Abstract
Objective
To prospectively examine associations between intakes of antioxidant vitamins, including vitamins E and C and carotenoids, and PD risk.
Methods
Cases were identified in two large cohorts: the Nurses’ Health Study and the Health Professionals Follow-up Study. Cohort members completed semi-quantitative food frequency questionnaires every four years.
Results
1036 PD cases were identified. Dietary intakes of vitamin E and carotenoids were not associated with PD risk; the multivariable-adjusted relative risk comparing extreme intake quintiles were 0.93 (95% CI 0.75-1.14) and 0.97 (95% CI 0.69-1.37), respectively. Dietary vitamin C intake was significantly associated with reduced PD risk (RR 0.81; 95% CI 0.65-1.01; ptrend 0.01); however, this result was not significant in a four-year lag analysis. For vitamins E and C, intake from foods and supplements combined were also unrelated to PD risk.
Conclusions
Our results do not support the hypothesis that intake of antioxidant vitamins reduces the risk of PD.
Keywords: Parkinson's disease, vitamin C, vitamin E, carotenoids, oxidative stress
Introduction
Multiple mechanisms may play a role in neurodegeneration in Parkinson's Disease (PD), including oxidative stress1-3. Since dietary antioxidants (e.g., vitamins E, C, and carotenoids) can prevent oxidative damage4, it has been hypothesized that these nutrients could protect against PD5. However, previous epidemiologic studies in this area have tended to be small and results have been inconsistent6-17. Therefore, we prospectively examined associations between intake of vitamin E, vitamin C, carotenoids, and risk of PD in the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). Results from these cohorts have been published previously11; however, we now present data after an additional 12 years of follow-up and over 1000 cases of PD.
Methods
Study population
The NHS cohort was established in 1976 when 121,700 female registered nurses aged 30-55 years completed a mailed questionnaire regarding their medical histories and health-related behaviors. The HPFS cohort was established in 1986 when 51,529 male health professionals aged 40-75 years responded to a similar questionnaire. Follow-up questionnaires have been sent to both cohorts every two years. We excluded individuals who reported implausible total energy intake at baseline (<660 or >3500 kcal/day for women and <800 or >4200 kcal/day for men), had a previous diagnosis of PD, or had missing baseline dietary information, leaving 80,750 women and 48,672 men for analysis.
Dietary assessment and other covariates
Participants completed validated FFQs18-21 that assessed how often over the past year they typically consumed a commonly used portion of each food. Responses ranged from “never” to “six or more times per day”. Nutrient intakes were computed by multiplying the frequency response by the nutrient content of the specified portion size, using data from the US Department of Agriculture and manufacturers. Data on supplemental vitamin use were also collected. For NHS diet was assessed in 1984 (baseline), 1986, and every four years thereafter. For HPFS, diet was assessed in 1986 (baseline) and every four years thereafter. Information on other covariates was also collected via self-report questionnaire.
Ascertainment of PD cases
PD cases were identified via biennial self-report questionnaires. We then contacted the treating neurologists and asked them to confirm the diagnosis or send a copy of the patients’ medical records. Prior to 2003, cases were confirmed if the treating neurologist or internist considered the diagnosis definite or probable, the medical record included a final diagnosis of PD by a neurologist, or the medical record indicated at least two of three cardinal signs of PD (resting tremor, rigidity, bradykinesia) in the absence of evidence for other diagnoses. Since 2003, the above procedure was used with the exception that medical records were requested from all cases and reviewed by a movement disorders specialist. Only confirmed cases were included in analyses.
