Abstract
In this issue of JEM, Chan et al. describe a novel way by which an investigational form of chemotherapy known as low-dose metronomic chemotherapy can inhibit tumor growth, which also has therapeutic implications for targeting tumor-initiating cells (TICs), the tumor stroma, and chemokine receptors, as well as invasion and metastasis.

Insight from Robert S. Kerbel and Yuval Shaked
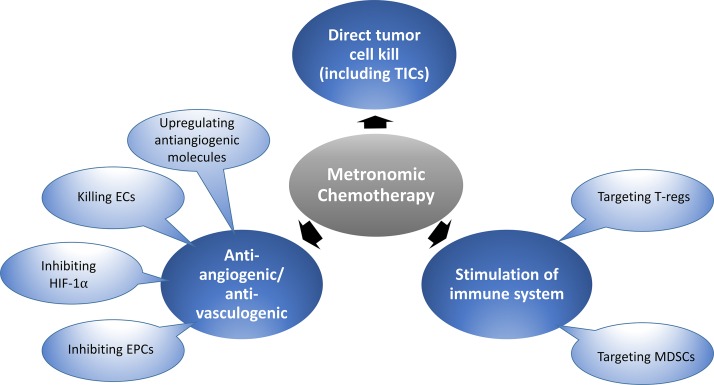
The concept of metronomic chemotherapy (Browder et al., 2000; Klement et al., 2000; Pasquier et al., 2010; Bocci and Francia, 2014) is now undergoing phase III clinical trial evaluation. In contrast to conventional chemotherapy, which is often administered at maximum tolerated doses (MTDs) separated by long break periods generally ranging from 2 to 3 wk to allow recovery from the toxic side effects, metronomic chemotherapy usually involves the close regular (even daily) administration of chemotherapy drugs administered at lower, less toxic doses per treatment. However, the cumulative dose over time may in fact be similar to the conventional MTD chemotherapy (Kerbel and Grothey, 2015) and is designed with the intention of being less toxic, but also to induce other biological mechanisms that can inhibit tumor growth and metastasis (Pasquier et al., 2010; Bocci and Francia, 2014). These additional mechanisms essentially convert a cytotoxic chemotherapy to the equivalent of a biological cytostatic therapeutic; the major ones implicated thus far mainly involve inhibition of angiogenesis (Browder et al., 2000; Klement et al., 2000), stimulation of the immune system (Ghiringhelli et al., 2007; Shaked et al., 2016), and also, to some extent, direct tumor cell killing (Folkins et al., 2009), as summarized in our first figure. There have been a few preliminary studies showing that metronomic chemotherapy may actually target the putative tumor-initiating cell (TIC) subpopulation (Folkins et al., 2009; Vives et al., 2013) in contrast to MTD chemotherapy, which is known to spare and even increase this subpopulation.
General mechanisms proposed to account for the antitumor effects of low-dose metronomic chemotherapy. Some of the effects illustrated are mediated preferentially or selectively by certain chemotherapy drugs, e.g., cyclophosphamide and gemcitabine, which can inhibit T regulatory (T-regs) cells or myeloid-derived suppressor cells (MDSCs), respectively, and hence stimulate antitumor immunity. Metronomic chemotherapy using several chemotherapy drugs can inhibit angiogenesis or vasculogenesis through direct endothelial cell (EC) killing or suppression of bone marrow–derived endothelial progenitor cells (EPCs). Low-dose topoisomerase II poisons, such as topotecan, can suppress HIF-1α expression, and low-dose cyclophosphamide can upregulate antiangiogenic endogenous molecules, e.g., TSP-1. The results presented in the report by Chan et al. (2016) implicate a new mechanism involving affecting fibroblastic elements of the tumor stroma, which in turn can prevent and even suppress the TIC subpopulation normally increased by conventional MTD chemotherapy.
The era of metronomic chemotherapy began in 2000 (Browder et al., 2000) and has progressed somewhat slowly, at least from a clinical development perspective, since then. Thus far, the most notable successes, or potential promise, would appear to be its use as a long-term maintenance therapy after patients have been treated upfront with conventional MTD chemotherapy, either with or without a biological agent such as an antiangiogenic drug. Metronomic chemotherapy has also been evaluated in phase III clinical trials in the adjuvant setting of early-stage as well as late-stage disease in breast cancer (Munzone and Colleoni, 2015; Colleoni et al., 2016) and in late-stage metastatic colorectal cancer (Kerbel and Grothey, 2015; Simkens et al., 2015).
