Kugler et al. show that systemic infection with Toxoplasma gondii triggers a long-term impairment in thymic function, which leads to an immunodeficient state reflected in decreased antimicrobial resistance.
Abstract
Because antigen-stimulated naive T cells either die as effectors or enter the activated/memory pool, continuous egress of new T lymphocytes from thymus is essential for maintenance of peripheral immune homeostasis. Unexpectedly, we found that systemic infection with the protozoan Toxoplasma gondii triggers not only a transient increase in activated CD4+ Th1 cells but also a persistent decrease in the size of the naive CD4+ T lymphocyte pool. This immune defect is associated with decreased thymic output and parasite-induced destruction of the thymic epithelium, as well as disruption of the overall architecture of that primary lymphoid organ. Importantly, the resulting quantitative and qualitative deficiency in naive CD4+ T cells leads to an immunocompromised state that both promotes chronic toxoplasma infection and leads to decreased resistance to challenge with an unrelated pathogen. These findings reveal that systemic infectious agents, such as T. gondii, can induce long-term immune alterations associated with impaired thymic function. When accumulated during the lifetime of the host, such events, even when occurring at low magnitude, could be a contributing factor in immunological senescence.
Introduction
The thymus plays a critical role in adaptive immunity as the site where hematopoietic progenitors give rise to naive T cells ready to respond to antigen stimulation and undergo effector differentiation. Each T lymphocyte, educated by the highly specialized microenvironment of the thymic epithelium, expresses a unique self MHC–restricted TCR selected to recognize an antigenic peptide (Klein et al., 2014). The T cell repertoire is estimated to comprise at least 108 different TCR specificities, enabling the host to defend itself against a wide range of pathogens and malignancies. The thymus grows rapidly during prenatal life and infancy, but then enters an involution phase, leading to decreased production of naive T cells, resulting in impaired immune function during aging (Ventevogel and Sempowski, 2013; Nikolich-Žugich, 2014).
Under steady-state conditions, the peripheral T cell compartment consists of a major population of naive lymphocytes with diverse TCR specificities and a minor population of activated/memory T cells with specificities determined by previous exposure to foreign antigen (Jenkins et al., 2010). In the case of the CD4+ T cell response to infection, clones normally undetectable under steady-state conditions rapidly proliferate and differentiate into different subsets of Th effectors. This vigorous expansion alters the ratio between naive and activated CD4+ T cells. However, fully differentiated CD4+ T effectors are short-lived and contract upon successful control of infection, thereby normalizing the ratio of naive versus antigen-experienced T cells. This type of infection-induced oscillation in the activated T lymphocyte pool has been well documented for a variety of different pathogens (Homann et al., 2001; Schiemann et al., 2003; McKinstry et al., 2010; Pepper et al., 2010). In contrast, the population of naive circulating T cells, essential for maintaining immune homeostasis, is considered to be relatively static because the rate of thymic output is independent of fluctuations in the peripheral T lymphocyte compartment (Gabor et al., 1997; Fink, 2013).
Although this homeostatic restoration is the norm, an imbalance in the frequency of activated versus naive T cells has been observed in certain diseases, such as rheumatoid arthritis and multiple sclerosis (Koetz et al., 2000; Hug et al., 2003; Haegert et al., 2011), and after acute control of hepatitis C, HTLV-1, and HIV viral infections (Yasunaga Ji et al., 2001; Yonkers et al., 2011; Lu et al., 2014), as well as after recovery from bacterial sepsis (Hotchkiss et al., 2009; Cabrera-Perez et al., 2015). In this regard, it is of interest that certain acute infections can lead to decreased numbers of lymphoid progenitors in the bone marrow and/or major reductions in immature T cells in thymus. Indeed, thymic atrophy is frequently observed in infections with viruses, bacteria, fungi, and parasites, and has been speculated to contribute to pathogen virulence (de Meis et al., 2012; Nunes-Alves et al., 2013).
Toxoplasma gondii is an intracellular protozoan parasite that triggers a potent IL-12–dependent Th1 response, which results in production of high levels of IFN-γ and TNF that efficiently control parasite replication in both hematopoietic and nonhematopoietic cells (Yap and Sher, 1999). Chronic infection is maintained by small numbers of parasite cysts localized in the CNS and contained by the residual T cell response (Suzuki et al., 1988). Regulation of the acute CD4 T lymphocyte response is an important aspect of the host–pathogen interaction, as it prevents clearance of the parasite while simultaneously protecting the host against T cell–mediated immune pathology (Gazzinelli et al., 1996; Villarino et al., 2003; Jankovic et al., 2007; Hall et al., 2012; Kugler et al., 2013). Interestingly, T. gondii is also known to induce thymic atrophy and does so in a variety of experimental animal models (Huldt et al., 1973), although the impact of this phenomenon on the host response to the endogenous infection or to resistance to heterologous pathogen challenge has not been addressed.
Here, we demonstrate that T. gondii infection rapidly triggers a profound and persistent reduction in the size of the peripheral naive CD4+ T cell pool. We further show that the resulting perturbation in T cell homeostasis is mechanistically associated with parasite-induced thymic atrophy and, more specifically, with a loss in the architectural integrity of the thymic epithelium. Moreover, this structural degeneration is accompanied by impaired TCR affinity maturation, as indicated by decreased CD5 expression on the few recent thymic emigrants (RTEs) that reach the periphery. Finally, we demonstrate that these alterations in the naive CD4+ T cell compartment lead to decreased host resistance to heterologous pathogen challenge and contribute to the maintenance of chronic T. gondii infection. Interestingly, the changes in thymic structure and function induced by toxoplasma closely resemble those associated with the thymic involution that occurs during aging, suggesting that infection-induced alterations in the thymus could be a factor promoting immunological senescence.
Results
T. gondii triggers a rapid and persistent loss in naive T lymphocytes in the periphery
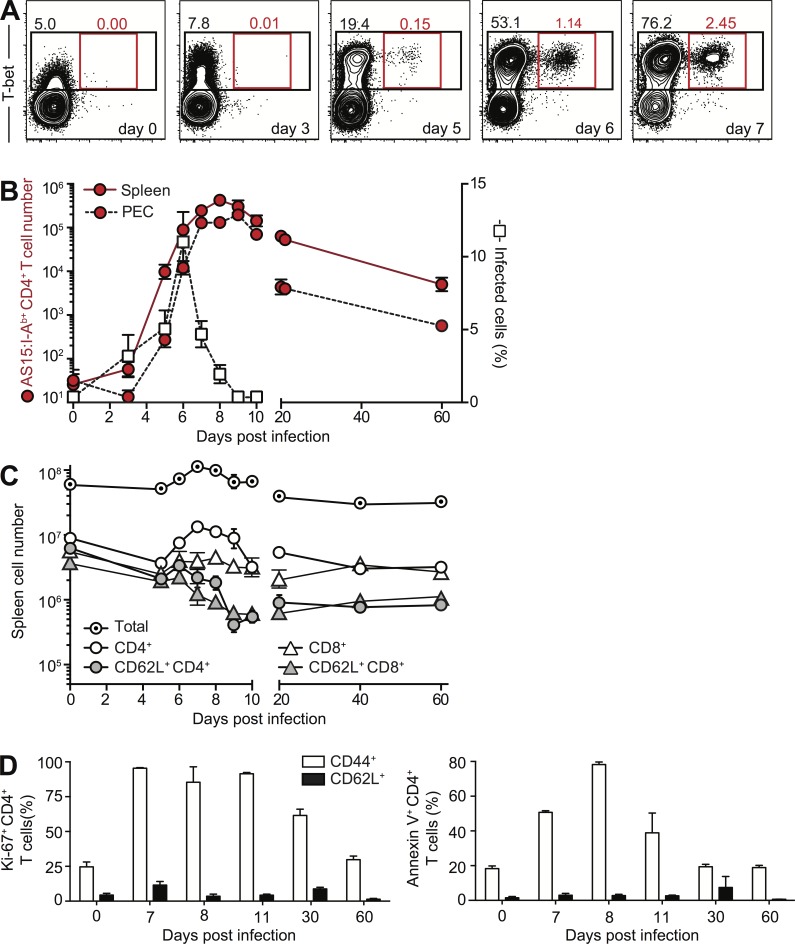
It has been established in numerous prior studies that acute T. gondii infection triggers activation of large numbers of CD4+ T cells, which rapidly acquire a Th1 phenotype. Using the AS15 tetramer, we found that the parasite-specific CD4 response peaks at day 7, greatly contracts as the acute infection is controlled, and persists at low levels into the chronic phase (Fig. 1, A and B). We further showed that the initial CD4 T cell expansion is the result of extensive expansion of activated Th1 effectors and is accompanied by apoptosis of the same cells (Fig. 1 D). In direct contrast, naive CD62L+CD44− CD4+ T lymphocytes examined in the same animals during the same period failed to display markers of either proliferation or death (Fig. 1 D). Nevertheless, when the absolute number of these cells was determined, a profound reduction in CD62L+CD44− CD4+ T cells was observed from day 9 onward, despite the contraction of the parasite-specific Th1 cell response during the same period (Fig. 1 C). The naive CD62L+CD44− CD8+ T cell population was also reduced in these infected animals (Fig. 1 C).
Figure 1.
Dynamics of activated parasite-specific CD4+ T cells and naive T cells after T. gondii infection. (A) Expansion of parasite-specific Th1 cells during T. gondii infection. Representative contour plots of T-bet versus AS15:I-Ab tetramer staining for splenic CD4+ T lymphocytes isolated from C57BL/6 mice on days 0, 3, 5, 6, and 7 after i.p. infection with ME-49 cysts. (B) Contraction of parasite-specific CD4+ T cells after control of acute infection. Number of AS15:I-Ab–specific CD4+ T lymphocytes (left, y axis) in spleen and PEC for individual animals at indicated time points during infection is superimposed with the frequency of infected cells in PEC (right, y axis) in the same mice. (C) Number of total splenocytes, CD4+ or CD8+ T lymphocytes, and naive CD62L+CD44− CD4 or CD8 T cells during the acute and chronic phases of infection. (D) Naive CD4+ T lymphocyte during T. gondii infection are not prone to apoptosis. Frequency of Ki67+ (left) or Annexin V+ (right) CD44+CD62L– and CD62L+CD44– splenic CD4+ T cells at indicated time points during infection. Each symbol (B and C) or bar (D) represents the mean ± SEM of the values from the individual animals (n = 4–8) pooled from two independent experiments.
