Abstract
Background
HIV-infected children receiving antiretroviral therapy (ART) have increased prevalence of hyperlipidemia and risk factors for cardiovascular disease. No studies have investigated the efficacy and safety of statins in this population.
Methods
HIV-infected youth aged 10 - < 24 years on stable ART with low-density lipoprotein-cholesterol (LDL-C) ≥130 mg/dL for ≥ 6 months initiated atorvastatin 10mg once daily. Atorvastatin was increased to 20mg if LDL-C efficacy criteria (LDL-C < 110 mg/dL or decreased ≥30% from baseline) were not met at week 4. Primary outcomes were safety and efficacy.
Results
Twenty-eight youth initiated atorvastatin; 7 were 10 - 15 years and 21 were 15 - 24 years. Mean baseline LDL-C was 161mg/dL (sd 19mg/dL). Efficacy criteria were met at week 4 by 17/27(63%). Atorvastatin was increased to 20mg in 10 participants. Mean LDL-C decreased from baseline by 30% (90% CI: 26%, 35%) at week 4, 28% (90% CI: 23%, 33%) at week 24, and 26% (90% CI: 20%, 33%) at week 48. LDL-C was less than 110 mg/dL in 44% at week 4, 42% at week 12, and 46% at weeks 24 and 48.Total cholesterol (TC), non-high-density lipoprotein (non-HDL)-C and apolipoprotein B (ApoB) decreased significantly, but IL-6 and high-sensitivity C-reactive protein did not. Two participants in the younger age group discontinued study for toxicities possibly related to atorvastatin.
Conclusions
Atorvastatin lowered TC, LDL-C, non-HDL-C and ApoB in HIV-infected youth with ART-associated hyperlipidemia. Atorvastatin could be considered for HIV-infected children with hyperlipidemia, but safety monitoring is important particularly in younger children.
Keywords: HIV, children, atorvastatin
Background
Accumulating evidence suggests that adults with human immunodeficiency virus (HIV) infection are at increased risk for early cardiovascular disease1-5. Statins have proven efficacy and safety for adults with HIV who meet criteria for treatment6
Guidelines from the National Heart, Lung and Blood Institute and the American Academy of Pediatrics7 provide recommendations for the use of statins in children and adolescents with hypercholesterolemia. HIV infection is one of the risk factors to be taken into account when determining the low-density lipoprotein cholesterol (LDL-C) level at which drug therapy should be considered. Elevated cholesterol levels are common in ART-treated HIV-infected children with greatest increases associated with use of protease inhibitors8-13; however, there are no clear recommendations for management of ART-associated hyperlipidemia in children and adolescents.
Atorvastatin is US Food and Drug Administration (FDA)-labeled for treatment of heterozygous familial hypercholesterolemia in adolescents and children as young as 10 years 14-18, but no studies of atorvastatin (or any statin) treatment have been reported in HIV-infected children and adolescents. The purpose of this study was to investigate the safety and efficacy of daily atorvastatin in HIV-infected children, adolescents and young adults with elevated LDL-C.
Methods
Participants
Subjects were HIV-infected children, adolescents and young adults aged 10 to < 24 years on stable antiretroviral regimens for at least six months prior to study entry (hereafter, the term “youth” will be used to indicate the entire age range). Participation required direct LDL-C ≥ 130 mg/dL at study screening and at least twice over the previous 6 months (fasted calculated LDL-C allowed for these measurements), with documented attempts at modifying diet and other risk factors for at least 3 months. Other inclusion criteria included: CD4% ≥ 15%, HIV-1 RNA ≤ 10,000 copies/mL and Tanner stage ≥ 2. Participants were excluded for laboratory values above grade 2 toxicity criteria (above grade 0 for liver enzymes and creatine kinase (CK)), past myopathy or neuromuscular disorder, chronic myositis, hepatitis, diabetes mellitus (DM), use of contraindicated medications, chemotherapy and pregnancy. The protocol was approved by the institutional review board at each participating site and all guardians and children, as appropriate, gave written consent.
Study Design
International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1063 was a phase I/II, single-arm safety and efficacy study of atorvastatin (Lipitor®) for the treatment of elevated LDL cholesterol in HIV-infected youth. Enrollment was stratified by age group, with a target of 20 participants 10 - < 15 years of age and 20 ≥ 15 - < 24 years of age. Enrollment was discontinued prematurely for administrative reasons after 28 participants enrolled. Participants started on atorvastatin at 10 mg once daily. If the participant's direct LDL-C was either reduced to <110 mg/dL or declined by ≥30% from baseline at week 4, the participant continued on the 10 mg daily dose. Otherwise, the participant's dose was increased to 20 mg once daily starting from week 8, provided that the participant did not develop any atorvastatin-related Grade ≥ 3 toxicity (or alanine amino-transferase (ALT) or aspartate amino-transferase (AST) ≥ Grade 2) by week 8. Study duration was 48 weeks.
Laboratory Assessments
Fasting (minimum 8 hours) sera were obtained at screening, entry, and weeks 4, 12, 24 and 48. Sera in serum separator tubes were allowed to clot for 30 minutes at ambient temperature and subsequently centrifuged within 1 hour. Aliquots were preserved at ≤ -70°C within 8 hours of collection.
Ultrafrozen sera aliquots were shipped to Quest Diagnostics, Baltimore for real-time determination of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), directly measured LDL-C and triglycerides which were assayed using commercially available FDA-cleared methods 19-22. Additional aliquots were stored at ≤ -70°C for end of study batch testing of specialty chemistry analytes. Apolipoproteins A1 and B and high sensitivity interleukin-6 (IL-6) were measured at Quest Diagnostics Nichols Institute21,23,24. High sensitivity C-reactive protein (hsCRP) was tested with an FDA-cleared, particle enhanced immunonephelometric assay at Quest Diagnostics, Baltimore23,25.