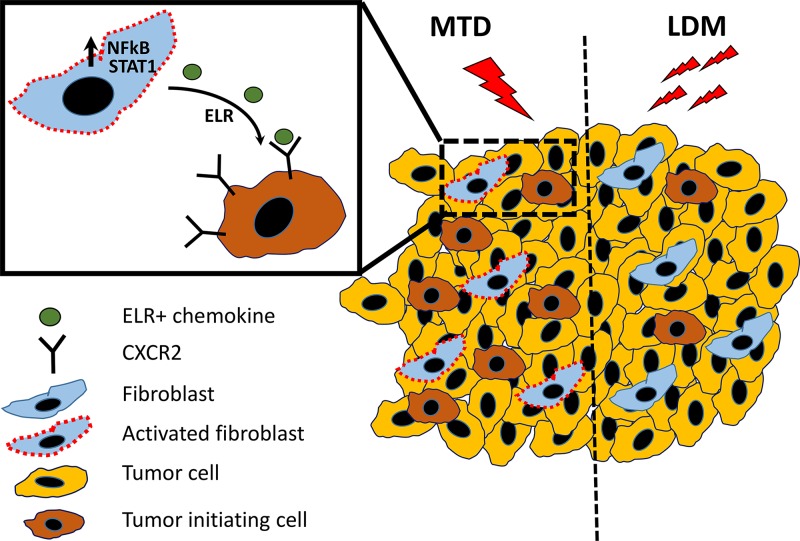
In this issue, Chan et al. undertook several parallel approaches to implicate carcinoma-associated fibroblasts (CAFs), NF-κB/STAT1 activation, carcinoma cell CXCR2 signaling, and impact on TICs in the therapy outcomes mediated by either conventional MTD or metronomic chemotherapy (see diagram in our second figure). First, they studied the interaction of human primary CAFs with human tumor cells under 3D cell co-culture conditions (Chan et al., 2016). The CAFs were treated with various concentrations of three well known chemotherapeutic drugs (doxorubicin, paclitaxel, and the active metabolite of cyclophosphamide), all of which are used to treat breast cancer patients. When exposed to MTD-like concentrations, CAFs significantly enhanced both the growth and invasive characteristics of the carcinoma cells. Moreover, similar growth-promoting effects were found in vivo when carcinoma cells were injected into mice with the MTD chemotherapy–treated CAFs. This was also accompanied by an increased rate of lymph node and pulmonary metastases. Subsequent experiments provided intriguing evidence that CAF-modulated carcinoma cells underwent a phenotypic shift such that they acquired several characteristics normally associated with the cancer stem cell/TIC subpopulation. These included changes in the expression of CD44 and CD24, altered aldehyde dehydrogenase activity, and sphere-forming capacity. Moreover, conversion of cancer cells into TICs was also demonstrated in vivo as a result of coinjection with MTD chemotherapy–treated CAFs. The authors showed that MTD-treated CAFs produced elevated levels of ELR-motif–positive chemokines, with some of the transcript levels increased by up to 200-fold. Such factors were also found in human tumor tissue specimens after neoadjuvant chemotherapy. ELR-motif stimulation was a result of STAT1 and NF-κB activation. Subsequent experiments revealed that these ELR+ MTD-treated CAFs can promote tumor angiogenesis and macrophage infiltration in vivo and appeared to do so by ELR+ chemokine–mediated signaling through CXCR-2 expressed by the carcinoma cells.
Differential effect of MTD versus low-dose metronomic (LDM) chemotherapy on CAF activation. In desmoplastic tumors, CAFs are especially abundant. Conventional MTD chemotherapy regimens can activate CAFs through increased activity of NF-κB and STAT1 transcription factors, which in turn causes the expression and release of ELR+ motif chemokines. These chemokines can bind to the chemokine receptor, CXCR2, expressed by tumor cells, causing a phenotypic conversion to TICs and increasing their relative numbers, thus paradoxically promoting malignant tumor progression. In contrast, by using an LDM chemotherapy regimen, CAFs are not activated or exhibit limited activation. Hence, a therapy-induced increase in TICs is not observed, while tumor growth is inhibited.
