Abstract
Objectives
Disturbances in sleep and waking patterns are highly prevalent during mood episodes in bipolar disorder. The question remains whether these disturbances persist during phases of euthymia and whether they are heritable traits of bipolar disorder. The current study investigates objective sleep measures in a large sample of bipolar I patients, non-affected siblings and controls.
Methods
A total of 107 bipolar disorder I patients, 74 non-affected siblings, and 80 controls were included. Sleep was measured with actigraphy over the course of 14 days. Seven sleep parameters were analyzed for group differences and their relationship with age at onset, number of episodes and psychotic symptoms using linear mixed model analysis to account for family dependencies.
Results
Patients had a longer sleep duration and later time of sleep offset compared to the non-affected siblings but these differences were entirely attributable to differences in mood symptoms. We found no difference between patients and controls or siblings and controls when the analyses were restricted to euthymic patients. None of the bipolar illness characteristics were associated with sleep.
Limitations
Medication use was not taken into account which may have influenced our findings and controls were younger compared to non-affected siblings.
Conclusions
In the largest study to date, our findings suggest that recovered bipolar I patients and their siblings do not experience clinically significant sleep disturbances. Sleep disturbances are primarily a reflection of current mood state, but are unrelated to the course of the disorder.
Keywords: Bipolar disorder, actigraphy, sleep disturbances
Introduction
Bipolar disorder is a chronic psychiatric disorder characterized by severe fluctuations in mood, which affects 1-2 % of the general population (Belmaker, 2004). According to the DSM-V criteria, one prominent manifestation of a mood episode is a shift in sleep-wake behavior (American Psychiatric Association, 2013). The majority of patients experience insomnia or hypersomnia during depression and a reduced need for sleep during mania (Harvey, 2008). These disturbances are thought to be a hallmark of a current mood episode, and often precede mood episodes, suggesting utility as a marker of prodromal symptoms (Jackson et al., 2003).
Studies focusing on bipolar patients have tried to delineate whether these sleep disturbances can be considered as more than a state marker of the disorder and represent a general deregulation of the endogenous circadian cycle independent from current episodes. In accordance with that idea, phase advances, phase delays and general phase instabilities have been reported for several measures of rhythmicity, such as body temperature, nocturnal cortisol levels and peak melatonin time in euthymic bipolar patients (Milhiet et al., 2011; Nurnberger et al., 2000). Sleep (the most evident behavioral reflection of circadian rhythms) has also been reported to be disturbed during non-clinical phases of the disorder. According to Harvey et al. (2005), 70% of euthymic patients reported clinically significant sleep problems, with 55% of patients also meeting the criteria for insomnia. Several other studies using self-report measures identified worse sleep quality and more disturbed sleep-timing preferences in non-clinical bipolar patients compared to controls (Cretu et al., 2016; Rocha et al., 2013; Seleem et al., 2015). Actigraphy has proven to be an indispensable tool in the objective assessment of sleep-wake parameters (Sadeh et al., 1995). Actiwatches are wrist-worn devices that continuously record movement and allow for measurements in a natural environment over several weeks. Validation showed better performance compared to observational measurements, sleep logs and diaries (Ancoli-Israel et al., 2003). When assessed in a bipolar sample, actigraphy showed high correlations with polysomnography and high to moderate correlations with subjective measures of sleep (Boudebesse et al., 2014). Nevertheless, studies using actigraphy in the analysis of sleep in bipolar disorder have come up with conflicting results. While some studies found differences between bipolar patients and controls on several sleep parameters (e.g. sleep duration, sleep onset latency, sleep efficiency) (Geoffroy et al., 2014; Harvey et al., 2005; Millar et al., 2004; Salvatore et al., 2008), other studies failed to replicate these findings (Jones et al., 2005; Kaplan et al., 2012; St-Amand et al., 2013). These contradictory results can potentially be explained by differences in methodology. Measurement periods varied between 2 and 54 nights and different diagnostic criteria may have resulted in heterogeneous patient samples. Moreover, sample sizes were generally small (<36 cases and controls), raising the possibility that some studies were underpowered. Recently, two meta-analyses concluded that bipolar patients differed from controls on measures of sleep duration, sleep onset latency and wake after sleep onset (Geoffroy et al., 2015a; Ng et al., 2014). Sleep efficiency of bipolar patients was significantly lower in only one of the two meta-analyses . According to the authors, the number of actigraphy studies in bipolar disorder is limited and lags behind similar research in depression and ADHD. Furthermore, Geoffroy et al. pointed to heterogeneity in methodologies and found that age matching, level of depressive symptoms and actigraphy device potentially influence the actigraphy analyses and should be taken into account in future research (Geoffroy et al., 2015a, 2015b). Moreover, associations between objective sleep parameters and bipolar illness characteristics have so far not been studied. If sleep disturbances indeed reflect a continuous aberration of the circadian rhythm, it is conceivable that these disturbances correlate with an unfavorable course of the disorder.