Adherence assessment
Adherence to ART and atorvastatin for the prior 3 days and for the last month was assessed using a standardized questionnaire at each study visit. Adherence was defined as excellent if the participants reported no missed doses in the previous 3 days.
Primary Efficacy Endpoint
Baseline LDL-C was defined as the mean of the direct LDL-C values at screening and entry. Subjects were identified as having an efficacious outcome if their LDL-C was < 110 mg/dL or if their LDL-C decreased by at least 30% from baseline at a given visit.
Toxicity Management
Safety labs were measured at each study visit. The study team reviewed all laboratory values ≥ Grade 2 for relatedness to atorvastatin and all adverse events were reviewed by the IMPAACT Study Monitoring Committee. Safety endpoints included any Grade ≥3 toxicity; AST and ALT ≥ Grade 2; total amylase and direct bilirubin ≥ 2 times the upper limit of normal (ULN).
Statistical Analyses
Baseline values for lipids and inflammatory markers were calculated by taking the mean of screening and entry (week 0) values. Intent-to-treat analysis of efficacy included all participants who started study drug; those without a fasting direct LDL-C at the relevant follow-up week were considered failures. The as-treated analysis of efficacy included those participants who were on study treatment and had direct LDL-C data available at the specified follow-up week. Exact 90% confidence intervals for proportions were computed based on the binomial distribution. Mean percent change from baseline and corresponding 90% confidence intervals were computed for normally distributed data, and median percent change from baseline and corresponding 90% confidence intervals were computed for highly skewed data. The use of a 90% level of confidence reflects the intent of the study to produce pilot estimates of safety and efficacy outcomes in this population for descriptive purposes rather than to conduct hypothesis testing. Statistical analysis and graphics were performed using SAS 9.4 (SAS Institute, Cary NC).
Results
Baseline Characteristics of Participants
All 28 participants initiated study treatment within three days of enrollment. The median age was 17 years (range 10-23), 68% of participants were female, and 64% were black non-Hispanic (Table 1). Median body mass index was 22.7 kg/m2. Median CD4 percent was 36% at screening. Plasma HIV-1 RNA was below the lower limit of quantitation of the local site assay in 71% of participants. ART regimens at study entry contained at least one non-nucleoside reverse transcriptase inhibitor (NNRTI) for 32% of participants and at least one protease inhibitor (PI) for 79% of participants.
Table 1. Baseline Characteristics of N=28 HIV-Infected Children, Adolescents and Young Adults with Elevated LDL Cholesterol.
| Characteristic | Number (%) | |
|---|---|---|
| Age at study entry (years) | Mean (s.d.) | 17 (4) |
| Min, Max | 10, 23 | |
| Median | 17 | |
| Age Group | ≥10 to <15 years old | 7 (25%) |
| 15 to <19 years old | 12 (43%) | |
| 19 to <24 years old | 9 (32%) | |
| Sex | Male | 9 (32%) |
| Female | 19 (68%) | |
| Race/Ethnicity | White Non-Hispanic | 4 (14%) |
| Black Non-Hispanic | 18 (64%) | |
| Hispanic (Regardless of Race) | 5 (18%) | |
| Asian, Pacific Islander | 1 (4%) | |
| BMI (kg/m2) at entry1 | Mean (s.d.) | 25.6 (9.5) |
| Min, Max | 14.7, 55.8 | |
| Median | 22.7 | |
| CD4 Percent at screening | 15 to <25% | 2 (7%) |
| ≥25% | 26 (93%) | |
| HIV-1 RNA (copies/mL)2 | ≥LLQ | 8 (29%) |
| <LLQ | 20 (71%) | |
| ARV regimen at entry | At least 1 PI3 and at least 1 NNRTI | 5 (18%) |
| At least 1 PI and no NNRTI | 17 (61%) | |
| At least 1 NNRTI and no PI | 4 (14%) | |
| Other ARV regimen | 2 (7%) |
Screening data were used when entry data were unavailable; BMI was missing for 1 participant at both screening and entry.
LLQ = Lower limit of quantification of assay
All PIs were boosted except for one participant
Application of Study Algorithm and Efficacy Endpoints
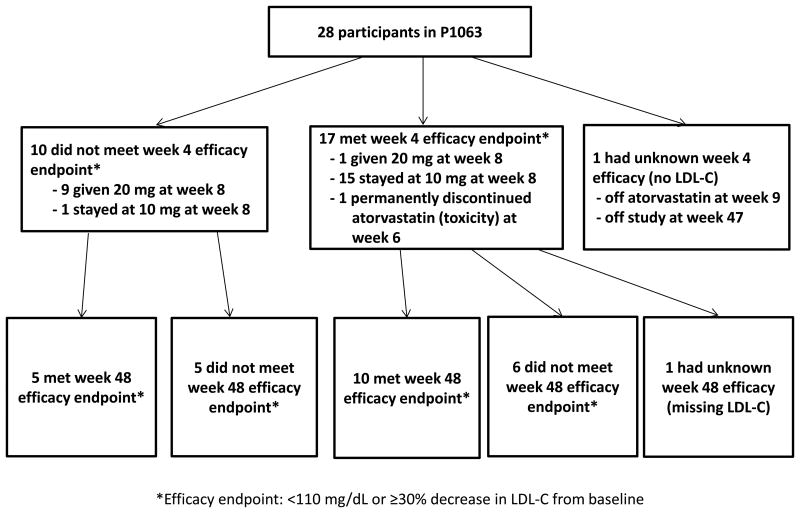
Seventeen of the 28 participants (61%) met the efficacy endpoint at week 4 (Figure 1) and were eligible to remain on the starting dose of 10 mg until they completed the study. Fifteen remained on the starting dose until week 48. One participant received an unintended dose increase to 20 mg from week 8 to week 48. Another participant experienced a toxicity potentially related to atorvastatin at week 6 and was taken off study treatment. The latter participant had a rebound in LDL-C at week 12 and had missing lipid evaluations for the remainder of the study. Ten of the 28 study participants (36%) did not meet the week 4 efficacy endpoint, and all except one of these received the protocol-prescribed dose increase to 20 mg from week 8 until week 48. The participant who was not dose-escalated (and continued on 10 mg until week 48) entered the study with a Grade 2 rash that worsened to a Grade 3 by week 4, prompting the family to refuse the dose increase. The rash ultimately resolved and was deemed to be unrelated to atorvastatin. Finally, one participant had unknown LDL-C at week 4, was non-adherent to study visits and medication, and was taken off study treatment at week 9 and off study at week 47. Mean baseline LDL-C was similar between those who met efficacy criteria at week 4 (161 mg/dL, SD 17 mg/dL) and those who required dose escalation (163 mg/dL, SD 24 md/dL).
