Abboud et al. reveal the striking and unexpected spatial organization of central- versus effector-like memory cells within the infected lung tissue and how cooperation between these two subsets contributes to host defense.
Abstract
How tissue-specific anatomical distribution and phenotypic specialization are linked to protective efficacy of memory T cells against reinfection is unclear. Here, we show that lung environmental cues program recently recruited central-like memory cells with migratory potentials for their tissue-specific functions during lethal respiratory virus infection. After entering the lung, some central-like cells retain their original CD27hiCXCR3hi phenotype, enabling them to localize near the infected bronchiolar epithelium and airway lumen to function as the first line of defense against pathogen encounter. Others, in response to local cytokine triggers, undergo a secondary program of differentiation that leads to the loss of CXCR3, migration arrest, and clustering within peribronchoarterial areas and in interalveolar septa. Here, the immune system adapts its response to prevent systemic viral dissemination and mortality. These results reveal the striking and unexpected spatial organization of central- versus effector-like memory cells within the lung and how cooperation between these two subsets contributes to host defense.
Introduction
Memory CD8 T cells have traditionally been classified as either central memory cells (CCR7hiCD62Lhi; Tcm) that recirculate through the blood and secondary lymphoid organs, or effector memory cells (CCR7loCD62Llo; Tem) that transit through blood and peripheral tissues, such as the lung (Sallusto et al., 1999; Masopust et al., 2001; Jameson and Masopust, 2009; Sathaliyawala et al., 2013; Thome et al., 2014). Recent studies performed in several experimental systems suggest that Tcm and Tem can be further separated into two major subsets based on their expression of CD27: CD27hi central-like (Tcm-l) and CD27lo effector-like (Tem-l) cells (Hikono et al., 2007; Olson et al., 2013). In the spleen, CD27hi cells predominantly reside in the T cell–rich areas of periarteriolar lymphocyte sheath (PALS) and exhibit optimal recall proliferative and self-renewal potential (Hikono et al., 2007; Jung et al., 2010). In contrast, CD27lo cells fail to undergo significant recall proliferation, but efficiently home to the red pulp and the marginal zone surrounding the white pulp (Hikono et al., 2007; Olson et al., 2013). The origins of these memory T cell subsets and how they relate to each other are still being elucidated. One possibility is that long-lived CD27lo Tem-l memory cells participate directly in the initiation of protective recall responses by rapidly producing cytolytic proteins at sites of pathogen entry, whereas activation of CD27hi Tcm-l memory cells is required for the generation of new rounds of effector memory T cells, and thus, may contribute to the maintenance and/or amplification of the overall response. Consistent with this idea, a recent study by Olson et al. (2013) demonstrated that despite their poor proliferative potential, CD27lo cells in the spleen provide superior protection against systemic (i.v.) infection with either Listeria monocytogenes or vaccinia virus (VACV), supporting the concept that to protect against rapidly replicating blood-borne pathogens, high-numbers of CD27lo Tem-l cells need to be present at the site of pathogen entry.
Pathogen-specific CD27lo memory cells also persist in mucosal tissues, such as the lung (Hikono et al., 2007). However, there is little information on how maturation, trafficking, and positioning of this subset of memory cells within specialized niches of the lung influence their ability to initiate a protective recall response to respiratory pathogens. This led us to investigate whether tissue-specific programs might exist at the cellular level, where different memory cell subsets specialize to elicit protective pathogen-specific recall responses.
Results and discussion
Phenotypic heterogeneity of memory CD8 T cells generated by intranasal VACV-WR infection
The presence of memory CD8 T cells in the lung has been associated with increased protection against respiratory virus infections (Kohlmeier and Woodland, 2009); however, it is unclear whether in situ immunity is caused by effector (CD27lo)- or central (CD27hi)-like memory cells trafficking to or resident in the lung tissue and airways. VACV is a good model pathogen for studying the mechanisms by which different memory subpopulations control and eliminate highly pathogenic respiratory viruses. In mice, i.n. infection with the mouse-adapted VACV Western Reserve strain (VACV-WR) causes striking local and systemic changes that, in many respects, mimic human smallpox infection (Chapman et al., 2010). Initial VACV replication occurs in bronchiolar and alveolar epithelial cells, followed by a transient viremia that disseminates the virus throughout the host (Chapman et al., 2010). A lethal respiratory infection with VACV-WR leads to extensive lung pathology, peribronchial and perivascular inflammation, alveoli destruction, hemorrhage, rapid weight loss, and eventual death by day 8 (Chapman et al., 2010; Goulding et al., 2012).
Our previous studies have shown that heterogeneous populations of VACV-WR reactive memory CD8 cells play a vital role in restricting lung pathology and virus dissemination to visceral tissues, and are necessary for complete clearance of virus and protection from death (Salek-Ardakani et al., 2008, 2011b,c). However, as the relative contribution of different memory cell subsets was not directly examined, particularly regarding the CD27hi and CD27lo subset demarcation, we were interested in determining whether control of acute respiratory VACV-WR infection correlated with the presence and/or recruitment of these subsets to the site of infection.
First, we asked whether CD27hi and CD27lo memory cell subsets were established in the lung and lymphoid tissues after recovery from a respiratory infection with VACV-WR. Cohorts of naive WT C57BL/6 mice were infected i.n. with sublethal inoculum of VACV-WR (1.25 × 104 PFU), and the kinetics of effector and memory CD8 T cell recruitment to the lung and spleen were determined by tracking the immunodominant B8R20–27/kb-reactive population (Tscharke et al., 2005). Consistent with published data (Hikono et al., 2007), respiratory VACV-WR infection resulted in the generation of virus-specific CD27hi and CD27lo cells in both the lung and spleen (Fig. S1, A and B), with lung memory cells exhibiting a higher proportion of CD27lo cells (Fig. S1 B). A time-course analysis showed that after the contraction phase of the primary immune response the frequency of CD27hi and CD27lo memory cells in the spleen remained relatively stable for at least 2 yr (Fig. S1 C), whereas they strongly declined in the lung (Fig. S1 D). These data confirmed that the phenotype, subset composition, and persistence of the primary (1°) memory pool elicited by respiratory VACV infection is comparable to that observed after Sendai virus (Hikono et al., 2007), influenza virus (Hikono et al., 2007), or systemic VACV infection (Olson et al., 2013).
Protective capacity of CD27hi and CD27lo memory subsets against lethal infection
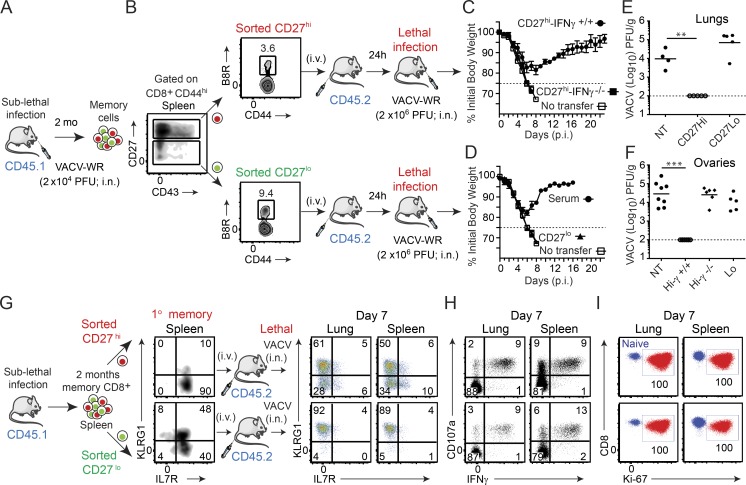
To investigate the relative importance of CD27hi and CD27lo cells to recall responses in the lung, we compared their capacity to protect against a lethal respiratory challenge with VACV-WR in an adoptive transfer model. To generate 1° memory T cells, cohorts of B6.SJL (CD45.1+) donor mice were infected i.n. with a sublethal inoculum of VACV-WR (Fig. 1 A). 60 d later, CD8+CD44hi cells from the spleen were FACS sorted into CD27hi and CD27lo subsets, normalized to ∼30,000 B8R20–27/kb tetramer+ cells, and injected i.v. into CD45.2+ congenically marked naive C57BL/6 recipients (Fig. 1 B). As a positive control, infected mice were bled at the same time, and VACV-immune serum was prepared and transferred i.p. into a separate group of naive mice. The following day, all groups were challenged i.n. with a lethal inoculum of VACV-WR (2 × 106 PFU) and monitored for signs of illness and survival (Fig. 1, C and D). As demonstrated previously (Salek-Ardakani et al., 2011a,b; Goulding et al., 2012), naive mice that did not receive any memory CD8 T cells exhibited rapid weight loss and death within 8 d, whereas 100% were protected by transfer of hyperimmune (anti-VACV IgG) serum (Fig. 1 D). Although splenic CD27lo Tem-l cells eliminated VACV after systemic viral challenge (Olson et al., 2013), the same cells were unable to mediate protection against a respiratory challenge, and all animals succumbed to the infection with kinetics similar to naive controls (Fig. 1 D). In striking contrast, all mice that received CD27hi Tcm-l cells survived the infection and exhibited only moderate (15–20%) loss of body weight (Fig. 1 C), closely mirroring the illness and recovery observed in control mice that received VACV-specific serum. These responses were paralleled by the virus titers detected in the lungs and ovaries, which were completely cleared in CD27hi recipient mice, but not in CD27lo recipients or naive controls (Fig. 1 E). These observations strongly suggest that the antiviral activity of CD27hi Tcm-l cells within the microenvironment of the lung is distinct from that of CD27lo Tem-l cells and support the idea that the route of infection and target tissue influence the use of distinct memory subsets.