Although the presence of sleep disturbances in bipolar patients has gained increasing attention, the question whether disturbances in sleep patterns can be considered as a heritable trait of bipolar disorder, has scarcely been investigated. As of yet, only two studies objectively studied the difference in sleep-wake behavior between bipolar patients and their relatives. Jones et al. (2006) studied children of bipolar patients and concluded that sleep onset latency and sleep fragmentation were lower in children of bipolar parents compared to control children. However, when affected bipolar offspring was excluded the effects were no longer significant. Pagani et al. (2015) analyzed 26 pedigrees ascertained for bipolar I disorder and showed that bipolar patients slept longer and woke up later compared to their non-affected relatives. The authors also provided evidence that a number of sleep measures are heritable. Extending this pedigree study by also including independent control subjects gives the opportunity to study the sleep-wake pattern in individuals who are genetically susceptible for the disorder, but lack the direct illness and its sequelae such as medication use. If first-degree relatives indeed show disturbed sleep patterns similar to probands, it would support the hypothesis that the sleep-wake pattern is a trait of bipolar disorder.
The current study aims at extending previous research in a large, homogenous sample of bipolar I patients using an elaborate collection of objective measures of sleeping behavior. First, the question will be addressed whether euthymic bipolar patients show differences in sleep pattern compared to controls and whether the non-affected siblings display similar patterns of sleeping behavior. Sleep duration, timing of sleep onset, timing of sleep offset, sleep onset latency, sleep efficiency, wake after sleep onset (WASO) and sleep inertia will be measured objectively using actigraphy. Subsequently, the association with current mood symptom level and life-time illness characteristics (i.e. age at onset, number of mood episodes, presence of psychotic symptoms and history of suicidal behavior) will be analyzed.
Methods
Sample
The current study is a follow up of the Dutch Bipolar Cohort (DBC) study, which is a collaboration between the University Medical Center Utrecht (UMCU), various health care institutes in the Netherlands and the University of California Los Angeles (UCLA). In short, the DBC study is designed to provide a deep-phenotype characterization of bipolar I patients and their first-degree relatives. The cohort included 1700 bipolar I patients, 586 relatives and 265 controls. After completion of the DBC protocol, a subgroup of patients, siblings and controls were re-approached to participate in the actigraphy protocol. Both the DBC study and the current study were approved by the medical ethical committee of the UMCU and were in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants prior to participation.
All participants had a minimum age of 18, at least three grandparents of Dutch descent. Exclusion criteria for all participants were self-reported major somatic illness (e.g. sleep apnea) and pregnancy. Inclusion criteria for patients was a bipolar I diagnosis, verified using the Structural Clinical Interview for DSM-IV (SCID-I) (First et al., 1997) and no current admission for their bipolar illness. None of the patients reported being in a current mood episode. However, 17 patients scored above the IDS-SR threshold for mild depressive symptoms. Additionally, 4 patients had an ASRM score indicative of a manic or hypomanic state. Siblings and control subjects with a diagnosis of bipolar disorder or other psychotic disorders were excluded, as were control subjects who had a first or second degree relative with such diagnoses. Both siblings and controls were assessed using the Mini-International Neuropsychiatric Interview (M.I.N.I) (Sheehan et al., 1998). In total, 466 eligible candidates were approached for participation via telephone, post or e-mail. 106 subjects did not respond to our invitation, 57 subjects refused to participate and 17 subjects did not show up for their appointment. In 5 participants with an appointment the Actiwatch never started recording and in 5 participants the data showed consecutive nights in which the minimum activity never reached 0, indicative of measurement errors. These 10 participants were excluded from further analyses. After excluding participants who did not meet the inclusion criteria (sleep apnea n=3, other somatic illness n=1, non-compliance to the protocol due to a mood episode n=3, sibling not meeting diagnostic criteria n=7, controls not meeting diagnostic criteria n=1) the sample consisted of 107 patients, 74 siblings and 80 controls.
