Abstract
Background
Insulin-like growth factor-1 (IGF-1) is neuroprotective after stroke and is regulated by insulin-like binding protein-3 (IGFBP-3). In healthy individuals, exercise and improved aerobic fitness (peak oxygen uptake; peak VO2) increases IGF-1 in circulation. Understanding the relationship between estimated pre-stroke aerobic fitness and IGF-1 and IGFBP-3 after stroke may provide insight into the benefits of exercise and aerobic fitness on stroke recovery.
Objective
The purpose of this study was to determine the relationship of IGF-1 and IGFBP-3 to estimated pre-stroke peak VO2 in individuals with acute stroke. We hypothesized that: 1) estimated pre-stroke peak VO2 would be related to IGF-1 and IGFBP-3; and 2) individuals with higher-than median IGF-1 levels will have higher estimated pre-stroke peak VO2 compared to those with lower-than median levels.
Methods
Fifteen individuals with acute stroke had blood sampled within 72 hours of hospital admission. Pre-stroke peak VO2 was estimated using a non-exercise prediction equation. IGF-1 and IGFBP-3 levels were quantified using enzyme-linked immunoassay.
Results
Estimated pre-stroke peak VO2 was significantly related to circulating IGF-1 levels (r = 0.60; p = .02), but not IGFBP-3. Individuals with higher-than median IGF-1 (117.9 ng/mL) had significantly better estimated aerobic fitness (32.4 ± 6.9 mL*kg−1*min−1) than those with lower-than median IGF-1 (20.7 ± 7.8 mL*kg−1*min−1; p = .03).
Conclusions
Improving aerobic fitness prior to stroke may be beneficial by increasing baseline IGF-1 levels. These results set the groundwork for future clinical trials to determine whether high IGF-1 and aerobic fitness are beneficial to stroke recovery by providing neuroprotection and improving function.
Introduction
Insulin-like growth factor-1 (IGF-1) is known to be neuroprotective after middle cerebral artery occlusion (MCAO) in animals1-4 and may even act as a neuronal rescue agent when given through intranasal delivery.2,5 When administered through intranasal delivery, core lesion size can be reduced up to 94%2 while also improving functional status compared to vehicle controls.4 In humans, those with high circulating levels of IGF-1 early after stroke have greater survival rates and fewer impairments, tested by the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS), at 3 and 24 months post-stroke compared to those with low IGF-1 levels.6-8 This suggests that IGF-1 may also have a neuroprotective effect in humans. Further, the molar ratio of IGF-1 and insulin-like growth factor binding protein-3 (IGFBP-3), IGF-1’s primary regulatory protein, has also been observed to be related to stroke recovery.7 Individuals with a high molar ratio of IGF-1 to IGFBP-3 exhibited better stroke outcomes at 3 months post-stroke, indicating that having low IGFBP-3 levels may also be beneficial to stroke recovery. Therefore, it is important to understand what may influence levels of IGF-1 and IGFBP-3 in individuals with stroke.
In healthy individuals, both circulating levels of IGF-1 and IGFBP-3 can be influenced by physical activity and aerobic fitness (peak oxygen consumption, peak VO2).9-13 Prior work has demonstrated that people who are more physically fit have better white matter integrity,14 regional brain volume,15 and brain blood flow.16 Therefore, aerobic fitness prior to a stroke may not only benefit overall brain health, but also provide some level of neuroprotection via circulating IGF-1, which has been shown to improve stroke outcomes.6-8 However, even though these studies have demonstrated that higher than median levels of circulating IGF-1 improve outcomes after stroke, there is lacking information regarding the relationship of pre-stroke aerobic fitness, IGF-1, and IGFBP-3 in individuals with acute stroke.
Assessing peak VO2 during the acute stroke hospital stay via a maximal exercise test and direct measurement of expired gases could be difficult if the person has unstable blood pressure, activity restrictions, or time constraints due to other standard of care testing. However, one potential alternative method for assessing pre-stroke aerobic fitness is through the use of a non-exercise prediction equation to estimate peak VO2. Current literature has shown that non-exercise equations to predict peak VO2 can be easily administered and provide useful alternatives to exercise testing in healthy adults and older adults.17,18 Further, we recently demonstrated the use of a non-exercise prediction equation18 to estimate pre-stroke peak VO2 during the acute stroke hospital stay.19 Quick, non-exercise, prediction equations would feasibly allow for the study of pre-stroke aerobic fitness (peak VO2) and its relationship to IGF-1 and IGFBP-3.
