Abstract
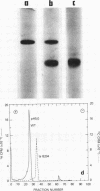
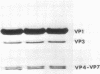
Intact wild-type simian virus 40 particles can be separated and resolved from a temperature-sensitive mutant and from a number of other viruses by agarose gel electrophoresis. The relative mobilities of the viruses appear to be a function of both virion size and surface composition. The virions of a temperature-sensitive strain of simian virus 40, tsB204, have significantly greater mobility than those of wild-type simian virus 40, when electrophoresis was conducted toward the cathode at pH 5.0. When electrophoresis was performed toward the anode at pH 7.0, TSB204 viruses have a slightly slower mobility as compared with that of the wild type. The data indicated that the virions of tsB204 have a greater positive charge at their surface than those of wild-type particles. No differences were detected in the finger print patterns of the tryptic peptides of VP1 and VP3 of these two virus strains. Although it was not possible to identify the structural polypeptide directly affected by the tsB204 mutation, we have shown that this mutation affects charge distribution on the surface of the virion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barban S. Electrophoretic differences in the capsid proteins of simian virus 40 plaque mutants. J Virol. 1973 Jun;11(6):971–977. doi: 10.1128/jvi.11.6.971-977.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban S., Goor R. S. Structural proteins of simian virus 40. J Virol. 1971 Feb;7(2):198–203. doi: 10.1128/jvi.7.2.198-203.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Products of complementation between temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):127–136. doi: 10.1128/jvi.15.1.127-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Edgell M. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XII. Phenotypic mixing between electrophoretic mutants of phi-X174. J Mol Biol. 1967 Feb 14;23(3):553–575. doi: 10.1016/s0022-2836(67)80125-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Lane L. C. The components of barley stripe mosaic and related viruses. Virology. 1974 Apr;58(2):323–333. doi: 10.1016/0042-6822(74)90068-3. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Two-dimensional polyacrylamide gel electrophoresis: an improved method for ribosomal proteins. Anal Biochem. 1974 Jan;57(1):200–210. doi: 10.1016/0003-2697(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Peterson J. I., Tipton H. W., Chrambach A. A gel slicer for the transverse sectioning of cyclindrical polyacrylamide gels. Anal Biochem. 1974 Nov;62(1):274–280. doi: 10.1016/0003-2697(74)90387-x. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R. J., Kaesberg P. Electrophoretic and other properties of bacteriophage Q : the effect of a variable number of read-through proteins. J Virol. 1973 Jan;11(1):116–128. doi: 10.1128/jvi.11.1.116-128.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S. Studies on electrophoretic heterogeneity in isometric plant viruses. Virology. 1966 Dec;30(4):698–704. doi: 10.1016/0042-6822(66)90174-7. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G., Casper R. Disc electrophoretic separation of elongated plant viruses in polyacrylamide-agarose gels. J Gen Virol. 1971 Sep;12(3):325–329. doi: 10.1099/0022-1317-12-3-325. [DOI] [PubMed] [Google Scholar]
- Wright P. J., Di Mayorca G. Virion polypeptide composition of the human papovavirus BK: comparison with simian virus 40 and polyoma virus. J Virol. 1975 Apr;15(4):828–835. doi: 10.1128/jvi.15.4.828-835.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]