Figure 1.
CD27hi memory CD8 T cells mediate optimal protection against lethal respiratory VACV-WR infection. (A) Cohorts of WT donor mice (B6.SJL; CD45.1+) were infected i.n. with VACV-WR (1.5 × 104 PFU/mouse). (B) Spleens were harvested 60 d later. Cells were enriched for CD8+ cells on negative selection columns and sorted into CD8+CD44hiCD27hi and CD8+CD44hiCD27lo subsets by FACS. The sorted CD27hi (C) and CD27lo (D) cells were then adjusted such that the number of B8R20–27/H-2Kb cells was equivalent (∼30,000 tetramer+ cells) and i.v. transferred into naive (CD45.2+) recipient mice. (D) Some groups received 250 µl VACV-immune serum i.p. 1 d after transfer, recipient mice were infected i.n. with a lethal inoculum of VACV-WR (2 × 106 PFU). Naive mice that did not receive memory cells were used as controls (No transfer [NT]). Animals were weighed daily and euthanized if weight loss was >25% of initial body weight for two consecutive days. (C and D) Mean percent of initial body weight is shown. On day 7 after challenge lung (E) and ovaries (F) from individual mice that survived the infection were collected and virus titers were determined by plaque assay as described in Materials and methods. Dotted line indicates limit of detection for the plaque assay. Each symbol represents one mouse. (G–I) Mice that received CD8+CD44hi CD27hi and CD27lo memory T cells were analyzed on day 7 after infection. Lung and spleen mononuclear cells were harvested and stained for CD8α, CD44, KLRG1, IL-7Rα, CD45.1, B8R20-27/Kb tetramer, intranuclear Ki-67, or stimulated with B8R peptide and subsequently stained for CD107a and intracellular IFN-γ. Representative plots for KLRG1/IL-7Rα (G), CD107a/IFN-γ (H), and Ki-67 (I) staining gated on CD8+CD44hiCD45.1+B8R20-27/Kb-tetramer+ (G and I) and CD8+CD44hiCD45.1+ (H) cells are shown. Numbers indicate percentages of positive cells within the gated population. Quadrant settings were based on controls, after gating on CD44lo cells in the same host for phenotypic analysis or using infected cells that were not stimulated with peptide and uninfected cells stimulated with B8R peptide for functional analysis (not depicted). Data show one representative experiment of at least four with same results with at least four mice per experimental group. Error bars, SEM (C and D). **, P < 0.01; ***, P < 0.001 (two-tailed Student’s t test).
CD27hi Tcm-l cells use IFN-γ to protect against the lethal effects of VACV-infection
Mice deficient for IFN-γ are highly susceptible to respiratory VACV-WR infection, and IFN-γ production, primarily by effector CD8 T cells (Goulding et al., 2014), is essential for host recovery from a primary infection (Goulding et al., 2014; Abboud et al., 2015). Therefore, our next set of experiments was designed to assess whether IFN-γ produced by the transferred CD27hi Tcm-l cells was responsible for the protection against secondary challenge. To generate IFN-γ−/− memory cells, we took advantage of the fact that IFN-γ−/− mice survive VACV-WR when infected by the i.p. route (Goulding et al., 2014), and that these mice maintain normal numbers of functional memory CD8 T cells in the spleen with unaltered immunodominance hierarchy (unpublished data). As before, CD27hi memory cells were FACS sorted from the spleen of VACV-WR–infected IFN-γ−/− mice or WT controls and adoptively transferred into naive WT recipients, followed by infection with a lethal inoculum of VACV-WR. Adoptive transfer of i.p. generated WT CD27hi memory cells afforded protection against death, which was comparable to that observed with i.n. generated cells (compare Fig. 1, E and F; and not depicted). In marked contrast, WT mice that received IFN-γ−/− CD27hi memory cells failed to control the virus (Fig. 1 F), developed clinical symptoms similar to those observed in nontransferred naive controls, and all succumbed to the infection by day 8 (Fig. 1 C). This finding suggests that similar to primary effector CD8 T cells (Goulding et al., 2014), CD27hi Tcm-l cells need to produce IFN-γ to protect against the lethal effects of secondary respiratory VACV-WR infection.
Functional characteristics of CD27hi and CD27lo CD8 T cell subpopulations
We sought to investigate the mechanism(s) underlying our observation that when compared with CD27lo memory cells, CD27hi memory cells were more protective against the lethal effects of respiratory VACV-WR infection. Our data pointed toward the release of IFN-γ as being a critical nonredundant component of CD27hi memory cell–mediated protection; thus, we hypothesized that IFN-γ production by CD27lo memory cells might be impacted by respiratory VACV-WR infection. At the time of peak response to VACV (7 d post-infection [p.i.]), analysis of B8R20–27/kb-tetramer+ cells in both the lung parenchyma and spleen showed that nearly all CD27lo memory cells had up-regulated KLRG1 and down-regulated IL-7R (Fig. 1 G). This phenotype is indicative of a highly activated and differentiated CD8 T cells (Hikono et al., 2007). Most importantly, we found that CD27hi and CD27lo cells produced equivalent levels of IFN-γ, and both populations had degranulated similarly upon antigen stimulation (Fig. 1 H). Collectively, these results demonstrate that the failure of CD27lo memory cells to control VACV was not due to either impaired priming or capacity to produce IFN-γ after trafficking to the lung tissue.
In situ accumulation of CD27hi Tcm-l and CD27lo Tem-l memory cells
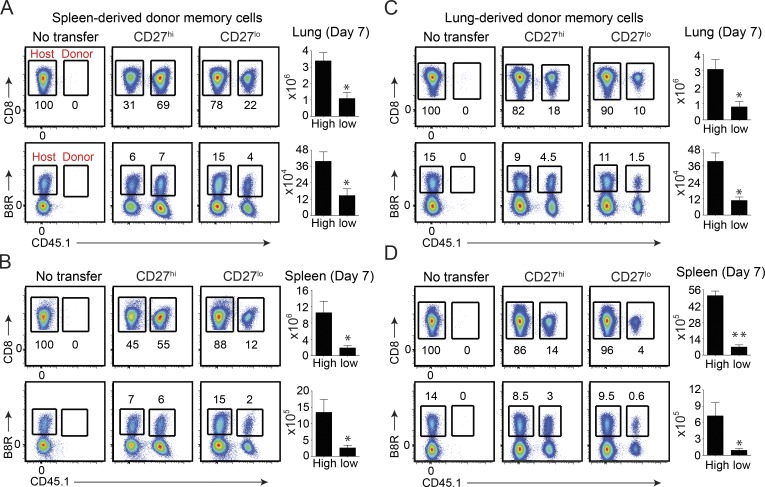
We next asked whether the recall proliferative and in situ accumulation differed between CD27hi and CD27lo memory populations. To this end, CD8+CD44hiCD27hi and CD8+CD44hiCD27lo cells were isolated from the spleen 2 mo after sublethal infection and equal numbers of B8R20–27/kb-tetramer+ cells were transferred separately into naive CD45.2 congenic recipients. In parallel experiments, we compared lung CD27hi and CD27lo memory cells from the same sets of mice. 1 d after transfer, recipient mice were infected i.n. with a lethal inoculum of VACV-WR and analyzed 8 d later. Our experimental design allowed comparison of primary (host; CD45.1−) and secondary (donor; CD45.1+) effector CD8 T cell responses in the absence of any preexisting VACV-specific antibodies. Moreover, transfer of total CD8+CD44hi cells allowed us to examine the recall response of both dominant and subdominant VACV-specific CD8 T cells. Interestingly, all of the adoptively transferred CD45.1+CD27lo and CD27hi cells trafficking to or within the lung parenchyma (Fig. 1 I, left), spleen (Fig. 1 I, right), and draining lymph nodes (not depicted) stained positive for the cell cycle–specific protein Ki67, indicating that both dominant and subdominant populations had proliferated in response to VACV infection. However, analysis of both CD45.1+ (total VACV-specific CD8 T cells; top) and B8R20–27/kb-tetramer+ cells (bottom) revealed that CD27lo cells weretwo to threefold less efficient at generating secondary (2°) effector T cells that could accumulate in the lung parenchyma (Fig. 2 A) and spleen (Fig. 2 B) compared with CD27hi cells, in agreement with earlier findings in the Sendai virus (Hikono et al., 2007) and systemic VACV (Olson et al., 2013) infection models. Notably, the same results were obtained when we used CD27hi Tcm-l and CD27lo Tem-l cells that were isolated from the lung (Fig. 2, C and D). Thus, regardless of their tissue of origin, CD27hi Tcm-l cells mediate stronger recall response than CD27lo Tem-l cells in terms their capacity to accumulate over time and protect.
