Hayakawa et al. show that distinctive B-lineage progression from B-1 development allows for generation of B1a cells with restricted BCRs and self-renewal capacity, both contributing to potential for CLL progression.
Abstract
In mice, generation of autoreactive CD5+ B cells occurs as a consequence of BCR signaling induced by (self)-ligand exposure from fetal/neonatal B-1 B cell development. A fraction of these cells self-renew and persist as a minor B1 B cell subset throughout life. Here, we show that transfer of early generated B1 B cells from Eμ-TCL1 transgenic mice resulted in chronic lymphocytic leukemia (CLL) with a biased repertoire, including stereotyped BCRs. Thus, B1 B cells bearing restricted BCRs can become CLL during aging. Increased anti-thymocyte/Thy-1 autoreactive (ATA) BCR cells in the B1 B cell subset by transgenic expression yielded spontaneous ATA B-CLL/lymphoma incidence, enhanced by TCL1 transgenesis. In contrast, ATA B-CLL did not develop from other B cell subsets, even when the identical ATA BCR was expressed on a Thy-1 low/null background. Thus, both a specific BCR and B1 B cell context were important for CLL progression. Neonatal B1 B cells and their CLL progeny in aged mice continued to express moderately up-regulated c-Myc and down-regulated proapoptotic Bmf, unlike most mature B cells in the adult. Thus, there is a genetic predisposition inherent in B-1 development generating restricted BCRs and self-renewal capacity, with both features contributing to potential for progression to CLL.
Introduction
In humans, B cell chronic lymphocytic leukemia (CLL) with CD5+ phenotype is a common form of adult leukemia with an incidence that increases with advancing age. A critical role of the BCR in development of CLL has been recognized by the presence of recurrent (stereotyped) BCRs, often with similar or identical Ig heavy chain third complementarity determining regions (HCDR3; Chiorazzi and Ferrarini, 2003; Stamatopoulos et al., 2007). BCR signaling is able to induce expression of CD5 (Wortis et al., 1995). About half of CLL patients express an unmutated IgVH, which is often a marker of cases with a poorer prognosis than cases with a mutated IgVH (Hamblin et al., 1999), and unmutated CLL BCRs have been shown to be autoreactive and polyreactive (Hervé et al., 2005). These findings led to a proposal of multistep leukemogenesis: first, the generation of autoantigen-experienced B cells; second, their persistence and proliferation resulting from cross-reactivity with pathogens; and third, events leading to transformation and progression to CLL without BCR mutation, as in cases with a more aggressive course (Chiorazzi and Ferrarini, 2011). However, it has long been debated how such autoreactive B cells with restricted BCRs are generated. Furthermore, recent data demonstrated that BCRs in CLLs often exhibit the capacity for autonomous signaling in the absence of an extracellular ligand, a feature not found in BCRs associated with other types of B cell lymphomas (Dühren-von Minden et al., 2012). This prompted the additional question of whether a stereotyped BCR plays a major role in B cell maintenance and/or transformation, independent of B cell context, once it is expressed.
In normal mice, generation of autoreactive mature CD5+ B cells, termed B1a, occurs as a positive outcome of fetal/neonatal B-1 B cell development from Lin28b+Let-7− B-lineage precursors. In contrast, Lin28b−Let-7+ B lineage precursors become predominant in adult B-2 B cell development, and mature CD5+ B cell generation declined (Hardy and Hayakawa, 2001; Yuan et al., 2012; Zhou et al., 2015). Because some B-1–derived B cells self-renew and are maintained throughout life as a minor B cell subset (Hayakawa et al., 1986) termed B1 B cells (also called B-1 B cells), this prompted the question of whether early generated CD5+ B cells can become CLL in aged mice. In most WT mouse strains, development of CLL is rare. However, aggressive CLLs in humans have higher levels of the T cell leukemia 1 (TCL1) oncogene, and transgenic expression of human TCL1 targeted to mouse B lineage cells (Eμ-hTCL1 Tg) leads to a high incidence of CD5+ CLLs during aging with biased utilization of unmutated BCRs (Bichi et al., 2002; Yan et al., 2006). One stereotyped BCR in mouse TCL1+CLL has an anti-nonmuscle myosin IIA autoreactivity, a feature also common to some human CLLs. Generation of mouse models with this autoreactive BCR by Ig transgenesis provided evidence that this particular BCR is restricted to the outcome of B-1 B cell development. Early generated B1 B cells with this BCR can develop CLL with aging, even without the TCL1 Tg, confirming that progression to CLL can occur from B-1–derived B1 B cells (Hayakawa et al., 2016). This Ig transgenic mouse model also demonstrated the importance of BCR structure, as not all early generated CD5+ B1 B cells with a similar BCR could become CLL; there was a requirement for particular CDR3s in the V/D/J and V/J junctions (Hayakawa et al., 2016).
Here, we show that B1 B cells also generate CLLs with other stereotyped BCRs commonly found in mouse CLL, and that progression to CLL by B1 B cells is not only a result of their ability to express specific BCRs. The proto-oncogene c-Myc (Myc), deregulated in most human cancers, is one of the critical transcription factors regulating normal cell proliferation, growth, and also apoptosis in development and cell maintenance (Pelengaris et al., 2002; Nilsson and Cleveland, 2003; Delgado and León, 2010). Myc is broadly expressed during embryogenesis, regulating hematopoiesis. In the adult, Myc expression is maintained by normal dividing cells at a relatively consistent moderate level (Pelengaris et al., 2002; Delgado and León, 2010). In cellular processes, Myc also plays a role in apoptosis, in that it targets the Bcl-2 network, suppressing the prosurvival Bcl-2 and Bcl-XL proteins (Nilsson and Cleveland, 2003). Bcl-2 family proteins are fundamental regulators of apoptosis, thus, the balance of anti- and proapoptotic proteins within the Bcl-2 family is important in determining cell survival.
Bim is a proapoptotic Bcl-2 family BH3-only protein expressed in a variety of cell types that is well known for playing various roles, including controlling the generation of autoreactive B and T cells during lymphocyte development (Bouillet et al., 1999, 2002; Enders et al., 2003). Bmf is also a BH3-only protein that was originally found as a regulator of anoikis in epithelial cells, similar to Bim (Puthalakath et al., 2001). Bmf is also expressed by hematopoietic cells, including B lymphocytes, where it controls the generation of autoreactive B cells (Labi et al., 2008). Both genes coding for Bim and Bmf proteins are located on chromosome 2 in mice, and the proteins share the ability to bind to cytoskeletal components through dynein light chain (DLC), sequestering Bim to the DLC1-microtubule and Bmf to the DLC2-actin–bound myosin V (Piñon et al., 2008). In response to certain cellular stress stimuli, Bim and/or Bmf are released from the cytoskeleton, localize to mitochondria, and neutralize prosurvival Bcl-2 family members to elicit apoptosis (Labi et al., 2008; Piñon et al., 2008; Grespi et al., 2010). Thus, reduction of Bim and/or Bmf can reduce apoptosis, promoting survival (Piñon et al., 2008; Labi et al., 2014). Although Bim continues to be expressed by immature through mature stage B cells, including by B1 B cells, we show that in B-1–derived B1 B cells, Myc is up-regulated and Bmf is down-regulated, in contrast to the majority of mature B cells generated from B-2 development. Thus, together with the ability to generate restricted autoreactive BCRs, the balance between proliferation and cell death in B1 B cells may confer a predisposition for CLL progression.
Results
T cell–independent CLL generation in Eμ-hTCL1 Tg mice
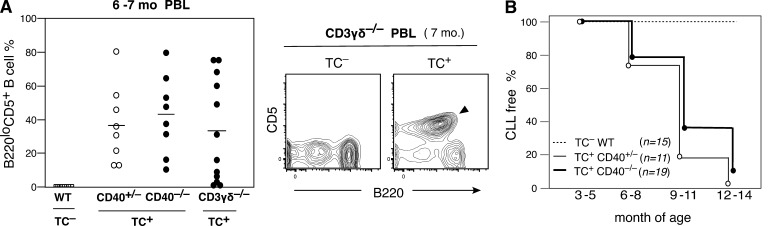
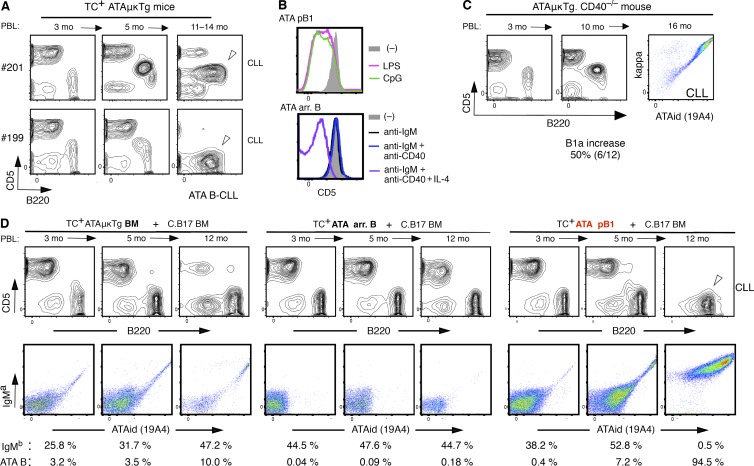
One potential feature in the evolution of CLL was T cell dependence, and germinal center (GC) generation through T cell CD40L–CD40 signaling was considered one possible mechanism (Zenz et al., 2010). To test this possibility, Eμ-hTCL1 (TC) transgenic mice were crossed with CD40−/− mice. As shown in Fig. 1 A, PBL analysis of TC+ CD40+/− and CD40−/− littermates at 6–7 mo of age, and CLL incidence comparison in Fig. 1 B, there was no strong reduction of CD5+B220lo CLL incidence on a CD40-deficient background. Moreover, development of CLL also occurred in TC+ mice lacking mature T cells as a result of CD3γδ deficiency (Fig. 1 A). Thus, the high incidence of CLL generation in TC+ mice is T cell independent, CD40 signal independent, and does not require GC formation.
Figure 1.
T cell–independent CLL in Eμ-TCL1 Tg mice. (A) Increase in CD5+B220lo B cell frequency in PBL of both CD40+/− and CD40−/− TC+/− littermates (C.B17 background) and CD3γδ−/− TC+/− mice (C.B17 and C57BL/6 mixed background), compared with WT (C.B17), at 6–7 mo. Mean is indicated by a bar. (right) B220loCD5+ B CLL in a 7-mo-old TC+/−CD3γδ−/− mouse (marked) compared with a TC−/− littermate. (B) Incidence of CLL over time. No strong reduction of CLL incidence occurred in CD40−/− background.