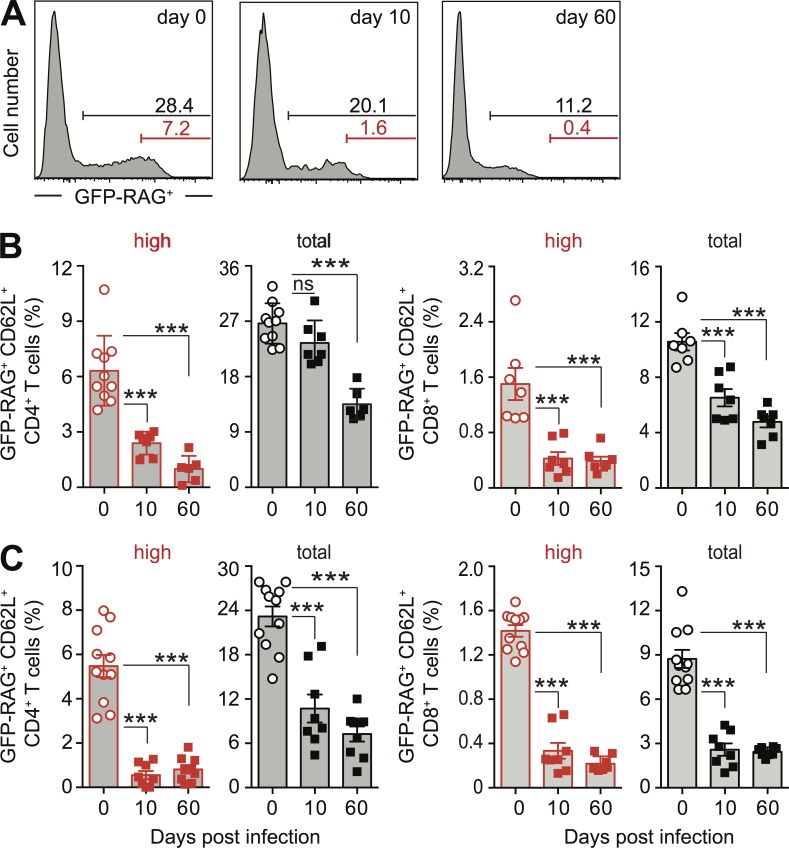
We therefore focused on the population of RTEs by using mice carrying a GFP transgene driven by the RAG2 promoter, in which RTE can be identified as GFP-RAG+ T cells (Berkley et al., 2013). Unexpectedly, the reduction in the naive CD4+ T lymphocyte pool size correlated with a decrease in the frequency of GFP-RAG+ RTE in T. gondii–infected reporter mice (Fig. 2 A). Initially, this decrease occurred preferentially within the brightest GFP-RAG+ CD4+ subpopulation, whereas in chronic phase, the entire population of RTE was affected both locally in spleen (Fig. 2, A–C) and lymph nodes (not depicted), as well as systemically in peripheral blood (Fig. 2 C). A similar decrease in the frequency of GFP-RAG+ CD8+ RTE was observed in the same tissue sites (Fig. 2, B and C; and not depicted).
Figure 2.
Reduced frequency of RTE after T. gondii infection. (A) Representative histograms of splenic CD44−CD62L+ CD4+ T lymphocytes in GFP-RAG reporter mice on day 0, 10, and 60 after T. gondii infection. (B and C) Bars represent the mean ± SEM frequencies of the brightest fraction (red) and the total (black) GFP-RAG+ population in CD44−CD62L+ CD4 (left) or CD8 (right) T cells per time point (n = 6–10 mice) pooled from at least two independent experiments in spleen (B) and peripheral blood (C). ***, P < 0.001.
T. gondii infection induces a persistent thymic atrophy that is largely glucocorticoid (GC) independent
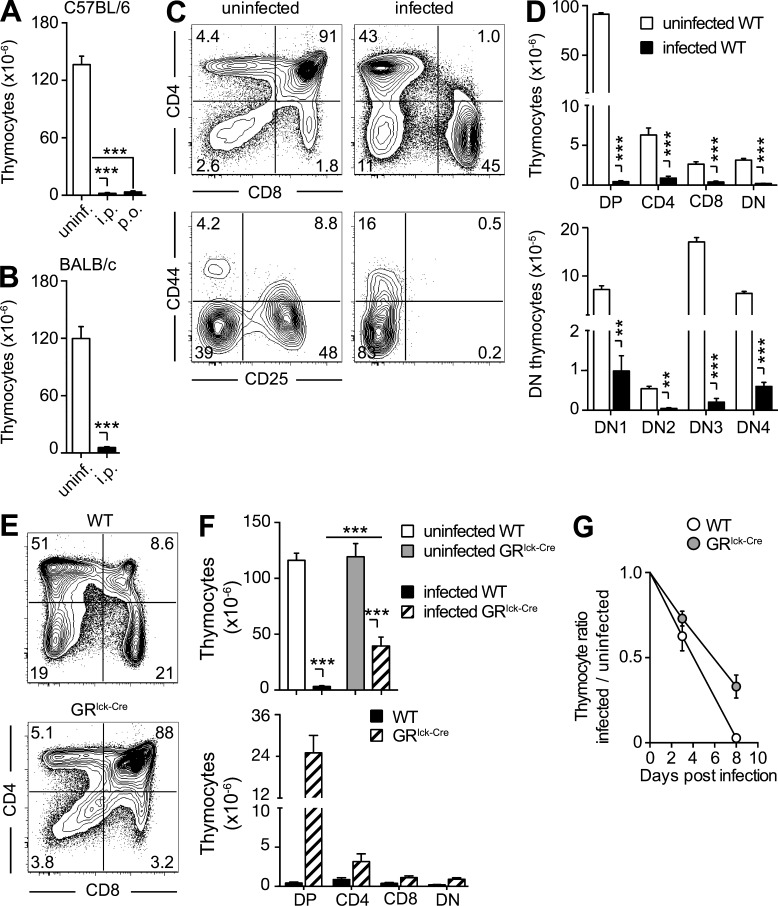
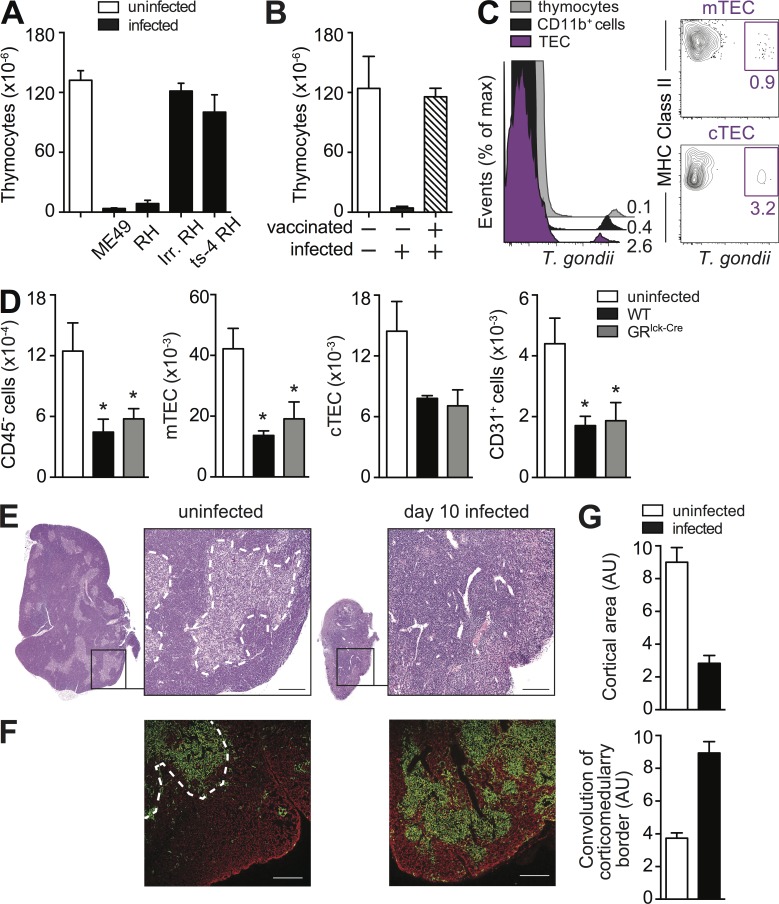
The observed decrease in RTE in T. gondii–infected mice suggested the involvement of the thymic atrophy previously described in these animals (Huldt et al., 1973). Indeed, when examined on day 10, infected mice displayed a marked (>95%) reduction in the number of thymocytes regardless of the route of parasite inoculation (Fig. 3 A), host genetic background (Fig. 3 B), or age of animals (8–12 wk vs. 5 mo vs. 7–8 mo; not depicted) at the time of infection.
Figure 3.
The thymic atrophy induced by toxoplasma infection is mouse strain and infection route independent, but only partially GC dependent. (A and B) The severe loss of thymocytes during acute toxoplasma infection is independent of both the route of exposure and host genetic background. Bars represent the mean ± SEM of thymocytes in C57BL/6 mice (n = 6–9) uninfected or infected for 10 d by either i.p. or gavage (p.o.; A), and in uninfected or 10 d i.p. infected BALB/c mice (n = 4; B) from one representative out of two experiments performed. (C and D) All thymocyte subsets are decreased on day 10 after T. gondii infection. (C) Representative dot-plots of total (B220− NK1.1− CD11b−) thymocytes stained with anti-CD4 and anti-CD8 antibody (top) and of double negative (DN) thymocytes stained with anti-CD44 and anti-CD25 antibody (bottom). (D) Bar graph showing the mean ± SEM of DP, CD4, CD8, and double-negative (DN) thymocytes (top) or DN1-4 populations (bottom) for individual animals (n = 6) pooled from two independent infections. (E–G) Lack of GR signaling only partially rescues thymic atrophy. (E) Representative dot plots of anti-CD4– and anti-CD8–stained thymocytes from 8-d infected WT or GRlck-Cre animals, and (F) bar graphs representing the mean ± SEM of total (top) and DP, CD4, CD8, and DN (bottom) thymocytes in each group (n = 10) pooled from three independent experiments. (G) Kinetics of thymocyte loss in infected WT or GRlck-Cre mice calculated as the ratio between number of thymocytes on day 0 and days 3 or 8 after infection (n = 4–8) from two time course experiments performed. **, P < 0.01; ***, P < 0.001.