Figure 2.
CD27hi memory CD8 T cells expand more than CD27lo subset regardless of their tissue of origin. Mice that received spleen- (A and B) or lung-derived (C and D) CD8+CD44hi CD27hi and CD27lo memory T cells were analyzed on day 7 after lethal infection. (top) Representative FACS plots showing total VACV-specific donor (CD45.1+) cells as a percentage of CD8+CD44hi cells. (bottom) Representative FACS plots showing B8R20-27/Kb tetramer+ cells from the host (CD45.1−) and each of the transferred (CD45.1+) CD27hi and CD27lo populations as indicated. Naive mice that did not receive any memory cells (NT) were used as controls. Numbers indicate percentages of positive cells within the gated population. Total lung (A and C) and spleen (B and D) CD45.1+ and B8R-reactive numbers presented as the mean ± SEM of three separate experiments with similar results containing three to four mice per group. *, P < 0.05; **, P < 0.01 (two-tailed Student’s t test).
CD27hi Tcm-l and CD27lo Tem-l cells localize to distinct anatomical niches within the lung
The data thus far implied that differential in situ accumulation of CD27hi and CD27lo memory cells, rather than their functionality, predicts protection against a lethal respiratory challenge with VACV-WR. In this regard, our previous studies (Salek-Ardakani et al., 2008, 2011b,c), which included direct adoptive transfer of memory CD8 T cells into the lungs, indicated that at least 10,000 memory cells needed to be present in the lung to allow the survival of the mice infected with a normally lethal inoculum of VACV-WR. This threshold was clearly achieved in mice that received CD27lo Tem-l cells. For example, on day 7 after infection, the total number of B8R20–27/kb-tetramer+ and total virus-specific (CD45.1+) effectors generated from CD27lo Tem-l donors in the lung was ∼105 and 106, respectively (Fig. 2 A). Thus, these data would argue against the absolute numbers of lung-infiltrating CD27lo cells as being the only contributing factor for their failure to control VACV. Indeed, when we transferred four times more CD27lo cells than CD27hi memory cells, which resulted in equal numbers of B8R20–27/kb-tetramer+ CD27lo and CD27hi cells (unpublished data), CD27lo cells still failed to protect against lethal VACV challenge (unpublished data).
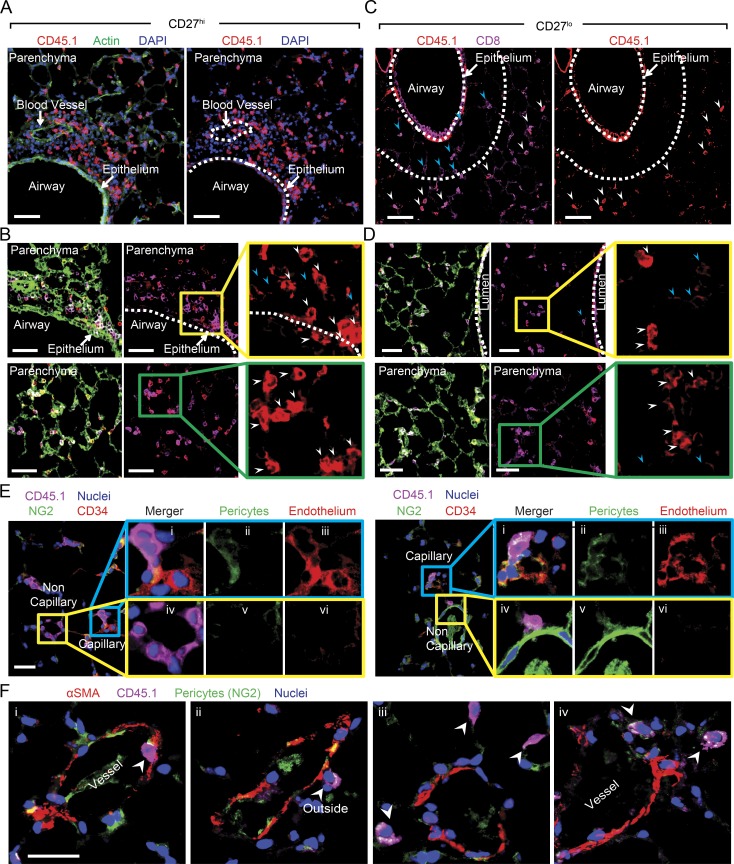
Immunofluorescence (IF) analysis of the distribution of CD27hi and CD27lo populations within the lung environment revealed striking and unexpected differences that help explain their differential contribution to viral clearance and recovery. In mice that received CD27hi memory cells, high numbers of CD45.1+ donor 2° effector cells could be readily visualized egressing from the bronchial arteries that supply the bronchial regions, i.e., connective tissue around the arteries (Fig. 3 A), bronchi, and the lamina propria of the bronchioles (Fig. 3, A and B). Most significantly, CD45.1+CD27hi donor cells were found in large numbers in direct contact with bronchiolar epithelium (Fig. 3 B, top and middle), where VACV infection and replication are commonly localized. Similar to CD45.1+CD27hi donor cells, the CD45.1+CD27lo 2° effectors were found in the connective tissue surrounding the arteries but showed a markedly different migration pattern through the bronchial regions. The majority of adoptively transferred CD45.1+CD27lo donor cells localized in high numbers in the peribronchoarterial areas and in interalveolar septa but were mostly excluded from the bronchiolar epithelium (Fig. 3, C and D; and Fig. S2, A and B). To definitively show that CD27lo cells were localized to the interalveolar septa, we used CD34 to identify alveolar capillary endothelial cells and NG2 (nerve/glial antigen 2, Cspg4) to mark pericytes associated with the pre- and postcapillary vessels. (Fig. 3 E). As expected, we observed CD45.1+CD27lo (donor) cells in the capillaries intimately associated with CD34+ and in NG2+ pulmonary capillaries (Fig. 3 E). Significantly, however, there were also abundant CD45.1+CD27lo donor cells that were not associated with the aforementioned capillary markers (Fig. 3 E), indicating that CD45.1+CD27lo 2° effectors are present within the vasculature as well as in the interalveolar septa. Next, we co-stained lung sections with NG2 and α-smooth muscle actin, which marks perivascular and peribronchiolar smooth muscle, as well as cells present discontinuously around the walls of the smallest venules. Importantly, and consistent with our other staining data, we could readily visualize CD45.1+CD27lo cells outside of the vessels (Fig. 3 F). Together, these data provide compelling evidence that VACV-specific CD27lo cells are truly outside of vasculature. They further establish that CD27lo 2° effector memory cells occupy a spatially distinct niche of the lung in a region distant from the initial site of virus infection and replication.
Figure 3.
Differential homing preference of CD27hi and CD27lo memory cells within the lung after lethal respiratory VACV-WR infection. Mice that received CD8+CD44hi CD27hi or CD27lo memory T cells in Fig. 1 were analyzed on day 7 after infection. IF analysis of frozen lung sections from VACV-infected CD8+CD44hi CD27hi (A and B) or CD8+CD44hi CD27lo (C and D) recipient mice stained for CD45.1 (red) and CD8 (magenta). DAPI (blue) and ActinGreen (green) were used to visualize nuclei and lung morphology, respectively. Bars, 50 µm. Lung parenchyma, airway lumen, bronchial arteries are delineated by broken lines. The marked areas are shown in higher magnification to the right of each panel. CD45.1+ (red) donor cells are delineated with white arrows. Endogenous CD8 T cells could also be identified (magenta; blue arrows). (E) IF analysis of lung sections from aforementioned CD27lo recipient mice stained with CD45.1 (magenta), NG2 (green), DAPI (blue), and CD34 (red) or (F) αSMA (red). Bars: (E and F) 10 µm. Results are representative of three experiments each with three mice per group.