Figure 1. Study profile.
LDL-C = low density lipoprotein cholesterol.
Based on an intent-to-treat analysis with all 28 participants who started study medication included in the denominator, 61% (90% CI: 44%, 76%) met efficacy criteria at week 4, 46% (90% CI: 30%, 63%) at week 12, 57% (90% CI: 40%, 73%) at week 24 and 54% (90% CI: 37%, 70%) at week 48. Among those participants who were on study treatment and had LDL-C data available at the specified time point, 63% (90% CI: 45%, 78%) met efficacy criteria at week 4, 50% (90% CI: 33%, 67%) at week 12, 62% (90% CI: 44%, 77%) at week 24, and 58% (90 % CI: 40%, 74%) at week 48.
Changes in Lipid Concentrations
Baseline mean direct LDL-C of the 28 participants and decreases by study week are detailed in Table 2 and Figure 2. The percentage of participants with LDL-C less than 110 mg/dL was 44% (90% CI: 28%, 62%) at week 4, 42% (90% CI: 26%, 60%) at week 12, and 46% (90% CI: 29%, 64%) at weeks 24 and 48. LDL-C concentrations after baseline were fairly stable over the 48 weeks of the study for the majority of participants, although some individuals had noticeable variation in their LDL-C concentrations at different study visits.
Table 2. Lipid and lipoprotein concentrations (in mg/dL) before atorvastatin treatment and changes from baseline during atorvastatin treatment.
| Baseline | Week 4 | Week 12 | Week 24 | Week 48 | ||
|---|---|---|---|---|---|---|
| (N=28) | (N=27) | (N=26) | (N=26) | (N=26) | ||
| LDL cholesterol (mg/dL)1 | Mean (s.d.) | 161 (19) | 112 (21) | 117 (30) | 116 (27) | 117 (27) |
| Median (Min, Max) | 162 (133, 207) | 113 (74, 165) | 114 (68, 179) | 110 (84, 173) | 115 (67, 164) | |
| Mean % change (90% CI) | -30.3 (-34.6, -26.1) | -27.3 (-33.3, -21.4) | -28.0 (-32.7, -23.4) | -26.4 (-33.0, -19.7) | ||
| HDL cholesterol (mg/dL) | Mean (s.d.) | 51 (13) | 51 (11) | 52 (15) | 52 (14) | 52 (13) |
| Median (Min, Max) | 50 (31, 77) | 49 (35, 80) | 50 (25, 88) | 48 (36, 90) | 53 (30, 84) | |
| Mean % change (90% CI) | 1.8 (-2.5, 6.1) | 2.2 (-1.4, 5.8) | 3.0 (-3.1, 9.1) | 4.2 (-3.5, 11.9) | ||
| Total Cholesterol (mg/dL) | Mean (s.d.) | 237 (27) | 182 (27) | 186 (34) | 184 (32) | 185 (29) |
| Median (Min, Max) | 240 (179, 285) | 178 (135, 235) | 187 (113, 249) | 176 (142, 261) | 185 (120, 248) | |
| Mean % change (90% CI) | -23.8 (-26.8, -20.8) | -21.8 (-25.8, -17.9) | -22.5 (-26.0, -19.0) | -21.5 (-26.4, -16.6) | ||
| Non-HDL cholesterol (mg/dL) | Mean (s.d.) | 187 (26) | 130 (27) | 133 (29) | 133 (29) | 133 (30) |
| Median (Min, Max) | 186 (136, 250) | 129 (86, 185) | 132 (79, 181) | 124 (99, 192) | 130 (74, 197) | |
| Mean % change (90% CI) | -30.6 (-34.3, -26.9) | -28.4 (-32.9, -23.9) | -28.9 (-33.0, -24.8) | -28.0 (-34.0, -22.1) | ||
| Triglycerides (mg/dL) | Mean (s.d.) | 160 (89) | 136 (64) | 143 (82) | 130 (50) | 142 (103) |
| Median (Min, Max) | 124 (61, 387) | 121 (38, 272) | 126 (32, 390) | 121 (57, 250) | 111 (44, 566) | |
| Mean % change (90% CI) | -9.5 (-20.4, 1.4) | -12.2 (-19.3, -5.1) | -11.3 (-22.8, 0.3) | -12.6 (-22.5, -2.7) | ||
|
| ||||||
| Baseline | Week 12 | Week 24 | Week 48 | |||
| (N=27) | (N=24) | (N=23) | (N=24) | |||
|
| ||||||
| Apolipoprotein A1 (mg/dL) | Mean (s.d.) | 140 (22) | N/A | 142 (28) | 143 (22) | 142 (22) |
| Median (Min, Max) | 142 (91, 195) | 139 (79, 198) | 141 (101, 206) | 146 (104, 172) | ||