Therefore, the purpose of this study was to determine the relationship of IGF-1 and IGFBP-3 to estimated pre-stroke aerobic fitness (peak VO2) in individuals with acute stroke. We hypothesized that: 1) estimated pre-stroke peak VO2 would be significantly and positively correlated to IGF-1 and inversely correlated to IGFBP-3; and 2) individuals with higher than median circulating IGF-1 levels would have significantly higher estimated pre-stroke peak VO2 compared to those with lower than median levels.
Methods
Study Design
Institution approval from the Human Subjects Committee at the University of Kansas (KU) Medical Center was obtained before beginning the study (HSC#00000972). Written informed consent was obtained by every individual or their surrogate decision maker prior to study participation.
Participants
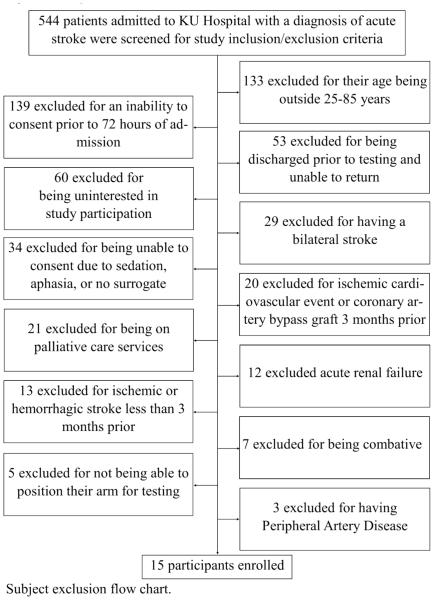
Individuals were a part of a larger, parent study and were therefore, eligible to participate if the met the parent study requirements. Individuals were eligible to be included if they had a confirmed diagnosis of acute ischemic or hemorrhagic stroke, were admitted to the certified Comprehensive Stroke units at the KU Hospital, and were between the ages of 25 and 85 years. Individuals were excluded from the study if they: 1) were not able to consent and begin data collection within 72 of admission; 2) had a diagnosis of bilateral stroke; 3) could not consent due to sedation, aphasia, and no surrogate consent present; 4) could not return for follow-up visit for the parent study; 5) could not position their arm for testing of the parent study; 6) had a diagnosis of acute renal failure; 7) a history of ischemic or hemorrhagic stroke, ischemic cardiovascular event, or coronary artery bypass graft (CABG) surgery less than 3 months prior to their current hospital admission; and 8) congestive heart failure; or 9) severe peripheral artery disease. Figure 1 describes the number of participants screened and their reasons for non-enrollment.
Figure 1.
Subject Exclusion Chart Subject exclusion chart
Estimated Pre-Stroke Peak VO2
Pre-stroke peak VO2 was estimated using the Jurca prediction equation.20 The Jurca prediction equation uses the following variables: sex, age, body mass index (BMI), resting heart rate (rHR), and a self-reported measure of physical activity. Using a questionnaire, participants or their surrogate decision maker were asked to estimate their average physical activity level prior to their stroke. The questionnaire consists of five categories that describe varying levels of physical activity over the course of a typical week. Participants (or their surrogate decision maker) were asked to select one of the five categories that most accurately describes their normal activities during a typical week prior to being admitted to the hospital. Each of the 5 categories is associated with a constant variable that is used in the prediction equation for calculation of estimated aerobic fitness (Level 1: 0.00 – Primarily sedentary; Level 2: 0.32 – Five days or more per week of light physical activity for 10 minutes or more at a time; Level 3: 1.06 – Moderate aerobic exercise for 20 to 60 minutes per week; Level 4: 1.76 – Moderate aerobic exercise for 1 to 3 hours per week; Level 5: 3.03 – Moderate aerobic exercise for over 3 hours per week).18 Other variables for the prediction equation (sex, age, BMI, rHR) were recorded immediately after consent into the study and within 72 hours of admission to the hospital. Resting heart rate was obtained after the participant was sitting or lying quietly for at least 30 minutes. Our previously published work showed that in a sample of individuals with acute stroke (n = 19), rHR was not significantly different between the rHR recorded during an outpatient, clinic visit from the electronic medical record prior to their stroke and the rHR recorded post-stroke.19 Further, we used electronic medical record to obtain height and weight, measured upon admission to KU Hospital, to calculate BMI. The equation to estimate peak VO2 is as follows: METs = [Sex, F = 0; M = 1 * 2.77) – (Age in years * 0.10) – (BMI * 0.17) – (rHR in bmp * 0.03) + (Physical Activity Constant * 1.00) + 18.07]. METs were then multiplied by 3.5 in order to obtain estimated pre-stroke peak VO2 (1 MET = 3.5 mL*kg−1*min−1).