Actigraphy recordings and sleep logs
Sleep-wake measurements were recorded with the Actiwatch 2 (Philips Respironics Inc, Murrysville, PA, USA). The Actiwatch has a solid state piezo-electric accelerometer and a lithium rechargeable battery. It records wrist movements and the sum of wrist movements is scored in epochs of 1 minute. All participants were instructed to wear the Actiwatch for a period of 14 consecutive days on their non-dominant wrist and only to remove it when exposed to water for long periods of time (e.g. swimming). During the 14-day recording period, participants kept a sleep diary in which bed times, nap times and off-wrist periods were noted. All Actiwatches were subjected to two calibration protocols prior to initial data collection and after every battery service (for details, see Pagani et al., 2015).
Actigraphy data analysis
To calculate the 7 sleep phenotypes, a series of algorithms in R statistical package (R Development Core Team, 2014) were used according to the procedure developed by Pagani et al. (2015). First, alterations had to be made for the recording days in which daylight saving time (DST) either set the clock back with one hour (fall) or set the clock forward with one hour (spring). After adjusting the time of the subsequent recordings so that it would match the new social time, the Saturday prior to DST, the Sunday of DST and the following Monday were discarded. This way, any jet lag caused by the shift in time was excluded from analyses.
In the next algorithm, the rest period was set for every 24-hr period. The starting point of rest was defined as the first moment after 6 PM when the participant started resting. A threshold was set (the median of overall activity, not exceeding 50 counts per epoch), which was used to identify consecutive epochs with low activity. The beginning of the rest interval was defined as the epoch that was preceded by consistent activity above the threshold, followed by a minimum of 15 epochs of activity below the threshold, allowing 2 minutes to be above threshold. The end of the rest interval was similarly defined as the epoch that was followed by consistent activity above the threshold and preceded by a minimum of 15 epochs of activity below the threshold, also allowing 2 minutes to be above threshold. The reported rest intervals from the sleep logs were used to cross-check the identified intervals set by R. To prevent daytime naps to get incorporated in the rest intervals, the script excluded intervals that had a rest period beginning 120 minutes before 6 PM. Also, when participants reported an off-wrist interval in their sleep logs, the recording was checked for 5 consecutive epochs or more with an activity count of 0 in the reported interval minus and plus 60 minutes. 24 hour-periods with more than 60 minutes defined as off-wrist were discarded.
After the rest period was defined, the sleep algorithm was used to calculate sleep duration, timing of sleep onset, timing of sleep offset, sleep onset latency, sleep efficiency (i.e. minutes asleep divided by minutes in bed), wake after sleep onset (WASO) and sleep inertia (wake interval between sleep offset and time out of bed). This algorithm was based on the Respironics Software Actiware.
Disease characteristics
Patients were assessed using several (semi)structured clinical interviews. The Questionnaire for Bipolar Disorder (Leverich et al., 2001; Suppes et al., 2001) was used to inquire age at onset of the disorder (defined as age of first medication). The presence of psychotic symptoms during mood episodes, the number of episodes and presence of rapid cycling in previous year were evaluated with the SCID-I (First et al., 1997). The Comprehensive Assessment of Symptoms and History (CASH) was used to screen for history of attempted suicide (Andreasen et al., 1992)
At the start of the actigraphy measurement period and after 7 days, depressive symptoms were assessed using the Inventory for Depressive Symptoms - Self Report (IDS-SR) (Rush et al., 2000) and manic symptoms were assessed using the Altman Self-Rating Mania Scale (ASRM) (Altman et al., 1997). For symptom measurement, the average of both time points was used in the analyses.
Statistical analysis
All analyses were carried out using either the R statistical package or IBM SPSS Statistics 21.0. Assumptions were checked and in case of significant outliers, sensitivity analyses were performed to assess the impact of the outliers on the overall model. Demographic data, group differences on continuous variables were analyzed using analysis of variance (ANOVA) with Bonferroni post hoc tests. Group differences on categorical variables were analyzed using chi-square analyses. Possible measurement bias of the sleep measurements by distribution of Actiwatch devices was assessed using multivariate analysis of variance (MANOVA).
To analyze differences in actigraphy sleep pattern between patients, siblings and controls, mixed-effects model analyses were conducted. Per outcome measure (means and standard deviations of sleep duration, sleep onset, sleep onset latency, WASO, sleep efficiency, sleep offset, sleep inertia) two separate models were used. One for the comparison of patients and siblings versus controls and one for patients and controls versus siblings. A random factor for relatedness was used, along with age and gender as covariates. A second series of analyses tested the possible confounding effect of depression and work by adding IDS-SR score and the number of workdays as covariate to the previous described mixed-effects models. The association between the illness characteristics and actigraphy sleep pattern was analyzed using linear regression.