Statistical analysis
Subjects contributed person-time from the age in months at the date of returning the baseline FFQ to the age in months at the date of first PD symptoms, death, last completed questionnaire, or end of follow-up (June 2010 for NHS and January 2010 for HPFS), whichever came first. Analyses were stratified by age in months at start of follow-up and calendar year of current questionnaire cycle. For nutrient analyses, PD incidence was related to cumulative updated average intake from all available FFQs up to the start of each two-year follow-up period, categorized by cohort-specific quintile of intake22. Secondary analyses were conducted using baseline nutrient intake levels. Models were fit for each exposure of interest: dietary vitamin E, dietary vitamin C, and dietary carotenoids. In addition, total vitamin E and vitamin C intake (including supplements) were investigated. Nutrients were adjusted for total energy using the residual method so that they would be uncorrelated with total energy intake22. Age-adjusted and multivariate-adjusted hazard ratios were calculated using Cox proportional hazards models. Multivariate models adjusted for pack years of smoking, coffee intake, body mass index, physical activity, alcohol intake, and total energy intake. Follow-up analyses separately examined each of five major carotenoids: lutein, lycopene, alpha-carotene, beta-carotene, and beta-cryptoxanthin. Results were pooled using random-effects methods. We conducted lagged analyses excluding the first four years of follow-up to address the possibility that participants could be experiencing PD symptoms at the time of questionnaire completion. Finally, we evaluated whether the association between vitamin E intake and PD risk was modified by dairy (high vs lower, based on median value), BMI (high vs lower), or the dietary urate index23 (comprised of intake of fructose, vitamin C, alcohol, and dairy protein; high vs lower) by including interaction terms in the models. We similarly evaluated potential effect modification between all exposures and caffeine intake (high vs lower). Statistical analyses were conducted using SAS (SAS Institute, Cary, NC).
Results
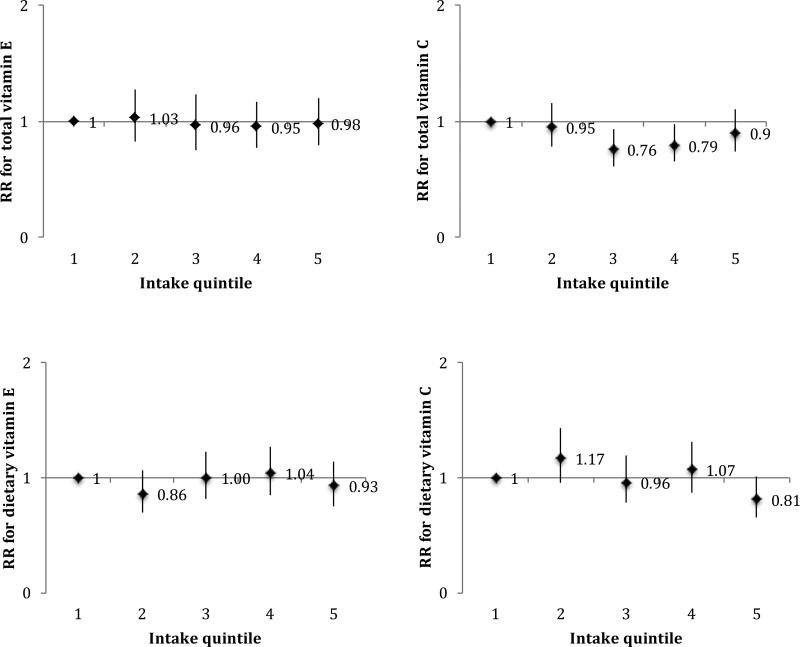
A total of 1036 cases (554 in HPFS and 482 in NHS) were observed. Baseline characteristics of each cohort are presented in Table 1. In pooled analyses, neither total vitamin E (multivariable-adjusted ptrend=0.82) nor total vitamin C (multivariable-adjusted ptrend=0.93) were significantly associated with risk of PD (Figure 1). Dietary vitamin E was also unassociated with risk of PD (multivariable-adjusted ptrend=0.93). Dietary vitamin C was inversely associated with PD risk (pooled RR comparing extreme quintiles=0.81; 95% CI 0.65-1.01; ptrend=0.01); however, this was no longer significant in a four-year lagged analysis (pooled RR 0.86; 95% CI 0.58-1.28; ptrend=0.30). We examined associations for dietary vitamins C and E after excluding supplement users and obtained similar results: the pooled RR for dietary vitamin C was 0.74 (95% CI, 0.55-0.98; ptrend=0.06) and for dietary vitamin E was 0.80 (95% CI, 0.62-1.03; ptrend=0.39). Again, results for dietary vitamin C were non-significant in a lagged analysis (pooled RR 0.85; 95% CI 0.51-1.42; ptrend=0.33).
Table 1.
