Abstract
Many adult stem cells divide asymmetrically, generating one stem cell (self-renewal) and one differentiating cell. Balancing self-renewal and differentiation is critical for sustaining tissue homeostasis throughout the life of an organism. Failure to execute asymmetric stem cell division can have profound impacts on tissue homeostasis, resulting in tissue degeneration or hyperplasia/tumorigenic overgrowth. Recent studies have expanded our understanding of both the extracellular and intracellular mechanisms that regulate, reinforce and ensure an asymmetric outcome following stem cell division. In this review, we discuss newly discovered aspects of asymmetric stem cell division that, in concert with well-established mechanisms, contribute to balancing self-renewal and differentiation.
Introduction: the most simplistic view of asymmetric stem cell division and its limitation
Adult stem cells have the capacity to both self-renew, by producing a new stem cell, and differentiate to produce a mature cell type such as neurons, epithelial cells and sperm. Adult stem cells contribute to tissue development and homeostasis through continuous production of differentiated cells. At the same time, stem cells need to self-renew or else risk exhausting the proliferative capacity of the tissue. This delicate balance between stem cell self-renewal and differentiation can be achieved through asymmetric stem cell division.
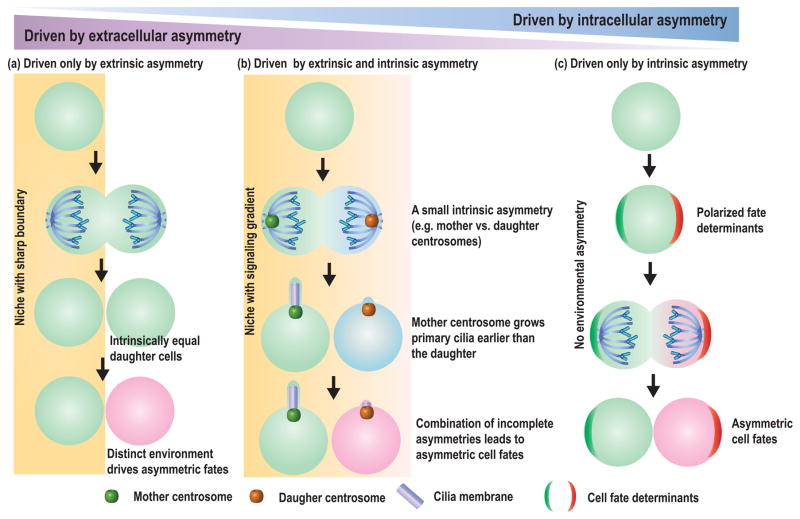
Asymmetric stem cell division is generally dictated by unequally distributed cell-extrinsic and/or -intrinsic fate determinants (Figure 1). In either case, spindle orientation plays a key role in achieving an asymmetric outcome after stem cell division by aligning the cell division plane with pre-established cell-extrinsic or -intrinsic asymmetries (Figure 1). The core machinery for orienting the spindle is evolutionarily conserved, and decades of study have provided critical insights into the molecular mechanisms of spindle orientation [1]. Although the detailed mechanisms regulating asymmetric cell division and spindle orientation have been elucidated largely using model organisms such as yeast, worm and flies, it has become clear that mammalian stem cells also utilize similar mechanisms [2,3]. Readers are directed to recent excellent reviews regarding the spindle orientation machinery [4,5], and further details of this machinery will not be discussed in this review.
Figure 1.
Mechanisms of asymmetric stem cell division. (a) Asymmetric stem cell division can be regulated by extrinsic fate determinants, such as those from the stem cell niche. The two daughters of the stem cell division will be placed in distinct cellular environments, either inside or outside the stem cell niche, resulting in the acquisition of different fates. (b) Asymmetric stem cell division can be modulated by intricate mechanisms that reinforce initial cell-extrinsic and/or -intrinsic asymmetries. For example, stem cell division might be barely asymmetric (e.g. slight differences due to the age of mother and daughter centrosomes and due to only a moderate gradient in signaling molecules). However, the mother centrosome grows a primary cilium earlier than the daughter, and as a result, the mother centrosome-containing cell might receive much higher levels of signal, leading to asymmetric cell fates. (c) Asymmetric stem cell division may rely on intrinsic fate determinants. Fate determinants are polarized in the dividing stem cells, leading to unequal distribution of these determinants following division and to two daughter cells with distinct fates.