Antiviral and inflammatory environment within the lungs of CD27hi Tcm-l– and CD27lo Tem-l–recipient mice
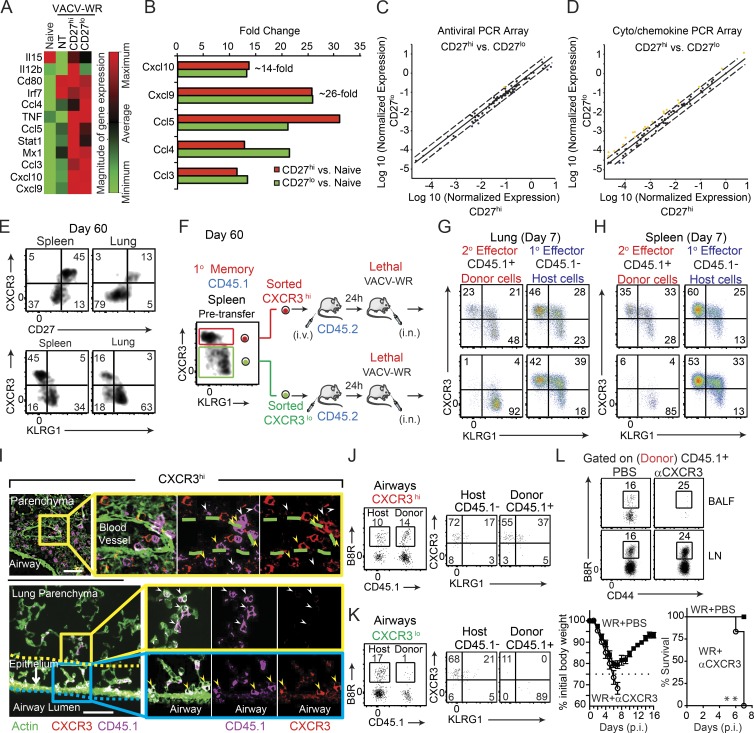
To understand the development of protective immune response to VACV and how homing preference of memory CD8 T cells within the lung is regulated, we compared the expression of 84 antiviral and inflammatory cytokine/chemokine genes from VACV-infected CD27hi and CD27lo recipient mice against naive mice, as well as VACV-infected WT mice that did not receive adoptive transfer of memory T cells. After disregarding unregulated, nondetectable gene products and presenting the relative levels of gene expression across all samples, we observed in adoptive transfer recipients (both CD27lo and CD27hi) a dramatic (10–25 fold) increase in the expression of mRNA for chemokines associated with the receptors CCR5 (CCL3, CCL4, and CCL5) and CXCR3 (CXCL9 and CXCL10; Fig. 4, A and B), which have been implicated in coordinating the migration of virus-specific memory CD8 T cells to the sites of infection (Kohlmeier et al., 2011; Kastenmüller et al., 2013; Hickman et al., 2015). Surprisingly, however, we found little or no difference in the expression of these chemokines after virus challenge when comparing CD27hi and CD27lo recipient mice (Fig. 4 B). There were also no major differences (less than threefold) between CD27hi and CD27lo recipient mice in terms of their overall antiviral (Fig. 4 C) and cytokine/chemokine (Fig. 4 D) mRNA expression profiles after infection. In line with these observations, both the frequency and absolute numbers of antiviral innate immune cells, including NK cells, neutrophils, and inflammatory monocytes recovered from the lung, were comparable between the two groups of mice (unpublished data).
Figure 4.
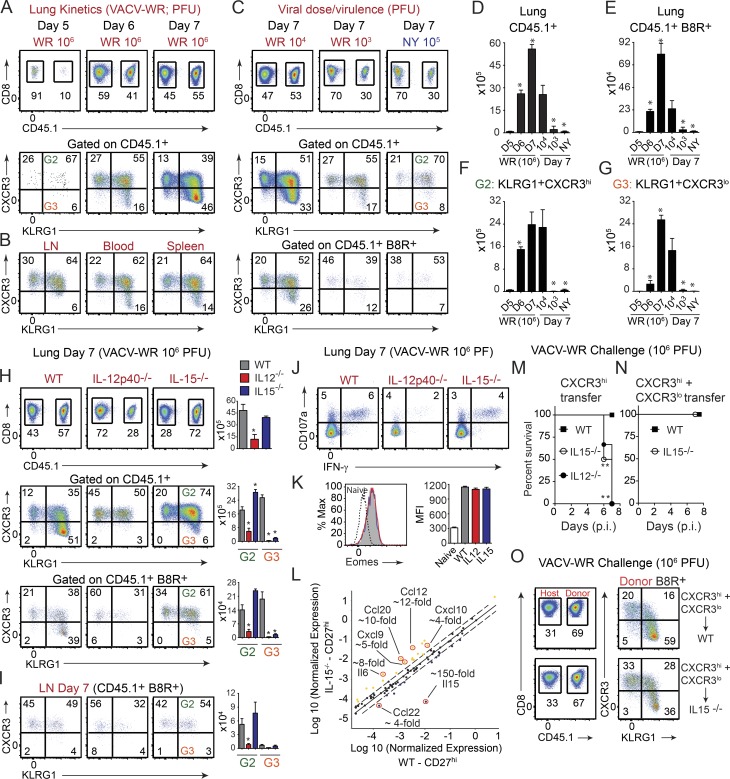
CD27lo effector cells are directly generated from CD27hi memory populations, and CXCR3 expression determines their differential localization and protective value. (A–D) Mice that received CD8+CD44hi CD27hi and CD27lo memory T cells in Fig. 1 were analyzed on day 7 after infection. One group of B6 mice did not receive any memory cell and used as a NT control. Total lung mRNA transcript levels of 84 antiviral and cytokine/chemokine genes were measured and presented as a heat map (A). Fold change in gene expression between CD27hi recipients versus naive (B) CD27lo recipients versus naive (B) CD27hi versus CD27lo recipient mice (C and D) is also presented. All array data are presented as the mean of gene expression level of four animals per group. (E) Representative FACS plots depict CD27/CXCR3 (top) and CXCR3/KLRG1 (bottom) expression profiles gated on B8R20-27/Kb tetramer+ memory cells recovered from the spleen and lung 60 d after primary infection. Spleen memory cells were obtained as in Fig. 1 and sorted into CXCR3hiKLRGlo and CXCR3loKLRGhi and i.v. injected into B6 (CD45.2+) recipients that were lethally infected the next day with VACV-WR (F). Representative FACS plots depict CXCR3/KLRG1 profile of B8R20-27/Kb tetramer-specific donor (secondary [2°] effectors) and host (primary [1°] effectors) cells in lungs (G) and spleens (H) 7 d after infection. (I) IFA of lung sections from CXCR3hi-recipient mice were stained for CXCR3 (red), CD45.1 (magenta), and ActinGreen (green). Bars, 50 µm. The marked areas are shown in higher magnification to the right of each panel. Donor cells coexpressing CXCR3+ (red) and CD45.1+ (magenta) are indicated with yellow arrows, whereas donor CD45.1+ cells that down-regulated CXCR3 expression is delineated with white arrows. Endogenous cells could also be identified as only expressing CXCR3 (red only). Donor cells in close proximity to the airways epithelium are double positive. Bronchoalveolar lavage fluid from CXCR3hi (J) and CXCR3lo (K) recipient mice was harvested and stained for CD8α, CD44, KLRG1, CXCR3, CD45.1, and B8R20-27/Kb tetramer. (left) Representative FACS plots showing B8R20-27/Kb tetramer+ cells from the host (CD45.1−) and each of the transferred (CD45.1+) CXCR3hi (J) and CXCR3lo (K) populations as indicated. (right) Representative FACS plots for CXCR3/KLRG1 staining gated on CD8+CD44hiB8R20-27/Kb-tetramer+ are shown. Numbers indicate percentages of positive cells within the gated population. (L) 30,000 B8R-tetramer+ CD8+CD44hiCXCR3hi cells were i.v. transferred into naive (CD45.2+) recipient mice that were infected 1 d later with a lethal inoculum of VACV-WR. Infected mice were treated i.p. with PBS or mAb against CXCR3 on days 1, 3, and 5. Animals were analyzed on day 7. (top) Representative FACS plots showing B8R20-27/Kb tetramer+ cells gated on the transferred CD45.1+ cells. (bottom) Animals were weighed daily and euthanized if weight loss was >25% of initial body weight for two consecutive days. Mean percent of initial body weight is shown. Data are from one representative experiment of two with at least three mice per group (A–D and L) or at least three experiments with three and four mice per group (E–J). **, P < 0.01 (two-tailed Student’s t test or Mantel-Cox test used in L).
Differential expression of CXCR3 on VACV-specific CD27hi and CD27lo memory CD8 T cells
We next asked whether virus-specific CD27hi Tcm-l and CD27lo Tem-l cells generated after acute respiratory VACV infection differentially expressed the chemokine receptors CXCR3 and CCR5. At steady state, <5% of naive (CD44lo) T cells in the spleen express CXCR3, and all are negative for CCR5 (unpublished data). After primary infection, all B8R20–27/kb-tetramer+ 1° effector cells in the spleen and lung up-regulated cell surface expression of CXCR3, whereas only a small fraction (<10%) expressed CCR5 (unpublished data). On day 60 after infection, a time point when memory cells were isolated for adoptive transfer experiments, the vast majority (>90%) of CD27hi B8R20–27/kb-tetramer+ memory cells recovered from the spleen and lung had retained high surface expression of CXCR3 (Fig. 4 E, top), but were negative for expression of KLRG1 (Fig. 4 E, bottom) and CCR5 (not depicted). In striking contrast, very few (<5%) CD27lo B8R20–27/kb-tetramer+ memory cells expressed CXCR3 in all sites sampled (Fig. 4 E, top). These findings are consistent with the hypothesis that differential expression of CXCR3 on CD27hi and CD27lo memory cells, rather than chemokine expression within the lung microenvironment, might be linked to their ability to home to airway epithelium and lumen.