Age-adjusted characteristics of the study population at baseline by quintile of total vitamin E intake
| Quintile of Vitamin E Intake | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Health Professionals Follow-up Study, 1986-2010 | n=10003 | n=9701 | n=9890 | n=9794 | n=9834 |
| Age, years* | 53.1(9.6) | 53.7(9.7) | 54.8(9.8) | 55.4(10.0) | 56.9(9.6) |
| Body mass index, kg/m2 | 25.7(3.3) | 25.7(3.3) | 25.7(3.6) | 25.4(3.3) | 25.2(3.2) |
| Current smoker, % | 13 | 10 | 8 | 9 | 9 |
| Past smoker, % | 42 | 44 | 44 | 46 | 44 |
| Caucasian, % | 95 | 96 | 95 | 96 | 96 |
| Activity, met-h/week | 15.4(22.8) | 17.5(24.4) | 19.6(26.9) | 20.0(26.6) | 21.3(29.3) |
| Coffee, servings/day | 1.5(1.7) | 1.4(1.6) | 1.3(1.6) | 1.3(1.5) | 1.1(1.5) |
| Alcohol, g/day | 13.6(18.4) | 10.7(14.5) | 10.4(14.0) | 10.9(14.7) | 11.3(15.3) |
| Total energy intake, kcal/day | 1730(529) | 1980(575) | 2171(634) | 2086(648) | 1961(614) |
| Nurses’ Health Study, 1984-2008 | n=15553 | n=16842 | n=16150 | n=16072 | n=16147 |
| Age, years* | 50.1(7.2) | 50.4(7.2) | 50.9(7.1) | 51.3(7.3) | 52.1(6.9) |
| Body mass index, kg/m2 | 25.8(5.0) | 26.1(5.1) | 26.3(5.1) | 25.7(4.8) | 25.4(4.7) |
| Current smoker, % | 32 | 25 | 22 | 22 | 21 |
| Past smoker, % | 27 | 30 | 33 | 34 | 35 |
| Caucasian, % | 98 | 98 | 97 | 98 | 98 |
| Activity, met-h/week | 10.7(16.8) | 12.6(18.7) | 14.8(21.2) | 15.5(22.1) | 16.7(24.2) |
| Coffee, servings/day | 1.9(1.8) | 1.9(1.8) | 1.8(1.8) | 1.7(1.7) | 1.6(1.7) |
| Alcohol, g/day | 7.8(13.4) | 6.5(10.4) | 6.5(10.3) | 6.8(11.0) | 6.9(11.0) |
| Total energy intake, kcal/day | 1495(447) | 1743(478) | 1935(534) | 1839(545) | 1702(528) |
Values are means(SD) or percentages and are standardized to the age distribution of the study population.
a Metabolic equivalents from recreational and leisure-time activities
Value is not age-adjusted
FIG. 1.
Associations of total and dietary vitamins E and C with PD according to intake quintiles, using cumulative average intake levels and adjusted for pack years of smoking, coffee intake, body mass index, physical activity, alcohol intake, and total energy intake. Median intake levels for each quintile of nutrients at baseline were as follows: for total vitamin E 6.0, 7.6, 9.3, 14.6, and 176.8 IU/day among women and 7.6, 9.6, 11.8, 18.8, and 193.6 IU/day among men; for total vitamin C 79, 130, 183, 302, and 825 mg/day among women and 95, 157, 228, 403, and 1159 mg/day among men; for dietary vitamin E 5.8, 6.8, 7.6, 8.5, and 10.2 IU/day among women, and 7.3, 8.7, 9.8, 11.1, and 13.7 IU/day among men; and for dietary vitamin C 67, 101, 128, 158, and 215 mg/day among women, and 78, 121, 154, 193, and 267 mg/day among men.