Early generated B-1–derived B1 B cells progress to CLL in aged mice
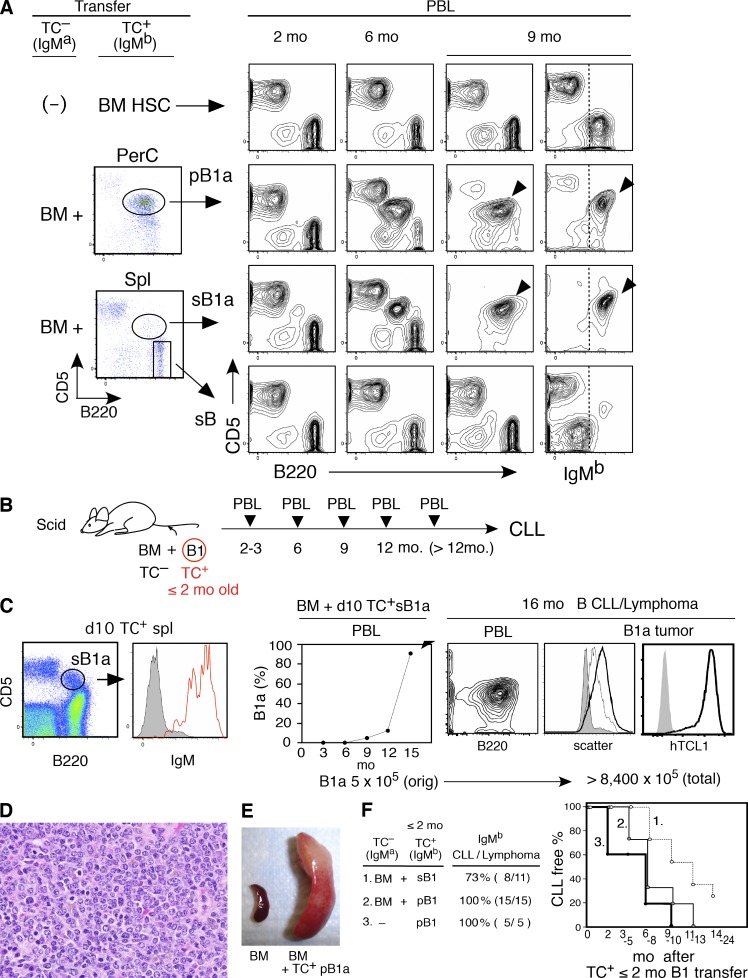
Induction of CD5 expression in B cells, yielding B1a cells, can occur as a consequence of BCR signaling, without requiring either CD40 signaling or T cell help (Wortis et al., 1995). To assess whether B1a cells in TC+ mice become CLL, we performed adoptive transfer experiments by cotransferring peritoneal B1a cells (pB1a), splenic B1a cells (sB1a), or splenic non-B1a B cells from TC+ mice, together with BM cells from TC− mice that differ in IgM allotype from the TC+ mice. As shown in Fig. 2 A, 2-mo-old TC+ mice had a small number of B1a cells (CD5+B220lo) in both the peritoneal cavity (PerC) and spleen that readily developed into CLL. In contrast, transferred cells derived from the dominant population of CD5− B cells (CD5–B220hi) did not develop tumors. For comparison, transfer of BM hematopoietic stem cells (HSCs) purified from the same TC+ mice used for the B1a transfer resulted in generation of a normal CD5− B cell population, but most recipients did not develop CLL within 12 mo after transfer (Fig. 2 A, top). Only 2/11 cases developed CLL after transfer of BM HSCs from 2–3-mo-old TC+ mice. Because the increase in B1a cells in PBL often began at 4–6 mo of age in TC+ mice, some started to develop CLL by 6–8 mo, and most had developed CLL at 12 mo (Fig. 1 B), it appears that a loss of preexisting B1a cells by BM transfer alone resulted in a reduced incidence of CLL.
Figure 2.
CLL development from early generated B-1–derived B cells. (A) Comparison of CLL incidence in C.B17.scid recipients transferred with BM HSC, B1a, and non-B1a B cells purified from 2 mo TC+ C.B17 mice. pB1a and sB1a (each 5 × 105/recipient), and non-B1a spleen B cells (sB; 3 × 106/recipient) were cotransferred with TC– BALB/c BM. 2 mo after transfer, PBL showed similar numbers of B and T cells (top, using TC+ HSC alone were all IgMb, others were predominantly IgMa). Increase in B220loCD5+ B cells of IgMb B1a and CLL development are marked. PerC, peritoneal cavity. Representative of six transfer cases using 2–3-mo-old mice. TC+ mice together with TC– mice. (B) Cotransfer of purified B1 B cells from ≤2-mo-old TC+C.B17 mice with TC– BALB/c BM into C.B17.scid mice, monitoring PBL after transfer. (C) Transfer of B1a cells from 10-d-old TC+/− mice and development of CLL. 106 purified sB1a B cells (5 × 105 TC+) from 12 10-d-old (TC+/− x CB17)F1 mice were transferred together with 5 × 106 TC– BM cells into CB.17. mice. (left) Surface IgM expression of sB1a cells. (right) Frequency of B1a cells in PBL of recipient after adoptive transfer (middle). Characteristic mouse CLL CD5+B220+ phenotype and lymphomatous spleen B cells (thick black line) compared with original transferred B1a cells (thin black line and CD5−B220+ B cells (gray). Expression of hTCL1 Tg in this CLL is visualized by cytoplasmic staining (gray, second step control). Total leukemia B cell number indicated as a sum of IgMb+ B cells in PBL (2 × 108), spleen (4.6 × 108), and peritoneal cavity (1.8 × 108), as compared with the size of the initial TC+ inoculum. Representative of 15 cases with CLL/lymphoma generation by ≤2-mo-old mice. TC+ B1 B cell transfer. (D) H&E staining of spleen section with CLL/lymphoma of day 10 B1a cell origin. (E) Splenomegaly 8 mo after TC+ pB1a transferred together with TC– BM cells, compared with a recipient of TC– BM alone. (F) Summary of CLL/lymphoma incidence by purified B1 B cell transfer from ≤2-mo-old TC+ mice.
To further assess the capacity of early generated B1 B cells to become CLL, B1a cells in spleen/PerC, or a mixture of B1a and B1b (CD5– B220lo B) cells in PerC, were purified from young (≤2 mo of age) TC+ mice, as B1 B; these cells were then cotransferred with TC– BM cells, and CLL development was monitored by analyses of PBL (Fig. 2 B). As shown in Fig. 2 (C–E) and summarized in Fig. 2 F, CLL generation occurred, including from day 10 neonatal B1a. In day 10 neonates, the majority of mature AA4 (CD93)– CD5+B220lo B1a cells were products of fetal/neonatal B-1 development (Hayakawa et al., 1985; Hardy and Hayakawa, 2001). Transfer of as few as 5 × 105 TC+ splenic IgM+ B1a cells from a day 10 neonatal TC+ littermate pool resulted in CD5+ CLL in aged recipients by 16 mo after transfer (Fig. 2 C). These B1a cell–derived neoplasms in aged mice became proliferative with a slight increase in cell size, as shown by scatter and retention of hTCL1 expression (Fig. 2 C). In most cases of CLL, in addition to predominance in PBL (Fig. 2, A and C), leukemias exhibited histological features of small lymphocytic lymphoma in spleens with parallels to human CLL (Morse et al., 2002; Fig. 2 D). Splenomegaly was common, presenting as aggressive CLL (Fig. 2 E). Transfer of B1 B cells from young TC+ mice consistently resulted in the development of CLL (Fig. 2 F). In addition, recipients of peritoneal B1 B cells (pB1) transferred without BM cells rapidly progressed to CLL as early as 2 mo (Fig. 2 F, right). Thus, early generated B1 B cells, predominantly with a B1a phenotype and progeny of B-1 development, can develop into CLL in aged mice.
CLLs of B1 B cell origin have stereotyped BCRs
Ig sequence analyses of 155 CLL samples from TC+ mice on a C.B17 background revealed biased VH usage without mutation from the germline (Tables S2 and S3, HCDR3 list). Among these, stereotyped BCRs with identical VH and VK usage comprised >50% of cases (Table 1, five BCR set). The VH gene nomenclature used here is based on Table S1, listed together with VBASE2 (Retter et al., 2005)names in parenthesis (100% identity). These stereotyped BCRs were found in CLLs generated in the absence of CD40 signaling, confirming their T cell and GC independence. Furthermore, CLLs generated from cell transfers of B1 B cells of ≤2-mo-old mice also exhibited stereotyped BCRs (Table 1 and Table S2). The finding that in set 5, the VH11/Vk9 BCR was not detected was likely a result of the limited number of transfer CLL cases analyzed (total 20), as B cells with this BCR, with anti-bromelain–treated mouse RBC (BrMRBC)/phosphatidylcholine autoreactivity, are known to derive from early generated B1 B cells (Hardy et al., 1989; Wasserman et al., 1998). Set 3 BCR, with identical CDR3 and J segments, is an anti–nonmuscle myosin IIA autoantibody, previously confirmed to be restricted to B1 B cell, as B-1 B, by generation of BCR transgenic/knock-in mouse models (Hayakawa et al., 2016). Thus, B1 B cells develop into CLL with biased unmutated BCRs, including stereotyped BCRs.
Table 1. Stereotyped BCRs in TCL1+ CLL in mice.
| CLL BCR set | Identical VH/VK BCR | TC+ | TC+ CD40–/– | ≤2 mo B1 B cell origin | |
|---|---|---|---|---|---|
| IgH | IgL | ||||
| VH genea | Vk geneb | n = 155 | n = 18 | n = 20 | |
| 1 | VH12-1 (V261) | Vk4-91 (kf4) | 40 (25.8 %) | 4 (22.2%) | 4 |
| 2 | J558-64 (V332) | Vk12-89 (fl12) | 19 (12.3 %) | 3 (16.6%) | 2 |
| 3 | Q52-1 (V222) | Vk9-96 (ce9) | 14 (9.0 %) | 1 (5.6%) | 2 |
| 4 | J558-67 (V328) | Vk1-117 (cr1) | 10 (6.5 %) | 1 (5.6%) | 3 |
| 5 | VH11-2 (V235) | Vk9-128 (br9) | 4 (2.6 %) | 1 (5.6%) | nd |
Presence of CLL stereotyped BCRs in TCL1+(TC+) CLL samples expressing identical VH and VL combination, including CD40 deficiency, and CLL developed by IgM+ B1 B cell transfer from TC+ neonatal/young mice (≤2 mo old). n, total sample number. nd, not detected.
VH gene (see Table S1 and Vbase2 for parentheses).
Vk gene (Thiebe et al., 1999).