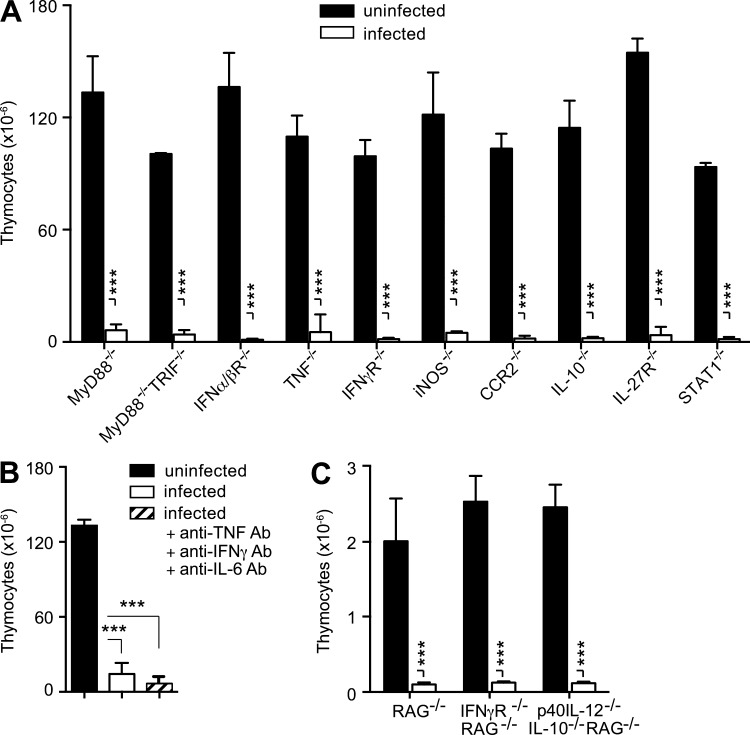
We have previously shown that GCs are induced during acute T. gondii infection (Kugler et al., 2013), and this type of stress response is known to lead to thymic atrophy caused by loss of CD4+CD8+ (double-positive; DP) thymocytes. Nevertheless, in T. gondii–infected mice, the loss in thymocytes was more general and not restricted to this DP population (Fig. 3, C and D). To directly evaluate the role of GC in toxoplasma-induced thymic atrophy, we selectively infected targeted knockout mice lacking the GC receptor (GR) in T cells. Although, as expected, the preferential loss in DP thymocytes was less pronounced in these animals (Fig. 3, E and F), overall the total number of thymocytes was still significantly decreased (>60%; Fig. 3 F) and had similar kinetics in WT animals (Fig. 3 G). In related experiments, T. gondii–induced thymic atrophy was found to occur normally in mice deficient in MyD88, MyD88/TRIF, TNF, IFN-αβR, IFN-γR, iNOS, CCR2, IL-10, IL-27R, or STAT1 (Fig. 4 A), as well as in WT mice treated simultaneously with neutralizing mAb against IL-6, TNF, and IFN-γ (Fig. 4 B). Similarly, T. gondii infection triggered a reduction in thymic cellularity in RAG−/−, IFN-γ−/−RAG−/−, and p40 IL-12−/−IL-10−/−RAG−/− mice comparable to that displayed by WT animals (Fig. 4 C). Thus, the loss of thymic function in toxoplasma infection does not appear to be dependent on the host cytokine response and is only partially dependent on GC signaling.
Figure 4.
T. gondii induces rapid thymic atrophy in the absence of critical elements of the host-protective immune response. Profound loss of thymocytes during acute toxoplasma infection in (A) C57BL/6 mice deficient in the indicated immune components (B) C57BL/6 mice treated simultaneously with neutralizing anti-TNF, anti–IFN-γ, and anti–IL-6 antibody, or (C) RAG−/−, double IFN-γ−/− RAG−/− and triple p40 IL-12−/−IL-10−/−RAG−/− mice. Bars represent the mean ± SEM of thymocytes in uninfected mice (n = 3–6) or i.p. infected animals on day 8–10 after infection. Data are representative of one of at least two independent experiments performed. ***, P < 0.001.
The thymic atrophy occurring during T. gondii infection is associated with a loss in architectural integrity of the thymic epithelium
During the acute phase, T. gondii disseminates via the blood stream to multiple tissue sites. Because the thymus is a heavily vascularized organ, we hypothesized that the observed damage to the thymic function might be related to the presence of the parasite. Indeed, the dissemination of live and replicating parasites was found to be required for T. gondii–induced thymic atrophy, as challenge with irradiated or temperature-sensitive mutant of the parasite that cannot establish active infection do not trigger thymic atrophy (Fig. 5 A). In addition, loss of thymocytes was not observed in previously vaccinated hosts challenged with wild-type parasites (Fig. 5 B). To identify the cell populations infected within the thymus, we exposed mice to mCherry labeled parasites and performed FACS analyses on total thymic cell preparations. Toxoplasma was detected at similar levels (MFI) in CD11b+ myeloid cells, thymocytes, and thymic epithelial cells (TECs). Although most of the infected cells were myeloid cells (73 ± 7%) and thymocyte (18 ± 5%), the TEC population was the most heavily infected on cell per cell basis (Fig. 5 C, left). Within the TEC population, both, cortical TEC (cTEC) and medullar TEC (mTEC) were found to harbor the parasite (Fig. 5 C, right) although at different ratios depending on the experiment.
Figure 5.
The thymic atrophy induced by T. gondii requires parasite replication and is associated with epithelial cell (TEC) disruption. (A and B) Thymic atrophy is observed after i.p. challenge with either type I (RH) or type II (ME49) strains of T. gondii but not with nonreplicating live-irradiated RH or a temperature sensitive mutant (ts-4) of that strain (A) or after challenge with WT parasites in previously vaccinated hosts (B). Bars represent the mean ± SEM of thymocytes assayed on day 8 in mice (A) infected i.p. with indicated strains of parasites (n = 4) or (B) challenged with RH after vaccination with ts-4 (n = 3). Data are representative of one of two independent experiments performed. (C) Phenotype of T. gondii–infected cells in the thymus. Mice were infected with mCherry-expressing tachyzoites and on day 3 FACS analyses were performed. Overlay histograms gated on total TEC population, CD11b+ myeloid cells, and thymocytes (left) and contour-plots gated on mTEC and CTEC (right) from one of three independent experiments with at least three infected mice each. (D) Loss of TEC during acute toxoplasma infection. TEC cells were isolated from uninfected WT mice, and day 8 infected WT and GRlck-Cre animals and were analyzed by FACS for mTEC (UEA+ I-Ab+), cTEC (Ly51+ I-Ab+), and CD31+cells. Bars represent the mean numbers (± SEM) of cTEC, mTEC, and CD31+ endothelial cells calculated from analyses performed on individual animals (n = 6–8) pooled from two independent experiments. (E) Cross section of thymus lobe from naive or day 8 infected WT mice stained with hematoxylin and eosin with indicated portion magnified 10×. Bar, 100 µm. (F) Image of a thymic section from uninfected and day 8 infected WT mice after immunostaining with anti-keratin 5 (mTEC) and anti-keratin 8 (cTEC) Antibodies, shown in green and red, respectively. Bar, 150 µm. (G) Quantitative representation of cortical area and convolution of corticomedulary border in thymii from uninfected and infected mice pooled from two experiments (n = 6). *, P < 0.05.
Normal thymic function is known to be influenced by interaction of thymocytes with thymic epithelium. To explore the possible role of this process in the decreased thymopioesis occurring in T. gondii infection, we enumerated the major cellular constituents of the thymic epithelium in mice exposed to the parasite 8 d previously. We observed major decreases (>50%) in the total numbers of TECs, as well as the individual Ly51+ cortical and UEA+ medullar populations when compared with uninfected control animals (Fig. 5 D). As expected the same alterations in TECs were observed in infected GRlck-cre mice.
Histological examination on day 8 after infection demonstrated that T. gondii infection leads to dramatic changes not only in the size but also in the architecture of the thymus (Fig. 5 E). In addition, staining with anti-keratin 5 and anti-keratin 8 mAbs revealed major alterations in the organization of the cortical and medullary areas of the thymus as indicated by decreased cortical surface area and increased convolution of the corticomedullary border (Fig. 5, F and G).
Persistent loss of thymic function during chronic T. gondii infection
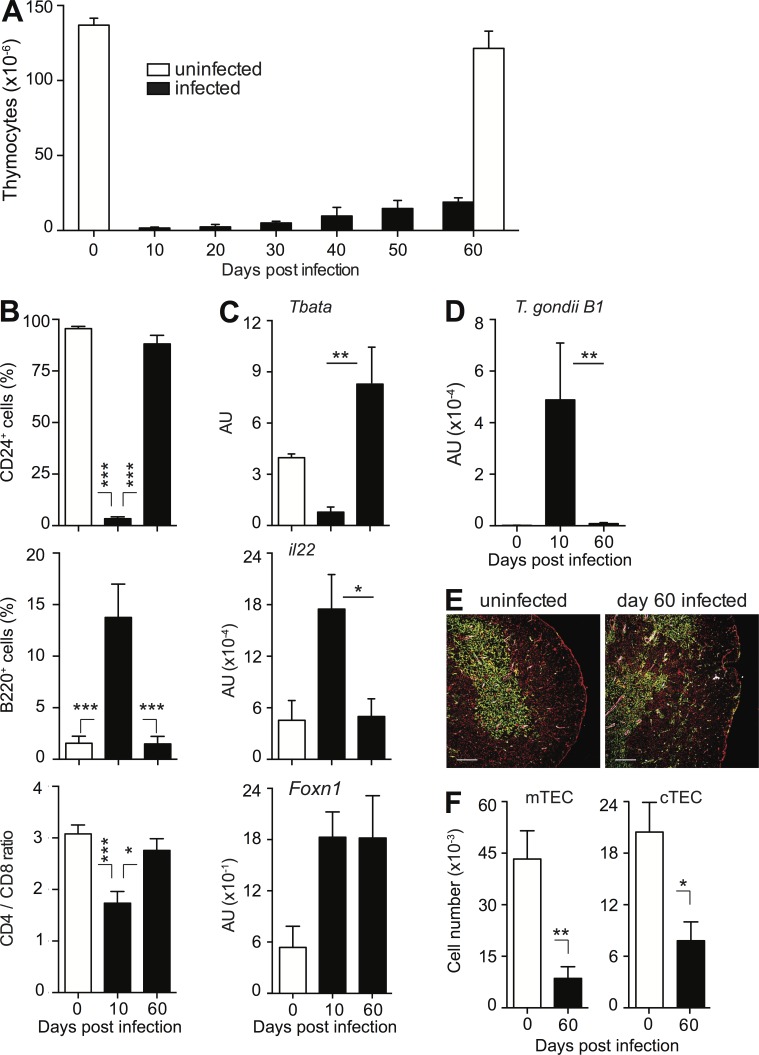
In most cases involving GC-dependent thymic atrophy, recovery of normal thymic function is restored once GC levels return to baseline. However, despite the previously documented transience of the acute GC response induced by T. gondii (Kugler et al., 2013), only limited recovery in thymocyte numbers in WT mice was observed during the chronic phase of infection (Fig. 6 A), although overall thymocyte composition appeared to normalize (Fig. 6 B), and parasite mRNA could no longer be detected in thymus (Fig. 6 D).
Figure 6.