CD27loCXCR3lo 2° effector cells are directly generated from CD27hiCXCR3hi memory populations
To address the role of CXCR3 in homing preference of memory cells more directly, we adoptively transferred equal numbers of FACS-sorted CD45.1+CXCR3hi (CD27hiKLRG1lo) and CD45.1+CXCR3lo (CD27loKLRG1hi) cells into naive WT recipients 1 d before lethal challenge (as illustrated in Fig. 4 F). At 7 d, when the adoptively transferred CD45.1+CXCR3lo donor Tem-l cells were reanalyzed for the expression of CXCR3, KLRG1, and CD27, all cells maintained their original CXCR3lo, CD27lo, and KLRG1hi phenotype, regardless of whether they were isolated from the lung tissue (Fig. 4 G, bottom left) or spleen (Fig. 4 H, bottom left), whereas the majority of host (CD45.1−) 1° effector cells were CXCR3hi (Fig. 4, G and H, bottom right). Intriguingly, when KLRG1 and CXCR3 were reanalyzed on the progeny of CD45.1+CXCR3hi Tcm-l donor cells, three major subsets could be identified in the lung parenchyma: a minor population that maintained their original KLRG1loCXCR3hi phenotype (Fig. 4 G, top left quadrants); an intermediate population defined as KLRG1hiCXCR3hi (Fig. 4 G, top right quadrants); and a terminally differentiated KLRG1hi population that exhibited a CXCR3lo phenotype (Fig. 4 G, bottom right quadrants). At the peak of the response to VACV, the terminally differentiated KLRG1hiCXCR3lo population comprised 50–60% of total lung-resident 2° effector cells, which was substantially higher fraction than was found in the spleen (compare Fig. 4, G and H, top left). Notably, all three effector populations had proliferated and exhibited hallmarks of activated cells, including up-regulation of CD43 (unpublished data), as well as down-regulation of IL-7R (unpublished data). Comparison of CXCR3 expression on 2° (donor) versus 1° (endogenous) B8R20–27/kb-tetramer+ effector populations in the same host revealed a significant shift from CXCR3hi to CXCR3lo phenotype in 2° effectors in all sites sampled, but particularly among the cells infiltrating the lung tissue. Together, these data indicate that CD27loCXCR3lo 2° effector cells are directly generated from CD27hiCXCR3hi Tcm-l populations that have undergone further rounds of differentiation upon secondary antigen encounter.
The finding that a significant proportion of CD27hiCXCR3hi memory cells become CD27loCXCR3lo 2° effectors offered us the opportunity to explore their anatomical localization in the same host. At 7 d after VACV-WR infection, high numbers of CD45.1+CXCR3hi donor 2° effector cells (Fig. 4 I; magenta [CD45.1], red stain [CXCR3]; double positive cells indicated by yellow arrows) could be visualized egressing from the bronchial arteries (Fig. 4 I, top) and in direct contact with airway epithelial cells (Fig. 4 I, bottom, yellow arrows). Notably, only CD45.1+CXCR3+, and not newly generated CD45.1+CXCR3lo, donor-derived B8R20–27/kb-tetramer+ effector cells were recovered from the bronchoalveolar lavage fluid (BALF; Fig. 4 J), suggesting that CXCR3 plays an important role in recruitment of memory CD8 T cells to the lung airways during respiratory VACV infection. In line, newly generated CD45.1+CXCR3lo 2° effectors were found in the connective tissue surrounding the arteries (Fig. 4 I, top, white arrows), but the majority of the cells showed reduced trafficking to the bronchiolar epithelium (Fig. 4 I, bottom, white arrows). Consistent with this pattern of migration, adoptively transferred CD45.1+CXCR3lo (CD27lo) donor cells localized in high numbers in interalveolar septa but were mostly excluded from the bronchiolar epithelium (Fig. S2 C). Again, this distribution was supported by flow cytometric observations in which very few CD45.1+CXCR3lo donor 2° effectors were recovered from the BALF, whereas host-derived CD45.1−CXCR3hi 1° effector CD8 T cells were readily visible in the same samples (Fig. 4 K).
To assess whether CXCR3 was responsible for the migration of CD27hi donor memory T cells to the airways after VACV infection, we performed several blocking experiments. Accordingly, CD27hiCXCR3hi recipients were injected i.p. on days 1, 3, and 5 with a blocking mAb to CXCR3 (clone CXCR3-173) or PBS as control and the influx of donor cells to the airways was assessed on day 7 after infection. As expected, high numbers of donor cells were recovered from in the BALF of control-treated CD27hiCXCR3hi recipients, whereas neutralization of CXCR3 almost completely ablated their ability to traffic to the airways and protect against the lethal effects of infection (Fig. 4 L), reproducing the migratory and protective phenotype of CD27loCXCR3lo cells. Most significantly, systemic neutralization of CXCR3 had little or no effect on the frequency of total (CD45.1+) and B8R20–27/kb-tetramer+ 2° effector cells in the lung draining LNs (Fig. 4 L), demonstrating that impaired T cell migration to the airways is not caused by depletion of CXCR3+ CD8 T cells. We also measured innate immune recruitment to the lungs and found that accumulation of NK cells, inflammatory monocytes, and neutrophils were not impacted by anti-CXCR3 mAb treatment (unpublished data). Collectively, these results provide evidence that donor CD8+ T cell recruitment to the airways is primarily dependent on CXCR3 receptor–ligand interactions.
To formally test the relationship between localization of CD27loCXCR3lo and their protective function in the lung, we transferred CD27loCXCR3lo memory cells directly into the airways of naive recipients by intratracheal (i.t.) injection and subsequently challenged them i.n. with a lethal inoculum of VACV-WR. As a control, we i.t. injected the same number of CD27hiCXCR3hi cells into parallel cohorts of mice (Fig. S2 D). As expected, mice that did not receive memory cells succumbed to the infection. In contrast, all of the mice were protected by direct airway transfer of CD27hiCXCR3hi memory cells (Fig. S2 D). Remarkably, mice that received CD27loCXCR3lo memory cells were also protected and all recovered from the lethal infection, albeit with delayed kinetics compared with CD27hiCXCR3hi cells (Fig. S2 D). Thus, CD27loCXCR3lo memory CD8 cells are fully functional and can protect against a highly lethal respiratory virus infection if localized to the initial sites of viral replication.
CD27loCXCR3lo 2° effector cells develop after entering the lung microenvironment
The appearance of KLRG1hiCXCR3lo Tem-l cells in the infected lungs at the peak of the response to VACV suggested one of two possibilities: either activated T cells spontaneously began to down-regulate CXCR3 as the immune response progressed, or alternatively, they received additional tissue-specific signals in the new lung microenvironment after initial priming in the draining LNs. To distinguish between these two possibilities, we analyzed the kinetics of KLRG1 and CXCR3 expression on total (CD45.1+) virus-specific (Fig. 5 A) and B8R20–27/kb-tetramer+ (unpublished data) CXCR3hi donor cells isolated from different organs. Starting at 5 d, the CXCR3hi Tcm-l donor cells underwent extensive expansion (Fig. 5 A, top), and the vast majority (∼60–80%) up-regulated KLRG1 expression, regardless of whether the cells were recovered from the lung tissue (Fig. 5 A, bottom left), draining lymph nodes, blood, or spleen (unpublished data). What is most noticeable is that, at this early time point, very few KLRG1+ donor cells in the lung were CXCR3lo (Fig. 5 A). CXCR3 expression began to decrease on a significant fraction (40–50%) of virus-specific KLRG1+ cells in the lung between days 5 and 7 p.i. (Fig. 5 A). Most significantly, a similar trend was not apparent for KLRG1+ donor cells recovered from the draining LNs at the same time point (Fig. 5 B). Moreover, very few KLRG1+ donor cells became CXCR3lo in the blood and spleen (Fig. 5 B). Together, these data provide compelling evidence that after leaving the draining LNs and migrating to the infected lung, a subset of KLRG1hiCXCR3hi Tcm-l cells receive secondary environmental cues that instructs them to complete a full program of differentiation. This ultimately results in loss of CXCR3 expression and subsequent migration arrest and clustering around the vessels and interalveolar septa.
Figure 5.