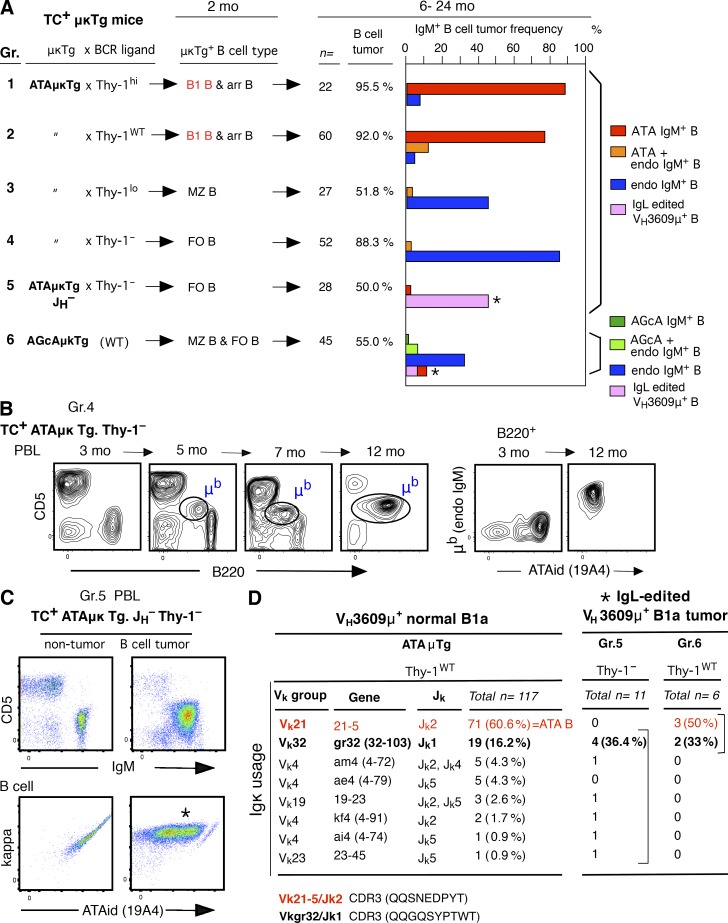
Increased ATA B cell generation by B1 B cells yields a high incidence of MBL/CLL
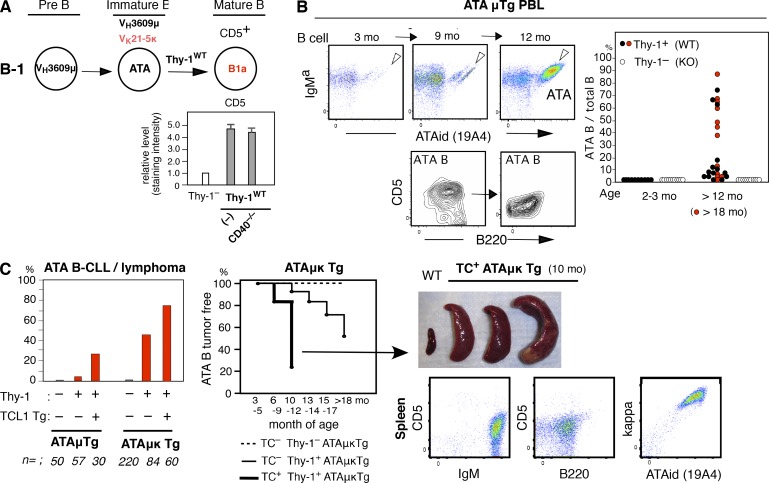
Without the TCL1 transgene, CLL is a rare neoplasm in most WT mouse strains. However, increased frequency of certain germline (unmutated) autoreactive BCRs by transgenesis in B1 B cells leads to an increased incidence of CLL without the TCL1 Tg, on a C.B17 background. Set 3 VHQ52/Vk9 BCR transgenesis was described previously (Hayakawa et al., 2016). VH3609/Vk21 ATA (anti-thymocyte/Thy-1 autoreactivity) BCR transgenesis also led to a high incidence of CLL/lymphoma. In a VH3609/D/JH2-μa Igμ transgenic mouse line, ATAμTg, mature CD5+ ATA B cells, as B1a, are generated by B-1 development, dependent on the presence of a self-Thy-1 glycoform predominantly expressed by immature thymocytes in WT mice (Thy-1WT; Gui et al., 1999; Hayakawa et al., 1999), as a positive selection. This is also true for VH3609/D/J knock-in mice (unpublished data). This selection is independent of CD40 signaling (Fig. 3 A). In the ATAμTg mouse line, the frequency of ATA B cells in total B cells often increased in PBL with age (detected by ATA idiotype antibody), with some becoming a monoclonal B cell lymphocytosis (MBL; >70% of total B cells; Fig. 3 B). A portion of these mice developed splenomegaly (unpublished data). Although most ATAμTg mice did not become leukemic without the TCL1 Tg, in the ATA Igμ/Igκ double-transgenic mouse line with dominance of ATA B cells among total B cells, total ATA B cell numbers increased in PBL, resulting in leukemia and splenomegaly as ATA B-CLL/lymphomas under the self-Thy-1 presence (TC– Thy-1+ATAμκTg mice; 46%; 39/84; Fig. 3 C, left and middle). The presence of the TCL1 Tg further promoted the incidence of ATA B-CLL with splenomegaly (TC+ Thy-1+ATAμκTg mice; 77%; 46/60; Fig. 3 C, left, middle, and right). Thus, an increased frequency of B1 B cells expressing this ATA BCR by μ or μκ transgenesis promoted the development of MBL/CLL.
Figure 3.
Increased incidence of MBL/CLL/lymphoma in B1 B cells with ATA BCR. (A) Self-Thy-1 antigen-dependent CD5 induction by ATA B cells through B-1 development does not involve CD40 signal. Comparative CD5 level by mature (AA4–) ATA B cells (ATAid+) in PerC in 2-mo-old ATAμκTg mice in Thy-1−/− versus WT (Thy-1+/+) and CD40−/− (n = 5; mean ± SE). (B) Increased ATA B cell frequency in PBL B cells in aging ATAμTg mice. (left) ATA B increased case (marked) as MBL at 12 mo, with further B220 reduction. CD5 reduction also occurred in around half the cases of increased ATA B cells. (C) ATA B-CLL/lymphoma incidence in ATAμTg and ATAμκTg mice with or without TCL1 Tg (left). Incidence over time by ATA μκTg mice (middle). (right) Splenomegaly by TC+ATAμκTg mice (>90% cases) together with CLL (top), and representative total spleen cell data analysis (bottom).
Role of BCR together with B1 B cell origin, not BCR alone, in CLL generation
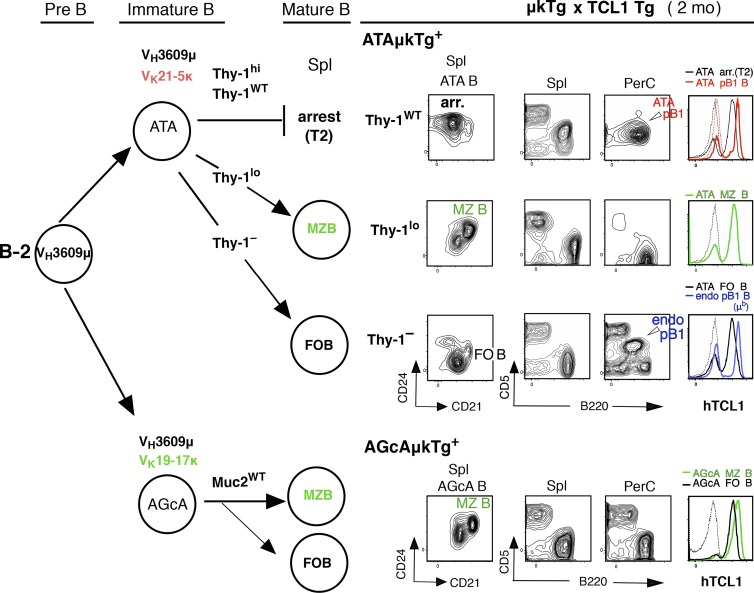
Using this strong TC+ ATAμκTg mouse model where ATA B cells increase, we next examined whether expression of the ATA BCR plays a major role in CLL development regardless of B cell context. As previously shown, and summarized in Fig. 4 (left; B-2 development), when ATAμκTg mice on a Thy-1 knockout background (Thy-1–) were crossed with Lck-Thy-1 transgenic mice expressing levels of Thy-1 different from the WT level, different B subsets were preferentially generated that all expressed the ATA BCR. Thy-1hi (Lck-Thy-130), Thy-1lo (Lck-Thy-10), and Thy-1– (Thy-1– background alone), respectively, generate arrested B cells (arr B), together with B1 B cells of B-1 origin, marginal zone B cells (MZ B), and follicular B cells (FO B; Fig. 4, top; Wen et al., 2005a). These ATAμκTg mice, including mice on a WT background (Thy-1WT), were each crossed with TCL1 Tg mice. At 2 mo of age, the majority of ATA B cells in spleen of Thy-1hi and Thy-1WT mice are arrested at the T2 stage as an outcome of B-2 development, in contrast to the dominant population of mature ATA B1 B cells in PerC that derive from B-1. On a Thy-1lo background, B cells are predominantly MZ B cells with a very few, nearly undetectable frequencies of B1a cells in both spleen and PerC. In Thy-1– mice, ATA B cells become FO B cells in spleen, and the B1a cells present in PerC have BCRs from endogenous Ig rearrangements (IgMb; Fig. 4, top right). These data from 2-mo-old adult mice were identical to those previously found with TC– mice (Hayakawa et al., 2003; Wen et al., 2005a). Cells in which the VH3609 IgH is associated with a different IgL, Vk19-17, become predominantly MZ B cells (CD21hiCD24medCD23lo/–CD1dhiCD5–), as well as FO B cells, with a different autoreactivity from ATA: an anti-intestinal goblet cell autoreactive (AGcA) BCR that primarily reacts with mucin 2 glycoprotein (Ichikawa et al., 2015; Fig. 4, bottom). These autoreactive AGcAμκTg mice were also crossed with TCL1 Tg mice as an example of BCRs with a different light chain, resulting in a B cell population dominated by MZ B (and FO B) cells, similar to the case with Thy-1lo mice. All these B cell subsets expressed hTCL1 protein in the cytoplasm at similar levels (Fig. 4, right).
Figure 4.
hTCL1 expression by all B cell subsets. (left) Summary of generation of different B cell subsets in spleen through B-2 development from BM; ATA B cell subset generation in ATAμκTg mice by different levels of self-Thy-1 antigen exposure, and AGcA B cell subsets (dominantly autoreactive to mucin 2) in AGcAμκTg mice, expressing different light chain from ATA BCR. (right) 2-mo-old TC+ ATAμκTg (with different Thy-1 levels) and AGcAμκTg mice. CD21/CD24 staining of ATA B or AGcA B cells in spleen (both constituting the dominant B cells among total B cells) to show arrested, MZ B, or FO B dominance, and B220/CD5 staining of total (lymphocyte) spleen and PerC. AA4 (CD93) and CD23 staining was also included to confirm each subset. Marked cell subsets for cytoplasmic hTCL1 staining analysis. CD21hiCD24med; MZ B, CD21medCD24lo; FO B. Thin dotted lines in hTCL1 staining are the data from TC– μκTg+ littermates as controls. Representative data of two to three samples each.
The CLL/lymphoma incidence data are summarized in Fig. 5 A. In mice with both Thy-1hi and Thy-1WT backgrounds, mature ATA B1 B cells are present and develop a high incidence of ATA B-CLL/lymphomas (Fig. 5 A, Gr. 1 and 2). In contrast, in mice with both Thy-1lo and Thy-1– backgrounds, CLL BCRs derived predominantly from B cells with endogenous rearrangements with a CD5+ B1a phenotype (Fig. 5 A, Gr. 3 and 4). A representative Thy-1– case is shown in Fig. 5 B. To assess whether a lack of competitive B cells expressing endogenous BCRs leads to ATA B CLL generation, ATAμκTg.Thy-1– mice were further crossed with JH knockout mice to maintain abundant ATA FO B cells into old animals. CLL B cells generated under this endogenous IgH– condition were predominantly IgL edited B cells (Fig. 5, A, Gr. 5, and C), and still not ATA B cells. Thus, surface expression of the ATA BCR alone did not result in a heightened incidence of CLL. In addition to the absence of CLL development from ATA BCR-expressing MZ B and FO B subsets, AGcA BCR+ MZ B and FO B cells also did not develop into CLL (Fig. 5 A, Gr. 6).
Figure 5.