Means and standard deviations from a recent meta-analysis by Geoffroy et al. (2015) were combined with the means and standard deviations from the current study in order to assess the effect sizes of sleep duration, sleep onset latency, WASO and sleep efficiency. Standardized mean differences (SMD) were calculated with Comprehensive Meta-Analysis 3.3.070 (Pierce, 2008), using random effects models. Only subjects from the current study with IDS-SR scores < 26 and ASRM scores < 6 were included in the meta-analysis.
Results
Participants
A total of 107 patients, 74 siblings and 80 controls were included with a mean measurement period of 14.4 days. Patients, siblings and controls had an equal male-female ratio, but groups differed on mean age, with siblings being significantly older than controls (F [2, 258] = 6.66, p = 0.001). All groups reported similarly low levels of manic symptoms. Current depressive symptoms were significantly higher in patients (F [2, 257] = 35.72, p < 0.001). The total number of workdays during the measurement period was lowest in patients, while siblings and controls worked an equal number of days (F [2, 258] = 4.05, p = 0.02). See table 1 for additional sample characteristics. Compared to the overall DBC cohort, the sample of patients, siblings and controls participating in the actigraphy study did not differ on age (F [1, 1501] = 0.21, p = 0.64 & F [1, 661] = 0.56, p = 0.46 & F [1, 344] = 0.14, p = 0.71) and gender (χ2 [1, 1503] = 0.02, p = 0.92 & χ2 [1, 663] = 0.20, p = 0.70 & χ2 [1, 346] = 0.01, p = 1.00).
Table 1.
Sample characteristics.
| Patients | Siblings | Controls | |
|---|---|---|---|
| N = 107 | N = 74 | N = 80 | |
| Gender male n (%) | 47 (43.9%) | 29 (39.2%) | 39 (48.8%) |
| Age M (sd)a | 50.3 (11.6) | 54.7 (12.1) | 46.8 (16.3) |
| Altman M (sd) | 1.9 (1.9) | 1.2 (1.4) | 1.6 (2.2) |
| IDS M (sd)b | 15.2 (11.1) | 6.9 (6.5) | 5.8 (4.9) |
| Waist circumference M (sd) | 95.8 (13.9) | 91.3 (12.3) | 91.4 (12.8) |
| Days Work M (sd)c | 4.1 (3.7) | 5.0 (3.6) | 5.6 (3.9) |
| Age at onset | 33.8 (13.3) | - | - |
| Number of episodes | 15.5 (24.5) | - | - |
| History of psychotic symptoms N (%) | 73 (69.5%) | - | - |
Difference siblings and controls significant (p < 0.01)
Difference patients and siblings and patients and controls significant (p < 0.001)
Difference patients and controls significant (p < 0.05)
At time of inclusion, 3 patients did not use any form of psychotropic medication, whereas the remaining patients used between 2 to 5 types of medication (median = 2). 58 patients received lithium, 36 patients received other mood stabilizers, 22 patients received antidepressants, 35 patients received antipsychotics (atypical = 29, typical = 6) and 29 patients received benzodiazepines. With the exception of 2 patients, all subjects used the same type of medication for at least 3 months. None of the subjects reported doing shift work during the measurement period.
Group differences
Table 2 shows the sleep parameters of the three groups and table 3 the summary of mixed-effect models. We found no differences between patients and controls on any of the sleep parameters. Compared to the non-affected siblings, patients slept significantly longer and had a later timing of sleep offset. Adjusting for depression symptom score by adding IDS-SR score as covariate, rendered the differences between patients and siblings non-significant at a Bonferroni-corrected significance level of 0.007. Adjusting for IDS-SR score in the remaining non-significant models (i.e. sleep onset, sleep onset latency, sleep efficiency, WASO and sleep inertia) did not change the results. Adding number of workdays as additional covariate in all previous models did not change any of the results. We subsequently analyzed whether the groups differed on variability in sleep measures, but found no significant differences between patients and controls, patients and siblings or siblings and controls (see supplementary table 1). Since Actiwatch device was not associated with the sleep measurements (V=0.73, F[192,1368]=0.99, p=0.54), we decided not to add it to the analyses as covariate. Supplementary figure 1 shows examples of actograms of a bipolar patient (1A), sibling (1B) and control (1C).