Both thymic atrophy and altered thymic architecture persist during chronic infection. (A) Impaired thymic recovery during chronic T. gondii infection. Bars represent the mean ± SEM of thymocytes assessed in infected mice in 10-d intervals and age-matched uninfected controls on day 0 and 60 (n = 6–10/time point) pooled from at least two kinetics experiments performed. (B) Normalization of thymocyte composition in the chronic phase of T. gondii infection. Bar graphs represent the mean frequencies (± SEM) of CD24+ thymocytes (top), B cells (middle), and the ratio CD4+ versus CD8+ thymocytes (bottom) in thymii of control and day 10 and day 60 infected mice (n = 6–8). Data are pooled from two or more independent experiments performed. (C) Normalization of TEC-specific gene expression during the chronic phase of infection. Tbata, il-22, and Foxn1 mRNA expression were measured by qRT-PCR in thymii isolated from uninfected and day 8 or 60 infected WT mice. Bars represent the mean (± SEM) values pooled from individual mice (n = 5–7) pooled from two independent experiments. (D) T. gondii levels in thymus during infection. The samples described in C were analyzed for mRNA expression of toxoplasma-specific gene B1, and the data were processed in the same manner. (E) The loss of corticomedullary demarcation and irregularity of the cortex persists during chronic infection. Thymii from naive or day 60 infected WT mice were stained with anti-keratin 5 (green) and anti-keratin 8 (red) plus anti-CD31 antibody (white) and analyzed by confocal microscopy. Bar, 150 µm. (F) Persistent decrease in number of mTEC and cTEC during T. gondii infection. TEC populations were isolated from mice described in E. Bars represent the mean numbers (± SEM) of cTEC and mTEC calculated from analyses performed on individual animals (n = 4–6) pooled from two independent infections. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To examine the status of thymic epithelium, we analyzed the expression of transcription factor Tbata (Flomerfelt et al., 2010) and the cytokine IL-22 (Dudakov et al., 2012), which are both known to be involved in the regulation of TEC expansion. When mRNA levels for these two proteins were measured by qRT-PCR in thymii on day 8 after infection low Tbata and high IL-22 levels were observed (Fig. 6 C). This suggested that the remaining TECs are responding to damage and attempting to undergo a regenerative process because Tbata inhibits, whereas IL-22 promotes, TEC growth. Interestingly, however, levels of Tbata and IL-22 were found to normalize when measured again on day 60 after infection despite the persistent atrophy (Fig. 6 C) indicating that the new equilibrium achieved in the chronic phase is stable. The observed changes in the expression of Tbata and IL-22 mRNA were not caused by differences in the total number of TEC because similar levels of FoxN1 mRNA were detected at both time points (Fig. 6 C). Nevertheless, the profound changes in the organization of the thymic epithelium as indicated by decreased cortical surface area and increased convolution of the corticomedullary border were found to persist into the chronic phase of infection (Fig. 6 E), as well as the significantly decreased cTEC and mTEC absolute numbers (Fig. 6 F).
T. gondii–induced thymic atrophy is directly dependent on parasite-induced alteration of the thymic epithelium
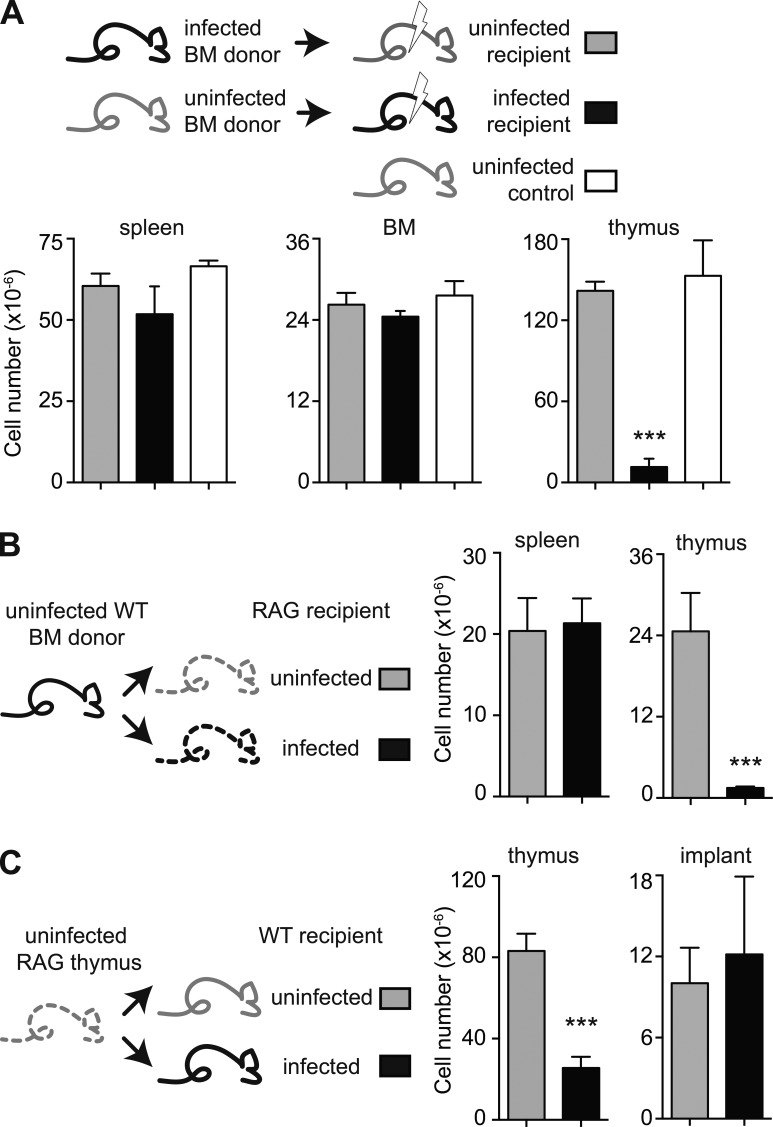
T. gondii infection triggers mobilization of BM progenitors, as well as BM hypoplasia (Petakov et al., 2002; Glatman Zaretsky et al., 2014). Indeed, we observed a partial and transient reduction in the number of BM cells in infected animals (unpublished data). To determine whether the persistent thymic atrophy observed during T. gondii infection is a consequence of an effect on the thymic epithelium or on the thymic precursors in BM, we performed experiments in which irradiated chronically infected mice were reconstituted with BM cells from uninfected animals or, reciprocally, irradiated naive mice were reconstituted with BM from chronically infected animals. After allowing reconstitution for 8 wk, we assessed thymic, BM, and splenic cellularity in both groups (Fig. 7 A). We found that the uninfected mice reconstituted with BM from infected donors displayed the same cellularity in all organs as intact uninfected C57BL/6 animals. In direct contrast, infected mice reconstituted with naive BM displayed normal levels of cells in BM and spleen, but a profound cellular deficiency in the thymus.
Figure 7.
The persistent thymic atrophy induced by T. gondii is a consequence of the disruption of thymic epithelium rather than a loss in bone marrow hematopoietic precursors. (A) BM cells from uninfected mice fail to restore thymic cellularity in infected recipients, whereas BM cells from the infected donors can regenerate the thymus in uninfected hosts. Numbers of cells in spleen, BM, and thymus were analyzed in an untreated age-matched control group of mice, as well as uninfected and 6-wk infected animals that were irradiated and reconstituted reciprocally with BM 8 wk prior. (B) Engraftment of BM cells reconstitutes the thymus in uninfected, but not T. gondii–infected, RAG2−/− mice. Splenic and thymic cellularity was determined in control and 7-d infected RAG2−/− animals that were i.v. injected with the same BM cells from uninfected WT animals 6 wk prior and maintained on antibiotic water during that period. (C) Thymic engraftment is equally successful in uninfected and infected WT mice. Uninfected and chronically infected animals received a thymus from 1-d-old RAG−/− mice and, 6 wk later, the number of cells in the endogenous and grafted thymii was evaluated in individual mice. (A–C) Bars represent the mean ± SEM cell number per organ in each group (n = 3–4) from one out of two independent experiments performed. ***, P < 0.001.
Because irradiation is known to induce thymocyte and TEC depletion, we performed an additional set of experiments using RAG−/− mice reconstituted with BM cells in the absence of irradiation. Once again, transfer of naive WT BM failed to reconstitute thymic cellularity in infected recipient, whereas fully reconstituting uninfected animals (Fig. 7 B).
The aforementioned experiments suggested that thymic atrophy in T. gondii–infected mice is caused by an effect on a nonhematopoietic component of that organ. To test whether thymic epithelium is the targeted cellular component, we implanted neonatal thymii from RAG−/− mice into naive or chronically WT infected animals. When examined 6 wk later, both the cellularity and histology of the thymic implants (unpublished data) were indistinguishable in the two sets of recipients, despite the expected significant difference in the size of the endogenous orthotopic thymii (Fig. 7 C).
Together these findings argued that the primary defect responsible for the persistent thymic atrophy observed during toxoplasma infection is a loss in epithelial integrity.
T cells display decreased CD5 levels during chronic T. gondii infection
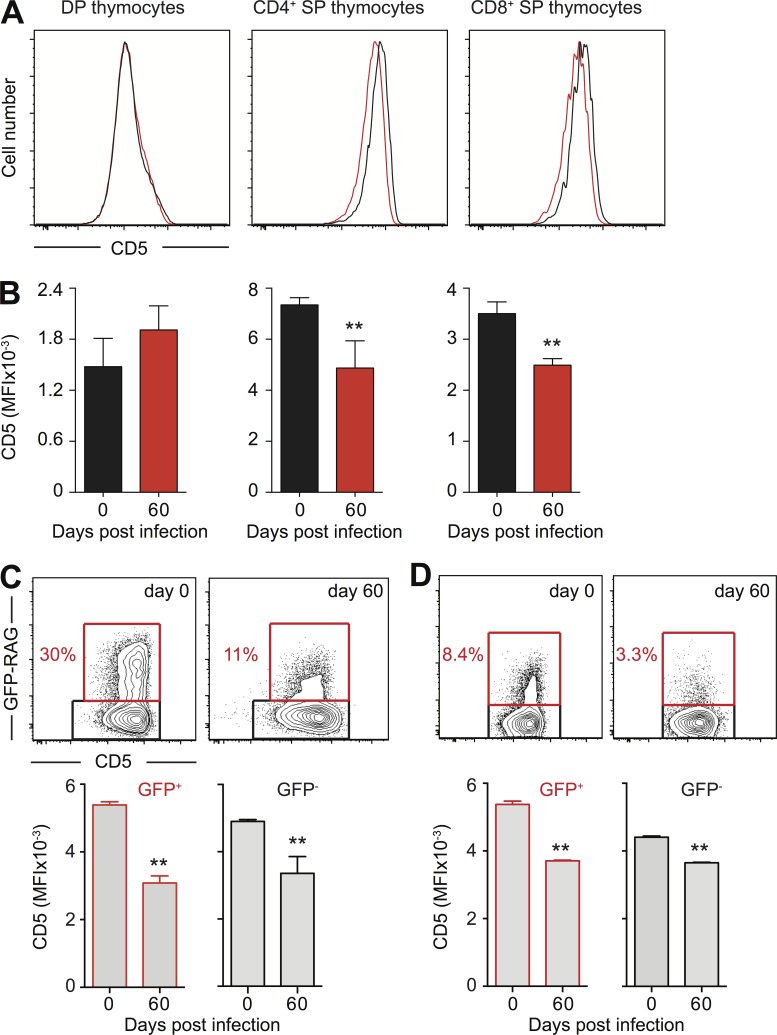
Because TEC provide a critical microenvironment for T cell selection, we questioned whether the thinning of the cortex, and loss of corticomedullary demarcation occurring in thymii of toxoplasma infected mice influences not only the number of thymocytes but also the immunocompetence of naive T cells. CD5 expression on naive T lymphocytes has proven to be a reliable read-out for the strength of the positively selecting TCR–peptide–MHC interaction in thymic cortex (Azzam et al., 1998; Klein et al., 2014). When assessed in our model, we found that the level of CD5 expression on single-positive CD4+ or CD8+ thymocytes was decreased in chronically infected mice when compared with same populations in uninfected mice (Fig. 8, A and B). Importantly, this reduction in CD5 expression was also evident in peripheral GFP-RAG+ RTE, as well as in the total population of naive CD4 and CD8 T cells in T. gondii chronically infected mice (Fig. 8, C and D). These results suggested that the thymic epithelial alterations seen in T. gondii–infected mice may not only affect the number of naive T cells generated (Fig. 1 C), but also result in the development/selection of T cells with lower TCR avidity for self-peptide–MHC. Consistent with the latter hypothesis, we observed a significantly decreased ratio of CD4/CD8 expression among mature TCRhigh thymocytes in chronically infected when compared with uninfected mice (2.29 ± 0.17 vs. 3.36 ± 0.24).