CLL/lymphoma incidence predominantly arises from B1 B cells. (A) Summary of IgM+ B cell tumor (CLL/lymphoma) incidence and frequency of tumor IgM with either original μκTg BCR, endogenous IgM (IgMb), or IgMb coexpressed with original μκTg BCR. In ATAμκTg.JH– Thy-1–, also in AGcA μκTg mice, IgL edited B cell tumor occurred together with original VH3609μ IgH expression (*). (B) Representative case of endogenous IgM (IgMb) increase as B220loCD5+ B cells in PBL of TC+ATAμκTg.Thy-1– mice (Gr. 4). As shown on right, original B220+CD5– cells at 3 mo in PBL were circulating FO B cells with IgMa+ ATA BCR, which did not develop B cell tumor. (C) TC+ATAμκTg.JH–Thy-1– mouse PBL at the same 14 mo, without or with B cell tumor development. The edited IgL by this tumor B cells (*) was Vk19-23, together with VH3609μ IgH. (D) Edited IgL in VH3609μ+ B tumors resemble normal mouse VH3609μ+ B1a Igκ. (left) Igκ single-cell sequence data by VH3609μ+ B cells (VH3609id)+kappa+ in 2–3-mo-old ATAμTg mouse pB1a. Summary of data from four mice (107 shown of the total 117 κ listed). (right) Igκ list of IgL-edited VH3609μ+ B1a (CD5+) tumors. 9/11 of Gr5 and 5/6 of Gr 6 edited Igκ were similar to normal mouse VH3609μ+ B1a, including identical CDR3 use by Vκ21/Jκ2 and Vκ32/Jκ1, the most frequently expressed by VH3609μ+ B1a (CDR3 amino acids are shown).
Stereotyped BCRs (Table 1) were detected among endogenous B1a cell CLLs (Fig. 5 A and not depicted). Furthermore, sequencing of edited IgL in Gr. 5 and 6 revealed that the majority of such BCRs are associated with a typical B1a repertoire with VH3609 IgH (Fig. 5 D). Among VH3609 IgH-expressing B1a in ATAμTg mice, Vk21-5 (Vk21C) IgL+ cells normally predominate as ATA B cells, followed by Vk32 (gr32) IgL (Fig. 5 D, left). In ATAμκTg mice under Thy-1– JH– condition (Gr. 5), and in AGcAμκTg mice on a WT self-Thy-1 background (Gr.6), edited IgLs were similar to the IgLs found in the B1a repertoire, including the ATA B CLLs found in AGcAμκTg mice (Fig. 5 D, right). In young adult TC+AGcAμκTg mice, 2 mo of age, the frequency of ATA B cells was very low; nevertheless, they were detected as B1a (unpublished data). As summarized in Table 2, these results reveal the importance of B1 B cell context, and not just BCR alone, in promoting progression to CLL.
Table 2. B1 B cell as a dominant origin of ATA μκTg+ B CLL/lymphoma.
| TC+ μκTg mice | μκTg BCR ligand | μκTg+ mature B cell | CLL/lymphoma in old aged μκTg B cell | CLL/lymphoma in old aged non-μκTg B cell |
|---|---|---|---|---|
| VH3609μ/Vk21-5κ μκTg | WT | B1 B | ++ | +/– |
| VH3609μ/Vk21-5κ μκTg | Thy-1hi | B1 B | ++ | +/– |
| VH3609μ/Vk21-5κ μκTg | Thy-1lo | MZ B > FO B | – | + B1a (endo IgM) |
| VH3609μ/Vk21-5κ μκTg | Thy-1– | FO B | – | ++ B1a (endo IgM) |
| VH3609μ/Vk21-5κ μκTg.JH– | Thy-1– | FO B | – | + B1a (IgL edited) |
| VH3609μ/Vk19-17κ μκTg | WT | MZ B and FO B | – | + B1a (endo IgM and IgL edited, including ATA B) |
VH3609μ/Vk21-5κ is ATA BCR, and VH3609μ/Vk19-17κ is AGcA BCR, both with the same IgH expression. On a WT background in TCL1+ μκTg mice, ATA B cells become mature B1 B cells and AGcA B cells become mature MZ B (and FO B) cells. CLL/lymphoma developed predominantly from ATA B1 B cells, not AGcA B cells. ATA B cells expressed by MZ B or FO B cells under Thy-1lo or Thy-1– conditions in ATA μκTg mice did not become tumors in contrast with endogenous IgM-expressing CD5+ B cells (B1a). IgL editing was permissive for the generation of B1a cells with the ability to become CLL/lymphoma, including generation of Vk21-5κ ATA B-CLL in AGcA μκTg mice.
B1 B cells are the major source for ATA B-CLL
In adult ATAμκTg mice, arrested B cells predominate in the splenic ATA B cell pool on Thy-1hi and Thy-1WT backgrounds. Thus, we asked whether these arrested B cells from B-2 development also became CLL. ATA B cells at the MBL/CLL stage often showed loss of CD5, in TC– mice as well, as in several TC+ CLL cases (Fig. 6 A, bottom, shows a TC+ littermate case). TLR signaling for cell proliferation is one possible reason for reduced expression of CD5, as CD5 reduction occurs in mature ATA B1 B cells stimulated with TLR in vitro for 3 d (Fig. 6 B, top). However, IL-4 exposure together with BCR and CD40 signals also results in reduced CD5 expression by arrested ATA B cells (Fig. 6 B, bottom), similar to what was found in humans (Gagro et al., 2000). Because a fraction of mature T cells in spleen express the ATA B-reactive Thy-1 glycoform initially expressed by immature thymocytes (Hayakawa et al., 1990), interaction between ATA B and T cells may take place. Furthermore, T cell CD40L–CD40 signaling can augment survival of autoantigen-binding tolerant B cells (Lesley et al., 2006). These results suggested the possibility that CD40 signaling from interacting T cells may promote survival of arrested ATA B cells. However, CD40 deficiency in ATAμκTg mice did not decrease ATA B-CLL incidence. ATA B cells increased in PBL of CD40−/− mice (50%) similar to CD40+/+ mice (46%; Fig. 3 C), and CLL incidence was unaffected (Fig. 6 C). Thus, ATA B cell generation and progression to CLL was independent of CD40 signaling (Fig. 6 C).
Figure 6.
ATA B-CLL generation from mature B1 B cells than arrested B cells. (A) CD5 down-regulation occurrance at CLL stage in TC+ATAμκTg mice. CD5+ and CD5– CLL (marked) found in two littermates, both with splenomegaly. CD5 down-regulation also occurred in TC– ATA B cells as shown in Fig. 3 B. (B) 3 d after stimulation of ATA B cells in peritoneal cavity (pB1) and arrested B cells in spleen of ATAμκTg mice. Cell proliferation by LPS and CpG with CD5 down-regulation. Increased viability and proliferation by anti-IgM + anti-CD40 + IL-4 stimulation by arrested ATA B cells. Similar outcome by two experiments. (C) Data from one representative mouse with spontaneous CLL generation in ATAμκTg.CD40−/− mice (6/12 cases). (D) Cotransfer of HSC enriched BM, arrested ATA B in spleen (arr. B), or ATA B in PerC (pB1) from TC+ATAμκTg (IgMa) mice, together with C.B17 mouse BM. Percentage of C.B17-derived B cells (IgMb) and ATA B in PBL are shown. ATA B-CLL generated by pB1 transfer is marked. Representative of five recipients each.
To further assess this conclusion, we performed cotransfer experiments similar to those shown in Fig. 2, using 2-mo-old TC+ ATAμκTg (IgMa) mice, either BM, AA4+ (arrested) B cells in spleen, or peritoneal B1 B cells, together with C.B17 (IgMb) BM (Fig. 6 D). Transfer of ATA pB1 cells of peritoneal origin showed consistent generation of CLL (5/5), often associated with loss of CD5 (Fig. 6 D right). In contrast, 3/5 cases of arrested ATA B transfer showed lack of CLL development, even after injection of threefold higher cell numbers than pB1 from the same mice (Fig. 6 D, middle). Though 2/5 cases of arrested B transfer showed cell number increases at >12 mo after transfer, this result was not readily interpreted, as it was possible the CLL originated from a small contamination of mature ATA B1 B cells in spleen that increased in aged mice by self-renewal. As shown in Fig. 3 B, continued ATA B increase in circulating PBL occurs from young adult to older age in the same mouse, rather than as an acute increase in older mice, and CLL progression does not require CD40 signaling. These results prompted us to conclude that the major source for ATA B-CLL is B1 B cells and not arrested/tolerant ATA B cells.
Increased c-Myc and low Bmf in B1 B cells continues through the CLL/lymphoma stage
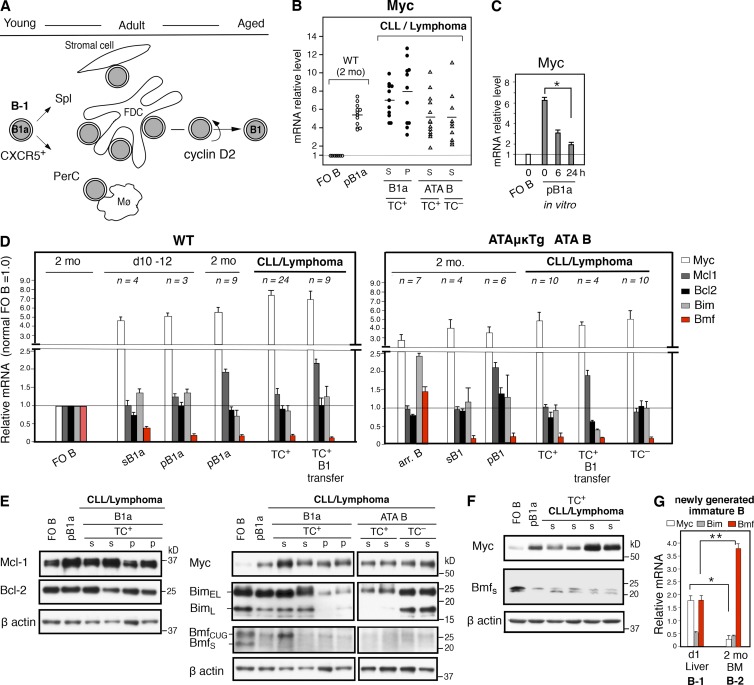
In human CLL, migration, survival, and proliferation of CLLs are regulated by the surrounding tissue microenvironment (Herishanu et al., 2011). In mice, after generation at the fetal/neonatal stage, B1 B cells migrate and circulate, and a fraction continues to self-renew, maintaining the B cell subset throughout life. As previously shown, early generated B1 B cells express the CXCR5 chemokine receptor, migrating to CXCL13-expressing compartments to interact in the peritoneal cavity with CXCR13+ macrophages (Ansel et al., 2002), in the spleen to interact with follicular DCs (FDCs; Wen et al., 2005b). Stromal cell interactions may also be important at the leukemic stage (Fig. S1). B1 B cells are cyclin D2 dependent (Solvason et al., 2000). Ccnd2, the gene encoding cyclin D2, together with Cdk4, are direct targets of c-Myc (Myc), thereby regulating the cell cycle (Pelengaris et al., 2002) with one distinctive feature of B1 B cells being moderately up-regulated Myc (Fig. 7 B). Various stimuli rapidly induce Myc, including BCR signaling; B1 B cells, with modestly increased cell size compared with FO B cells (Hayakawa et al., 1983), constitutively maintain up-regulated Myc, relative to FO B cells. All TC+ B CLL/lymphomas, including TC– spontaneous ATA B CLL/lymphomas, express Myc mRNA at levels similar to or higher than early generated nonneoplastic B1 B cells (Fig. 7 B). Myc up-regulation in B1 B cells was also confirmed by elevated protein levels (Fig. 7, E and F). For B1 B cells at the preneoplastic stage, up-regulated Myc mRNA declines when the cells are cultured in medium with fetal calf serum alone in vitro (Fig. 7 C). This indicates the importance of continuous interaction by B1 B cells with the microenvironment.
Figure 7.