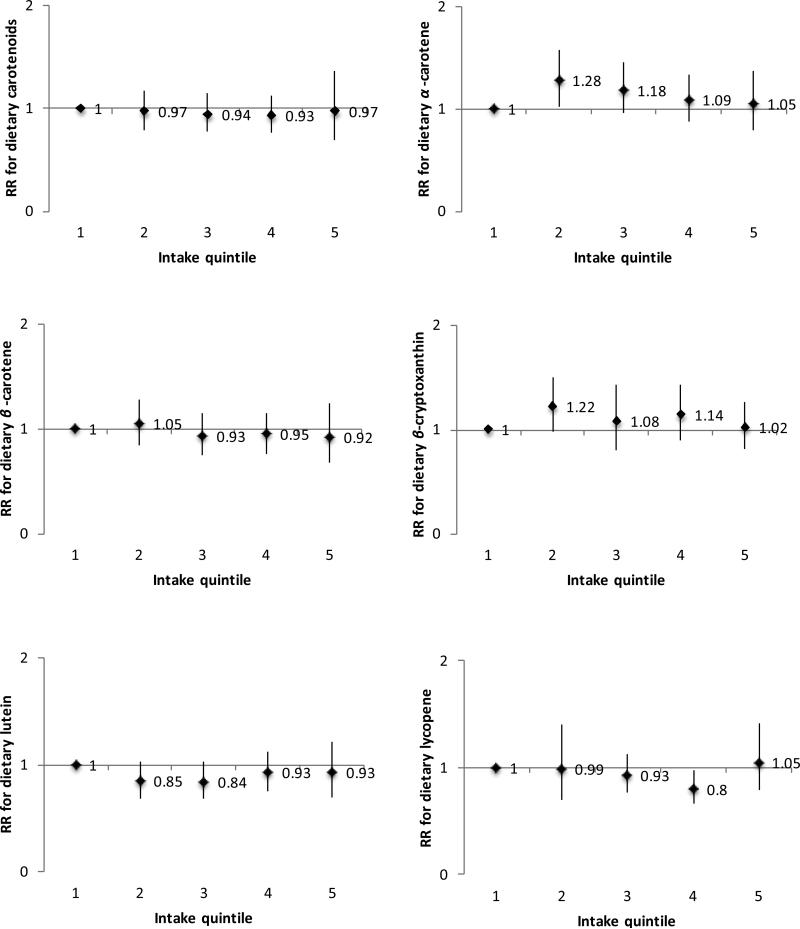
For dietary carotenoids, we found no significant association with PD risk (multivariable-adjusted ptrend=0.82). We also found no significant associations for individual carotenoids (Figure 2). Results were similar when using baseline nutrient intakes, as well as when restricting to non-smokers and to definite PD cases. Results were not substantially affected by conducting a lag analysis or after further adjustment for flavonoids, dairy, the Alternate Healthy Eating Index24 (comprised of fruit, vegetables, nuts, and other dietary components), the dietary urate index, NSAIDs, or multivitamin use. We also investigated duration of supplemental vitamin C or E use in relation to PD risk. We found no significant associations for duration of supplemental vitamin E use (compared with non-users, pooled multivariable-adjusted RR among users for ≥15 years=0.96 [95% CI, .76-1.20; ptrend=0.59]) or duration of supplemental vitamin C use (RR for ≥15 years of use=0.88 [95% CI, 0.73-1.07; ptrend=0.14). Finally, we observed no significant interactions between caffeine and any of the exposures, or between vitamin E and BMI, dairy, or dietary urate index (p>0.05 for all).
FIG. 2.
Associations of dietary carotenoids with PD according to intake quintiles, using cumulative average intake levels and adjusted for pack years of smoking, coffee intake, body mass index, physical activity, alcohol intake, and total energy intake. Median intake levels for each quintile of nutrients at baseline were as follows: for dietary carotenoids 7502, 10561, 13238, 16567, and 23008 mg/day among women and 7902, 11602, 14854, 18947, and 26940 mg/day among men; for b-cryptoxanthin 62, 115, 170, 228, and 328 mg/day among women, and 66, 126, 188, 256, and 384 mg/day among men; for acarotene 248, 407, 541, 837, and 1661 mg/day among women, and 269, 458, 617, 995, and 1933 mg/day among men; for b-carotene 1712, 2616, 3516, 4759, and 7292 mg/day among women, and 1902, 2935, 3958, 5383, and 8238 mg/day among men; for lutein 1137, 1903, 2576, 3383, 5165 mg/day among women and 1263, 2108, 2866, 3802, and 5838 mg/day among men; and for lycopene 2783,4302, 5553, 7223, 11001 mg/day among women and 2592, 4534, 6125, 8293, and 13426 mg/day among men.
Discussion
Our results suggest that greater intake of antioxidant vitamins may not reduce the risk of Parkinson's disease. Strengths of this study include the prospective design, high follow-up rates in both cohorts, the use of validated dietary assessment measures, and the large number of cases, which reduces the risk of false negative results.
Our study has some limitations. First, some degree of misclassification of nutrient intake is inevitable. Because of the prospective design, we expect that misclassification would be non-differential and result in bias toward the null, which could explain our findings. However, previous research suggests that our nutrient assessment method reflects long-term nutrient intakes reasonably well18-21, 25-27. In addition, we expect errors to be reduced by the use of repeated measures. Another limitation is the possibility that early PD symptoms influenced responses. However, we used first PD symptoms as our outcome to minimize this possibility. Additionally, the results of our lagged analysis suggest that reverse causation is unlikely.