Figure 8.
Decreased expression of CD5 on mature thymocytes and naive T cells in T. gondii chronically infected mice. (A and B) CD5 levels on DP or single-positive CD24+ GFP+ thymocytes in T. gondii chronically infected mice. (A) Representative overlay histograms of CD5 staining from control (black line) and day 60 infected GFP-RAG animals (red line). (B) Bars represent the mean ± SEM of CD5 MFI for each group (n = 4) from one of two experiments performed. (C and D) CD5 levels on naive CD4+ and CD8+ T cells in spleen of T. gondii chronically infected mice. Representative contour plots of CD5 versus GFP-RAG staining on splenic CD62L+CD44− CD4+ (C) or CD8+ (D) T lymphocytes isolated from control and day 60 infected GFP-RAG animals. Bars represent the mean ± SEM of CD5 MFI on GFP+ (left) and GFP− (right) naive CD4+ (C) or CD8+ (D) T cells for each group (n = 4) from one of two experiments performed. **, P < 0.01.
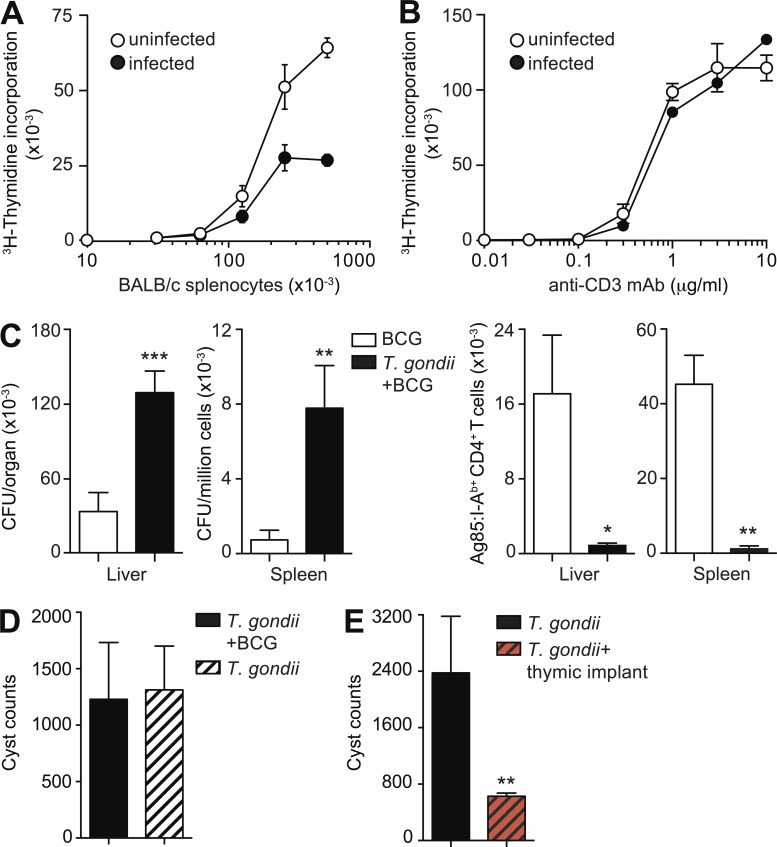
T lymphocytes expressing low levels of CD5 are known to be poorly responsive to foreign antigens (Mandl et al., 2013; Persaud et al., 2014). Indeed, an attenuated response was observed when naive splenic CD4+ T lymphocytes from 60 d T. gondii–exposed mice were stimulated in an allogeneic mixed lymphocyte reaction using irradiated BALB/c splenocytes (Fig. 9 A). In contrast, we demonstrated that the same cell population displayed normal responsiveness when polyclonally activated with anti-CD3 mAb (Fig. 9 B).
Figure 9.
The antigen-dependent responsiveness of CD4+ T cells is compromised during chronic T. gondii infection. (A and B) Decreased antigen responsiveness of naive T cells in chronically infected mice. 3H-thymidine incorporation of CD62+CD44− CD4+ T lymphocytes isolated from mice on day 0 and 60 after infection and cultured for 60 h with indicated number of irradiated BALB/c splenocytes (A) or stimulated with increasing concentrations of anti-CD3 mAb (B). Data are shown as mean ± SD cpm from duplicate cultures from one representative out of two (A) and three (B) experiments performed. (C) Number of BCG CFU (left) and Ag85+ CD4+ T cells (right) in liver and spleen of control and 50 d T. gondii–infected mice challenged in parallel with BCG for 25 d. Bars represent the mean ± SEM of values for individual mice (n = 6–10) pooled from two independent experiments. (D) BCG challenge does not affect the parasite burden in chronically infected mice. Data are shown as mean ± SEM of cyst number/brain from individual mice (n = 5) from one representative out of two experiments performed. (E) Increased host resistance of T. gondii chronically infected mice after RAG−/− thymic implant. Bars represent the mean ± SEM of values for individual mice (n = 4–12) from two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The contraction of the naive T cell pool in T. gondii infection is associated with decreased immunity to heterologous microbial challenge
To assess the in vivo responsiveness of naive CD4 T cells in mice chronically infected with T. gondii, we used a model of heterologous microbial challenge. Bacillus Calmette–Guérin (BCG) was chosen for this purpose because it produces a self-limiting infection that, similar to T. gondii, stimulates a Th1 immune response without triggering thymic atrophy. In addition, in this model, mycobacteria-specific CD4+ T cell responses can be readily tracked using an I-Ab-Ag85b tetramer.
When assessed 25 d after BCG challenge, mice chronically infected with toxoplasma displayed fourfold higher bacterial CFU in both liver and spleen (Fig. 9 C). This increase in bacterial load was accompanied by a dramatic reduction in the number of Ag85b-specific CD4+ T cells in both organs. The impaired BCG response did not appear to be the result of defective APC function, as irradiated splenocytes from T. gondii–infected mice were able to efficiently prime TCR transgenic P25 CD4+ T cells specific for Ag85b (unpublished data). In addition, no differences in parasite cyst burden were observed between unchallenged and BCG-challenged T. gondii–infected mice (Fig. 9 D), excluding an indirect effect of secondary mycobacterial exposure on the primary protozoan infection as an explanation for the altered response to BCG.
Restoration of thymic function enhances control of chronic T. gondii infection
The aforementioned findings raised the interesting possibility that thymic atrophy contributes to parasite persistence itself. In this scenario, acute infection with T. gondii would lead to suppression of the response to stage-specific surface antigens expressed by the parasite during the chronic phase (Kim and Boothroyd, 2005). To test this hypothesis, we examined the effect of thymic grafting on parasite burdens in chronically infected mice. We found that 60-d T. gondii exposed animals implanted with thymii from naive RAG−/− mice displayed a significant threefold reduction in brain cyst loads compared with nonimplanted controls (Fig. 9 E). The latter finding supports the concept that parasite-induced thymic involution promotes chronic infection.
Discussion
The thymus plays a major role in the immune system by providing a continuous source of naive T lymphocytes with a wide variety of TCR specificities that recognize a broad range of foreign antigens (Miller, 2011). As the body reaches adulthood, the gradual involution of the thymus accelerates likely reflecting the increasing need to prevent tolerance to foreign antigen and, conversely, the decreasing need to delete self-antigen–specific T cells (Dowling and Hodgkin, 2009; Shanley et al., 2009). In an ideal situation, host exposure to infections and other antigenic challenges should not affect the normal homeostatic function of the thymus in maintaining T cell availability and diversity. Nevertheless, a wide variety of pathogens trigger an atrophic state in which the thymus undergoes an accelerated decrease in cellularity that in some (Ross et al., 2012), but not all, situations (Douek et al., 1998; Bonzon and Fan, 1999; Borges et al., 2012) rebounds to steady-state levels after acute infection. This infection-induced thymic atrophy has been postulated to be a mechanism promoting pathogen virulence. However, the impact of infectious exposures on the normal age-dependent process of thymic involution is poorly understood.
In this study, we describe a long-term effect of T. gondii infection on the ability of the host to generate naive T cells. This immune alteration is associated with a profound and persistent thymic atrophy that reduces not only the size of the naive T lymphocyte pool but also the functional competence of those cells that successfully emerge. The resulting immunocompromised state manifests in decreased resistance to both heterologous pathogen challenge and established T. gondii infection itself.
Previous studies on thymic atrophy have identified GR-mediated apoptosis of DP thymocytes as an important mechanism underlying this process (Savino, 2006; Pérez et al., 2007). In contrast, the data presented here demonstrate that T cell–intrinsic GR signaling is not the major trigger of T. gondii–induced thymic atrophy. Instead, thymic atrophy was closely associated with dramatic changes in the architecture of the thymic epithelium. Interestingly, several clinical reports describe similarly altered thymic architecture in autopsied T. gondii–infected humans (Frenkel and Friedlander, 1951; Yermakov et al., 1982).