Continued up-regulated c-Myc and down-regulated Bmf by B1 B cells to become CLL. (A) CXCR5 up-regulated B1a cell migration, interaction, and renewal/maintenance from young to aged, with cyclin D2 dependence. (B) Myc mRNA levels by B1 B cells at normal and tumor stage, including TC– ATA B cell tumors, relative to FO B cells. Mean percentages indicated in lines. S, spleen; p, peritoneal cavity. (C) Reduction of Myc mRNA by normal mouse (C.B17) B1a during 24-h culture. After plating in a 96-well U-plate for culture (with 10% FCS), the immediately harvested samples before 37°C incubation was defined as 0 h. FO B Myc mRNA levels were similar before and after culture (as 1.0). n = 4; mean ± SE; *, P = 0.0007. (D) Myc, Mcl-1, Bcl-2, Bim, and Bmf mRNA levels by B1 B cells at TC– nontumor and TC+ (and TC–) tumor stage relative to 2-mo-old FO B cells. (left) WT (C.B17 mice). (right) ATA B cells in ATAμκTg mice, including arrested ATA B cells in spleen. Values are mean ± SE. (E) Western blot of samples of FO B and pB1a cells in 2-mo-old WT mice together with TC+ CLL/lymphomas stained for Myc, Mcl-1, Bcl-2, Bim, Bmf, and β-actin. Another set of WT/ATA B cell tumors was assembled to compare Myc, Bim, Bmf, and β-actin. WT FO B and pB1a sample data were similar to WT data on left (not depicted). Representative data of two to three experiments. (F) Western blot using rabbit anti-Bmf, in comparison with Myc. (G) Myc, Bim, and Bmf mRNA levels in immature B cells (CD19+B220+AA4+IgM+IgD–) in day 1 liver and 2 mo BM. 2-mo-old spleen FO B cells in 1.0. C.B17 mouse. n = 4; mean ± SE; *, P = 0.016; **, P = 0.001.
In playing a role in homeostasis, Myc not only regulates the cell cycle, but apoptosis as well, targeting the Bcl-2 network (Nilsson and Cleveland, 2003). Thus, to understand the function of up-regulated Myc in B1 B cell self-renewal and sensitivity to CLL progression, Bcl-2 family genes were compared, initially by microarray analysis using nontumor pB1a and sB1a cells, in comparison to splenic FO B cells. The results showed no significant differences in expression of the majority of Bcl-2 network genes (antiapoptotic Bcl-2, Bcl-XL, A1; proapoptotic Bax, Bak1, and Bok; and BH3-only proapoptotic Bad, Bid, Bim, Noxa, and Puma), except for slightly increased expression of antiapoptotic Mcl-1 in pB1a, and the consistently lowest level of BH3-only proapoptotic Bmf in both spleen and peritoneal cavity B1a cells. This led us to further analyze Bcl-2, Mcl-1, Bim, and Bmf, comparing mRNA levels between adult FO B cells and B1a cells during their initial generation in neonates (day 10–12), in adults (2 mo), and at the CLL/lymphoma stage, including tumor samples generated by B1 B cell transfer (Fig. 7 D, left). For ATA B cells, arrested B cells from adult mice were also included for comparison with B1 B cells (Fig. 7 D, right). As shown in Fig. 7 D, the major difference was up-regulated Myc together with down-regulated Bmf, consistently found in normal B1a cells, ATA B1 B cells, and in CLL/lymphoma samples, including the TC–ATA B cell/lymphomas. Bcl-2 levels were not up-regulated in normal B1a cells and CLL/lymphomas. Although Mcl-1 was slightly higher in pB1a- and also in B1 B–transferred cases, this was not consistently found in CLL/lymphomas. Arrested B cells showed a clear difference in Bmf mRNA levels from B1 B cells. BCR signaling is known to induce Myc and Bim (Craxton et al., 2005; Delgado and León, 2010), and expression of Bim and Bmf controls autoreactive B cell generation. Self-antigen–exposed-arrested ATA B cells, predominantly at the T2 stage, showed higher Myc and Bim mRNA, and Bmf mRNA was also slightly higher than for mature FO B cells in wild type mice. In contrast, Bmf mRNA was low in B1 B cells from the same ATAμκ Tg mice.
As shown in Fig. 7 E, transcript levels of Myc, Mcl-1, and Bcl-2 were confirmed at the protein levels. For the BH3-only proteins, Bim and Bmf, transcriptional regulation, generation of multiple isoforms, and post-translational modifications occur (Piñon et al., 2008). Alternate splicing for Bim generates three Bim isoforms, and BimEL and BimL are widely expressed where BimEL plays a major role, including in B cells (Craxton et al., 2005). Normal pB1a cells in adult showed similar (or slightly lower) Bim protein level compared with FO B cells, confirming the mRNA data. However, some B cells at the tumor stage showed sharply reduced levels of Bim protein that were not reflected at the mRNA level. Thus, reduction of Bim occurred in B1 B cells in a proportion of CLLs due to post-transcriptional modification. For Bmf, two major isoforms, BmfCUG and Bmfs, are generated in mice by an alternative start site detected by monoclonal anti-Bmf antibody (Grespi et al., 2010), and both isoforms are present in FO B cells (Labi et al., 2008). Bmfs at 21 kD is the originally described Bmf protein (Puthalakath et al., 2001), and normal pB1a and sB1a, and all CLL/lymphomas showed low levels of Bmfs. BmfCUG was also low in the majority of samples, although one tumor case showed no reduction (Fig. 7 E, and 2/12 cases in total). By using a polyclonal anti-Bmf antibody which detects mouse Bmfs, we confirmed low Bmf in both B1 B cells and at the tumor stage in conjunction with up-regulated Myc (Fig. 7 F).
In addition to these Myc and Bmf data obtained with mature B1a cells in secondary sites (spleen and peritoneum), the newly generated immature (AA4+ IgM+IgD–) B cells in the primary site of B-1 cell development, day 1 liver, already showed higher Myc and lower Bmf mRNA, relative to immature B cells in adult BM (Fig. 7 G). These immature B cells in primary sites are the immediate products from the pre–B cell stage to the stage controlling autoreactive B cell development. Thus, genetic predisposition difference between early fetal B-1 and adult B-2 development is continuing. As an outcome, fetal/neonatal B-1 development generated CD5 up-regulated autoreactive B1 B cells with restricted BCRs, and with further up-regulated Myc and down-regulated Bmf cells that can self-renew and progress to CLL.
Discussion
Fetal/neonatal B-1 B cell development in mice generates B1 B cells, and part of them renew throughout life. Here, we show that progression to CLL without BCR mutation can occur from B1 B cells, including cells initially generated during neonatal life. Self-renewal of B1 B cells is T cell independent, with continued expression of moderately up-regulated c-Myc and low Bmf, with the potential for CLL development in aged mice, being dependent on expression of certain BCRs expressed by B1 B cells. The importance of B1 B cells able to express particular BCRs for CLL/lymphoma development is shown by comparison between TC+ ATAμκTg and AGcAμκTg mice. ATA B1 B cells arise from B-1 development associated with a high incidence of CLL/lymphoma. When an Ig light chain is different from that of ATA BCR, such as AGcA BCR, expression of this BCR results primarily in development of MZ B cells, together with FO B cells, from B-2 development; these AGcA BCR-expressing B cells did not become CLL. Thus, the BCR is critical for development of CLL. Furthermore, we found that when we direct ATA BCR expressing cells into the MZ or FO B cell pools from B-2 development by regulating the level of Thy-1 autoantigen, CLL with ATA BCR did not occur. Thus, these experiments permit the conclusion that possible autonomous signaling ability by biased BCRs is not the only factor leading to CLL progression. Instead, two factors promote CLL generation by B1 B cells, the capability to express specific BCRs, and BCR expression in the context of B1 B cells.
In adult B-2 B cell development, which is responsible for generating the majority of mature B cells, expression of the proapoptotic protein Bmf is up-regulated at the pre–B cell stage in the BM and expression continues at the immature and transitional stages, thereby controlling generation of autoreactive B cells, together with Bim (Labi et al., 2008). After maturation, most B cells maintain expression of both Bmf and Bim. Reduction in Bmf and/or Bim levels promotes survival. Loss of Bmf (Bmf−/−) in mice has an effect on B cells; immature and circulating B cells increase, there is a competitive survival advantage for mature B cells, and Bmf−/− mice develop age-dependent, B cell–restricted lymphadenopathy (Labi et al., 2008). In contrast to such B-2 development generating mature B cells with normal levels of Bmf expression, B1 B cells generated by early B-1development have the lowest levels of Bmf and continue to maintain low levels throughout life. Furthermore, we show here that, after initial mature B1a cell generation from B-1 development, which is known to be dependent on Myc together with N-Myc (Habib et al., 2007), B1 B cells continue to express moderately up-regulated Myc in vivo together with low expression of Bmf. In B-2 development, Myc plays a role at the early pre–BCR stage during association of IgH and surrogate light chain (SLC), inducing proliferation. However, Myc down-regulation occurs at the IgM– pre–B cell stage (Yasuda et al., 2008) together with Bmf induction (Labi et al., 2008) before the initiation of IgM+ immature B cell development. Thus, distinctive B-lineage progression is occurring, generating B1a cells with restricted BCRs from B-1 development.
When Myc is overexpressed by the Eμ-Myc transgene in mouse B-lineage cells, it promotes age-related development of pre–B and B cell tumors that, together with altered expression of the Bcl-2 family members, strongly accelerates B cell lymphomagenesis (Strasser et al., 1990; Egle et al., 2004). One such Bcl-2 members is Bmf. Bmf deficiency alone does not result in B cell tumors, but when combined with the Eμ-Myc transgene, the deficiency results in increased B lymphomagenesis, including leukemia accompanied by splenomegaly (Frenzel et al., 2010). Down-regulated expression of Bmf and moderately heightened expression of Myc are normal characteristics of B1 B cells with the capacity to become CLL. Development of CLL is not random among B1 B cells, but rather depends on restricted expression of specific BCRs. As in human CLLs, mouse CLLs show biased BCR usage, including stereotyped (recurrent) BCRs. Stereotypic BCRs were expressed by >50% of TC+ CLL cases in mice we analyzed, and cell transfer experiments revealed that early generated B1 B cells developed CLLs expressing these BCRs. In addition to the stereotyped IgVH/VL BCRs, several IgVH with similar HCDR3 sizes were also commonly found in TC+CLL as biased VH groups, and some were also confirmed to be of B1 B cell origin by adoptive transfer (Table S3). These BCRs were not selected solely by nonphysiological overexpression of hTCL1 nor restricted to the C.B17 mouse background. Mouse strains that develop CD5+ B cell leukemia/lymphoma with aging on particular genetic backgrounds (Pennell et al., 1988), or mice with chromosomal deletions that included miR15a/16-1 controlling cell proliferation (Klein et al., 2010), both showed VHs shared with TC+ CLL VH, including stereotyped BCRs (Table S2). A further example is provided by Eμ-Bcl-1 (cyclin D1) transgenic mice on a C57BL/6 background. When Bcl-1 is overexpressed, CD5+ B cells increase during aging (Fig. S2). These B cells in aged mice show biased VH usage, including all stereotyped BCR sets listed in Fig. S2 as potential B lymphoma precursors. Thus, CD5+ CLL/lymphoma in aged mice, promoted by genetic background or by transgenesis/gene knockout, show common recurrent unmutated BCRs, where B1 B cells with restricted BCRs are likely to be involved.