Quantification of Biomarkers
Blood for quantification of IGF-1 and IGFBP-3 was obtained after an overnight fast, between the hours of 7:30 am and 9:00 am, and within 72 hours of stroke admission. Ten milliliters (mL) of blood from each participant was collected in a sodium heparinized tube from BD Biosciences (Becton, Dickinson and Company: Franklin Lakes, NJ). Collection tubes were immediately put on ice and transferred to the laboratory for processing. Within one hour of collection, blood samples were centrifuged to obtain plasma, aliquotted into 3, 1.5-mL polypropylene tubes, and frozen at −80 degrees Celsius until assaying. New aliquots were used for each assay to avoid multiple freeze/thaw cycles.
After all samples were collected, total IGF-1 (Alpco: Salem, NJ; cat#22-IGFHU-E01) and IGBP-3 (Alpco; Salem, NJ; cat#22-BP3HU-E01) were quantified using enzyme-linked immunoassays (ELISAs). To avoid protein shock, samples were slowly thawed to room temperature with ice prior to assaying. Procedures for assaying were performed exactly to the recommendations of the manufacturer in the manual provided with the ELISA kits.
Stroke Severity and Lesion Size
NIHSS was obtained by the admitting stroke physician and taken from the patients’ electronic medical record (EMR). A radiologist, and study team member, measured and calculated lesion volume from structural magnetic resonance imaging (MRI) performed upon admission to the hospital. Established, standardized techniques of neuroimaging using an ellipsoidal assumption of lesion shape was used.21,22 When multiple lesions were present, the largest lesion volume (cm3) was reported and used for analysis.
Data Analysis
Shapiro-Wilk tests were performed to determine whether estimated pre-stroke peak VO2 and IGF-1 levels were normally distributed. Pearson correlations were performed to determine the relationship of proteins (IGF-1 and IGFBP-3) to estimated pre-stroke peak VO2. Median levels of IGF-1 and IGFBP-3 were determined using descriptive statistics. T-tests were performed to determine the difference in estimated pre-stroke peak VO2 between above and below median values for both IGF-1 and IGFBP-3. All statistical tests were considered significant at the alpha-level of .05 and were performed using IBM ® SPSS ® Statistics Version 22 (SPSS, Inc., Chicago, IL). Pearson correlations were also performed to determine the association of lesion volume, stroke severity, and BMI to circulating IGF-1 and IGFBP-3 levels.
Results
Fifteen individuals with acute stroke (8 males, 61.1 ± 10.7 years) were enrolled into the study and completed data collection within 72 hours of hospital admission. Thirteen individuals completed data collection within 48 hours of admission. Table 1 describes the demographics of all participants, mean levels of IGF-1 and IGFBP-3, and the results of the non-exercise pre-stroke peak VO2 estimation equation.
Table 1.