Lung entry affects CXCR3 expression on central-like memory cells in a time- and virulence-dependent fashion and is cytokine driven. Memory CD27hiCXCR3hi CD8 T cells were sorted from spleens of CD45.1+ SJL mice, i.v. transferred into naive (CD45.2+) WT (A–G), IL-12p40−/− (H–K and N), and IL-15−/− (H–O) recipient mice that were i.n. infected next day with VACV-WR (106, 104, and 103 PFU) or attenuated VACV-NYCBOH (NY; 105 PFU) as indicated. Lung mononuclear cells were harvested on day 5 (A), 6 (A), and 7 (A–C and H–K) after infection and stained for CXCR3 and KLRG1 expression as in Fig. 4 F. (A, top) Representative plots of gated CD8+CD44+ T cells staining for CD45.1 on the indicated days after infection; (bottom) Representative plots showing CXCR3 and KLRG1 expression gated on CD8+CD44+CD45.1+ donor cells. (B) Representative plots showing CXCR3 and KLRG1 expression gated on CD8+CD44+CD45.1+ donor cells recovered from the LNs, blood, and spleen. (C and H) Representative plots of gated CD8+CD44+ T cells staining for CD45.1 (top) and CXCR3/KLRG1 expression gated on total CD45.1+ (middle) and CD45.1+ B8R20-27/Kb-tetramer+ (bottom) cells on day 7. Total numbers of CD45.1+ (D), CD45.1+ B8R20-27/Kb-tetramer+ (E), CD45.1+KLRG1+ (gate 2; F), and CD45.1+KLRG1+CXCR3lo (gate 3; G) presented as the mean ± SEM of two separate experiments containing three mice per group. (I) Representative plots of gated CD8+CD44+CD45.1+ B8R20-27/Kb-tetramer+ LN cells staining for CXCR3/KLRG1 expression on day 7. Representative plots for CD107a/IFN-γ (J) and Eomesodermin (K) staining gated on CD8+CD44hiCD45.1+ cells recovered from the lung. (L) Total lung mRNA transcript levels of 84 cytokine and chemokine genes were measured from the same groups as in H and presented as scatter plot. Fold change in gene expression between CD27hiCXCR3hi WT and IL-15−/− recipients is presented. (M and N) CD27hiCXCR3hi (M) and total CD8+CD44+ CD8 T cells (containing a mixture of CD27hiCXCR3hi and CD27loCXCR3lo cells; N) were sorted from spleens of CD45.1+ SJL mice, i.v. transferred into naive (CD45.2+) WT, IL-12p40−/−, and IL-15−/−-recipient mice that were i.n. infected the next day with a lethal inoculum of VACV-WR. Mean percent survival curves are shown. (O) Representative plots of gated CD8+CD44+ T cells staining for CD45.1 (left) and CXCR3/KLRG1 expression (right) gated on CD45.1+ B8R20-27/Kb-tetramer+ lung cells on day 7. Results are mean (n = 6–8 per group) from two experiments with similar results. *, P < 0.05; **, P < 0.01 (two-tailed Student’s t test or Mantel-Cox test used in M and N).
CD27loCXCR3lo 2° effector cells develop in response to high antigen load and viral virulence mechanisms
The significance of generating CD27loCXCR3lo Tem-l cells in the lung after respiratory VACV infection is not clear, though it may be related to host fitness. Indeed, VACV has the capacity to infect multiple innate immune cells, including monocytes and dendritic cells that have infiltrated or are in close proximity to the infectious foci and thereby facilitate rapid viral dissemination throughout the host. Interestingly, after infection with certain strains of Sendai and influenza viruses that only replicate locally, CD27hi Tcm-l cells did not down-regulate CXCR3 upon entering the lung parenchyma (Hikono et al., 2007). We speculated that differential anatomical distribution of CD27hiCXCR3hi Tcm-l and CD27loCXCR3lo Tem-l populations provides layered immunological defense against highly virulent respiratory pathogens that have the capacity to spread rapidly beyond the initial site of infection. We considered the possibility that the inflammatory microenvironment created by less virulent viruses (or during low dose infections) may not provide the necessary co-signals that are needed to allow modulation of CXCR3 on lung-infiltrating T cells. Perhaps a certain threshold of antigen exposure and/or inflammation is required to allow the full program of differentiation to be initiated. To test this, we infected CD27hiCXCR3hi Tcm-l recipient mice with 100- or 1,000-fold lower inoculum of VACV-WR and monitored the expression of KLRG1 and CXCR3 on donor cells (Fig. 5 C). As an additional test of our hypothesis, we used a replication-competent highly attenuated New York City Board of Health (NYCBOH) strain used as a smallpox vaccine in the United States (Dryvax; Wyeth). Whereas even a low dose of VACV-WR (104 or 103 PFU) was not controlled well, resulting in weight loss, 105 PFU NYCBOH was not pathogenic, and little to no weight loss was observed (Salek-Ardakani et al., 2011b; and unpublished data). At 7 d after infection, the frequency of total virus-specific and B8R20–27/kb-tetramer+ donor cells in both the lung (Fig. 5, D and E) and secondary lymphoid organs (not depicted) was 2–30-fold greater in mice infected with 106 PFU WR than in those infected with 104 PFU, 103 PFU, or 105 PFU of NYCBOH, supporting our data that enhanced CD8 T cell expansion is related to the extent of viral load (Salek-Ardakani et al., 2011b). Analysis of CXCR3 expression showed that loss of cell surface expression on virus-specific donor cells was likely to be explained by altered inflammatory environment during secondary infection, as the extent of CXCR3 down-regulation directly correlated with the initial inoculum and virulence of VACV (Fig. 5 C, middle and bottom), again, mirroring the rate of virus clearance from the lung. Notably, lower frequencies (Fig. 5, F and G) of CXCR3lo Tem-l cells after low dose/virulence infection was not caused by impaired activation of donor CD8 T cells in that KLRG1 (Fig. 5 C, middle and bottom) and CD43 (not depicted) were similarly elevated. Furthermore, the ability to produce IFN-γ and TNF on a per cell basis was almost identical between WR- and NYCBOH-infected groups over a wide range of viral doses (not depicted), suggesting that exposure to varying levels of viral antigen primarily affects the frequency and tissue-homing imprinting signals in the lung microenvironment, rather than the functional activity of individual cells.
Formation of CD27loCXCR3lo 2° effector cells requires IL-12 and IL-15
The experiments described thus far indicated that a subset of recently recruited CD27hiCXCR3hi 2° Tcm-l cells within the lung may be exposed to a specific array of virus-induced inflammatory co-signals, and this may have stimulated their full program of differentiation to CD27loCXCR3lo Tem-l cells. In this regard, it has been shown in other experimental systems that induction of IL-12 and IL-15 can, through both direct and indirect mechanisms, provide activation signals to effector and memory cells that alter their differentiation and homing patterns in vivo. To investigate a role for IL-12 and IL-15 signaling in the development of CD27loCXCR3lo Tem-l cells, we transferred WT CD27hiCXCR3hi 1° memory cells into congenically marked IL-12– and IL-15–deficient recipient mice that we then infected with VACV-WR. At day 7 p.i., both percentages and numbers of total (CD45.1+) virus-specific (Fig. 5 H, top) and B8R20–27/kb-tetramer+ CXCR3hi cells (not depicted) were strongly reduced in both lungs and draining LNs (Fig. 5 I) of IL-12−/− recipient mice when compared with cells in WT mice, and VACV-specific CD8 T cells that up-regulated KLRG1 (Fig. 5 H, middle and bottom), CD107a (Fig. 5 J), IFN-γ (Fig. 5 J), and T-bet (not depicted), were reduced by ∼50–70%. Concurrently, few if any CXCR3lo donor cells were present in the lungs (Fig. 5 H) and LNs (Fig. 5 I) of the IL-12−/− recipient mice. In striking contrast, both the percentage and absolute numbers of VACV-specific CXCR3hi donor cells were comparable between WT and IL-15−/− mice (Fig. 5, H and I). Most significantly, KLRG1 (Fig. 5, H and I), CD107a (Fig. 5 J), IFN-γ (Fig. 5 J), and Eomes (Fig. 5 K) were similarly elevated on VACV-reactive effector CD8 T cells in the lung of IL-15−/− mice as compared with WT mice, demonstrating that CD8 T cells were primed, able to migrate to the lung, and functional in the absence of IL-15. Despite this, only a small fraction (<10%) of activated KLRG1hi cells fully differentiated to become CXCR3lo effectors. Thus, IL-15 did not affect the size of the virus-specific effector population in the lung but reduced the expression of CXCR3.
One interpretation of these data could be that induction of Cxcl9 and Cxcl10 is impaired in IL-15−/− mice. To address this, the effect of IL-15 deficiency on the induction of total lung cytokine and chemokine mRNA levels was examined by gene pathway array. Interestingly, we found that Cxcl9 and Cxcl10, as well as several other chemokine genes, including Ccl12, Ccl2, Ccl20, Ccl4, Cxcl13, and Cxcl3, were all up-regulated in IL-15−/− recipient mice as compared with WT mice (Fig. 5 L). The elevated levels of Cxcl9 and Cxcl10 correlated with a marked influx of VACV-reactive donor cells within infected airway lumen (Fig. S3, A and B) and bronchiolar epithelium (Fig. S3 C). Comparable (<2.5-fold change) IFN-γ, IFN-α, IL-12, IL-18, IL-2, IL-23a, IL-7, and TNF mRNA levels were observed in the lungs of IL-15−/− and WT mice. As expected, no IL-15 mRNA expression was detected in lungs isolated from VACV-infected IL-15−/− mice. Collectively, these data suggest that early after infection, IL-12 predominantly dictates the frequency and initial differentiation of donor cells in the draining LNs, whereas IL-15 directs the secondary programming and homing preference of newly recruited T cells within the lung microenvironment.