| Mean % change (90% CI) | 0.8 (-3.2, 4.8) | 2.4 (-2.6, 7.5) | 0.3 (-4.8, 5.4) | |||
| Apolipoprotein B (mg/dL) | Mean (s.d.) | 127 (20) | N/A | 92 (19) | 94 (19) | 95 (23) |
| Median (Min, Max) | 128 (88, 176) | 95 (58, 127) | 87 (68, 131) | 90 (58, 135) | ||
| Mean % change (90% CI) | -27.2 (-32.1, -22.4) | -25.1 (-29.6, -20.7) | -23.8 (-30.5, -17.2) | |||
| Apolipoprotein B/A1 Ratio | Mean (s.d.) | 0.93 (0.22) | N/A | 0.66 (0.15) | 0.67 (0.17) | 0.69 (0.21) |
| Median (Min, Max) | 0.92 (0.61, 1.56) | 0.70 (0.34, 0.90) | 0.67 (0.43, 1.05) | 0.65 (0.42, 1.29) | ||
| Mean % change (90% CI) | -27.3 (-32.3, -22.3) | -25.6 (-31.5, -19.7) | -23.5 (-30.1, -16.9) | |||
Primary efficacy measure s.d.= standard deviation CI= confidence interval
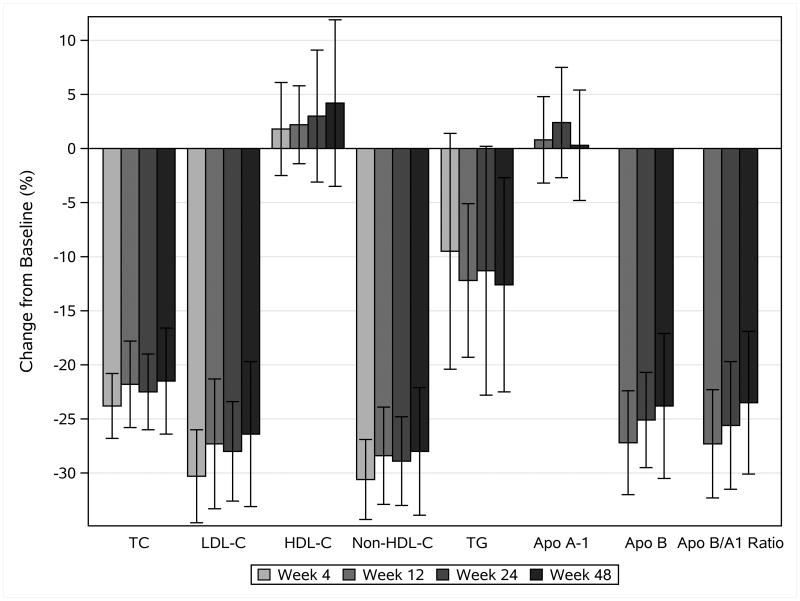
Figure 2.
Mean percent change in lipid and lipoprotein concentrations from baseline during atorvastatin treatment. Error bars show 90% confidence intervals. TC= total cholesterol, LDL-C = low density lipoprotein cholesterol, HDL-C= high density lipoprotein cholesterol, TG= triglyceride, Apo A-1= apolipoprotein A-1, Apo B= apolipoprotein B
Table 2 and Figure 2 also show changes in other fasting lipids and apolipoproteins by study week. Mean HDL-C did not change substantially over the course of the study. Mean TC, mean non-HDL-C, and mean triglyceride concentrations demonstrated similar patterns of change over time, declining from baseline to week 4 and then remaining relatively constant for the remainder of follow-up.
Mean ApoA1 did not change throughout the study, while mean ApoB and ApoB/A1 declined by 27% (90% CI: 22%, 32%) from baseline to Week 12 and were steady for the remainder of study follow-up.
Safety Events and Drug Toxicity
Two participants [7%; 90% CI: 1% - 21%] discontinued study treatment due to toxicities possibly related to atorvastatin by week 48. Both participants were in the younger age group (10-<15 years old), were receiving zidovudine/lamivudine/lopinavir/ritonavir at study entry and were receiving 10mg atorvastatin. One participant had a Grade 3 creatinine elevation at week 6 with no changes in ARV regimen prior to week 6; another had Grade 4 ALT and AST elevations at the final study visit and also received a diagnosis of drug-induced hepatitis. This participant's ARV regimen had been changed to abacavir/lamivudine/atazanavir at week 24. Both participants had normal values at baseline.
Six other participants experienced non-treatment related Grade 3 events: one elevated total bilirubin level at week 8 (attributed to atazanavir); one elevated total amylase at week 35 (ibuprofen overdose); one elevated CK at week 9 (high levels of physical activity); one elevated fasting glucose level at week 3; and one non-allergic rash at week 4 (exudative pharyngitis). No other Grade 4 events and no deaths occurred.
Changes in Markers of Inflammation
Descriptive statistics for serum IL-6 and hs-CRP and their percent change over time are shown in Table 3. Median IL-6 was 1.66 pg/mL at baseline and fluctuated over time with no clear trend. Median percent change in IL-6 was also variable, and the corresponding confidence intervals were wide. Median hs-CRP was 1.20 mg/L at baseline and 1.00 mg/L at week 12 before declining to 0.50 mg/mL at week 24 and 0.60 mg/L at week 48; however, the ranges of hs-CRP values at all weeks were relatively large.