Participant Demographics
| Characteristics, n = 15 | Number or Sample Mean (SD) | Range |
|---|---|---|
| Male | 8 | |
| Beta Adrenergic Receptor Blockers | 5 | |
| Age (years) | 61.1 (10.7) | (38-75) |
| Body Mass Index | 30.3 (5.7) | (22.8-45.7) |
| National Institutes of Health Stroke Scale | 8.5 (8.0) | (1-22) |
| Type of Stroke | ||
| Ischemic | 13 | |
| Hemorrhagic | 2 | |
| Blood Levels (ng/mL) | ||
| Median IGF-1 | 117.9 | (40.6-217.6) |
| Median IGFBP-3 | 2464 | (636-2937) |
| Estimated Pre-Stroke Peak VO2 (mL*kg−1*min−1) | 27.0 (9.3) | (8.8-45.9) |
| Resting Measures | ||
| Heart Rate (bpm) | 70 (12) | (53-99) |
| Systolic Blood Pressure (mmHg) | 145 (32) | (115-222) |
| Diastolic Blood Pressure (mmHg) | 73 (19) | (46-110) |
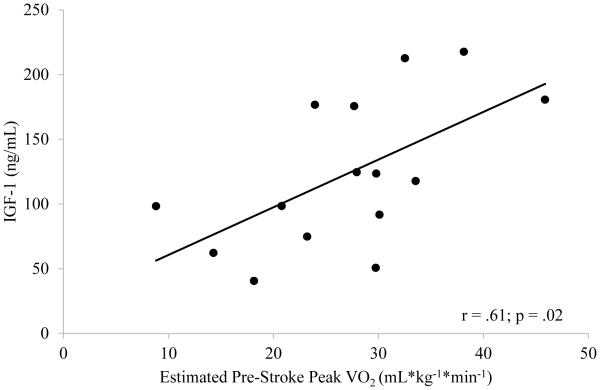
Shapiro-Wilk tests revealed that estimated pre-stroke peak VO2 (p = .93) and IGF-1 levels (p = .40) were both normally distributed. Estimated pre-stroke peak VO2 was significantly and positively related to IGF-1 levels sampled within 72 hours of stroke (r = .60; p = .02) (Figure 2), but not significantly related to IGFBP-3 (r = −.09; p = .75). Median level of IGF-1 and IGFBP-3 was 117.9 and 2464 ng/mL, respectively. Individuals with higher than median levels of IGF-1 during acute stroke had significantly better estimated peak VO2 (n = 8; 32.4 ± 6.9 mL*kg−1*min−1) compared to individuals with lower than median IGF-1 levels (n = 7; 20.7 ± 7.8 mL*kg−1*min−1; p = .03). There were no significant differences in estimated peak VO2 between higher- (n = 8; 29.7 ± 8.0 mL*kg−1*min−1) and lower-than median (n = 7; 23.8 ± 10.3 mL*kg− 1*min−1; p = .24) levels of IGFBP-3.
Figure 2. Relationship of Circulating IGF-1 and Estimated Pre-Stroke Peak VO2.
Individuals with higher estimated pre-stroke peak VO2 have elevated circulating IGF-1 levels during acute stroke.
Finally, circulating IGF-1 levels were not significantly related to lesion volume (r = −.15; p = .6), admitting NIHSS (r = −.36; p = .19), nor BMI (r = .02; p = .95). Circulating IGFBP-3 levels were also not significantly related to lesion volume (r = −.10; p = .74), admitting NIHSS (r = −.41; p = .13), nor BMI (r = .36; p = .18).
Discussion
Our hypotheses were partially supported in that estimated pre-stroke peak VO2 was related to circulating levels of IGF-1 early after stroke, but not to levels of IGFBP-3. Further, we found that individuals with higher than median levels of IGF-1 had greater estimated aerobic fitness (peak VO2) when compared to individuals with lower than median levels. The results of this work have important implications for future studies to investigate whether circulating IGF-1 levels and aerobic fitness are related to functional recovery following a stroke. These studies would be important for further development of future rehabilitation techniques to improve functional recovery in individuals with stroke.