The thymus is a highly vascularized organ, but is deficient in immune effector cells relative to secondary lymphoid tissue. This may favor the accumulation of pathogen-infected CD11b cells, which unchecked by the immune response can serve as Trojan horses delivering intracellular microbes to thymocytes and TEC. T. gondii promiscuously invades multiple cell types and, as described here, can infect thymic epithelium during its acute blood-borne dissemination. Nevertheless, the subsequent permanent changes in TEC do not appear to depend on the continued physical presence of the parasite in the tissue. Moreover, our attempts to regenerate the thymus in T. gondii–exposed mice by administering keratin growth factor, and IL-22 were unsuccessful (unpublished data). Based on these observations, we speculate that the persistent thymic atrophy occurring during toxoplasma infection may stem from parasite invasion and the permanent destruction of TEC progenitor cells.
Although not well studied, the thymic atrophy occurring in other infections may also involve related GC-independent mechanisms of TEC impairment. For example, the GC-independent thymic involution seen in mice exposed to Mycobacterium avium is associated with the presence of infected macrophages which accumulate in that organ during the chronic phase (Morrison et al., 1982; Borges et al., 2012). Similarly, in murine influenza (PR8) the tropism of the virus for epithelium may contribute to the thymic atrophy seen in this infection because damage of thymic epithelium was observed even in adrenalectomized mice (Garaci et al., 1974). Likewise, measles infection has been shown to induce terminal differentiation of cortical TEC in vitro (Numazaki et al., 1989) and, importantly, apoptosis of human TEC in a SCID-hu mouse model (Auwaerter et al., 1996). In addition, during chronic LCMV infection, infectious virus has been detected in both thymocytes and cortical stromal cells (King et al., 1992). Consistent with the latter findings, we have observed significant reductions in the numbers of splenic CD44−CD62+ CD4+ and CD8+ T lymphocytes in LCMV-infected mice on day 40 after infection (unpublished data).
Regardless of the mechanism of its induction, infection-triggered thymic atrophy has been postulated to play a role in promoting pathogen persistence. For example, infection of the thymus with M. avium has been proposed to induce a state of central tolerance to antigens of the pathogen, thereby facilitating its long-term survival in the host (Nobrega et al., 2010, 2013). Alternatively, by causing a wholesale decrease in thymic output, atrophy may result in a generalized immunosuppression affecting both pathogen-specific and irrelevant effector T cell responses. Our findings are consistent with the latter outcome.
T lymphocyte expression of CD5 in the periphery is known to correlate with the affinity of TCR for self-antigen and indirectly for foreign antigen, a property imprinted during T cell differentiation in the thymus. It was therefore of interest that both CD4 and CD8 thymocytes, the initial RTE appearing after thymic involution during acute infection (unpublished data) and the subsequent pool of naive T cells present during the chronic phase, express decreased levels of CD5. These observations suggest that the damage to the thymic epithelium induced by T. gondii results in impaired positive selection of the thymocyte repertoire that lessens the ability of the host to mount an adequate immune response. Indeed, chronically infected mice display an increased susceptibility to co-infection with a heterologous pathogen, as well as improved survival of the primary infection. In addition, we have observed a highly significant negative correlation between cyst burden and thymocyte numbers in mice chronically infected with T. gondii (unpublished data), an observation also consistent with a role for thymic involution in parasite persistence.
An interesting aspect of the alterations in thymic architecture and function described here in murine T. gondii infection is that they resemble the changes in this organ observed during natural aging. Thus, senescence of the immune system is associated with gradual structural deterioration of the thymic epithelium (Gui et al., 2007; Kim et al., 2015) and a concurrent decline in the frequency of peripheral naive T lymphocytes (Fagnoni et al., 2000; Naylor et al., 2005; Schulz et al., 2015). These parallel manifestations of infection and senescence raise the intriguing possibility that exposure during childhood to pathogens that, along with T. gondii, target the thymus could accelerate the natural aging process, rendering the host more susceptible to new infections. A proposed link between prior infectious exposure and the kinetics of immune senescence is supported by several observations (Gavazzi and Krause, 2002; Deeks, 2011; Derhovanessian et al., 2014). Perhaps the best studied example is the association of seropositivity for cytomegalovirus (CMV), a pathogen known to infect TEC (Mocarski et al., 1993; Price et al., 1993), and accelerated aging of the immune system (Pawelec et al., 2010). Although this phenomenon has been attributed to memory T cell inflation (Arens et al., 2015), the results presented here raise virus-induced thymic disruption as a possible alternative explanation of the CMV infection data. Future studies that analyze in depth the longitudinal relationship between age, infection history, and thymic output could be used to evaluate the contribution of this proposed mechanism to immune senescence.
Materials and methods
Experimental animals
C57BL/6 and BALB/c mice were purchased from Taconic Farms, whereas C57BL/6 CD45.2 RAG1−/−, CD45.1 RAG1−/−, IL-10−/−, and IFNAR (IFN-α/βR−/−) mice were provided by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Supply Contract at Taconic Farms. TNF−/−, IFN-γR−/−, and CCR2−/− animals were obtained from The Jackson Laboratory; STAT1−/− animals (Durbin et al., 1996) were provided by D. Woodland (Trudeau Institute, Saranac Lake, NY); MyD88−/−, MyD88−/−TRIF−/− double-deficient (Yamamoto et al., 2003), IL-27R−/− (Batten et al., 2008), GFP-RAG (Yu et al., 1999), and GRlck-Cre (Mittelstadt et al., 2012) mice were bred in our facilities. IFN-γ−/−RAG−/− and p40−/−IL-10−/−RAG−/− animals were generated by intercrossing mice with the respective single gene deficiency. All animals were maintained at an American Association for the Accreditation of Laboratory Animal Care–accredited and specific pathogen–free facility at the NIAID/National Institutes of Health or National Cancer Institute (NCI)/National Institutes of Health. All procedures were performed in accordance with the protocols outlined in the Guide for the Care and Use of Laboratory Animals and described in an animal study proposal approved by the NIAID Animal Care and Use Committee. 8–12-wk-old age- and sex-matched experimental and littermate control mice were used in all experiments, except when noted otherwise.
T. gondii infection and parasite burden determination
Type II avirulent strain ME-49 cysts were obtained from the brains of chronically infected C57BL/6 mice. Cyst preparations were pepsin treated to eliminate potential contamination with host cells and mice were inoculated i.p. with an average of 15 cysts. Parasite burden was assessed by enumerating infected cells in cytospin-smears of PEC (105 cells) during the acute phase of infection or by counting cysts in brain homogenates during the chronic phase. Tachyzoites of the RH-88 type I strain of T. gondii, its temperature sensitive mutant, ts-4 (Pfefferkorn and Pfefferkorn, 1976), and an mCherry+ expressing recombinant (Koshy et al., 2010), were cultured and harvested from infected monolayers of human foreskin fibroblasts.
Vaccination experiments
Groups of mice were vaccinated by two biweekly i.p. injections of 2 × 105 ts-4 tachyzoites in 0.5 ml PBS, while a control group was injected with PBS alone. Both groups were challenged in parallel 2 wk later by subcutaneous injection of 1,000 virulent RH strain tachyzoites.
BCG cultures, infections, and quantification
M. bovis BCG (strain Pasteur) were expanded to log phase in Middlebrook 7H9 liquid medium (Difco) supplemented with OADC (Difco), 0.05% glycerol (Invitrogen), and 0.05% Tween-80 (Thermo Fisher Scientific), washed, aliquoted in PBS, and stored at −80°C until further use. Animals were inoculated i.v. with 5 × 106 CFU. Quantification of bacterial stocks and the numbers of viable bacteria in whole-organ homogenates on day 25 after infection were measured by plating serial dilutions supplemented Middle-brook 7H11 nutrient agar (Difco) with OADC and 0.5% glycerol. Plates were incubated for 20 d at 37°C before counting bacterial colony formations.
In vivo mAb treatment
To block TNF, IFN-γ, and IL-6 function mice were injected i.p. with the combination of 1 mg each of neutralizing antibody XT3-11, XMG-1.2, and MP5-20F3 (BioXcell) on days −1, 1, 3, 5, and 7 after infection. Control groups of animals received an equivalent amount of rat mAb GL113.
Cell preparation and flow cytometry analyses
At the indicated time points, single-cell suspensions were prepared from peritoneal exudate cell (PEC), spleen, liver, thymus, or BM from individual naive and infected animals, and after counting nucleated cells were used for further analysis (Jankovic et al., 2007). Samples from individual animals were stained with Fixable Viability Dye (eBioscience) and an appropriate combination of mAb specific for CD4 (RM4-5), CD8 (53–6.7), CD44 (IM7), CD62L (MEL14), TCRβ (H57-597), T-bet (O4-46), CD5 (53–7-3), CD24 (M1/69) B220 (RA3-6B2), NK1.1 (PK136), CD90.1 (HIS51), CD90.2 (53–2.1), CD45.1 (A20), or CD45.2. (104) purchased from eBioscience, BioLegend, or BD. Parasite-specific CD4+ T cells were determined using a fluorescently labeled MHC class II tetramer bound to T. gondii antigenic peptide AS15 (Grover et al., 2012), whereas BCG-specific CD4+ lymphocytes were detected with a fluorescently labeled Ag85b280-294–loaded I-Ab tetramer (Vogelzang et al., 2014), both provided by the National Institutes of Health Tetramer Core Facility (Bethesda, MD).
Proliferation assay
Splenic naive Th lymphocytes were isolated as CD62L+CD44− CD4+ T cells by sorting total splenocytes on a FACSAria III (BD) and gating out B220+, NK1.1+, I-A/I-Eb+, and CD8+ T cells. Naive CD4+ T lymphocytes (2 × 105/well) were stimulated with indicated number of irradiated BALB/c splenocytes. Alternatively, naive CD4+ T cells (7.5 × 104/well) were cultured in 96-well plates coated with 1 µg of anti-CD28 (37–51) and increasing amounts of anti-CD3 mAbs (2C11). After 48 h of incubation, cultures were pulsed with 3H-thymidine for an additional 18 h before harvesting.
TEC isolation
Thymii from uninfected and infected mice were processed individually by mincing in a Mickle Tissue Homogenizer, followed by incubation at 37°C for 15 min and shaking at 50 RPM 37°C in 0.5 ml RPMI containing 20 µl of Liberase TH (28 U/ml) and DNase1 (5 mg/ml). Phenotypic analysis of thymic epithelial cells was performed on CD45neg cells stained with anti-EpCAM (G8.8) Ulex Europaeus Agglutinin I (Vector Laboratories), anti-Ly51 antibody (BP-1), anti-CD31 antibody (390), and anti-MHC class II antibody (M5/114). The TEC-gating strategy as described previously (Xing and Hogquist, 2014) is shown in Fig. S1.