Among the five stereotyped BCRs listed, heavy chains of three BCRs (sets 3–5) generally do not associate strongly with SLC: VHQ52-1 (Ox-1); all (Hayakawa et al., 2016), VHJ558-67; 9/12 (unpublished data), VH11; all (Wasserman et al., 1998), indicating a B-1 origin, as intense SLC association is not necessary in B-1 development for progression to the immature B cell stage (Wasserman et al., 1998). Set 1 VH12 is known to be a B1a-associated BCR. As found in set 3 BCR, this VH12 BCR also requires a restricted CDR3, and all VH12+ CLLs expressed the glycine (G)-containing HCDR3 (Table S2). Such restricted VH12-expressing B cells were already detected in day 1 newborn liver (Clarke and McCray, 1993). Set 2 VH J558-64 (V332, 165.1) was also found in normal mouse B1a cells with identical CLL VH/D/J sequences (unpublished data), and transfer of CD5+ B cells from aged mice into newborn also generated CD5+ B cell lymphoma with this VH, without the TCL1 transgene (Förster et al., 1988). In aged Eμ-Bcl-1 transgenic mice, B1a cells expressed all these stereotyped BCRs (Fig. S2). Thus, in addition to B1 B cell transfer experiments using ≤2 mo mice, CLLs with these stereotyped BCRs clearly confirm continued presence of B1 B cells, from young to old, with the capacity to eventually progress to CLL/lymphoma. Although it remains unknown whether all CD5+ CLLs with unmutated biased VH groups in mice are of B1 B origin, we show here that majority of TC+ CLLs we analyzed developed independent of CD40 signaling, not only CLLs with restricted stereotyped BCRs.
ATA BCR used in μκTg mice with IgVH3609/D/J IgH and Vk21-5/J IgL is not among the readily detected stereotyped BCRs listed in Table 1. However, the presence of B cells with anti-thymocyte/Thy-1 autoreactivity (ATA) is well known, including in 8-d-old BALB/c mice (Underwood et al., 1993). Though such B cells are not abundant normally, increased ATA CD5+ B cells and antibody production occurs in certain mouse strain backgrounds such as NZB and SM/J mice (Shirai and Mellors, 1971; Hayakawa et al., 1984, 1990). When we increased the frequency of cells expressing this ATA BCR by μ Ig transgenesis on a C.B17 background (BALB/c with congenic C57BL/Ka IgM allele), predominant expression of ATA BCR by B1 B cells occurred, including at the neonatal B1a stage (Hayakawa et al., 1999), and ATA μκTg exhibited a higher incidence of CLL/lymphoma. This was also illustrated by set 3 VHQ52-1/Vk9 anti-nonmuscle myosin IIA BCR among stereotyped BCRs; this BCR is rare among B1 B cells, but fostering progression to CLL by Ig transgenesis (Hayakawa et al., 2016). Thus, expression of specific BCR, rather than frequency itself among the self-renewing B1 B cell pool, is key for progression to CLL by B1 B cells.
After the initial BCR selection to become mature B1a cells from B-1 development, the requirement for self-renewal and/or transformation may drive further BCR selection. Because unmutated germline BCRs have conformational flexibility (Wedemayer et al., 1997), polyreactivity can occur. Cross-reactivity differences are recognized among BCRs with similar autoreactivity, such as between VH12 and VH11 anti-phosphatidylcholine BCRs (Conger et al., 1991). Though present in both stereotyped BCR sets, the mice with VH12 BCR exhibited a much higher incidence of CLL with splenomegaly. Thus, we suggest the importance of certain cross-reactivity, possibly combined with autonomous signaling, comprise roles that the restricted BCR contributes to CLL progression. Further, as for B1 B cell context, moderately up-regulated Myc and low Bmf by B1 B cells appear to play an important role. Continuous interaction occurs between B1 B cells and the surrounding tissue microenvironment, including FDCs and stromal cells. Tumor necrosis receptor family members BAFF and APRL provided by the microenvironment are known to promote B cell survival, and also increase the incidence of CLL in both mice and humans (Tsukada et al., 2002; Planelles et al., 2004; Enzler et al., 2009). Without BAFF, normal mature B cells are lost, whereas Bim and Bmf double deficiency promotes B cell survival in the absence of BAFF (Woess et al., 2015). It is likely that down-regulated Bim and Bmf, as found in a portion of mouse CLLs (Fig. 7 E), promotes survival. Thus, low Bmf and up-regulated Myc by B1 B cells combined with interactions with the microenvironment together with BCR signaling would promote survival, self-renewal, and/or increase in population numbers. Reduced expression and loss of CD5 by some B1 B cells, originally CD5+B1a, occurring during development of CLL suggests that TLR signaling is also likely to be involved in proliferation. Mouse B1 B cells are known to be the B cells capable of producing IL-10 (O’Garra et al., 1992), and this cytokine regulates the immune system. B1 B cells are a part of regulatory B cell population, and low-dose LPS TLR treatment in vivo rapidly induced IL-10 in the majority of TC+ mice (DiLillo et al., 2013). These suggest that an inflammatory signaling may also be involved to promote CLL/lymphoma development.
In human CLL, about half of patients express unmutated IgVH with more possibility for poor outcome than mutated IgVH. How mouse CLL relates to human CLL, particularly the CLL with unmutated BCR, is an ongoing issue that needs to be addressed. An issue related to the relevance mouse CLL to human CLL is the occurrence of loss of a chromosomal region with synteny to human 13q14 including miR15a/16-1, an abnormality commonly found in human CLL (Hayakawa et al., 2016). This occurred dominantly in set 3 anti-nonmuscle myosin IIA BCR CLL, but also occurred in some CLLs with other stereotyped BCRs (such as set 1 and 2), particularly with cases showing infiltration of leukemic cells into subcutaneous tissues in mice. In addition to biased BCR usage in both human and mouse CLLs, overexpression of CXCR5 and cyclin D2 has been detected in human CLL (Delmer et al., 1995; Bürkle et al., 2007), resembling mouse B1 B cells. Although Myc was originally thought to be low in human CLL based on analysis of PBL, CLL present in the tissue environment, such as in lymph node, show detectable Myc, with an increased level in aggressive CLL (Herishanu et al., 2011). The role of Bmf in human B cells is not clear. However, three spliced variants of Bmf have been reported to be present in human B cells, including CLL, with two transcripts lacking the BH3 domain, different from mouse Bmf, and one of them with ability to counteract the proapoptotic function (Morales et al., 2004). Interestingly, Burkitt’s lymphoma was originally described as a B cell neoplasm with a role for enhanced expression of Myc induced by chromosomal translocation, and these lymphoma cells also showed low levels of Bmf (Frenzel et al., 2010). This may indicate a prosurvival role for reduced Bmf in humans, as in mice.
Importantly, the differential Lin28b expression patterns between mouse B-1 and B-2 B-precursors are also found in human fetal and adult hematopoietic cells (Yuan et al., 2012). Thus, it will be interesting to know whether early generated CD5+ B cells in humans are also the outcome of restricted early generated B cell development with distinct control of the balance between cell proliferation and death by members of the Bcl-2 family. In mice, there is a clear distinction at the pro–pre–BCR stage; Lin28b+ Let-7– in B-1 B cell development contrasts with Lin28b– Let-7+ in adult B-2 B cell development (Zhou et al., 2015). Considering that the miR Let-7 down-regulates Myc (Sampson et al., 2007), low/negative Let-7 levels at the pro–pre–BCR stage in B-1 development may play a role for distinctive B-lineage progression, including pre–B and immature stages that allow generation of autoreactive B cells associated with CD5 induction, different from B-2 development. Several other miR and protein differences are also recognized (Zhou et al., 2015). The differing Myc and Bmf levels in newly generated immature B cells, as shown here, is likely to result from such distinctive development. Understanding this early B-lineage process will be critical for testing this hypothesis as to how B cells can self-renew and be maintained throughout life, with an ability to become CLL.
Materials and methods
Mice
Eμ-hTCL1 Tg mice were backcrossed onto the C.B17 mouse background for more than six generations. Transgenic mouse lines, VH3609μ (ATAμTg), VH3609/Vk21-5 μκ (ATAμκTg; 3369) and VH3609/Vk19-17 μκ (AGcAμκTg; EP67), Thy-1 knockout, lck-Thy-1 transgenic lines, JH knockout, all on C.B17 mouse background, have been previously described (Hayakawa et al., 1999, 2003; Wen et al., 2005a; Ichikawa et al., 2015). C.B17, BALB/c, CD40−/−.C.B17, and C.B17.scid, were all bred and maintained in our laboratory animal facility. CD3γδ−/− mice were a gift from D. Kappes, originally provided by C. Terhorst (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; Wang et al., 1998). All animal experiments were conducted under a protocol (#00-10) approved by the FCCC Institutional Animal Care and Use Committee (IACUC).
Flow cytometry analysis, sorting, and reagents
Multicolor flow cytometry analysis, sorting, and monoclonal antibody reagents, including rat anti–mouse Ig idiotype antibodies, P9-19A4 (anti-ATA idiotype), together with anti-κ to detect ATA B cells, and P9-10C7 (anti-VH3609 idiotype) together with P9-13H8 (anti-Vk21 and Vk19) to detect AGcA B cells. These have been described previously (Hayakawa et al., 2003; Ichikawa et al., 2015). Anti-hTCL1 antibody (27D6/20) originally provided by C. Croce (Bichi et al., 2002) was conjugated to PE (phycoerythrin) for cytoplasmic staining.
B cell leukemia/lymphoma analysis, diagnosis, and purification
PBL analysis was performed every 2–3 mo for each mouse. A predominance of B1a (CD19+B220loCD5+) in TC+ Tg mice and/or ATA B (ATAid+ IgMb–) in TC+ and TC– ATAμκTg mice in PBL (>70% of B cells), with significant increase of total PBL number (>10-fold) was defined as CLL stage. The majority of CLL samples expanded by >100-fold increased B cells in PBL compared with normal B cells, often with splenomegaly. These CLL samples were used for tissue analysis and B cell purification. For Ig sequencing, gene expression analysis, and Western blotting, leukemia/lymphoma B cells (all IgM+) in TC+ mice and TC+ B cell–transferred mice were purified as CD19+B220loCD5+/lo by flow cytometry sorting. For ATA CLL (TC+ and TC–), B220loCD5+/lo ATAid (19A4)+ IgMb–B cells were purified. Histological diagnoses were made using formalin-fixed, paraffin-embedded sections that were stained with H&E using established criteria (Morse et al., 2002).
Ig sequencing
Sequencing Igμ and Igκ variable regions of tumor B cell BCRs by μ or κ PCR with consensus V primers was performed using standard procedures in most cases. When sequences were not recovered, specifically for Vk9-96 (ce9) and Vk1-117 (cr1) κ, 5′ RACE cloning was done using the SMARTer RACE cDNA amplification kit (Takara Bio Inc.). For single-cell sequencing, individual cells were deposited using a FACSAriaII (BD), directly onto either AmpliGrid AG480F slides (Beckman Coulter), or onto 96-well plates prepared with SuperScript III RT kit (Life Technologies) as previously described (Ichikawa et al., 2015; Hayakawa et al., 2016). The efficiency of obtaining sequences was 70–90%. κ-chain single-cell sequencing of VH3609μ+ peritoneal CD5+ B cells in ATAμTg mice was done by staining with P9-10C7 (anti-VH3609id) together with anti-κ as previously described (Ichikawa et al., 2015); the Vk usage list is a summary of data from three mice.
Mouse V gene nomenclature
The VH gene family name is based on Johnston et al. (2006). VH gene nomenclature was made based on BALB/mouse IgM VH gene analysis, with >99% match with genes in Vbase2 for the majority (Table S1); listed CLL BCR data are a 100% match. Table S1 shows total VH gene nomenclature list together with the matches from Vbase2, IMGT, and GenBank. Because C.B17 mouse is a congenic mouse line with IgH locus of C57BL/Ka, on a BALB/c mouse background, VH gene sequencing data showed VH allele representing C57BL. Vk gene nomenclature is based on Thiebe et al. (1999).
