source-space analysis. NeuroImage. 2015;122:417–426. doi: 10.1016/j.neuroimage.2015.07.055. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS). IEEE Transactions on Signal Processing. 2005;53:3359–3372. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping. 2007;28:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi R. Signal-space projection method for separating MEG or EEG into components. Medical & Biological Engineering & Computing. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Weathers F, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment. 1999;11:124–133. [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, McDermott TJ, Santamaria PM, Gendelman HE, Wilson TW. Quiet connections: Reduced fronto-temporal connectivity in nondemented Parkinson's disease during working memory encoding. Human Brain Mapping. 2016;37(9):3224–3235. doi: 10.1002/hbm.23237. [DOI] [PMC free article] [PubMed] [Google Scholar]