Table 3. Absolute inflammatory marker concentrations before atorvastatin treatment and changes during atorvastatin treatment.
| Baseline | Week 12 | Week 24 | Week 48 | ||
|---|---|---|---|---|---|
| IL-6 (pg/mL) | N | 27 | 24 | 23 | 24 |
| Mean (s.d.) | 1.83 (1.53) | 2.27 (1.71) | 1.53 (1.09) | 2.63 (4.92) | |
| Median (Min, Max) | 1.66 (0.47, 7.94) | 1.94 (0.51, 6.97) | 1.36 (0.42, 4.41) | 1.15 (0.56, 24.56) | |
| Mean % change (90% CI) | 62.1 (11.6, 112.6) | -1.3 (-22, 19.4) | 40.2 (-6, 86.4) | ||
| Median % change (90% CI) | -1 (-32, 110) | -19 (-32, -5) | -11.5 (-34, 46) | ||
| hs-CRP (mg/L) | N | 27 | 25 | 23 | 24 |
| Mean (s.d.) | 4.32 (5.97) | 4.14 (6.18) | 1.91 (3.92) | 1.88 (4.05) | |
| Median (Min, Max) | 1.20 (0.20, 22.00) | 1.00 (0.20, 20.50) | 0.50 (0.20, 18.90) | 0.60 (0.20, 19.30) | |
| Mean % change (90% CI) | 132.2 (23.5, 240.9) | -9.8 (-43.3, 23.7) | 20.4 (-33.8, 74.6) | ||
| Median % change (90% CI) | 0 (-35, 44) | -20 (-67, 0) | 0 (-78, 17) |
IL-6= interleukin-6 hs-CRP= highly sensitive C-reactive protein s.d.= standard deviation CI= confidence interval
Virologic Changes
The percentage of participants with HIV-1 RNA viral load (VL) below the lower limit of quantitation was 71% at week 0, 69% at week 12, 62% at week 24, and 69% at week 48. Of those with undetectable VL at baseline and follow-up data available, 80% had undetectable VL at week 48 and 55% had undetectable VL at all study weeks. Of those with detectable virus at baseline, median plasma HIV-1 RNA concentration was low (2.5 log10 copies/mL) and unchanging over time.
Discussion
The HIV-infected youth participating in P1063 experienced a significant decline in plasma lipid concentrations after initiating atorvastatin. The overall decline in LDL-C and TC was somewhat less than that seen in children with familial hypercholesterolemia15,16 but similar to that in children with Kawasaki disease18, Type 1 diabetes26 and systemic lupus17. LDL-C and TC decreased by a mean of 39% and 31%, respectively, between baseline and 26 weeks in the 140 children randomized to atorvastatin in a placebo-controlled trial in children and adolescents with familial or severe hypercholesterolemia15. The larger decrease may have been due in part to the study design which required dose escalation if LDL-C <130 mg/dL was not achieved at 4 weeks on 10 mg of atorvastatin. In contrast, participants in P1063 did not dose escalate if their LDL-C decreased by ≥ 30% regardless of actual concentration achieved. Similarly, studies in adults have found somewhat lower responses to statins in HIV-infected vs uninfected adults27-29.
Some individuals demonstrated considerable variability in LDL-C concentrations during the study suggesting that adherence to atorvastatin may not have been as consistent as was indicated through self-report. Alternatively, dietary intake may have been variable. One participant, who changed from a boosted-PI to an unboosted-PI at week 24, had lower LDL-C at week 48. Two additional participants had ARV changes during the study period which did not appear to affect their LDL-C.
In addition to LDL-C, ApoB, non-HDL-C and ApoB/Apo A-1 ratio decreased significantly from baseline to week 48. ApoB, a measure of total atherogenic particle number, and non-HDL-C, which includes very low density lipoprotein cholesterol as well as LDL-C, are additional measures of cardiovascular disease risk30-33. Several studies have shown ApoB concentrations to be a better predictor of cardiovascular disease risk than LDL-C concentrations in HIV-uninfected adults treated with statins34-36.
The association between childhood risk factors including LDL-C37,38and adult cardiovascular disease has been demonstrated through longitudinal epidemiologic studies37-39. Similarly, evidence is accumulating for increased cardiovascular risk in HIV-infected children and adolescents, including elevated cholesterol concentrations particularly in those on PI-based ART8,9,12,13 and increased Pathobiological Determinants of Atherosclerosis in Youth scores40. Increased carotid artery intima-media thickness (CIMT) has been demonstrated in some pediatric HIV-infected cohorts41-43 but not others44. The finding of increased risk of development of coronary heart disease in young HIV infected adults between the ages of 18-24 years45 highlights the potential risk for early cardiovascular disease even in children with perinatal HIV infection.
While no studies directly demonstrate a decrease in mortality related to cardiovascular disease in HIV-infected adults treated with statins, there is evidence that statins decrease subclinical cardiovascular disease: including decreased CIMT46, improved endothelial function47, decreases in markers of vascular inflammation48,49 and decreased non-calcified coronary artery plaque volume50. Statin therapy reduces risk for cardiovascular disease by effectively decreasing both cholesterol concentrations and chronic inflammation. While the immune mechanisms resulting in increased atherosclerosis are complex, hs-CRP has been shown to be a relevant surrogate marker for immune activation and is a significant independent risk factor for myocardial infarction and peripheral vascular disease51-53. Statins lower hs-CRP concentrations in adults and the clinical efficacy in reducing cardiovascular disease is independently related to the ability of statins to reduce both blood lipid concentrations and hs-CRP54-57. Both elevated hs-CRP and HIV infection were found to be independent risk factors for risk of acute myocardial infarction in adults58 and increased hs-CRP concentrations have been associated with increased CIMT in HIV-infected adults59 and children60. Statin use resulted in decreased hs-CRP in HIV-infected adults in some studies61,62 but not others63. Although both hs-CRP and IL-6 concentrations appeared to decrease in this study after initiation of atorvastatin, the variability was high for both analytes. As IL-6 and hs-CRP are acute phase reactants and participants were not required to be free of minor symptoms such as upper respiratory tract infections, the concentrations may have been affected by the clinical status of the participant at the time of the study visits. Therefore this study suggests, but cannot confirm, a decrease in inflammatory markers after initiation of atorvastatin treatment in HIV-infected youth.