Collectively, these effects suggest that MTD chemotherapy, in addition to having the desirable effect of potentially killing or inhibiting the growth of cancer cells, can act as a double-edged sword, possibly promoting the growth as well as malignant characteristics of the surviving cells. Such effects may act to reduce or even nullify the overall antitumor efficacy of MTD chemotherapy (summarized in Shaked [2016]).
In contrast to the aforementioned undesirable effects of MTD chemotherapy on CAFs and the impact they can have on the TIC subpopulation and tumor growth, administering the same drugs in lower concentrations in vitro or lower doses in vivo—but at cumulatively similar doses in vivo to the MTD schedule—prevented these MTD effects. This could explain why, counterintuitively, lower doses of chemotherapy, at least if administered in a long-term metronomic-like regimen, may cause antitumor effects that are similar or even superior compared with conventional MTD chemotherapy (Browder et al., 2000; Munoz et al., 2006). The effects reported by Chan et al. (2016) on carcinoma stromal fibroblast may provide metronomic chemotherapy regimens with a particular advantage when treating desmoplastic tumors that have a high CAF content, such as certain types of breast cancer or pancreatic cancer.
These findings are important in part because they impact so many critical areas of tumor biology and therapy. Moreover, they serve to link different cellular elements of the tumor microenvironment and add to the multi-modality mechanisms ascribed to metronomic chemotherapy (Pasquier et al., 2010). What remains less clear is how the results can be translated to the clinic and improve the prospects of metronomic chemotherapy, at least for desmoplastic types of cancer. Can the MTD chemotherapy activation effects on stromal cells be reversed by follow-up metronomic chemotherapy, e.g., using maintenance chemotherapy? The problem remains how to select an optimal “low” metronomic dose as well as treatment schedule for metronomic chemotherapy and the lack of any predictive biomarker to select patients likely to benefit from receiving metronomic chemotherapy. Despite these remaining questions, the results of Chan et al. (2016) provide an additional rationale for the clinical development and assessment of metronomic chemotherapy treatments, particularly as possible maintenance therapies for desmoplastic cancers.
References
- Bocci G., and Francia G., editors. 2014. Metronomic Chemotherapy: Pharmacology and Clinical Applications. 10.1007/978-3-662-43604-2 [DOI] [Google Scholar]
- Browder T., et al. 2000. Cancer Res. 60:1878–1886. [PubMed] [Google Scholar]
- Chan T.-S., et al. J. Exp. Med. 2016 doi: 10.1084/jem.20151665. [DOI] [Google Scholar]
- Colleoni M., et al. J. Clin. Oncol. 2016 doi: 10.1200/JCO.2015.65.6595. [DOI] [Google Scholar]
- Folkins C., et al. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [Google Scholar]
- Ghiringhelli F., et al. Cancer Immunol. Immunother. 2007 doi: 10.1007/s00262-006-0225-8. [DOI] [Google Scholar]
- Kerbel R.S., and Grothey A. Nat. Rev. Clin. Oncol. 2015 doi: 10.1038/nrclinonc.2015.89. [DOI] [PubMed] [Google Scholar]
- Klement G., et al. J. Clin. Invest. 2000 doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz R., et al. Cancer Res. 2006 doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [Google Scholar]
- Munzone E., and Colleoni M. Nat. Rev. Clin. Oncol. 2015 doi: 10.1038/nrclinonc.2015.131. [DOI] [PubMed] [Google Scholar]
- Pasquier E., et al. Nat. Rev. Clin. Oncol. 2010 doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- Shaked Y. Nat. Rev. Clin. Oncol. 2016 doi: 10.1038/nrclinonc.2016.57. [DOI] [PubMed] [Google Scholar]
- Shaked Y., et al. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-0136. [DOI] [Google Scholar]
- Simkens L.H.J., et al. Lancet. 2015 doi: 10.1016/S0140-6736(14)62004-3. [DOI] [Google Scholar]
- Vives M., et al. Int. J. Cancer. 2013 doi: 10.1002/ijc.28259. [DOI] [Google Scholar]