Table 2.
Means (standard deviations) for sleep measures
| Patients | Siblings | Controls | |
|---|---|---|---|
| Sleep duration | 475.16 (82.68) | 444.00 (55.47) | 452.10 (44.53) |
| Sleep onset | −3.54 (76.22) | −16.86 (54.45) | 9.59 (74.13) |
| Sleep onset latency | 5.32 (5.50) | 5.33 (7.91) | 6.01 (13.00) |
| Sleep efficiency | 86.20 (6.32) | 85.95 (6.42) | 87.17 (4.41) |
| WASO | 54.72 (23.15) | 50.95 (16.99) | 49.60 (18.15) |
| Sleep offset | 471.63 (79.52) | 427.14 (44.90) | 461.69 (77.64) |
| Sleep inertia | 5.89 (5.78) | 6.87 (7.63) | 4.92 (5.36) |
Table 3.
Analysis of differences in sleep parameters between groups in crude model vs model adjusted for current level of depression
| A | ||||||
|---|---|---|---|---|---|---|
| Patients vs Siblings | Patients vs Controls | Siblings vs Controls | ||||
| β | p | β | p | β | p | |
| Sleep duration | 31.75 | 0.003* | 22.20 | 0.03 | 9.55 | 0.37 |
| Sleep onset | 7.34 | 0.49 | −8.02 | 0.44 | 15.36 | 0.17 |
| Sleep onset latency | 0.01 | 1.00 | −0.70 | 0.61 | 0.70 | 0.64 |
| Sleep efficiency | 0.48 | 0.59 | −1.18 | 0.18 | 1.66 | 0.08 |
| WASO | 0.56 | 0.83 | 5.03 | 0.11 | −4.47 | 0.18 |
| Sleep offset | 40.30 | < 0.001* | 15.15 | 0.16 | 25.15 | 0.03 |
| Sleep inertia | −1.27 | 0.19 | 1.22 | 0.20 | −2.49 | 0.02 |
| B | ||||||
|---|---|---|---|---|---|---|
| Patients vs Siblings | Patients vs Controls | Siblings vs Controls | ||||
| β | p | β | p | β | p | |
| Sleep duration | 10.90 | 0.28 | 0.63 | 0.95 | 10.27 | 0.31 |
| Sleep onset | 12.60 | 0.27 | −2.18 | 0.85 | 14.77 | 0.19 |
| Sleep onset latency | 0.30 | 0.84 | −0.37 | 0.81 | 0.67 | 0.66 |
| Sleep efficiency | 0.33 | 0.73 | −1.35 | 0.17 | 1.68 | 0.08 |
| WASO | −2.02 | 0.48 | 2.13 | 0.52 | −4.15 | 0.20 |
| Sleep offset | 24.47 | 0.03 | −0.23 | 0.98 | 24.70 | 0.03 |
| Sleep inertia | −1.11 | 0.29 | 1.50 | 0.16 | −2.60 | 0.01 |
Beta (β) and p-values of patients vs siblings, patients versus controls and siblings versus controls;
Table A: Random factor: relatedness; Covariates: age and gender
Table B: Random factor: relatedness; Covariates: age, gender and IDS-SR score
Significant at Bonferroni-corrected significance level of 0.007
Relation current depressive symptoms with sleep
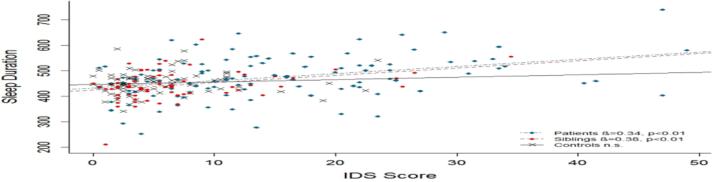
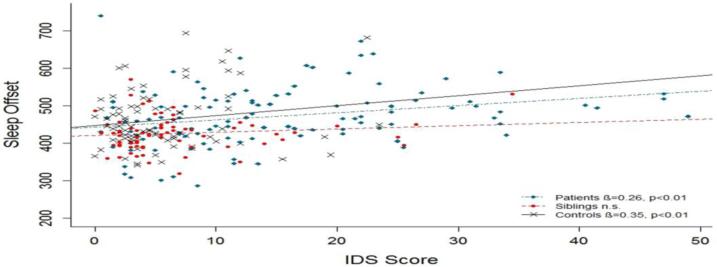
The direct association between sleep duration and IDS score was tested in linear regression analyses with age and gender as covariates. For patients and siblings, a significant association was found (see figure 1). To prevent circular reasoning, the analyses were repeated after questions regarding sleep were eliminated from the IDS-SR score. The association between depression score and sleep duration remained significant in both patients and sibling (β=0.28, t=3.01, p=0.003 and β=0.33, t=2.90, p=0.005). Linear regression analyses testing the direct relation between sleep offset and IDS score showed a significant association in patients and controls (see figure 2). The association also remained significant after elimination of the sleep questions from the IDS score (β=0.21, t=2.25 p=0.03 and β=0.29, t=2.75, p=0.008).