Bone marrow reconstitution
In one set of experiments, 48-d T. gondii–infected and uninfected age-matched C57BL/6 mice were lethally irradiated with 950 rad and subsequently reconstituted with 2 × 106 congenic whole bone marrow cells using mismatched CD45.1/2 markers from either uninfected or 48-d T. gondii–infected donors, respectively. Alternatively, 8-d T. gondii–infected and uninfected age-matched RAG2−/− mice were injected i.v. with 107 syngeneic bone marrow cells from naive WT donors after depletion of T lymphocytes by negative selection on CD90.2 MACS columns (Miltenyi Biotec). All BM recipients were maintained on antibiotic TMS water containing Trimethoprim (0.13 mg/ml), Sulfadiazine (0.5 mg/ml), and 0.67 mg/ml Sulfamethoxazole (0.67 mg/ml) throughout the duration of the experiment 8 and 6 wk for the WT and RAG2−/− recipients, respectively. In addition to their bactericidal activity, these antibiotics are known to prevent T. gondii replication, and thus rescued the infected RAG2−/− mice from lethality.
Histology and immunofluorescent microscopy
Tissues were fixed in Bouin's fixative, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For immunofluorescence, sections of a tissue embedded in OCT freezing media were prepared on a CM3050s cryostat (Leica), adhered to Superfrost Plus slides (VWR), and subsequently permeabilized and blocked in PBS containing 0.3% Triton X-100 (Sigma-Aldrich) plus 10% normal mouse serum (Jackson ImmunoResearch Laboratories). Staining was performed with rabbit anti-K5 mAb (EP1601Y; Abcam) and rat anti-K8 mAb (TROMA-I; DSHB). Histological sections were evaluated (based on a 1–10 scoring system) for two parameters: cortical area that measures the distance from the thymic capsule to the corticomedulary border and convolution of the corticomedulary border that measures the length of the junction between cortex and medulla.
Confocal immunofluorescence microscopy
Isolated mouse thymii were sliced into 8–10-µm sections using a cryostat and stained with fluorescently labeled anti-K5, K8, and CD31 mAb. Confocal microscopy of immunostained sections was performed using a Leica SP8 inverted five-channel confocal microscope equipped with 2 HyD ultra-sensitive detectors (Leica) and a broad range of visible lasers. The microscope configuration was adjusted for three-dimensional analysis (x, y, z) of cell segregation within tissue sections, and 20-µm z stacks were collected.
Thymus grafting
Thymii were recovered from newborn congenitally marked C57BL/6 CD45.1 RAG1−/− donor mice. Recipient 48-d T. gondii–infected and uninfected age-matched C57BL/6 animals were shaved and scrubbed, and a suitable incision was made in the right shoulder blade in which the donor thymus was inserted. The topical analgesic bupivacaine was applied into the incision for immediate post-surgical analgesia, and the wound was closed with two sterile wound clips.
Quantitative RT-PCR
Total RNA was isolated (RNeasy Mini kit; QIAGEN) and reverse-transcribed (SuperScript II Reverse transcription; Invitrogen). Gene expression analysis was performed using SYBRGreen-based real-time quantitative PCR (RT-qPCR) on an ABI Prism 7900HT analyzer (Applied Biosystems). Arbitrary units represent the ratio of tested mRNA levels compared with hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA levels. The following primer pairs were used: Tbata: 5′-TCAGGGACGAGTTCTCTCTGT-3 (forward) and 5′-GGTTCCGATTGGACTGTGGA-3′ (reverse); il22: 5′-TCTGGATGTTCTGGTCGTCA-3′ (forward) and 5′-TTTCCTGACCAAACTCAGCA-3′ (reverse); Foxn1: 5′-CATCTACCAGGCCCAAGG-3′ (forward) and 5′-AGCAGCTAGAGCTCTCTGTTCAC-3′ (reverse); B-1: 5′-TCCCCTCTGCTGGCGAAAAGT-3′ (forward) and 5′-AGCGTTCGTGGTCAACTATCGATTG-3′ (reverse; Burg et al., 1989).
Statistical analysis
The statistical significance of differences between data means was evaluated using an unpaired, two-tailed Student’s t test and, in the case of qRT-PCR data, by nonparametric ANOVA (Kruskal-Wallis) with multiple-comparisons.
Online supplemental material
Fig. S1 shows the gating strategy for FACS analysis of TEC.
Supplementary Material
Acknowledgments
We thank Calvin Eigsti for performing FACS sorting, Sandy White, Sara Hieny, and Deborah Surman Depew for technical help with toxoplasma infections, and Sharon Evans for assistance with thymus grafting. We are also grateful to Dr. Rima McLeod for discussions and input concerning human congenital toxoplasmosis.
This work was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health and by an Intramural AIDS Research Fellowship (to D.G. Kugler).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- cTEC
- cortical TEC
- DP
- double positive
- GC
- glucocorticoid
- GR
- GC receptor
- mTEC
- medullary TEC
- PEC
- peritoneal exudate cell
- RTE
- recent thymic emigrant
- TEC
- thymic epithelial cell
References
- Arens R., Remmerswaal E.B., Bosch J.A., and van Lier R.A.. 2015. 5th International Workshop on CMV and Immunosenescence - A shadow of cytomegalovirus infection on immunological memory. Eur. J. Immunol. 45:954–957. 10.1002/eji.201570044 [DOI] [PubMed] [Google Scholar]
- Auwaerter P.G., Kaneshima H., McCune J.M., Wiegand G., and Griffin D.E.. 1996. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam H.S., Grinberg A., Lui K., Shen H., Shores E.W., and Love P.E.. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301–2311. 10.1084/jem.188.12.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M., Kljavin N.M., Li J., Walter M.J., de Sauvage F.J., and Ghilardi N.. 2008. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 180:2752–2756. 10.4049/jimmunol.180.5.2752 [DOI] [PubMed] [Google Scholar]
- Berkley A.M., Hendricks D.W., Simmons K.B., and Fink P.J.. 2013. Recent thymic emigrants and mature naive T cells exhibit differential DNA methylation at key cytokine loci. J. Immunol. 190:6180–6186. 10.4049/jimmunol.1300181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzon C., and Fan H.. 1999. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J. Virol. 73:2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges M., Barreira-Silva P., Flórido M., Jordan M.B., Correia-Neves M., and Appelberg R.. 2012. Molecular and cellular mechanisms of Mycobacterium avium-induced thymic atrophy. J. Immunol. 189:3600–3608. 10.4049/jimmunol.1201525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg J.L., Grover C.M., Pouletty P., and Boothroyd J.C.. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Perez J., Condotta S.A., James B.R., Kashem S.W., Brincks E.L., Rai D., Kucaba T.A., Badovinac V.P., and Griffith T.S.. 2015. Alterations in antigen-specific naive CD4 T cell precursors after sepsis impairs their responsiveness to pathogen challenge. J. Immunol. 194:1609–1620. 10.4049/jimmunol.1401711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S.G. 2011. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 62:141–155. 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis J., Aurélio Farias-de-Oliveira D., Nunes Panzenhagen P.H., Maran N., Villa-Verde D.M., Morrot A., and Savino W.. 2012. Thymus atrophy and double-positive escape are common features in infectious diseases. J. Parasitol. Res. 2012:574020 10.1155/2012/574020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E., Maier A.B., Hähnel K., McElhaney J.E., Slagboom E.P., and Pawelec G.. 2014. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J. Immunol. 193:3624–3631. 10.4049/jimmunol.1303361 [DOI] [PubMed] [Google Scholar]
- Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F., Polis M.A., Haase A.T., Feinberg M.B., Sullivan J.L., et al. . 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature. 396:690–695. 10.1038/25374 [DOI] [PubMed] [Google Scholar]
- Dowling M.R., and Hodgkin P.D.. 2009. Why does the thymus involute? A selection-based hypothesis. Trends Immunol. 30:295–300. 10.1016/j.it.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Dudakov J.A., Hanash A.M., Jenq R.R., Young L.F., Ghosh A., Singer N.V., West M.L., Smith O.M., Holland A.M., Tsai J.J., et al. . 2012. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 336:91–95. 10.1126/science.1218004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin J.E., Hackenmiller R., Simon M.C., and Levy D.E.. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 84:443–450. 10.1016/S0092-8674(00)81289-1 [DOI] [PubMed] [Google Scholar]
- Fagnoni F.F., Vescovini R., Passeri G., Bologna G., Pedrazzoni M., Lavagetto G., Casti A., Franceschi C., Passeri M., and Sansoni P.. 2000. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood. 95:2860–2868. [PubMed] [Google Scholar]
- Fink P.J. 2013. The biology of recent thymic emigrants. Annu. Rev. Immunol. 31:31–50. 10.1146/annurev-immunol-032712-100010 [DOI] [PubMed] [Google Scholar]
- Flomerfelt F.A., El Kassar N., Gurunathan C., Chua K.S., League S.C., Schmitz S., Gershon T.R., Kapoor V., Yan X.Y., Schwartz R.H., and Gress R.E.. 2010. Tbata modulates thymic stromal cell proliferation and thymus function. J. Exp. Med. 207:2521–2532. 10.1084/jem.20092759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J.K., and Friedlander S.. 1951. Toxoplasmosis: pathology of neonatal disease, pathogenesis, diagnosis, and treatment. United States Government Printing Office, Washington. 12-35 pp. [Google Scholar]
- Gabor M.J., Scollay R., and Godfrey D.I.. 1997. Thymic T cell export is not influenced by the peripheral T cell pool. Eur. J. Immunol. 27:2986–2993. 10.1002/eji.1830271135 [DOI] [PubMed] [Google Scholar]
- Garaci E., Caliò R., and Djaczenko W.. 1974. Effect of influenza virus PR8 infection on thymus in intact and adrenalectomized mice. Experientia. 30:358–360. 10.1007/BF01921662 [DOI] [PubMed] [Google Scholar]
- Gavazzi G., and Krause K.H.. 2002. Ageing and infection. Lancet Infect. Dis. 2:659–666. 10.1016/S1473-3099(02)00437-1 [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kühn R., Müller W., Trinchieri G., and Sher A.. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798–805. [PubMed] [Google Scholar]
- Glatman Zaretsky A., Engiles J.B., and Hunter C.A.. 2014. Infection-induced changes in hematopoiesis. J. Immunol. 192:27–33. 10.4049/jimmunol.1302061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover H.S., Blanchard N., Gonzalez F., Chan S., Robey E.A., and Shastri N.. 2012. The Toxoplasma gondii peptide AS15 elicits CD4 T cells that can control parasite burden. Infect. Immun. 80:3279–3288. 10.1128/IAI.00425-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J., Zhu X., Dohkan J., Cheng L., Barnes P.F., and Su D.M.. 2007. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 19:1201–1211. 10.1093/intimm/dxm095 [DOI] [PubMed] [Google Scholar]