Many of the statins currently in use are metabolized by cytochrome P450 (CYP) 3A4 enzymes; therefore the risk for drug interactions with PIs is significant. Simvastatin and lovastatin are contraindicated with PIs due to significantly increased statin levels64,65. Atorvastatin concentrations are affected less by concomitant PI administration65 and atorvastatin has been used safely in HIV-infected adults6,66. Atorvastatin should not be co-administered with tipranavir and the dose should not exceed 20 mg when co-administered with darunavir, fosamprenavir/ritonavir or saquinavir/ritonavir65,67. In contrast, administration of atorvastatin does not affect PI drug concentrations64,65. Currently, atorvastatin is FDA-labeled for use in children down to age 10 years with heterozygous familial hyperlipidemias 68.
A systematic review of the literature on the safety and tolerability of atorvastatin found a low incidence of elevated liver enzymes, CK and myalgias, with no clear dose-dependency69. Serious muscle-related side effects have been rare across clinical studies. While myalgia occurred in close to 5% of participants, the rate of persistent increase in CK (>10xULN) ranged from 0.1% to 0.4%, with few cases of rhabdomyolysis 69-71. Drug-induced rhabdomyolysis after the concomitant use of boosted PI, macrolide antibiotic therapy (lopinavir/ritonavir together with clarithromycin) and atorvastatin has been reported72. There have been reports of increased risk for type 2 DM in adults treated with statins. The increased risk across all statins is low73 and largely associated with high-dose statin therapy74. No adolescents or children were included in these studies.
A meta-analysis of statin use in children showed an overall favorable safety profile similar to that in adults, with increased risk of adverse events mainly with higher doses and co-administration of interacting drugs metabolized by the CYP system. Five studies reported on elevations of AST, ALT, and CK, with no substantial elevations in 2 studies for AST and 3 studies for ALT and CK75. However, due to the limited sample size and short study duration, the authors drew no definite conclusions on liver- and muscle-related adverse effects and recommended monitoring muscle and liver safety markers in pediatric patients. Treatment-related hepatic (1 possibly related) and muscle (0) toxicity were uncommon in our study despite use of PIs in 79% of participants at study entry, consistent with the reported safety of statins in adults with HIV6. Renal toxicity has not been reported for atorvastatin and only one report of creatinine increase with concomitant uric acid serum level elevation is in the literature76.
Conclusion
Management options for TC and/or LDL-C elevations in HIV-infected children include life-style and dietary changes, or switching to ARVs less likely to elevate lipid concentrations77-81. If lipid concentrations do not improve with these interventions or for individuals in whom such interventions are not feasible, our data suggest that treatment with atorvastatin appears to be safe and effective for HIV-infected youth on ART.
Acknowledgments
Atorvastatin (Lipitor®) for this study was provided by Pfizer, Inc. We gratefully acknowledge the contributions of the site investigators and site staff who conducted the P1063 study:
Texas Children's Hospital: Chivon McMullen-Jackson, RN, BSN, CCRP, Norma Cooper, RN, BSN, MS, Mary Paul, MD, William Shearer, MD, PhD; Lurie Children's Hospital of Chicago: Ruth Williams, RN, Lynn Heald, PNP, Margaret Ann Sanders, MPH, Ram Yogev, MD; University of Miami Pediatric Perinatal HIV/AIDS: Charles D. Mitchell, MD, Gwendolyn B. Scott, MD, Monica Stone, MD, Patricia Bryan, RN, BS, MPH; Metropolitan Hospital: Mahrukh Bamji, MD, Santa Paul, MD; Boston Medical Center Pediatric HIV Program: Ellen R Cooper MD, Diana Clarke PharmD, Deb McLaud, RN, C Moloney, RN, PNP, MEd; New York University: Nagamah Deygoo, MS, Siham Akleh, RN, Aditya Kaul, MD, Sulachni Chandwani MD; University of South Florida – Tampa: Carina A. Rodriguez, MD, Patricia J. Emmanuel, MD, Denise Casey, RN, Elizabeth Enriquez-Bruce, MD; University of Colorado School of Medicine and Children's Hospital Colorado: Suzanne Paul, BSN, MSN, FNP-C, Hannah Bernath, MPH, Jason Child PharmD, Jenna Wallace, MSW, CCRP; Tulane University: Margarita Silio, MD, Thomas Alchediak, MD, Russell Van Dyke, MD; Bronx-Lebanon Hospital: Mary Elizabeth Vachon, MPH, Ana Maria Emeh, MD, Murli Udharam Purswani, MD; St Jude's Children's Hospital: Nehali Patel, MD, Sandra Boyd, PNP, Thomas Wride, MS, Aditya Gaur, MD.
Source of Funding: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and Contract No. HHSN272200800014C, with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: No authors report conflicts of interest.