Figure 1.
Association between mean sleep duration and IDS score
Figure 2.
Association between mean sleep offset and IDS score
Differences in sleep parameters compared to euthymic patients only
To account for the relatively large dispersion in current mood symptomatology, the analyses were repeated in participants scoring under the cut-off indicative of mild depressive and manic symptoms (IDS-SR < 26 and ASRM < 6)(Altman et al., 2001; Karsten et al., 2010). This subsample consisted of 87 patients, 71 siblings and 75 controls. Current depression symptom score was not significantly different between groups (IDS-SR mean ± sd ; patients: 11.8 ± 7.3, siblings: 6.2 ± 5.2 and controls: 5.7 ± 5.0). Also, no group differences on manic symptoms were found (ASRM mean ± sd ; patients: 1.6 ± 1.5, siblings: 1.1 ± 1.2 and controls: 1.2 ± 1.5), nor on the number of days work (patients 4.4 ± 3.8, siblings 5.2 ± 3.6, 5.6 ± 4.0). When comparing the groups on the sleep parameters, the only significant difference we found was between patients and siblings on timing of sleep offset (β=38.66, t=3.61 p=0.002). However, after adjusting for IDS-SR score, the group difference in the sleep offset model did not reach the required Bonferroni-corrected significance level of 0.007 (β= 23.30, t=2.06, p = 0.05). Adjusting for IDS-SR score in the 6 remaining models (i.e. sleep duration, sleep onset, sleep onset latency, sleep efficiency, WASO and sleep inertia) did not change the results. Adding number of work days as covariate to all previous models did not change any of the results.
Life-time illness characteristics
None of the actigraphy sleep pattern variables was significantly associated with age at onset of the bipolar illness. Also, a history of psychotic symptomatology during episodes was not related with the sleep pattern variables. Total number of mood episodes was not related to the sleep pattern variables, as were number of episodes by polarity and presence of rapid cycling. Only WASO was significantly associated with history of suicidal behavior. See table 4 and supplementary table 2 for a summary of the regression models. Analyses in the euthymic subsample resulted in a non-significant association between WASO and suicidal behavior (β=0.26, p=0.05) and similar non-significant associations between sleep and the remaining illness characteristics.
Table 4.
Association of sleep with illness characteristics
| Age at onset | Psychosis | Total nr of episodes | Suicidal behavior | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Sleep duration | 0.12 | 0.31 | −0.05 | 0.63 | −0.04 | 0.71 | 0.10 | 0.39 |
| Sleep onset | −0.15 | 0.19 | 0.02 | 0.85 | −0.01 | 0.92 | 0.04 | 0.75 |
| Sleep onset latency | 0.08 | 0.52 | −0.09 | 0.39 | −0.19 | 0.08 | −0.07 | 0.57 |
| Sleep efficiency | 0.11 | 0.33 | 0.08 | 0.41 | 0.23 | 0.03 | −0.26 | 0.03 |
| WASO | −0.09 | 0.44 | −0.13 | 0.19 | −0.22 | 0.05 | 0.38 | 0.001* |
| Sleep offset | −0.03 | 0.83 | −0.03 | 0.75 | −0.06 | 0.61 | 0.15 | 0.20 |
| Sleep inertia | 0.05 | 0.66 | 0.01 | 0.90 | −0.17 | 0.12 | 0.13 | 0.27 |
Beta (β) and p-values of association between illness characteristics and sleep pattern.
Significant at Bonferroni-corrected significance level of 0.007.