The relation between dietary antioxidants and PD risk has been examined in several cross-sectional and retrospective studies. Vitamin E was inversely associated with PD risk in two studies8, 12, although in one study this association was only significant among women. Beta-carotene was also inversely associated with PD12. Another cross-sectional study reported an inverse association between PD risk and vitamin C and a non-significant inverse association with beta-carotene6. However, a positive association with PD risk has been reported for lutein13. In other case-control studies no associations were found between PD and vitamin C8, 9, 12, 14, 15, 16, vitamin E6, 9, 14, 15, 16, and carotenoids8, 12, 14, 15, 16 . These results, however, are difficult to interpret because of the potential impact of recall and selection bias.
While few prospective studies have been conducted examining these associations, results have tended to be null. Investigators from the Honolulu Heart Study Cohort identified 84 cases over 30 years and found no association between vitamin E and PD risk7. Results from another study in which 395 cases were identified over 17 years showed no association between vitamin C and PD risk10. In a Chinese cohort including 157 incident PD cases over 12 years, vitamin E intake was associated with lower PD risk; however, no association was found for vitamin C or carotenoids17. In a large, double-blind, placebo-controlled clinical trial, a daily dose of 2000 IU of alpha-tocopherol, a biologically active component of vitamin E, was not found to significantly improve the rate of progression of PD28. Previous results from our cohorts suggested a reduced risk of PD associated with high dietary vitamin E intake, but no association for supplemental vitamin E or for vitamin C or carotenoids. Our updated results suggest that the previous finding for dietary vitamin E intake could be due to chance.
It is important to note that our null findings do not invalidate the role of oxidative stress in PD. Importantly, urate has been found in prospective studies to be associated with a reduced risk of PD29-32, 36 and higher serum and cerebrospinal fluid concentrations of urate have been associated with slower rates of clinical decline33, 34; these associations may be attributable to urate's antioxidant properties. In addition, our group reported an inverse association between intake of flavonoids and PD risk, particularly among men, in the NHS and HPFS cohorts35. Although various mechanisms could drive this association, including regulation of mitochondrial function or inflammation, flavonoids may also have antioxidant properties that could have contributed to these results.
In conclusion, our results from two large prospective cohort studies provide evidence that intake of vitamin E, vitamin C, and carotenoids, at least at the levels consumed in our cohorts, does not substantially affect the risk of PD.
Supplementary Material
Acknowledgments
Study funding: This study was supported by NIH grants UM1 CA186107, UM1 CA167552, and R01 NS061858, and by Department of Defense grant W81XWH-14-0131.
Dr. Gao has served on committees of the Sleep Research Society, American Academy of Sleep Medicine, and Parkinson Study group and received funding from the NIH/NINDS. He serves on the editorial boards of Advances in Nutrition and Journal of Human Genetics.
Dr. Wang receives research support from the NIAID, NIEHS, NCI, NIDCD, NIDDK, NIAMS, American Institute for Cancer Research, and the Breast Cancer Research Foundation.
Dr. Weisskopf serves on the scientific advisory board for the Kaiser Permanente Research Biobank and the NIEHS GuLF Study. He has been funded by NIH grants R01ES 019188,R01ES024165, R21ES024700, R21ES019982, and R56NS082105. He has also received research support from the Muscular Dystrophy Association. He serves as an Associate Editor for Environmental Health Perspectives and the co-Editor in Chief of Current Environmental Health Reports.
Dr. Schwarzschild was a consultant for Biotie Therapies. He serves on the Scientific Advisory Board of CBD Solutions. He is funded NIH grants #NS090259 and #NS090246, DOD grants # W81XWH-11-1-0150 and #W81XWH-14-1-0131, the Parkinson's Disease Foundation, Target ALS, and the Michael J. Fox Foundation for Parkinson's Research.
Dr. Ascherio receives research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Department of Defense and served on a medical advisory board for Bayer HealthCare.
Footnotes
Statistical Analysis conducted by Katherine Hughes, Harvard T. H. Chan School of Public Health
Author Contributions:
Drafting/revising the manuscript (KCH, XG, IYK, ER, MW, MGW, MAS, AA), Study concept/design (AA, XG, MAS), analysis or interpretation of data (KCH, XG, ER, MW, MGW, MAS, AA), acquisition of data (XG, MAS, AA), study supervision (AA), obtaining funding (AA)
Disclosures:
Ms. Hughes reports no disclosures.