References
- 1.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A Systematic Review of the Usefulness of Statin Therapy in HIV-Infected Patients. Am J Cardiol. 2015;115:1760–6. doi: 10.1016/j.amjcard.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melvin AJ, Lennon S, Mohan KM, Purnell JQ. Metabolic abnormalities in HIV type 1-infected children treated and not treated with protease inhibitors. AIDS Res Hum Retroviruses. 2001;17:1117–23. doi: 10.1089/088922201316912727. [DOI] [PubMed] [Google Scholar]
- 9.Lainka E, Oezbek S, Falck M, Ndagijimana J, Niehues T. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics. 2002;110:e56. doi: 10.1542/peds.110.5.e56. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Zaoutis T, Chu J, Zhao H, Rutstein R. Effects of highly active antiretroviral therapy (HAART) on cholesterol in HIV-1 infected children: a retrospective cohort study. Pharmacoepidemiology and drug safety. 2009;18:589–94. doi: 10.1002/pds.1755. [DOI] [PubMed] [Google Scholar]
- 11.Brewinski M, Megazzini K, Hance LF, et al. Dyslipidemia in a cohort of HIV-infected Latin American children receiving highly active antiretroviral therapy. J Trop Pediatr. 2011;57:324–32. doi: 10.1093/tropej/fmq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhoads MP, Lanigan J, Smith CJ, Lyall EG. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr. 2011;57:404–12. doi: 10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- 13.Tassiopoulos K, Williams PL, Seage GR, 3rd, et al. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–14. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamaida N, Capuano E, Pinto L, Capuano E, Capuano R, Capuano V. The safety of statins in children. Acta paediatrica. 2013;102:857–62. doi: 10.1111/apa.12280. [DOI] [PubMed] [Google Scholar]
- 15.McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr. 2003;143:74–80. doi: 10.1016/S0022-3476(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 16.Gandelman K, Glue P, Laskey R, Jones J, LaBadie R, Ose L. An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia. Pediatr Cardiol. 2011;32:433–41. doi: 10.1007/s00246-011-9885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schanberg LE, Sandborg C, Barnhart HX, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 2012;64:285–96. doi: 10.1002/art.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedra E, Chahal N, Manlhiot C, Yeung RS, McCrindle BW. Atorvastatin safety in Kawasaki disease patients with coronary artery aneurysms. Pediatr Cardiol. 2014;35:89–92. doi: 10.1007/s00246-013-0746-9. [DOI] [PubMed] [Google Scholar]
- 19.Dube MP, Parker RA, Tebas P, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–18. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 20.Shikuma CM, Yang Y, Glesby MJ, et al. Metabolic effects of protease inhibitor-sparing antiretroviral regimens given as initial treatment of HIV-1 Infection (AIDS Clinical Trials Group Study A5095) J Acquir Immune Defic Syndr. 2007;44:540–50. doi: 10.1097/QAI.0b013e318031d5a0. [DOI] [PubMed] [Google Scholar]
- 21.Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–38. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–72. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan K, Harris DR, Monte D, et al. Obesity and dyslipidemia in behaviorally HIV-infected young women: Adolescent Trials Network study 021. Clin Infect Dis. 2010;50:106–14. doi: 10.1086/648728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervia JS, Chantry CJ, Hughes MD, et al. Associations of proinflammatory cytokine levels with lipid profiles, growth, and body composition in HIV-infected children initiating or changing antiretroviral therapy. Pediatr Infect Dis J. 2010;29:1118–22. doi: 10.1097/INF.0b013e3181ed9f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:461–8. doi: 10.1089/aid.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canas JA, Ross JL, Taboada MV, et al. A randomized, double blind, placebo-controlled pilot trial of the safety and efficacy of atorvastatin in children with elevated low-density lipoprotein cholesterol (LDL-C) and type 1 diabetes. Pediatr Diabetes. 2015;16:79–89. doi: 10.1111/pedi.12245. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150:301–13. doi: 10.7326/0003-4819-150-5-200903030-00006. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Willig JH, Mugavero MJ, et al. Comparative Effectiveness and Toxicity of Statins Among HIV-Infected Patients. Clin Infect Dis. 2011;52:387–95. doi: 10.1093/cid/ciq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend ML, Hollowell SB, Bhalodia J, Wilson KH, Kaye KS, Johnson MD. A comparison of the effectiveness of lipid-lowering therapy between HIV- and non-HIV-infected subjects with hyperlipidaemia. Int J STD AIDS. 2007;18:851–5. doi: 10.1258/095646207782716974. [DOI] [PubMed] [Google Scholar]
- 30.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–61. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–33. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB, Zdrojewski T, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. 2015 doi: 10.1177/2047487315569411. [DOI] [PubMed] [Google Scholar]
- 33.Emerging Risk Factors C. Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotto AM, Jr, Whitney E, Stein EA, et al. Relation between baseline and on-treatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Circulation. 2000;101:477–84. doi: 10.1161/01.cir.101.5.477. [DOI] [PubMed] [Google Scholar]
- 35.Thanassoulis G, Williams K, Ye K, et al. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc. 2014;3:e000759. doi: 10.1161/JAHA.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lennep JE, Westerveld HT, van Lennep HW, Zwinderman AH, Erkelens DW, van der Wall EE. Apolipoprotein concentrations during treatment and recurrent coronary artery disease events. Arterioscler Thromb Vasc Biol. 2000;20:2408–13. doi: 10.1161/01.atv.20.11.2408. [DOI] [PubMed] [Google Scholar]
- 37.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 39.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol. 2002;90:3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 40.Patel K, Wang J, Jacobson DL, et al. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation. 2014;129:1204–12. doi: 10.1161/CIRCULATIONAHA.113.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuliano Ide C, de Freitas SF, de Souza M, Caramelli B. Subclinic atherosclerosis and cardiovascular risk factors in HIV-infected children: PERI study. Coron Artery Dis. 2008;19:167–72. doi: 10.1097/MCA.0b013e3282f6dffb. [DOI] [PubMed] [Google Scholar]