Meta-analysis
To facilitate the interpretation of our findings we pooled the data of all available studies using actigraphy. We calculated the standardized mean differences (SDM) between patients and controls of sleep duration, sleep onset latency, WASO and sleep efficiency. The meta-analysis of sleep duration (10 studies; N= 289), showed a significant difference in SMD between bipolar patients and controls of 0.52 (SE=0.13, z=3.86, p<0.001). Sleep onset latency (8 studies; N=239) was higher in bipolar patients compared to controls with an SMD of 0.31 (SE=0.09, z=3.38, p=0.001). Sleep efficiency was lower in patients compared to controls (8 studies, N=239), with an SMD of −0.30 (SE=0.09, z=-3.17, p=0.002). Lastly, bipolar patients had a higher WASO compared to controls (8 studies; N=239) with an SMD of 0.24 (SE=0.09, z=2.61, p=0.009). Current levels of manic and depressive symptoms were not accounted for in this meta-analysis.
Discussion
Investigating a large bipolar I sample with a long actigraphy measurement period of 14 days, the current study aimed to expand previous findings on sleep disturbances in bipolar disorder. We found a longer sleep duration and later timing of sleep offset in patients compared to non-affected siblings, but these findings were attributable to differences in current depressive symptoms. Euthymic bipolar patients and healthy controls did not differ on any of the sleep parameter, nor was there a relation between sleep pattern and bipolar illness characteristics after adjusting for depressive symptoms.
When combining our data with results from the recent meta-analysis by Geoffroy et al. (2015) the overall evidence points in the direction of a lower sleep efficiency and higher sleep duration, WASO and sleep onset latency in bipolar patients. This would indicate that bipolar disorder patients do show trait-like differences in sleep pattern compared to controls. Nevertheless, the effect sizes from the meta-analysis are small, with the exception of sleep duration that showed a medium effect size. Whether such longer sleep duration is indicative of a sleep disorder is questionable, considering that the mean sleep duration never exceeded 9 hours, which is still within the range of recommended sleep for adults (Hirshkowitz et al., 2015). A previous meta-analysis by Ng et al. (2014) found similar effect sizes for sleep duration, WASO and sleep onset latency, but no difference in sleep efficiency between bipolar patients and controls. Also, bipolar patients did not show any deviations on the polysomnography measures, suggesting that sleep of remitted bipolar patients has similar restorative qualities as compared to controls. These results suggest that any differences in sleep pattern between bipolar patients and controls, is of disputable clinical importance. Of note is that a retrospective study found persisting sleep disturbances across all phases of bipolar disorder, including the inter-episode months (Kanady et al., 2015). However, as this study selectively included bipolar patients with a comorbid insomnia diagnosis, these results may not reflect the sleep pattern of bipolar patients in general.
The direct association between measures of depression and sleep pattern in patients suggests that sleep disturbances are a state-dependent phenomenon. Given that sleep disturbances are a core feature of depression (Harvey, 2008), it is not surprising that we found depressive symptoms to be associated with longer sleep duration and later sleep offset. These associations also held after removing sleep symptoms items from depression assessment. The direct relationship between sleep and mood symptomatology is in keeping with previous actigraphy studies: Gershon et al. (2012) found coupling of sleep disturbances and negative affect in interepisodic patients and Bauer et al. (2006) found changes in sleep and bed rest to precede changes in mood, with an average latency of one day. Moreover, a meta-analysis of actigraphy in patients with depression concluded that these patients showed higher sleep efficiency and lower sleep duration after treatment of depressive symptoms (Burton et al., 2013). The coupling between sleep duration and depressive symptoms appears stronger for bipolar patients and their siblings as compared to controls, which is in accordance with the longitudinal measures by Gershon et al. (2012). However, a similar association between sleep offset and depressive symptoms was absent in siblings, suggesting that mood and sleep are not indisputably related in siblings.
By including non-affected siblings of bipolar patients, we addressed the question whether sleep disturbances are a heritable trait of bipolar disorder. Bipolar disorder has as an estimated heritability of roughly 80% and several disorder characteristics are expressed at a higher rate in non-affected relatives than in the general population (Barnett and Smoller, 2009). The extensive pedigree analysis by Pagani et al. (2015) revealed that several sleep phenotypes, among which sleep duration and sleep offset, are significantly heritable. In agreement with our observations, they also found that bipolar patients slept longer and woke up later compared to non-affected relatives. However, we found no difference between patients, non-affected siblings and controls, after adjustments for current mood symptom levels. These findings suggest that the sleep phenotypes, although being heritable traits, are mood state-related rather than a trait of bipolar disorder.