In the most simplistic view, cell-extrinsic or -intrinsic fate determinants in combination with spindle orientation should suffice to explain asymmetric stem cell divisions: that is, if a master regulator of stem cell identity or differentiation is polarized within the stem cell, and the spindle is aligned such that 100% of the master regulator is inherited by only one daughter, one need not assume that influence by the extracellular environment affects asymmetric stem cell division (Fig 1). Vice versa, if the extracellular environment is set up such that spindle orientation would place the two daughter cells in distinct environments, which dictate either stem cell identity or differentiation, cells would not need intrinsic fate determinants. However, recent studies have illuminated the importance of intricate mechanisms that modulate and reinforce both cell-extrinsic and -intrinsic asymmetries in order to achieve a bipolar outcome following stem cell division. Such intricate mechanisms enable asymmetric divisions by solving problems inherent to “simplistic views” of asymmetric division described above. For example, the oriented spindle can place cells only one cell diameter away from each other, thus two daughter cells are placed right next to each other. How the tissue can ensure that these two daughter cells are placed in distinct signaling environments? In this review, we summarize critical aspects of asymmetric cell division with a particular focus on these and other emerging mechanisms that reinforce and ensure an asymmetric outcome of stem cell division.
Setting up and refining unequal environments
Many stem cells are specified by signals originating from their stem cell niche, the specialized microenvironment essential for stem cell identity. To divide asymmetrically within such a niche, one of the daughters of stem cell division must be placed inside of the niche while the other is placed outside of the niche. This is often achieved through spindle orientation (Figure 1). The two daughter cells of a stem cell division may not be necessarily intrinsically divergent, but they may acquire distinct fates through differential exposure to niche signaling.
The niche is thought to provide a short-range signaling environment to limit the number of stem cells in a tissue. To achieve asymmetric stem cell division through spindle orientation, the range of the niche signaling must be tightly controlled within a one cell diameter deviation. This way, when the stem cell divides with correct spindle orientation, one daughter cell will be placed outside the range of niche signaling (Figure 1). In this regard, Notch signaling is ideally suited for stem cell niches because its juxtacrine nature inherently requires cell-cell contact for signal transduction, limiting its range to one cell diameter. As such, it is utilized by many stem cells for self-renewal [6]. However, many other niche-derived ligands are known to diffuse over a long distance (many cell diameters) in other contexts. For example, in both the Drosophila male and female germlines, niche cells support germline stem cell (GSC) self-renewal by secreting a cytokine-like ligand Upd, and BMP (bone morphogenic protein) ligands Dpp/Gbb, both of which can diffuse far away from the source cells [7]. Similarly, other diffusible ligands including Wnt, Hh, Egf serve as niche factors in mammalian and Drosophila tissues [8]. Some of these ligands can diffuse over a long range (~500 μm) in certain contexts, such as when they function as morphogens [9]. Therefore, limiting the signaling range of niche ligands is vital for achieving asymmetric stem cell division by spindle orientation and concordantly, regulating the size of the stem cell population. Accordingly, a mechanism to precisely regulate the range of the niche signaling must be in place.
Recent studies have illuminated the involvement of cellular protrusions in delivering the niche ligand in a manner that is exclusive to stem cells, thereby limiting the range of the niche signaling. It was shown that Drosophila male GSCs form microtubule-based nanotubes (MT-nanotubes), which are inserted into the niche cells [10]. The interaction between the BMP ligand Dpp and its receptor Tkv is conducted predominantly on the surface of the MT-nanotubes. Therefore, differentiating cells are not exposed to niche-derived Dpp. In the Drosophila female GSC niche, a niche component known as a cap cell extends a cytoneme, a specialized filopodia, to locally deliver Hh ligands to adjacent escort cells, which in turn regulate the expression of BMP ligands to maintain GSCs [11].
Interestingly, another niche-derived ligand, Upd, is not regulated by the MT-nanotubes [10]. Instead, Upd’s signaling range might be regulated by its association with the extracellular matrix (ECM), which restricts its diffusion [12]. The ECM also regulates BMP signaling. ECM components, including Dally, Dally-like and likely Magu, function to concentrate the BMP ligand Dpp to the stem cell niche [13–15]. It is unknown how the MT-nanotube and the ECM collaborate to achieve a desirable mode of BMP signaling and therefore control the number of stem cells within the niche. Also, it remains elusive why the signaling range of two ligands (Upd and Dpp), secreted from the same source, to regulate the same stem cell population, needs to be regulated differently. Recently, it was shown that Wnt3 ligand secreted from Paneth cells, the niche component for mammalian intestinal stem cells, exists mainly in a membrane-bound form and does not diffuse away from the source, creating a limited niche space [16]. Altogether, these findings underscore the importance of strictly controlling the range of niche signaling such that the appropriate number of stem cells is maintained whereas the appropriate number of stem cell daughters leaves the niche for differentiation.