- 42.Chanthong P, Lapphra K, Saihongthong S, et al. Echocardiography and carotid intima-media thickness among asymptomatic HIV-infected adolescents in Thailand. AIDS. 2014;28:2071–9. doi: 10.1097/QAD.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McComsey GA, O'Riordan M, Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–7. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 44.Ross AC, Storer N, O'Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–8. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 45.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 46.Calza L, Manfredi R, Colangeli V, et al. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Res Hum Retroviruses. 2013;29:547–56. doi: 10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- 47.Hurlimann D, Chenevard R, Ruschitzka F, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92:110–2. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209:1156–64. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 52.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–7. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 53.Toprak A, Kandavar R, Toprak D, et al. C-reactive protein is an independent predictor for carotid artery intima-media thickness progression in asymptomatic younger adults (from the Bogalusa Heart Study) BMC Cardiovasc Disord. 2011;11:78. doi: 10.1186/1471-2261-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 56.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet J, McPherson R, Tedgui A, et al. Comparative effects of 10-mg versus 80-mg Atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther. 2008;30:2298–313. doi: 10.1016/j.clinthera.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–73. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:340–4. doi: 10.1097/QAI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 60.Ross AC, O'Riordan MA, Storer N, Dogra V, McComsey GA. Heightened inflammation is linked to carotid intima-media thickness and endothelial activation in HIV-infected children. Atherosclerosis. 2010;211:492–8. doi: 10.1016/j.atherosclerosis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Aslangul E, Fellahi S, Assoumou LK, Bastard JP, Capeau J, Costagliola D. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS. 2011;25:1128–31. doi: 10.1097/QAD.0b013e328346be29. [DOI] [PubMed] [Google Scholar]
- 62.Calza L, Trapani F, Bartoletti M, et al. Statin therapy decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-alpha in HIV-infected patients treated with ritonavir-boosted protease inhibitors. HIV Clin Trials. 2012;13:153–61. doi: 10.1310/hct1303-153. [DOI] [PubMed] [Google Scholar]
- 63.Fichtenbaum CJ, Evans SE, Aberg JA. High-sensitivity C-reactive protein levels do not decrease with the use of statins in all persons with HIV infection. AIDS. 2011;25:2053. doi: 10.1097/QAD.0b013e32834b9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–77. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 65.Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52:815–31. doi: 10.1007/s40262-013-0075-4. [DOI] [PubMed] [Google Scholar]
- 66.Soler A, Deig E, Guil J, Rodriguez-Martin M, Guelar A, Pedrol E. [Effectiveness and tolerance of atorvastatin for antiretroviral therapy-secondary dyslipemia] Med Clin (Barc) 2006;127:250–2. doi: 10.1157/13091265. [DOI] [PubMed] [Google Scholar]
- 67.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Table 19a. (Accessed September 22, 2015. [Google Scholar]
- 68.Drugs@FDA: FDA Approved Drug Products. [September 22, 2015];US Food and Drug Administration. at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 69.Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Atorvastatin: safety and tolerability. Expert opinion on drug safety. 2010;9:667–74. doi: 10.1517/14740338.2010.495385. [DOI] [PubMed] [Google Scholar]
- 70.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 71.Escobar C, Echarri R, Barrios V. Relative safety profiles of high dose statin regimens. Vasc Health Risk Manag. 2008;4:525–33. doi: 10.2147/vhrm.s2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mah Ming JB, Gill MJ. Drug-induced rhabdomyolysis after concomitant use of clarithromycin, atorvastatin, and lopinavir/ritonavir in a patient with HIV. AIDS Patient Care STDS. 2003;17:207–10. doi: 10.1089/108729103321655854. [DOI] [PubMed] [Google Scholar]
- 73.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 74.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 75.Avis HJ, Vissers MN, Stein EA, et al. A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2007;27:1803–10. doi: 10.1161/ATVBAHA.107.145151. [DOI] [PubMed] [Google Scholar]
- 76.Kakafika A, Liamis G, Elisaf M, Mikhailidis D. Effect of atorvastatin on serum creatinine levels. Curr Med Res Opin. 2001;17:230–1. doi: 10.1185/0300799039117071. [DOI] [PubMed] [Google Scholar]
- 77.Vigano A, Aldrovandi GM, Giacomet V, et al. Improvement in dyslipidaemia after switching stavudine to tenofovir and replacing protease inhibitors with efavirenz in HIV-infected children. Antivir Ther. 2005;10:917–24. [PubMed] [Google Scholar]
- 78.Fabiano V, Giacomet V, Vigano A, et al. Long-term body composition and metabolic changes in HIV-infected children switched from stavudine to tenofovir and from protease inhibitors to efavirenz. European journal of pediatrics. 2013;172:1089–96. doi: 10.1007/s00431-013-2018-3. [DOI] [PubMed] [Google Scholar]
- 79.Martinez E, D'Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26:2315–26. doi: 10.1097/QAD.0b013e328359f29c. [DOI] [PubMed] [Google Scholar]
- 80.Xiang N, James M, Walters S, Bamford A, Foster C. Improved serum cholesterol in paediatric patients switched from suppressive lopinavir-based therapy to boosted darunavir or atazanavir: an 18-month retrospective study. HIV Med. 2014;15:635–6. doi: 10.1111/hiv.12180. [DOI] [PubMed] [Google Scholar]
- 81.Fabbiani M, Mondi A, Colafigli M, et al. Safety and efficacy of treatment switch to raltegravir plus tenofovir/emtricitabine or abacavir/lamivudine in patients with optimal virological control: 48-week results from a randomized pilot study (Raltegravir Switch for Toxicity or Adverse Events, RASTA Study) Scand J Infect Dis. 2014;46:34–45. doi: 10.3109/00365548.2013.840920. [DOI] [PubMed] [Google Scholar]