B1 B cell and bone marrow cell cotransfer
≤2 mo B1 B cell transfer
For days 10–13 TC+ B1 B cell transfer, CD5+ B220lo B1a B cells or total B cells (B220+κ+ including B1a) were purified from mixed spleens of (TC+/− x CB17) F1 littermates (30–50% of littermates were TC Tg+). For 2–4 wk, B1 B transfer, B1a B cells from spleen or peritoneal cavity (or total B cells in peritoneal cavity) were used. From 5–8 wk mice, TC+/− B1a B cells from spleen and peritoneal cavity were sorted. These purified B1 B cells, either 1–10 × 105 cells from 10 d to 4 wk or 5–15 × 105 cells from 5–8 wk donors were injected intravenously into each recipient, transferred alone or with total bone marrow cells (5 × 106) from 2-mo-old TC– BALB/c mice into C.B17 scid recipients (lightly irradiated, 3 Gy, 1 d prior).
2 mo B1 B cell transfer
BM HSC–enriched fraction, B1a, and non-B1a B cells (B220hiCD5– in spleen) were purified from 2 mo TC+ C.B17 mouse (pool of two mice). pB1a and sB1a (each 5 × 105/recipient), and non-B1a spleen B cells (sB; 3 × 106/recipient) were cotransferred with TC– BALB/c BM (5 × 106/recipient) to C.B17 scid recipients. For TC+ bone marrow stem cell transfer alone, stem cell/progenitor enriched fractions (CD19– IgM– CD5– and low side scatter) were sorted from 2–3-mo-old TC+ mice, and transferred (5 × 105 cells per scid recipient). Cellular origins of B cells in recipients were distinguished by anti-IgM allotype staining.
ATA B and bone marrow cell cotransfer
2–4-mo-old TC+ ATAμκTg (IgMa Tg) mice were used for purification, and then cotransferred with 2–3 mo C.B17 mouse (IgMb) BM into C.B17 scid mice by intravenous injection. Per recipient: bone marrow stem cell/progenitor enriched fraction (CD19–IgM–CD5–; 106), arrested ATA B cells (B220+AA4+ IgMb–,1.5 × 106), or peritoneal ATA B cells (6B2+AA4–IgMb–; 5 × 105), together with total C.B17 mouse bone marrow cells (5 × 106). PBL was analyzed by staining with (CD19, B220, CD5, ATAid, and IgMa) and (CD19, B220, ATAid, IgMa, and IgMb).
ATA B cell stimulation in vitro
Cell sorter purified ATA B1a cells (B220+CD5+IgMb–) in peritoneal cavity (pB1) and arrested cells (B220+AA4+IgMb–) in spleen in ATA μκTg mice were cultured in a 96-well U-plate at 2 × 105 cells in 150 µl medium (with 10% FCS)/well (in duplicate), with or without stimulation. Stimulating reagents: LPS (Sigma-Aldrich) at 20 µg/ml, CpG (InvivoGen) at 3 µg/ml, goat F(ab')2 anti-IgM (The Jackson Laboratory) at 10 µg/ml, anti-CD40 (eBioscience) at 5 µg/ml, and recombinant murine IL-4 (PeproTech) at 10 ng/ml.
Quantitative RT-PCR assay
Gene expression was quantitated by real-time PCR, using TaqMan assays from Applied Biosystems, an ABI 7500 real-time thermal cycler, and ABI software (Life Technologies). Relative gene expression levels were normalized using β-actin values as a standard.
Western blotting
FO B cells (B220+CD21medCD23+AA4–) and pB1a (CD19+B220loCD5+) from 2-mo-old C.B17 mice and CLL/lymphoma (CD19+B220loCD5+/lo in TC+ WT or B220loCD5+/lo ATAid+ in TC– or TC+ ATAμκTg mice) were purified by cell sorting (2 × 106 cells/tube), and cell lysates were subjected to SDS-PAGE. Anti–c-Myc, anti–Mcl-1, and HRP goat anti–rabbit IgG antibodies were all obtained from Cell Signaling Technologies. Anti–Bcl-2 (BioLegend) with HRP rat anti–mouse IgG1 (Southern Biotech) was also used. Rat anti-Bim 3C5 and rat anti-Bmf 17A9 were both purchased from Enzo Life Sciences, together with HRP-goat anti–rat (Cell Signaling Technology). Rabbit anti-Bmf (Abcam; ab9655) was used together with HRP goat anti–rabbit IgG (Cell Signaling Technology). Anti-b actin was obtained from Bethyl Labs.
Online supplemental material
Fig. S1 shows TC+ CLL-stromal cell interaction. Fig. S2 shows BCR usage by B1a, increased in aged Eμ-Bcl-1 transgenic mice. Table S1 lists mouse VH gene nomenclature. Table S2 lists BCR-stereotyped sets with HCDR3 and LCDR3 used by TC+ CLLs (total 155 samples). Table S3 lists BCR-stereotyped sets with other VH/CDR3 used by TC+ CLLs (total 155 samples). Tables S1–S3 are available as Excel files.
Supplementary Material
Acknowledgments
We thank Carlo M. Croce for providing Eμ-hTCL1 transgenic mice. CD3γδ knockout mice originally made by C. Terhorst were provided by D. Kappes. Eμ-Bcl-1 transgenic mice provided by M. Smith. We thank Y. Nakao for analysis of tumor mice, L. Wen for hTCL1 cytoplasmic staining, and A. Purdy for photomicrograph of cultured tumor B cells. Also, we acknowledge several Fox Chase Cancer Center (FCCC) Facilities (Lab Animal, Flow Cytometry, DNA Sequencing, and Histopathology) for technical support and K. Campbell for comments on the manuscript.
This work was supported by National Institutes of Health (NIH) grants R01 CA129330 (K. Hayakawa), R01 AI049335 (K.H. Hayakawa), and R01 AI11320 (R.R. Hardy), and the FCCC Blood Cell Development and Cancer Keystone program, and in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- ATA
- anti-thymocyte/Thy-1 autoreactive
- ATAμTg
- VH3609μ transgene
- ATAμκTg
- VH3609μ/Vk21κ transgene
- AGcA
- anti-intestinal goblet cell autoreactive
- CLL
- chronic lymphocyte leukemia
- FO B
- follicular B cell
- GC
- germinal center
- HCDR3
- heavy chain third complementarity determining region
- HSC
- hematopoietic stem cell
- MZ B
- marginal zone B cell
- pB1a
- peritoneal B1a
- PBL
- peripheral blood lymphocyte
- PerC
- peritoneal cavity
- sB1a
- splenic B1a
- SLC
- surrogate light chain
- TCL1
- T cell leukemia 1
References
- Ansel K.M., Harris R.B., and Cyster J.G.. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 16:67–76. 10.1016/S1074-7613(01)00257-6 [DOI] [PubMed] [Google Scholar]
- Bichi R., Shinton S.A., Martin E.S., Koval A., Calin G.A., Cesari R., Russo G., Hardy R.R., and Croce C.M.. 2002. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl. Acad. Sci. USA. 99:6955–6960. 10.1073/pnas.102181599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C., Tarlinton D.M., Kay T.W., Köntgen F., Adams J.M., and Strasser A.. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. 10.1126/science.286.5445.1735 [DOI] [PubMed] [Google Scholar]
- Bouillet P., Purton J.F., Godfrey D.I., Zhang L.C., Coultas L., Puthalakath H., Pellegrini M., Cory S., Adams J.M., and Strasser A.. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. 10.1038/415922a [DOI] [PubMed] [Google Scholar]
- Bürkle A., Niedermeier M., Schmitt-Gräff A., Wierda W.G., Keating M.J., and Burger J.A.. 2007. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 110:3316–3325. 10.1182/blood-2007-05-089409 [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., and Ferrarini M.. 2003. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu. Rev. Immunol. 21:841–894. 10.1146/annurev.immunol.21.120601.141018 [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., and Ferrarini M.. 2011. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 117:1781–1791. 10.1182/blood-2010-07-155663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.H., and McCray S.K.. 1993. VH CDR3-dependent positive selection of murine VH12-expressing B cells in the neonate. Eur. J. Immunol. 23:3327–3334. 10.1002/eji.1830231240 [DOI] [PubMed] [Google Scholar]
- Conger J.D., Sage H.J., Kawaguchi S., and Corley R.B.. 1991. Properties of murine antibodies from different V region families specific for bromelain-treated mouse erythrocytes. J. Immunol. 146:1216–1219. [PubMed] [Google Scholar]
- Craxton A., Draves K.E., Gruppi A., and Clark E.A.. 2005. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J. Exp. Med. 202:1363–1374. 10.1084/jem.20051283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.D., and León J.. 2010. Myc roles in hematopoiesis and leukemia. Genes Cancer. 1:605–616. 10.1177/1947601910377495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer A., Ajchenbaum-Cymbalista F., Tang R., Ramond S., Faussat A.M., Marie J.P., and Zittoun R.. 1995. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood. 85:2870–2876. [PubMed] [Google Scholar]
- DiLillo D.J., Weinberg J.B., Yoshizaki A., Horikawa M., Bryant J.M., Iwata Y., Matsushita T., Matta K.M., Chen Y., Venturi G.M., et al. 2013. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 27:170–182. 10.1038/leu.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühren-von Minden M., Übelhart R., Schneider D., Wossning T., Bach M.P., Buchner M., Hofmann D., Surova E., Follo M., Köhler F., et al. 2012. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 489:309–312. 10.1038/nature11309 [DOI] [PubMed] [Google Scholar]
- Egle A., Harris A.W., Bouillet P., and Cory S.. 2004. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc. Natl. Acad. Sci. USA. 101:6164–6169. 10.1073/pnas.0401471101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders A., Bouillet P., Puthalakath H., Xu Y., Tarlinton D.M., and Strasser A.. 2003. Loss of the proapoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119–1126. 10.1084/jem.20030411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzler T., Kater A.P., Zhang W., Widhopf G.F. II, Chuang H.Y., Lee J., Avery E., Croce C.M., Karin M., and Kipps T.J.. 2009. Chronic lymphocytic leukemia of Emu-TCL1 transgenic mice undergoes rapid cell turnover that can be offset by extrinsic CD257 to accelerate disease progression. Blood. 114:4469–4476. 10.1182/blood-2009-06-230169 [DOI] [PubMed] [Google Scholar]
- Förster I., Gu H., and Rajewsky K.. 1988. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 7:3693–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A., Labi V., Chmelewskij W., Ploner C., Geley S., Fiegl H., Tzankov A., and Villunger A.. 2010. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood. 115:995–1005. 10.1182/blood-2009-03-212670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagro A., McCloskey N., Challa A., Holder M., Grafton G., Pound J.D., and Gordon J.. 2000. CD5-positive and CD5-negative human B cells converge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology. 101:201–209. 10.1046/j.1365-2567.2000.00098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grespi F., Soratroi C., Krumschnabel G., Sohm B., Ploner C., Geley S., Hengst L., Häcker G., and Villunger A.. 2010. BH3-only protein Bmf mediates apoptosis upon inhibition of CAP-dependent protein synthesis. Cell Death Differ. 17:1672–1683. 10.1038/cdd.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui M., Wiest D.L., Li J., Kappes D., Hardy R.R., and Hayakawa K.. 1999. Peripheral CD4+ T cell maturation recognized by increased expression of Thy-1/CD90 bearing the 6C10 carbohydrate epitope. J. Immunol. 163:4796–4804. [PubMed] [Google Scholar]
- Habib T., Park H., Tsang M., de Alborán I.M., Nicks A., Wilson L., Knoepfler P.S., Andrews S., Rawlings D.J., Eisenman R.N., and Iritani B.M.. 2007. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J. Cell Biol. 179:717–731. 10.1083/jcb.200704173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin T.J., Davis Z., Gardiner A., Oscier D.G., and Stevenson F.K.. 1999. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 94:1848–1854. [PubMed] [Google Scholar]