Ms. Kim reports no disclosures.
Dr. Rimm reports no disclosures.
References
- 1.Olanow CW. A radical hypothesis for neurodegeneration. Trends in neurosciences. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 2.Prasad KN, Cole WC, Hovland AR, et al. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Current opinion in neurology. 1999;12:761–770. doi: 10.1097/00019052-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Annals of the New York Academy of Sciences. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 4.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Annals of the New York Academy of Sciences. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt CR, Weber HK. Parkinson's disease: a chronic, low-grade antioxidant deficiency? Medical hypotheses. 1994;43:111–114. doi: 10.1016/0306-9877(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 6.Hellenbrand W, Boeing H, Robra BP, et al. Diet and Parkinson's disease. II: A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47:644–650. doi: 10.1212/wnl.47.3.644. [DOI] [PubMed] [Google Scholar]
- 7.Morens DM, Grandinetti A, Waslien CI, Park CB, Ross GW, White LR. Case-control study of idiopathic Parkinson's disease and dietary vitamin E intake. Neurology. 1996;46:1270–1274. doi: 10.1212/wnl.46.5.1270. [DOI] [PubMed] [Google Scholar]
- 8.de Rijk MC, Breteler MM, den Breeijen JH, et al. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Archives of neurology. 1997;54:762–765. doi: 10.1001/archneur.1997.00550180070015. [DOI] [PubMed] [Google Scholar]
- 9.Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Movement disorders : official journal of the Movement Disorder Society. 1999;14:21–27. doi: 10.1002/1531-8257(199901)14:1<21::aid-mds1006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Paganini-Hill A. Risk factors for parkinson's disease: the leisure world cohort study. Neuroepidemiology. 2001;20:118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 11.Zhang SM, Hernan MA, Chen H, Spiegelman D, Willett WC, Ascherio A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161–1169. doi: 10.1212/01.wnl.0000028688.75881.12. [DOI] [PubMed] [Google Scholar]
- 12.Miyake Y, Fukushima W, Tanaka K, et al. Dietary intake of antioxidant vitamins and risk of Parkinson's disease: a case-control study in Japan. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:106–113. doi: 10.1111/j.1468-1331.2010.03088.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson's disease. International journal of epidemiology. 1999;28:1102–1109. doi: 10.1093/ije/28.6.1102. [DOI] [PubMed] [Google Scholar]
- 14.Scheider WL, Hershey LA, Vena JE, Holmlund T, Marshall JR, Freudenheim Dietary antioxidants and other dietary factors in the etiology of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 1997;12:190–196. doi: 10.1002/mds.870120209. [DOI] [PubMed] [Google Scholar]
- 15.Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Jr., Swanson PD, Checkoway H. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology. 2003;60:1761–1766. doi: 10.1212/01.wnl.0000068021.13945.7f. [DOI] [PubMed] [Google Scholar]
- 16.Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Annals of neurology. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 17.Tan LC, Koh WP, Yuan JM, et al. Differential effects of black versus green tea on risk of Parkinson's disease in the Singapore Chinese Health Study. American journal of epidemiology. 2008;167:553–560. doi: 10.1093/aje/kwm338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 20.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. The Journal of nutrition. 1992;122:1792–1801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 21.Michaud DS, Giovannucci EL, Ascherio A, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7:283–290. [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American journal of epidemiology. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. American journal of epidemiology. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. The American journal of clinical nutrition. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett WC. Nutritional Epidemiology. 2 ed. Oxford University Press; New York: 1998. Reproducibility and Validity of Food-Frequency Questionnaires. [Google Scholar]
- 26.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. American journal of epidemiology. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 27.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 28.The Parkinson Study Group Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Ton TG, Boudreau RM, et al. The risk of Parkinson disease associated with urate in a community-based cohort of older adults. Neuroepidemiology. 2011;36:223–229. doi: 10.1159/000327748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. American journal of epidemiology. 2009;169:1064–1069. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Annals of neurology. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 32.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. American journal of epidemiology. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Archives of neurology. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Archives of neurology. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology. 2012;78:1138–1145. doi: 10.1212/WNL.0b013e31824f7fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X, O'Reilly EJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. 2016;86:520–6. doi: 10.1212/WNL.0000000000002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.