CD27hiCXCR3hi memory cells fail to control lethal VACV infection in the absence of CD27loCXCR3lo cells
Lastly, we addressed the significance of the spatial organization of Tem-l and Tcm-l memory cells in the lung. From our results in Fig. 1, it was clear that Tem-l CD27loCXCR3lo cells in isolation could not orchestrate protective recall responses in the absence of the Tcm-l CD27hiCXCR3hi subset, consistent with the notion that for memory cells to completely clear VACV from the lungs, they must mobilize from the circulation to the infected airway lumen and bronchiolar epithelium foci. Because we found that a significant fraction of Tcm-l CD27hiCXCR3hi cells become CD27loCXCR3lo after entering the lung microenvironment, it was unclear whether Tcm-l cells could recapitulate the full response required for protection in the absence of Tem-l subset or whether some degree of cooperation between these two subsets was necessary for immune protection.
To address this we examined IL-15−/− mice that contain Tcm-l CD27hiCXCR3hi cells but lack Tem-l CD27loCXCR3lo 2° effectors. As before (Fig. 1), adoptive transfer of CD27hiCXCR3hi into WT recipients afforded protection against death in this model (Fig. 5 M). Contrary to our expectations, IL-15−/− mice that received Tcm-l CD27hiCXCR3hi cells failed to control the virus and all succumbed to infection by day 7 (Fig. 5 M). As expected, IL-12−/− recipient mice, which have reduced numbers of Tcm-l CXCR3hi and Tem-l CXCR3lo cells in the lung, were also found to be highly susceptible to infection, but exhibited more severe cachexia and immobility as compared with IL-15−/− mice (Fig. 5 M).
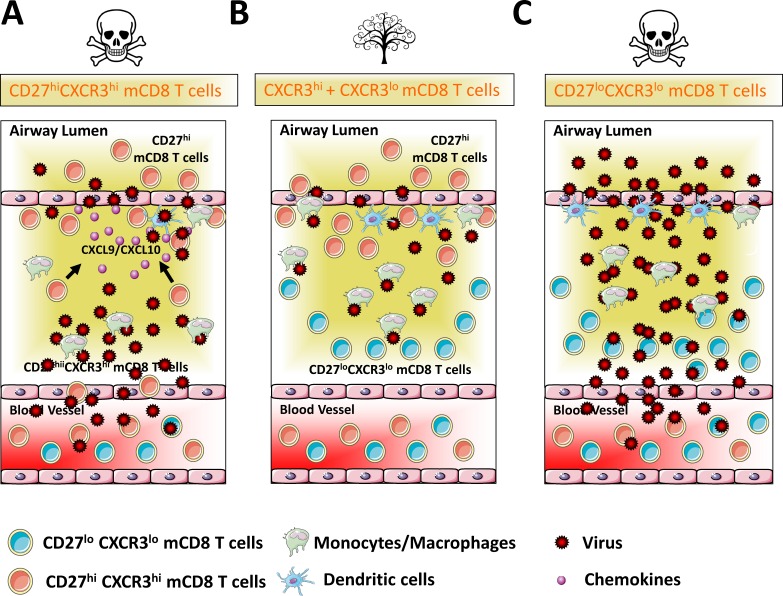
Consistent with the elevated chemokine mRNA levels observed in the lungs of VACV-infected IL-15−/− recipient mice (Fig. 5 L), we found that the recruitment and accumulation of neutrophils and inflammatory monocytes was enhanced in the absence of IL-15 (Fig. S3 D). As expected, no NK cells were observed in the lungs of VACV-infected IL-15−/− recipients (Fig. S3 D). We considered the possibility that lack of NK cells, rather than Tem-l CXCR3lo cells per se, might have been responsible for the enhanced susceptibility of IL-15−/− mice to lethal challenge. However, in our earlier study (Abboud et al., 2015), we had shown that absence of NK cells has little or no effect on the ability of mice to recover from a respiratory VACV-WR infection. Therefore, the interpretation that we favored is that the lack of protection observed in IL-15−/− recipients is caused by the absence of Tem-I cells in the infected lungs. Definitive proof for this came from adoptive co-transfer of CXCR3hi and CXCR3lo memory CD8 T cells into IL-15−/− mice (Fig. 5 N). In contrast to CXCR3hi single transfer group (Fig. 5 M), all IL-15−/− mice that received both CXCR3hi and CXCR3lo populations were protected from the lethal effects of infection (Fig. 5 N). The presence of VACV-specific CXCR3lo donor cells in the lungs of IL-15−/− mice confirmed that CD8 T cells were primed and able to migrate to the site of infection in the absence of IL-15 (compare Fig. 5, H and O, third row). Together, these data provide compelling evidence that protective immunity provided by virus-specific memory CD8 T cells requires coordinated action of Tem-l and Tcm-l subsets. In this study, we propose a novel model for respiratory immunity (Fig. 6) in which CD27hiCXCR3hi Tcm-l memory cells efficiently localize to the epithelial foci and are able to control virus replication at the initial sites of pathogen exposure, but they are likely unable to eliminate mobile virus-infected immune cells. Thus, we propose that CD27loCXCR3lo 2° Tem-l cells, localized in high numbers in the peribronchoarterial areas and in interalveolar septa, form a second layer of defense, which can engage and kill infected cells that escape initial clearance to prevent systemic viral dissemination and eventual death.
Figure 6.
Memory CD8 T cell subsets form tissue-specific layered defense to protect against lethal respiratory virus infection. Airway epithelial cells serve as the primary location for virus-replication and spread after respiratory virus infection. (A) CD27hiCXCR3hi memory CD8 T cells mobilize from the circulation into the lung parenchyma and airway epithelial foci cell and function as the first line of defense against pathogen exposure. (B) If mobile-infected cells successfully breach this first line of defense, the host possesses a second layer of protection, the CD27loCXCR3lo memory subsets, which are generated directly from CD27hiCXCR3hi in situ in response to pathogen-specific inflammatory co-signals. Here, the immune system adapts its response to engage and kill infected cells to prevent systemic viral dissemination and eventual death. (C) CD27loCXCR3lo cannot mobilize to the epithelial foci and are eventually overwhelmed by the invading virus. The concept of layered immunity allows the adaptive immune system a certain level of plasticity to mount faster and stronger attacks each time the virus is encountered.
These findings are noteworthy, as much of what is known regarding the relative contribution of memory populations during recall responses to respiratory viruses is related to their proliferative potential rather than their protective capacity (Ely et al., 2003; Roberts and Woodland, 2004; Roberts et al., 2005; Hikono et al., 2007). Although CD27hi central-like memory cells indeed exhibit greater capacity for recall proliferative responses, effector-like cells have been claimed to provide superior protection, primarily by engaging pathogens at the initial sites of exposure. Indeed, as previously mentioned, the protective capacity of CD27hi Tcm-l and CD27lo Tem-l subpopulations was observed within the spleen after systemic infection with VACV (Olson et al., 2013). Here, CD27lo but not CD27hi cells were found to preferentially localize in the red pulp (the primary points of contact between CD8 T cells and blood-borne pathogens) and were more effective at preventing VACV replication than CD27hi cells. Our finding that CD27lo effector-like memory cells tend to congregate in clusters far from airway epithelium, together with their apparent failure to clear virus from the lung tissue, challenges this paradigm. Instead, it appears that, in the respiratory tract, CD27hi Tcm-l cells function as frontline defenses by rapidly mobilizing to the initial sites of viral entry and infectious foci. Together, these studies highlight the complexities of antiviral immunity and reinforce the idea that phenotypic and functional heterogeneity in the memory pool provides the host certain level of plasticity in terms of its capacity to combat pathogens encountered via different routes.
Our current study has important implications for vaccine design. Previous studies suggest that peripheral prime-boost regimens, to induce secondary and possibly tertiary effector-like memory CD8 T cells, are required for long-lasting immunity (Woodland, 2004; Butler et al., 2011; Nolz and Harty, 2011). Although this is likely true for elimination of blood-borne pathogens in certain lymphoid tissues, the predominance of CD27lo Tem-l cells in the memory pool that frequently occurs after repetitive antigen encounters will likely provide insufficient protection against respiratory pathogens. Thus, the challenge for the future will be to develop strategies that induce localization of pathogen-specific Tcm-l CD27hi CD8 memory cells within the lung epithelium and, at the same time, shift the balance of cell differentiation from a CD27hi to a CD27lo phenotype, allowing for layered immunological defense against highly virulent respiratory viruses.
Materials and methods
Mice
8–12-wk-old female C57BL/6 (CD45.2), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1/Ly5.1), and B6.129S7-Ifngtm1Ts/J mice were either purchased from The Jackson Laboratory or bred and maintained at the University of Florida animal facility. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida (Gainesville, FL; OLAW Assurance # A3377-01).