- Hardy R.R., and Hayakawa K.. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621. 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Carmack C.E., Shinton S.A., Riblet R.J., and Hayakawa K.. 1989. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 142:3643–3651. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Parks D.R., and Herzenberg L.A.. 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 157:202–218. 10.1084/jem.157.1.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Honda M., Herzenberg L.A., Steinberg A.D., and Herzenberg L.A.. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 81:2494–2498. 10.1073/pnas.81.8.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Herzenberg L.A., and Herzenberg L.A.. 1985. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161:1554–1568. 10.1084/jem.161.6.1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Stall A.M., Herzenberg L.A., and Herzenberg L.A.. 1986. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur. J. Immunol. 16:1313–1316. 10.1002/eji.1830161021 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Carmack C.E., Hyman R., and Hardy R.R.. 1990. Natural autoantibodies to thymocytes: origin, VH genes, fine specificities, and the role of Thy-1 glycoprotein. J. Exp. Med. 172:869–878. 10.1084/jem.172.3.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Allman D., Stewart C.L., Silver J., and Hardy R.R.. 1999. Positive selection of natural autoreactive B cells. Science. 285:113–116. 10.1126/science.285.5424.113 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Wen L.J., Dashoff J., and Hardy R.R.. 2003. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J. Exp. Med. 197:87–99. 10.1084/jem.20021459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Formica A.M., Colombo M.J., Shinton S.A., Brill-Dashoff J., Morse Iii H.C., Li Y.S., and Hardy R.R.. 2016. Loss of a chromosomal region with synteny to human 13q14 occurs in mouse chronic lymphocytic leukemia that originates from early-generated B-1 B cells. Leukemia. 30:1510–1519. 10.1038/leu.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herishanu Y., Pérez-Galán P., Liu D., Biancotto A., Pittaluga S., Vire B., Gibellini F., Njuguna N., Lee E., Stennett L., et al. 2011. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 117:563–574. 10.1182/blood-2010-05-284984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé M., Xu K., Ng Y.S., Wardemann H., Albesiano E., Messmer B.T., Chiorazzi N., and Meffre E.. 2005. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J. Clin. Invest. 115:1636–1643. 10.1172/JCI24387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa D., Asano M., Shinton S.A., Brill-Dashoff J., Formica A.M., Velcich A., Hardy R.R., and Hayakawa K.. 2015. Natural anti-intestinal goblet cell autoantibody production from marginal zone B cells. J. Immunol. 194:606–614. 10.4049/jimmunol.1402383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.M., Wood A.L., Bolland D.J., and Corcoran A.E.. 2006. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J. Immunol. 176:4221–4234. 10.4049/jimmunol.176.7.4221 [DOI] [PubMed] [Google Scholar]
- Klein U., Lia M., Crespo M., Siegel R., Shen Q., Mo T., Ambesi-Impiombato A., Califano A., Migliazza A., Bhagat G., and Dalla-Favera R.. 2010. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 17:28–40. 10.1016/j.ccr.2009.11.019 [DOI] [PubMed] [Google Scholar]
- Labi V., Erlacher M., Kiessling S., Manzl C., Frenzel A., O’Reilly L., Strasser A., and Villunger A.. 2008. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J. Exp. Med. 205:641–655. 10.1084/jem.20071658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V., Woess C., Tuzlak S., Erlacher M., Bouillet P., Strasser A., Tzankov A., and Villunger A.. 2014. Deregulated cell death and lymphocyte homeostasis cause premature lethality in mice lacking the BH3-only proteins Bim and Bmf. Blood. 123:2652–2662. 10.1182/blood-2013-11-537217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley R., Kelly L.M., Xu Y., and Cyster J.G.. 2006. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc. Natl. Acad. Sci. USA. 103:10717–10722. 10.1073/pnas.0601539103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A.A., Olsson A., Celsing F., Osterborg A., Jondal M., and Osorio L.M.. 2004. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia. 18:41–47. 10.1038/sj.leu.2403183 [DOI] [PubMed] [Google Scholar]
- Morse H.C. III, Anver M.R., Fredrickson T.N., Haines D.C., Harris A.W., Harris N.L., Jaffe E.S., Kogan S.C., MacLennan I.C., Pattengale P.K., and Ward J.M.. Hematopathology subcommittee of the Mouse Models of Human Cancers Consortium . 2002. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 100:246–258. 10.1182/blood.V100.1.246 [DOI] [PubMed] [Google Scholar]
- Nilsson J.A., and Cleveland J.L.. 2003. Myc pathways provoking cell suicide and cancer. Oncogene. 22:9007–9021. 10.1038/sj.onc.1207261 [DOI] [PubMed] [Google Scholar]
- O’Garra A., Chang R., Go N., Hastings R., Haughton G., and Howard M.. 1992. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 22:711–717. 10.1002/eji.1830220314 [DOI] [PubMed] [Google Scholar]
- Pelengaris S., Khan M., and Evan G.. 2002. c-MYC: more than just a matter of life and death. Nat. Rev. Cancer. 2:764–776. 10.1038/nrc904 [DOI] [PubMed] [Google Scholar]
- Pennell C.A., Arnold L.W., Haughton G., and Clarke S.H.. 1988. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J. Immunol. 141:2788–2796. [PubMed] [Google Scholar]
- Piñon J.D., Labi V., Egle A., and Villunger A.. 2008. Bim and Bmf in tissue homeostasis and malignant disease. Oncogene. 27(Suppl 1):S41–S52. 10.1038/onc.2009.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles L., Carvalho-Pinto C.E., Hardenberg G., Smaniotto S., Savino W., Gómez-Caro R., Alvarez-Mon M., de Jong J., Eldering E., Martínez-A C., et al. 2004. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 6:399–408. 10.1016/j.ccr.2004.08.033 [DOI] [PubMed] [Google Scholar]
- Puthalakath H., Villunger A., O’Reilly L.A., Beaumont J.G., Coultas L., Cheney R.E., Huang D.C., and Strasser A.. 2001. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 293:1829–1832. 10.1126/science.1062257 [DOI] [PubMed] [Google Scholar]
- Retter I., Althaus H.H., Münch R., and Müller W.. 2005. VBASE2, an integrative V gene database. Nucleic Acids Res. 33:D671–D674. 10.1093/nar/gki088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson V.B., Rong N.H., Han J., Yang Q., Aris V., Soteropoulos P., Petrelli N.J., Dunn S.P., and Krueger L.J.. 2007. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 67:9762–9770. 10.1158/0008-5472.CAN-07-2462 [DOI] [PubMed] [Google Scholar]
- Shirai T., and Mellors R.C.. 1971. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc. Natl. Acad. Sci. USA. 68:1412–1415. 10.1073/pnas.68.7.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason N., Wu W.W., Parry D., Mahony D., Lam E.W., Glassford J., Klaus G.G., Sicinski P., Weinberg R., Liu Y.J., et al. 2000. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int. Immunol. 12:631–638. 10.1093/intimm/12.5.631 [DOI] [PubMed] [Google Scholar]
- Stamatopoulos K., Belessi C., Moreno C., Boudjograh M., Guida G., Smilevska T., Belhoul L., Stella S., Stavroyianni N., Crespo M., et al. 2007. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 109:259–270. 10.1182/blood-2006-03-012948 [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Bath M.L., and Cory S.. 1990. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 348:331–333. 10.1038/348331a0 [DOI] [PubMed] [Google Scholar]
- Thiebe R., Schäble K.F., Bensch A., Brensing-Küppers J., Heim V., Kirschbaum T., Mitlöhner H., Ohnrich M., Pourrajabi S., Röschenthaler F., et al. 1999. The variable genes and gene families of the mouse immunoglobulin kappa locus. Eur. J. Immunol. 29:2072–2081. [DOI] [PubMed] [Google Scholar]
- Tsukada N., Burger J.A., Zvaifler N.J., and Kipps T.J.. 2002. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 99:1030–1037. 10.1182/blood.V99.3.1030 [DOI] [PubMed] [Google Scholar]
- Underwood J.R., Csar X.F., Veitch B.A., and Hearn M.T.. 1993. Characterization of the specificity of a naturally-occurring monoclonal anti-thymocyte autoantibody derived from an unimmunized, neonatal Balb/c mouse. Thymus. 21:199–219. [PubMed] [Google Scholar]
- Wang B., Wang N., Salio M., Sharpe A., Allen D., She J., and Terhorst C.. 1998. Essential and partially overlapping role of CD3gamma and CD3delta for development of alphabeta and gammadelta T lymphocytes. J. Exp. Med. 188:1375–1380. 10.1084/jem.188.7.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R., Li Y.S., Shinton S.A., Carmack C.E., Manser T., Wiest D.L., Hayakawa K., and Hardy R.R.. 1998. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J. Exp. Med. 187:259–264. 10.1084/jem.187.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemayer G.J., Patten P.A., Wang L.H., Schultz P.G., and Stevens R.C.. 1997. Structural insights into the evolution of an antibody combining site. Science. 276:1665–1669. 10.1126/science.276.5319.1665 [DOI] [PubMed] [Google Scholar]
- Wen L., Brill-Dashoff J., Shinton S.A., Asano M., Hardy R.R., and Hayakawa K.. 2005a Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 23:297–308. 10.1016/j.immuni.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Wen L., Shinton S.A., Hardy R.R., and Hayakawa K.. 2005b Association of B-1 B cells with follicular dendritic cells in spleen. J. Immunol. 174:6918–6926. 10.4049/jimmunol.174.11.6918 [DOI] [PubMed] [Google Scholar]
- Woess C., Tuzlak S., Labi V., Drach M., Bertele D., Schneider P., and Villunger A.. 2015. Combined loss of the BH3-only proteins Bim and Bmf restores B-cell development and function in TACI-Ig transgenic mice. Cell Death Differ. 22:1477–1488. 10.1038/cdd.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortis H.H., Teutsch M., Higer M., Zheng J., and Parker D.C.. 1995. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc. Natl. Acad. Sci. USA. 92:3348–3352. 10.1073/pnas.92.8.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.J., Albesiano E., Zanesi N., Yancopoulos S., Sawyer A., Romano E., Petlickovski A., Efremov D.G., Croce C.M., and Chiorazzi N.. 2006. B cell receptors in TCL1 transgenic mice resemble those of aggressive, treatment-resistant human chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 103:11713–11718. 10.1073/pnas.0604564103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Sanjo H., Pagès G., Kawano Y., Karasuyama H., Pouysségur J., Ogata M., and Kurosaki T.. 2008. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 28:499–508. 10.1016/j.immuni.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Yuan J., Nguyen C.K., Liu X., Kanellopoulou C., and Muljo S.A.. 2012. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 335:1195–1200. 10.1126/science.1216557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenz T., Mertens D., Küppers R., Döhner H., and Stilgenbauer S.. 2010. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat. Rev. Cancer. 10:37–50. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Li Y.S., Bandi S.R., Tang L., Shinton S.A., Hayakawa K., and Hardy R.R.. 2015. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J. Exp. Med. 212:569–580. 10.1084/jem.20141510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.