- Haegert D.G., Hackenbroch J.D., Duszczyszyn D., Fitz-Gerald L., Zastepa E., Mason H., Lapierre Y., Antel J., and Bar-Or A.. 2011. Reduced thymic output and peripheral naïve CD4 T-cell alterations in primary progressive multiple sclerosis (PPMS). J. Neuroimmunol. 233:233–239. 10.1016/j.jneuroim.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Hall A.O., Beiting D.P., Tato C., John B., Oldenhove G., Lombana C.G., Pritchard G.H., Silver J.S., Bouladoux N., Stumhofer J.S., et al. . 2012. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 37:511–523. 10.1016/j.immuni.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D., Teyton L., and Oldstone M.B.. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919. 10.1038/90950 [DOI] [PubMed] [Google Scholar]
- Hotchkiss R.S., Coopersmith C.M., McDunn J.E., and Ferguson T.A.. 2009. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 15:496–497. 10.1038/nm0509-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug A., Korporal M., Schröder I., Haas J., Glatz K., Storch-Hagenlocher B., and Wildemann B.. 2003. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J. Immunol. 171:432–437. 10.4049/jimmunol.171.1.432 [DOI] [PubMed] [Google Scholar]
- Huldt G., Gard S., and Olovson S.G.. 1973. Effect of Toxoplasma gondii on the thymus. Nature. 244:301–303. 10.1038/244301a0 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., and Sher A.. 2007. Conventional T-bet+Foxp3- Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.K., Chu H.H., McLachlan J.B., and Moon J.J.. 2010. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu. Rev. Immunol. 28:275–294. 10.1146/annurev-immunol-030409-101253 [DOI] [PubMed] [Google Scholar]
- Kim S.K., and Boothroyd J.C.. 2005. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 174:8038–8048. 10.4049/jimmunol.174.12.8038 [DOI] [PubMed] [Google Scholar]
- Kim M.J., Miller C.M., Shadrach J.L., Wagers A.J., and Serwold T.. 2015. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. J. Immunol. 194:4784–4795. 10.4049/jimmunol.1403158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.C., Jamieson B.D., Reddy K., Bali N., Concepcion R.J., and Ahmed R.. 1992. Viral infection of the thymus. J. Virol. 66:3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L., Kyewski B., Allen P.M., and Hogquist K.A.. 2014. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14:377–391. 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetz K., Bryl E., Spickschen K., O’Fallon W.M., Goronzy J.J., and Weyand C.M.. 2000. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 97:9203–9208. 10.1073/pnas.97.16.9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy A.A., Fouts A.E., Lodoen M.B., Alkan O., Blau H.M., and Boothroyd J.C.. 2010. Toxoplasma secreting Cre recombinase for analysis of host-parasite interactions. Nat. Methods. 7:307–309. 10.1038/nmeth.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler D.G., Mittelstadt P.R., Ashwell J.D., Sher A., and Jankovic D.. 2013. CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection. J. Exp. Med. 210:1919–1927. 10.1084/jem.20122300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu I., Eberhard J., Ahmad F., Bhatnagar N., Behrens G., Jacobs R., Schmidt R.E., and Meyer-Olson D.. 2014. Elevated CD57 and CD95 expressions are associated with lower numbers of CD4+ recent thymic emigrants in HIV-1 infected immune responders following antiretroviral treatment. Immunol. Lett. 158:1–6. 10.1016/j.imlet.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Mandl J.N., Monteiro J.P., Vrisekoop N., and Germain R.N.. 2013. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 38:263–274. 10.1016/j.immuni.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry K.K., Strutt T.M., and Swain S.L.. 2010. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol. Rev. 236:110–124. 10.1111/j.1600-065X.2010.00921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.F. 2011. The golden anniversary of the thymus. Nat. Rev. Immunol. 11:489–495. 10.1038/nri2993 [DOI] [PubMed] [Google Scholar]
- Mittelstadt P.R., Monteiro J.P., and Ashwell J.D.. 2012. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J. Clin. Invest. 122:2384–2394. 10.1172/JCI63067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E.S., Bonyhadi M., Salimi S., McCune J.M., and Kaneshima H.. 1993. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. USA. 90:104–108. 10.1073/pnas.90.1.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W.I., Murray M., and Hinson C.A.. 1982. The response of the murine lymphoid system to a chronic infection with Trypanosoma congolense. II. The lymph nodes, thymus and liver. J. Pathol. 138:273–288. 10.1002/path.1711380308 [DOI] [PubMed] [Google Scholar]
- Naylor K., Li G., Vallejo A.N., Lee W.W., Koetz K., Bryl E., Witkowski J., Fulbright J., Weyand C.M., and Goronzy J.J.. 2005. The influence of age on T cell generation and TCR diversity. J. Immunol. 174:7446–7452. 10.4049/jimmunol.174.11.7446 [DOI] [PubMed] [Google Scholar]
- Nikolich-Žugich J. 2014. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J. Immunol. 193:2622–2629. 10.4049/jimmunol.1401174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega C., Roque S., Nunes-Alves C., Coelho A., Medeiros I., Castro A.G., Appelberg R., and Correia-Neves M.. 2010. Dissemination of mycobacteria to the thymus renders newly generated T cells tolerant to the invading pathogen. J. Immunol. 184:351–358. 10.4049/jimmunol.0902152 [DOI] [PubMed] [Google Scholar]
- Nobrega C., Nunes-Alves C., Cerqueira-Rodrigues B., Roque S., Barreira-Silva P., Behar S.M., and Correia-Neves M.. 2013. T cells home to the thymus and control infection. J. Immunol. 190:1646–1658. 10.4049/jimmunol.1202412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki K., Goldman H., Wong I., and Wainberg M.A.. 1989. Replication of measles virus in cultured human thymic epithelial cells. J. Med. Virol. 27:52–58. 10.1002/jmv.1890270112 [DOI] [PubMed] [Google Scholar]
- Nunes-Alves C., Nobrega C., Behar S.M., and Correia-Neves M.. 2013. Tolerance has its limits: how the thymus copes with infection. Trends Immunol. 34:502–510. 10.1016/j.it.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G., Akbar A., Beverley P., Caruso C., Derhovanessian E., Fülöp T., Griffiths P., Grubeck-Loebenstein B., Hamprecht K., Jahn G., et al. . 2010. Immunosenescence and Cytomegalovirus: where do we stand after a decade? Immun. Ageing. 7:13 10.1186/1742-4933-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M., Linehan J.L., Pagán A.J., Zell T., Dileepan T., Cleary P.P., and Jenkins M.K.. 2010. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 11:83–89. 10.1038/ni.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez A.R., Roggero E., Nicora A., Palazzi J., Besedovsky H.O., Del Rey A., and Bottasso O.A.. 2007. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav. Immun. 21:890–900. 10.1016/j.bbi.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Persaud S.P., Parker C.R., Lo W.L., Weber K.S., and Allen P.M.. 2014. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 15:266–274. 10.1038/ni.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petakov M., Stojanović N., Jovcić G., Bugarski D., Todorović V., and Djurković-Djaković O.. 2002. Hematopoiesis during acute Toxoplasma gondii infection in mice. Haematologia (Budap.). 32:439–455. [PubMed] [Google Scholar]
- Pfefferkorn E.R., and Pfefferkorn L.C.. 1976. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 39:365–376. 10.1016/0014-4894(76)90040-0 [DOI] [PubMed] [Google Scholar]
- Price P., Olver S.D., Gibbons A.E., Teo H.K., and Shellam G.R.. 1993. Characterization of thymic involution induced by murine cytomegalovirus infection. Immunol. Cell Biol. 71:155–165. 10.1038/icb.1993.18 [DOI] [PubMed] [Google Scholar]
- Ross E.A., Coughlan R.E., Flores-Langarica A., Lax S., Nicholson J., Desanti G.E., Marshall J.L., Bobat S., Hitchcock J., White A., et al. . 2012. Thymic function is maintained during Salmonella-induced atrophy and recovery. J. Immunol. 189:4266–4274. 10.4049/jimmunol.1200070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. 2006. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2:e62 10.1371/journal.ppat.0020062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann M., Busch V., Linkemann K., Huster K.M., and Busch D.H.. 2003. Differences in maintenance of CD8+ and CD4+ bacteria-specific effector-memory T cell populations. Eur. J. Immunol. 33:2875–2885. 10.1002/eji.200324224 [DOI] [PubMed] [Google Scholar]
- Schulz A.R., Mälzer J.N., Domingo C., Jürchott K., Grützkau A., Babel N., Nienen M., Jelinek T., Niedrig M., and Thiel A.. 2015. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J. Immunol. 195:4699–4711. 10.4049/jimmunol.1500598 [DOI] [PubMed] [Google Scholar]
- Shanley D.P., Aw D., Manley N.R., and Palmer D.B.. 2009. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 30:374–381. 10.1016/j.it.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M.A., Schreiber R.D., and Remington J.S.. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 240:516–518. 10.1126/science.3128869 [DOI] [PubMed] [Google Scholar]
- Ventevogel M.S., and Sempowski G.D.. 2013. Thymic rejuvenation and aging. Curr. Opin. Immunol. 25:516–522. 10.1016/j.coi.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino A., Hibbert L., Lieberman L., Wilson E., Mak T., Yoshida H., Kastelein R.A., Saris C., and Hunter C.A.. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 19:645–655. 10.1016/S1074-7613(03)00300-5 [DOI] [PubMed] [Google Scholar]
- Vogelzang A., Perdomo C., Zedler U., Kuhlmann S., Hurwitz R., Gengenbacher M., and Kaufmann S.H.E.. 2014. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guérin ΔureC:hly vaccine’s superior protection against tuberculosis. J. Infect. Dis. 210:1928–1937. 10.1093/infdis/jiu347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., and Hogquist K.A.. 2014. Isolation, identification, and purification of murine thymic epithelial cells. J. Vis. Exp. 90:e51780 10.3791/51780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., and Akira S.. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. 10.1126/science.1087262 [DOI] [PubMed] [Google Scholar]
- Yap G.S., and Sher A.. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083–1092. 10.1084/jem.189.7.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga Ji T., Sakai K., Nosaka S. Etoh Ki S. Tamiya S. Koga M. Mita H. Uchino, Mitsuya, and Matsuoka M.. 2001. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 97:3177–3183. 10.1182/blood.V97.10.3177 [DOI] [PubMed] [Google Scholar]
- Yermakov V., Rashid R.K., Vuletin J.C., Pertschuk L.P., and Isaksson H.. 1982. Disseminated toxoplasmosis. Case report and review of the literature. Arch. Pathol. Lab. Med. 106:524–528. [PubMed] [Google Scholar]
- Yonkers N.L., Sieg S., Rodriguez B., and Anthony D.D.. 2011. Reduced naive CD4 T cell numbers and impaired induction of CD27 in response to T cell receptor stimulation reflect a state of immune activation in chronic hepatitis C virus infection. J. Infect. Dis. 203:635–645. 10.1093/infdis/jiq101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., and Nussenzweig M.C.. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687. 10.1038/23287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.