Neuroprotection
IGF-1 is known to be neuroprotective after stroke.1-4 In humans, individuals with higher than median levels of IGF-1 during the early phase of stroke have higher survival rates and less severe impairments at 3, 6, and 24 months post-stroke compared to those with lower than median IGF-1 levels.6-8 Denti and colleagues examined IGF-1 and IGFBP-3 levels in 85 acute stroke participants.8 Stroke severity was assessed by the Barthel Index (BI) at 3 and 6 months post-stroke. Individuals with acute stroke who had IGF-1 levels less than 60 ng/mL had higher stroke severity and lower survival rates at 3 and 6 months post-stroke.8 DeSmedt further demonstrated the neuroprotective quality of baseline IGF-1 by examining IGF-1 and IGFBP-3 levels and stroke severity with the NIHSS and mRS.7 The results showed that even when controlling for cardiac risk factors, such as hypertension, diabetes, atrial fibrillation, coronary artery disease, heart failure, smoking, and previous stroke, lower than median IGF-1 levels were related to increased stroke severity and lower survival rates at 3 and 6 months post-stroke when compared to those with higher than median levels of IGF-1.7 There were no associations between IGFBP-3 levels and stroke severity. However, individuals with higher molar ratios of IGF-1 to IGFBP-3 had better stroke outcomes at 3 months post-stroke,7 indicating that IGFBP-3 may not directly be related to stroke recovery, but plays a role in regulating the availability of IGF-1, and is therefore important to consider. The studies by DeSmedt and Denti have demonstrated that higher than median circulating IGF-1 improves outcomes after stroke and, along with IGFBP-3, is important to stroke recovery. However, there is missing knowledge regarding the potential influence of factors such as pre-stroke peak VO2 and its relationship to IGF-1 and IGFBP-3 in individuals with acute stroke.
Aerobic Fitness
Aerobic fitness, tested by peak aerobic capacity (peak VO2), has been directly related to circulating IGF-1 levels in 846 healthy men.13 Blood was collected in the morning after an overnight fast for the quantification of IGF-1. Individuals then underwent a graded maximal exercise test on a cycle ergometer with direct gas analysis to obtain peak VO2. A repeated squats test was also performed to assess leg strength. Individuals with higher peak VO2 exhibited higher levels of circulating baseline IGF-1. Further, better aerobic fitness was associated with increased performance on the repeated squats test, indicating a greater functional performance in the lower extremities.13 Nindl’s study in healthy men could potentially have very large implications for individuals post-stroke. However, the relationship of exercise performance, specifically, aerobic fitness, to IGF-1 levels had not yet been tested in individuals with stroke prior to the current study, but gives important insight for which future studies could be based.
Testing aerobic fitness in individuals with stroke can be extremely difficult due to neuro-motor limitations in the lower extremities. In the acute setting, it is particularly challenging because of various factors such as time constraints for standard of care diagnostic assessments, a lack of trained professionals and specialized equipment, and extreme fatigue of the participant. However, our previous work displays feasibility of the use of a non-exercise prediction equation to estimate pre-stroke peak VO2 in individuals admitted to the hospital with a diagnosis of acute stroke.19 Our work showed that estimated pre-stroke peak VO2 was easily administered. The results of the current study corroborates these findings, but also shows that this easily-administered tool to estimate pre-stroke aerobic fitness may also influence IGF-1 levels in individuals with acute stroke.
The results of the current study are novel and important as we found a strong and significant relationship between estimated pre-stroke aerobic fitness (peak VO2) and IGF-1. Although this requires further exploration, it is plausible that pre-stroke aerobic fitness may have a potential benefit following stroke. In healthy men, those with higher peak VO2 and greater lower extremity functional ability, had higher levels of circulating baseline IGF-1.13 In our study, we also report that individuals with higher estimated pre-stroke peak VO2 have above median levels of IGF-1 when compared to individuals with lower than median levels of IGF-1. However, the current study does not answer questions regarding IGF-1 levels and functional performance of the lower extremity, but provides strong rationale for further investigation. Further, we did not observe a significant relationship between estimated pre-stroke peak VO2 and circulating IGFBP-3 levels. The lack of the hypothesized relationship could be due to many factors. In the study by DeSmedt, IGFBP-3 was not directly related to stroke outcomes, but the ratio of IGF-1 to IGFBP-3 was.7 It is possible that IGFBP-3 can be influenced by exercise short term, but baseline levels may not be directly related to aerobic fitness. Further, changes in IGFBP-3 circulating levels may depend on the type or duration of exercise.23
Limitations
We must acknowledge that the participants, or their surrogate decision maker, may report higher or lower activity levels, and thereby, influence the estimated pre-stroke peak VO2 estimation. From our prior work using the Jurca non-exercise estimation equation in acute stroke19 and others using the equation in community-dwelling adults and older adults with several cardiovascular risk factors,18,24 we believe this is a feasible and safe alternative in individuals who are within 72 hours of stroke
Further, the current study was limited by a small sample size and the results should be interpreted with caution. Despite this, we believe the results of our study give rise to further questions regarding estimated pre-stroke aerobic fitness, IGF-1, and functional recovery. Future work should address whether pre-stroke aerobic fitness and IGF-1 in acute stroke influence functional recovery and stroke outcomes.