Intracellular asymmetries dictate unequal cell fates
Biased distribution of cell-intrinsic fate determinants within stem cells also contributes to asymmetric stem cell division (Figure 1). A classical model of this is found in the Drosophila neuroblast, which divides asymmetrically by segregating classical fate determinants such as Numb, Prospero and Brat to differentiating daughters [17].
However, recent studies have revealed that partitioning of fate determinants is not the sole cell-intrinsic method for dictating asymmetric stem cell division. Indeed, “fate modulators” of many forms have proven to be crucial in achieving an asymmetric outcome following cell division. These modulators may not determine cell fate directly but instead collaborate with extracellular signals to influence asymmetric stem cell division. For example, biased partitioning of Sara (Smad anchor for receptor activation) endosomes mediates differential endocytosis of the ligand Delta and the receptor Notch in the two daughter cells to modulate their Notch signaling levels, thus conferring different fates to the two daughter cells [18]. Asymmetric segregation of Sara endosomes plays an important role in the asymmetric divisions of sensory organ precursors and intestinal stem cells in Drosophila as well as the neural precursors in the zebrafish spinal cord [18–20]. This process requires spindle asymmetry, which is generated by microtubule motors, to distribute Sara endosomes asymmetrically [21].
Centrosomes have emerged as another organelle capable of modulating cell fate. Biased segregation of mother and daughter centrosomes has been observed in the asymmetric cell divisions of several stem cell systems. For instance, Drosophila male GSCs and mouse radial glial progenitor cells consistently inherit the mother centrosome, while Drosophila neuroblasts and female GSCs consistently inherit the daughter centrosome [22–26]. As centrosome asymmetry is associated with the differential ability of nucleating/anchoring microtubules [22], it is possible that the sole purpose for centrosomal asymmetry is proper spindle orientation. However, it has been shown that inheritance of the mother or daughter centrosome can lead to differential receptivity to extrinsic signaling in culture cell system [27]. It was shown that the mother centrosome grows a primary cilia much earlier than the daughter centrosome upon cell division, conferring a higher sensitivity to hedgehog (Hh) signaling to the mother centrosome-containing cell [27]. More recently, it was shown in mouse embryonic neocortical stem cell division that ciliary membrane is endocytosed together with the mother centriole at mitotic onset, providing “head-start” to the mother centriole to grow primary cilia in the next cell cycle [28]. This led to differential timing in accumulation of Smo in the primary cilia between two daughter cells, likely activating Hh signaling differentially in stem cells. Therefore, even if the mother or daughter centrosome does not confer cell fate on its own, it may lead to asymmetric cell fate by reinforcing asymmetries provided by the signaling environment (Fig 1). Such a strategy can achieve on/off fate determination, even if the ligand field is not sharp enough to discriminate between two juxtaposing cells.
At the interface of intracellular and extracellular asymmetries
Although the importance of extracellular and intracellular mechanisms for asymmetric stem cell division is well appreciated, significant gaps exist in our understanding of how intracellular machineries that orient the cell division are linked to the asymmetries provided by the extracellular environment. One simple mechanism is to utilize the interface between stem cells and the niche as a cue. As cells adhere to the niche, where they naturally/inevitably form cell-cell or cell-ECM adhesions, cell adhesion sites can be used as a cue for orienting the stem cells. Indeed, cell adhesions molecules play important roles for oriented cell divisions in many systems. For example, both cadherins and integrins have been reported to regulate spindle orientation in Drosophila and mammalian systems [29–32].