Virus and peptides
VACV-WR was purchased from the ATCC, grown in HeLa cells, and subsequently titered on VeroE6 cells as described previously (Salek-Ardakani et al., 2008). The VACV-WR peptide B8R (20–27; TSYKFESV; Tscharke et al., 2005; Moutaftsi et al., 2006) was purchased From A & A Labs. Major MHC/peptide tetramers containing the B8R peptide (20–27; TSYKFESV)/H-2Kb, conjugated to allophycocyanin (APC), were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Flow cytometric and FACS analysis
Preparation of cells, extracellular/intracellular staining, data acquisition, and data analysis were performed as described previously (Salek-Ardakani et al., 2011b; Flynn et al., 2013; Goulding et al., 2014).
IF studies
At the indicated times VACV-infected lungs were inflated with 500 µl 50:50 OCT/PBS embedding solution and immediately snap frozen on dry ice. Frozen blocks were cut into 6-µm sections, fixed in acetone, blocked in a 5% BSA PBS solution for 1 h, and stained with DAPI (Invitrogen) and antibodies specific for CD45.1 (clone A20), CD8 (clone 53–6.7), CXCR3 (CXCR3-173), and actin Green 488 Ready Probes. Analysis of microscopy images was performed as previously described (Flynn et al., 2013; Goulding et al., 2014).
Statistical analysis
Tests were performed using Prism 5.0 software (GraphPad). Statistics were done using two-tailed, unpaired Student’s t test with 95% confidence intervals unless otherwise indicated. Two-way ANOVA was used to determine differences in weight loss profiles and the Mantel-Cox test was used for survival analysis. Unless otherwise indicated, data represent the mean ± SEM; P < 0.05 considered statistically significant.
Online supplemental material
Fig. S1 shows the composition and kinetics of splenic and lung memory CD8+ T cell subsets after sublethal respiratory VACV-WR infection. Fig. S2 shows the method of enumerating VACV-specific donor cells in the peribronchiolar regions of the lungs and the localization of CD27lo (CXCR3lo) within the lungs at day 7 after lethal infection, as well as the weight loss curve obtained with the two CD8 memory subsets when provided directly in the airways followed by a lethal VACV infection. Fig. S3 shows the recruitment of CD27hi (CXCR3hi) memory CD8+ T cell subset to the airway lumen and epithelium in IL-15−/− mice as well as the lung innate immune response after lethal respiratory VACV infection.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC/peptide tetramer containing the B8R peptide (20–27; TSYKFESV)/H-2Kb, conjugated to APC.
This work was supported by National Institutes of Health grant AI087734 to S. Salek-Ardakani. V. Tahiliani was supported by National Institutes of Health grant T32 AR007603-15. P. Desai was supported through The American Association of Immunologists Careers in Immunology Fellowship Program.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- 1°
- primary
- 2°
- secondary
- BALF
- bronchoalveolar lavage fluid
- IF
- immunofluorescence
- NT
- no transfer
- NYCBOH
- New York City Board of Health
- p.i.
- post-infection
- Tcm-l
- central-like T cell
- Tem-l
- effector-like T cell
- VACV
- vaccinia virus
- WR
- Western Reserve strain
References
- Abboud G., Tahiliani V., Desai P., Varkoly K., Driver J., Hutchinson T.E., and Salek-Ardakani S.. 2015. Natural killer cells and innate interferon gamma participate in the host defense against respiratory vaccinia virus infection. J. Virol. 90:129–141. 10.1128/JVI.01894-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler N.S., Nolz J.C., and Harty J.T.. 2011. Immunologic considerations for generating memory CD8 T cells through vaccination. Cell. Microbiol. 13:925–933. 10.1111/j.1462-5822.2011.01594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J.L., Nichols D.K., Martinez M.J., and Raymond J.W.. 2010. Animal models of orthopoxvirus infection. Vet. Pathol. 47:852–870. 10.1177/0300985810378649 [DOI] [PubMed] [Google Scholar]
- Ely K.H., Roberts A.D., and Woodland D.L.. 2003. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J. Immunol. 171:3338–3342. 10.4049/jimmunol.171.7.3338 [DOI] [PubMed] [Google Scholar]
- Flynn R., Hutchinson T., Murphy K.M., Ware C.F., Croft M., and Salek-Ardakani S.. 2013. CD8 T cell memory to a viral pathogen requires trans cosignaling between HVEM and BTLA. PLoS One. 8:e77991 10.1371/journal.pone.0077991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding J., Bogue R., Tahiliani V., Croft M., and Salek-Ardakani S.. 2012. CD8 T cells are essential for recovery from a respiratory vaccinia virus infection. J. Immunol. 189:2432–2440. 10.4049/jimmunol.1200799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding J., Abboud G., Tahiliani V., Desai P., Hutchinson T.E., and Salek-Ardakani S.. 2014. CD8 T cells use IFN-γ to protect against the lethal effects of a respiratory poxvirus infection. J. Immunol. 192:5415–5425. 10.4049/jimmunol.1400256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman H.D., Reynoso G.V., Ngudiankama B.F., Cush S.S., Gibbs J., Bennink J.R., and Yewdell J.W.. 2015. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity. 42:524–537. 10.1016/j.immuni.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikono H., Kohlmeier J.E., Takamura S., Wittmer S.T., Roberts A.D., and Woodland D.L.. 2007. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204:1625–1636. 10.1084/jem.20070322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson S.C., and Masopust D.. 2009. Diversity in T cell memory: an embarrassment of riches. Immunity. 31:859–871. 10.1016/j.immuni.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.W., Rutishauser R.L., Joshi N.S., Haberman A.M., and Kaech S.M.. 2010. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J. Immunol. 185:5315–5325. 10.4049/jimmunol.1001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmüller W., Brandes M., Wang Z., Herz J., Egen J.G., and Germain R.N.. 2013. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 38:502–513. 10.1016/j.immuni.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier J.E., and Woodland D.L.. 2009. Immunity to respiratory viruses. Annu. Rev. Immunol. 27:61–82. 10.1146/annurev.immunol.021908.132625 [DOI] [PubMed] [Google Scholar]
- Kohlmeier J.E., Reiley W.W., Perona-Wright G., Freeman M.L., Yager E.J., Connor L.M., Brincks E.L., Cookenham T., Roberts A.D., Burkum C.E., et al. 2011. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J. Exp. Med. 208:1621–1634. 10.1084/jem.20102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Marzo A.L., and Lefrançois L.. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- Moutaftsi M., Peters B., Pasquetto V., Tscharke D.C., Sidney J., Bui H.H., Grey H., and Sette A.. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24:817–819. 10.1038/nbt1215 [DOI] [PubMed] [Google Scholar]
- Nolz J.C., and Harty J.T.. 2011. Strategies and implications for prime-boost vaccination to generate memory CD8 T cells. Adv. Exp. Med. Biol. 780:69–83. 10.1007/978-1-4419-5632-3_7 [DOI] [PubMed] [Google Scholar]
- Olson J.A., McDonald-Hyman C., Jameson S.C., and Hamilton S.E.. 2013. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity. 38:1250–1260. 10.1016/j.immuni.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.D., and Woodland D.L.. 2004. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 172:6533–6537. 10.4049/jimmunol.172.11.6533 [DOI] [PubMed] [Google Scholar]
- Roberts A.D., Ely K.H., and Woodland D.L.. 2005. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 202:123–133. 10.1084/jem.20050137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S., Moutaftsi M., Crotty S., Sette A., and Croft M.. 2008. OX40 drives protective vaccinia virus-specific CD8 T cells. J. Immunol. 181:7969–7976. 10.4049/jimmunol.181.11.7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S., Choi Y.S., Rafii-El-Idrissi Benhnia M., Flynn R., Arens R., Shoenberger S., Crotty S., Croft M., and Salek-Ardakani S.. 2011a B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J. Immunol. 186:5294–5303. 10.4049/jimmunol.1100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S., Flynn R., Arens R., Yagita H., Smith G.L., Borst J., Schoenberger S.P., and Croft M.. 2011b The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J. Clin. Invest. 121:296–307. 10.1172/JCI42056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S., Moutaftsi M., Sette A., and Croft M.. 2011c Targeting OX40 promotes lung-resident memory CD8 T cell populations that protect against respiratory poxvirus infection. J. Virol. 85:9051–9059. 10.1128/JVI.00619-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Förster R., Lipp M., and Lanzavecchia A.. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J.J., Bickham K.L., Lerner H., Goldstein M., Sykes M., et al. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 38:187–197. 10.1016/j.immuni.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J.J., Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T., Kato T., Lerner H., Shen Y., and Farber D.L.. 2014. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 159:814–828. 10.1016/j.cell.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke D.C., Karupiah G., Zhou J., Palmore T., Irvine K.R., Haeryfar S.M., Williams S., Sidney J., Sette A., Bennink J.R., and Yewdell J.W.. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95–104. 10.1084/jem.20041912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D.L. 2004. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 25:98–104. 10.1016/j.it.2003.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.