Another potential limitation of the study was that lesion volume may be associated with circulating IGF-1 and IGFB-3 levels. However, in our sample, we did not see any significant relationships between IGF-1 and IGFBP-3 to lesion volume or stroke severity. Further, dietary intake, BMI, percent body fat, and body fat distribution could be related to circulating IGF-1 and IGFBP-3 levels. However, while we did not control for dietary intake or percent body fat/body fat distribution, our results showed no relationship between BMI and IGF-1 levels nor IGFBP-3 levels. Additionally, a study by Cappon and colleagues showed that increases in IGF-1 after a short bout of exercise were not significantly different after drinking a high fat meal, high-glucose meal, or a non-caloric placebo meal.10
Finally, blood was sampled, on average, within 48 hours of stroke admission. The sampling window used was more narrow than one study which sampled blood anywhere between 1 and 10 days post-stroke.6 Although we sampled blood within at most 72 hours, our time window for sampling blood was wider than previous studies examining IGF-1 and stroke outcomes.7,8 However, we believe that this would have minimal effect on our study as there is no suggested optimal time to obtain blood for the quantification of IGF-1 and IGFBP3.
Conclusion
This study investigated the relationship between IGF-1 and IGFBP-3 levels and pre-stroke estimated peak VO2 in individuals with acute stroke. We hypothesized that 1) pre-stroke estimated peak VO2 would be positively and significantly related to IGF-1 and negatively related to IGFBP-3; and 2) individuals with higher than median circulating IGF-1 levels would have significantly better estimated pre-stroke peak VO2 compared to individuals with lower than median levels of IGF-1. Our results partially supported this hypothesis in that we observed a significant, positive relationship between pre-stroke peak VO2 and circulating IGF-1 levels, but a non-significant relationship with IGFBP-3. Further, our second hypothesis was supported and demonstrated that individuals with higher than median levels of IGF-1 had higher pre-stroke estimated peak VO2, while individuals with lower than median levels of IGF-1 had a lower pre-stroke estimated peak VO2. The results of this study are novel and significant to the field in that they provide support for future studies for examining how aerobic fitness, IGF-1, and IGFBP-3 levels are related to recovery and physical function after stroke.
ACKNOWLEDGEMENTS
SAB was supported in part by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. AEM was supported in part by T32HD0577850 from the National Institutes of Health and in part by award number 14PRE20040026 from the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development nor American Heart Association. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund. The authors would also like to thank the KU hospital stroke unit for their support, participants, and their families for their time and dedication to this study.