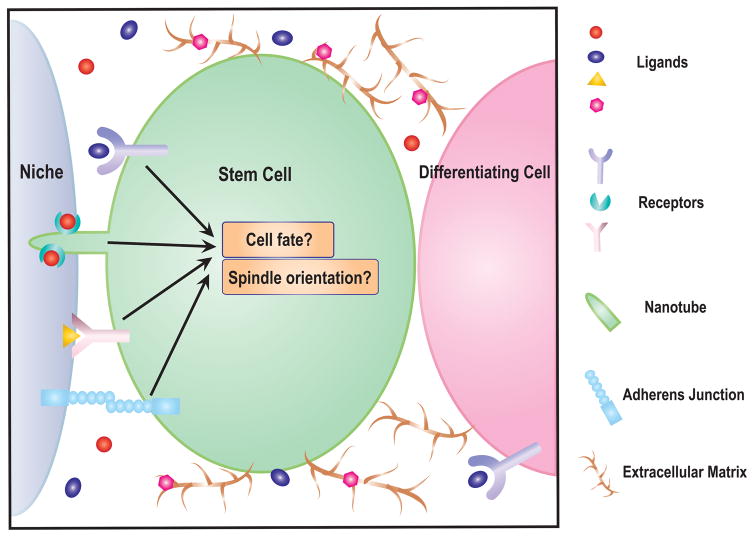
In addition, in cases where stem cells are adhered to the niche, one might wonder whether niche-derived signaling also contributes to stem cell-intrinsic asymmetries/spindle orientation (Fig 2). Such a mechanism would be ideally suited to link stem cell identity and asymmetric division. In C. elegans embryos, asymmetric microtubule organization and spindle orientation during the division of the EMS cell is controlled by Wnt ligand expressed in the neighboring cell [33]. A recent study has shown that spatially immobilized Wnt3a ligand on beads is sufficient to orient the division plane of mitotic mouse embryonic stem cells in in vitro culture [34]. The localized Wnt3a signal is capable of inducing asymmetric participation of Wnt pathway components, such as the receptor LRP6 and associated β-catenin, as well as biased inheritance of centrosomes. It remains unclear whether spindle orientation is regulated by changes in gene expression downstream of Wnt signaling or whether the ligand-receptor directly binds the cytoskeleton. This question will be difficult to unambiguously address, because loss of the signaling pathway will likely lead to loss of cell identity, which can indirectly influence spindle orientation.
Figure 2.
Various modes of regulation of niche signaling range. Niche-derived signaling molecules can be enriched in the vicinity of stem cells either by ligand-receptor interactions or by extracellular matrix components in order to generate a signal gradient across stem cells and their differentiating daughters for cell fate determination. Alternatively, niche signals can be precisely controlled by specialized subcellular structures including nanotubes and cytonemes in the stem cells (cytonemes are specialized filopodia. Although not drawn in the figure, cytonemes also concentrate ligands and receptors on their surface to allow very specific intercellular signaling events).
The mechanisms that ensure asymmetric stem cell division
As described above, many intracellular and extracellular factors function to achieve asymmetric cell divisions. It follows that a cell needs to be able to recognize when it is correctly polarized in order to ensure proper asymmetric division. Indeed, exquisite orientation control can be ensured by checkpoint mechanisms that function to dictate coordinated progression through the cell division cycle. The spindle position checkpoint (SPOC) in budding yeast is one such surveillance mechanism that specifically monitors spindle orientation and blocks mitotic exit if the spindle is misaligned [35]. A similar orientation checkpoint mechanism also exists in Drosophila male GSCs that monitors correct centrosome positioning before mitotic entry (centrosome orientation checkpoint (COC) [36–39]. The orientation checkpoint mechanisms are not well documented in other systems: however, it is conceivable that similar mechanisms ensure correct orientation in other stem cell systems, given the importance of asymmetric stem cell division.
Concluding Remarks
Recent discoveries have tremendously enriched our knowledge about how stem cells function to maintain tissue homeostasis. Accumulating knowledge highlights the importance of intracellular and extracellular mechanisms for asymmetric stem cell division. As we summarized here, asymmetric cell division is the outcome of many interlinked biological processes, which include setting up, modulating and reinforcing asymmetries using cell intrinsic and extrinsic mechanisms. Although each modular mechanism may be simple and comes with limited variation, combinatory use of those modular mechanisms likely allows distinct stem cell populations to adopt carefully tailored mechanisms of asymmetric cell divisions. This would allow context-dependent modulation of asymmetric cell divisions, such as the degree of flexibility in employing symmetric stem cell division and dedifferentiation, to maintain robust tissue homeostasis.