REFERENCES
- 1.Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY. Insulin-like growth factor I signaling for brain recovery and exercise ability in brain ischemic rats. Med Sci Sports Exerc. 2011;43(12):2274–2280. doi: 10.1249/MSS.0b013e318223b5d9. [DOI] [PubMed] [Google Scholar]
- 2.Guan J, Williams C, Gunning M, Mallard C, Gluckman P. Effects of IGF1 treatment after hypoxic-ischemic brain injury in adult rats. Journal of Cerebral Blood Flow and Metabolism. 1993;13:609–616. doi: 10.1038/jcbfm.1993.79. [DOI] [PubMed] [Google Scholar]
- 3.Guan J, Williams CE, Skinner SJ, Mallard EC, Gluckman PD. The effects of insulin-like factor (IGF)-1, IGF-2, and des-IGF on neuronal loss after hypoxic-ischemic brain injury in adult rats: evidence for a role for IGF binding proteins. Endocrinology. 1996;137(3):893–898. doi: 10.1210/endo.137.3.8603600. [DOI] [PubMed] [Google Scholar]
- 4.Zheng HQ, Zhang LY, Luo J, et al. Physical exercise promotes recovery of neurological function after ischemic stroke in rats. Int J Mol Sci. 2014;15(6):10974–10988. doi: 10.3390/ijms150610974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypasses blood-brain barrier and protects against focal cerebral ischemic damage. Journal of the Neurological Sciences. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 6.Aberg D, Jood K, Blomstrand C, et al. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab. 2011;96(7):E1055–1064. doi: 10.1210/jc.2010-2802. [DOI] [PubMed] [Google Scholar]
- 7.De Smedt A, Brouns R, Uyttenboogaart M, et al. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42(8):2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- 8.Denti L, Annoni V, Cattadori E, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. The American Journal of Medicine. 2004;117(5):312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Variation in Plasma Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Protein-3: Personal and Lifestyle Factors (United States) Cancer Causes & Control. 2005;16(8):917–927. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 10.Cappon J, Brasel JA, Mohan S, Cooper DM. Effect of brief exercise on circulating insulin-like growth factor 1. Journal of Applied Physiology. 1994;76(6):2490–2496. doi: 10.1152/jappl.1994.76.6.2490. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low-and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. Journal of Clinical Endocrinology & Metabolism. 1996;81(10):3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- 12.Eliakim A, Brasel J, Mohan S, Barstowy T, Berman N, Cooper D. Physical fitness, endurance training, and the growth hormone-insulin-like-growth factor 1 system in adolescent females. J Clin Endocrin Metab. 1996;81(11):3986–3992. doi: 10.1210/jcem.81.11.8923848. [DOI] [PubMed] [Google Scholar]
- 13.Nindl BC, Santtila M, Vaara J, Hakkinen K, Kyrolainen H. Circulating IGF-I is associated with fitness and health outcomes in a population of 846 young healthy men. Growth Horm IGF Res. 2011;21(3):124–128. doi: 10.1016/j.ghir.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Tseng B, t. G, Khan M, et al. White matter integrity in physically fit older adults. Neuroimage. 2013:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng BY, Uh J, Rossetti HC, et al. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J Magn Reson Imaging. 2013;38(5):1169–1176. doi: 10.1002/jmri.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu N, Jacobs D, Schreiner P, et al. Cardiorespiratory fitness and brain volume and white matter integrity. Neurology. 2015;84:2347–2353. doi: 10.1212/WNL.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The rapid assessment of physical activity (RAPA) among older adults. Preventing Chronic Disease. 2006;3(4):1–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Jurca R, Jackson AS, LaMonte MJ, et al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29(3):185–193. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Mattlage A, Redlin S, Rosterman L, et al. Use of a non-exercise estimate for pre-stroke peak vo2 during the acute stroke hospital stay. Cardiopulm Phys Ther J. 2016 [PMC free article] [PubMed] [Google Scholar]
- 20.Juul A. Low Serum Insulin-Like Growth Factor I Is Associated With Increased Risk of Ischemic Heart Disease: A Population-Based Case-Control Study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 21.Majersik JJ, Cole JW, Golledge J, et al. Recommendations from the international stroke genetics consortium, part 1: standardized phenotypic data collection. Stroke. 2015;46(1):279–284. doi: 10.1161/STROKEAHA.114.006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman GC. Clarification of abc/2 rule for ICH volume. Stroke. 2007;38(3):862. doi: 10.1161/01.STR.0000257309.50643.0a. [DOI] [PubMed] [Google Scholar]
- 23.Chadan S, Dill R, Vanderhoeck K, Parkhouse W. Influence of physical activity on plasma IGF-1 and IGFBPs in healthy older women. Mech Ageing Dev. 1999;109:21–34. doi: 10.1016/s0047-6374(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 24.Mailey EL, White SM, Wojcicki TR, Szabo AN, Kramer AF, McAuley E. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. BMC Public Health. 2010;10:59. doi: 10.1186/1471-2458-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]