Acknowledgments
The research in the Yamashita laboratory is supported by the Howard Hughes Medical Institute and the National Institutes of Health (R01GM118308). J.F. is supported by a training grant from the University of Michigan Program in Cellular and Molecular Biology [T32 GM007315].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Inaba M, Yamashita YM. Asymmetric stem cell division: precision for robustness. Cell Stem Cell. 2012;11:461–469. doi: 10.1016/j.stem.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Seldin L, Muroyama A, Lechler T. NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife. 2016;5 doi: 10.7554/eLife.12504. This paper demonstrates that MuMA’s dynein-independent interaction with microtubules is critical for spindle orientation in mouse epidermis and hair follicle stem cells. This study clearly demonstrated the role of spindle orientation in morphogenesis of mammalian complex tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 5.Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan LJ, de Cuevas M, Matunis E. Genetics of gonadal stem cell renewal. Annu Rev Cell Dev Biol. 2015;31:291–315. doi: 10.1146/annurev-cellbio-100913-013344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller P, Rogers KW, Yu SR, Brand M, Schier AF. Morphogen transport. Development. 2013;140:1621–1638. doi: 10.1242/dev.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Inaba M, Buszczak M, Yamashita YM. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature. 2015;523:329–332. doi: 10.1038/nature14602. This study reveals that a previously unrecognized structure, the MT-nanotube, mediates selective BMP signaling for stem cell specification in the Drosophila male GSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. This paper reports cap cells extend cytonemes to deliver Hedgehog signals locally to neighboring escort cells for stem cell maintenance in the Drosophila female GSC niche. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12:3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Q, Wang Y, Vargas E, DiNardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–210. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. This study demonstrated that Wnt3 ligand secreted from Paneth cells does not diffuse, thereby creating a restricted niche space in the mammalian intestinal stem cell niche. [DOI] [PubMed] [Google Scholar]
- 17.Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139:4297–4310. doi: 10.1242/dev.080515. [DOI] [PubMed] [Google Scholar]
- 18.Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature. 2009;458:1051–1055. doi: 10.1038/nature07854. [DOI] [PubMed] [Google Scholar]
- 19.Montagne C, Gonzalez-Gaitan M. Sara endosomes and the asymmetric division of intestinal stem cells. Development. 2014;141:2014–2023. doi: 10.1242/dev.104240. [DOI] [PubMed] [Google Scholar]
- 20.Kressmann S, Campos C, Castanon I, Furthauer M, Gonzalez-Gaitan M. Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat Cell Biol. 2015;17:333–339. doi: 10.1038/ncb3119. [DOI] [PubMed] [Google Scholar]
- 21••.Derivery E, Seum C, Daeden A, Loubery S, Holtzer L, Julicher F, Gonzalez-Gaitan M. Polarized endosome dynamics by spindle asymmetry during asymmetric cell division. Nature. 2015;528:280–285. doi: 10.1038/nature16443. This paper identified key molecular players for generating spindle asymmetry in order to achieve asymmetric distribution of Sara endosomes in Drosophila sensory organ precursors. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol. 2010;20:2187–2192. doi: 10.1016/j.cub.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 24.Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzmann V, Chen C, Chiang CY, Tiyaboonchai A, Mayer M, Yamashita YM. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell. 2014;25:267–275. doi: 10.1091/mbc.E13-09-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Paridaen JT, Wilsch-Brauninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell. 2013;155:333–344. doi: 10.1016/j.cell.2013.08.060. This paper links cell fate determination with the signaling activity of the primary cilium by showing asymmetric segregation of the ciliary membrane into the stem cell daughter during embryonic neocortical stem cell division. [DOI] [PubMed] [Google Scholar]
- 29.Le Borgne R, Bellaiche Y, Schweisguth F. Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol. 2002;12:95–104. doi: 10.1016/s0960-9822(01)00648-0. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Minan A, Martin-Bermudo MD, Gonzalez-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 31.den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell. 2009;20:3740–3750. doi: 10.1091/mbc.E09-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petridou NI, Skourides PA. A ligand-independent integrin beta1 mechanosensory complex guides spindle orientation. Nat Commun. 2016;7:10899. doi: 10.1038/ncomms10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugioka K, Mizumoto K, Sawa H. Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell. 2011;146:942–954. doi: 10.1016/j.cell.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caydasi AK, Ibrahim B, Pereira G. Monitoring spindle orientation: Spindle position checkpoint in charge. Cell Div. 2010;5:28. doi: 10.1186/1747-1028-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan H, Chiang CY, Cheng J, Salzmann V, Yamashita YM. Regulation of cyclin A localization downstream of Par-1 function is critical for the centrosome orientation checkpoint in Drosophila male germline stem cells. Dev Biol. 2012;361:57–67. doi: 10.1016/j.ydbio.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inaba M, Venkei ZG, Yamashita YM. The polarity protein Baz forms a platform for the centrosome orientation during asymmetric stem cell division in the Drosophila male germline. Elife. 2015:4. doi: 10.7554/eLife.04960. [DOI] [PMC free article] [PubMed] [Google Scholar]