Limitations
The current findings need to be interpreted in the context of several limitations. First, although sleep in bipolar patients was not affected in our study, we cannot conclude that general circadian rhythmicity is not at all altered in bipolar patients. Clinical guidelines on the maintenance treatment of bipolar disorder recommend restoring behavioral rhythmicity and sleep by, for example, prescribing sleep-promoting medication and social rhythm therapy (Hirschfeld, 2005). Perhaps, our sample of bipolar patients consisted of successfully treated bipolar patients who do not show overt signs of circadian rhythm disturbances, while other manifestations of circadian rhythms (nocturnal body temperature, sleep architecture, hormone secretion) may still show irregularities. Future studies could address this question by combining both behavioral and biological measures of circadian rhythmicity.
Second, the effect of pharmacological treatment is one of the hardest hurdles to overcome when studying sleep in bipolar disorder. Bipolar patients, even after being functionally recovered, are advised to continue psychotropic medication to prevent relapse (Hirschfeld, 2005). Possibly, bipolar patients did not differ from controls due to the sedative side-effects of antipsychotic medication and benzodiazepines or the normalizing effect of lithium on the 24-hour endogenous rhythmicity (Klemfuss, 1992). Given that the majority of patients used more than one type of medication, delineating the specific effect per medication type was not possible. Studying non-affected siblings could serve as a reliable way of overcoming the problem of medication. It is noteworthy that these genetically susceptible individuals were not using lithium or antipsychotic medication and also did not show any signs of sleep disturbances. A third limitation could be the selection of patients in our sample. While this is an inherent problem of all cross-sectional studies, our sample consisted of patients who were fit to participate in an elaborate assessment and were willing to participate in a follow-up actigraphy study. Although we cannot rule out selection bias, there is also no reason to assume this, as the patients in the current actigraphy study did not differ from the overall sample of DBC-participants on demographic characteristics. Furthermore, a potential limitation is the difference in age between siblings and controls. Age is known to influence sleep characteristics, with older subjects experiencing a lower sleep efficiency and a higher rate of insomnia (Ancoli-Israel, 2009). However, it is unlikely that this age difference has masked an effect in the sibling-control comparison, since controls were on average younger compared to the average age of siblings. We also need to point out that other than sleep apnea, we did not inquire other somatic or psychological conditions that may have resulted in distorted sleep in the participants. Finally, by selectively including patients with bipolar disorder type I we studied sleep in a clinically homogeneous sample. We cannot, however, extend our findings to other types of mood disorders.
Conclusion
In a large homogeneous sample of bipolar I sample, we did not identify persisting sleep disturbances in bipolar patients other than related to increased depressive symptoms. The meta-analysis points in the direction of longer sleep duration, WASO and sleep onset latency and lower sleep efficiency in bipolar patients. The majority of these differences in the meta-analysis were however small, the overall number of participants limited and the question remains whether they reflect clinically significant sleep disturbances. Furthermore, non-affected siblings showed normal sleep-wake patterns. Overall in our data sleep disturbances are a state marker of mood symptomatology which underscores the need for further study of the persistence of disturbance in sleep-wake patterns in euthymic bipolar disorder patients and in patients before disease onset.
Supplementary Material
Highlights.
We found no difference in sleep pattern between patients, non-affected siblings and controls, after adjustments for current mood symptom levels.
We found no relation between sleep pattern and bipolar illness characteristics.
Measures of depression and sleep pattern were directly associated in patients.
Our data suggests that sleep disturbances are primarily a state marker of mood symptomatology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman E, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biol. Psychiatry. 2001;50:468–471. doi: 10.1016/s0006-3223(01)01065-4. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. The altman self-rating mania scale. Biol. Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; Washington DC: 2013. [Google Scholar]
- Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(SUPPL 1):S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164:331–343. doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N. Engl. J. Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Boudebesse C, Geoffroy PA, Bellivier F, Henry C, Folkard S, Leboyer M, Etain B. Correlations between objective and subjective sleep and circadian markers in remitted patients with bipolar disorder. Chronobiol. Int. 2014;2:698–704. doi: 10.3109/07420528.2014.895742. [DOI] [PubMed] [Google Scholar]
- Burton C, McKinstry B, Szentagotai Tatar A, Serrano-Blanco A, Pagliari C, Wolters M. Activity monitoring in patients with depression: A systematic review. J. Affect. Disord. 2013;145:21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Cretu JB, Culver JL, Goffin KC, Shah S, Ketter T. a. Sleep, residual mood symptoms, and time to relapse in recovered patients with bipolar disorder. J. Affect. Disord. 2016;190:162–166. doi: 10.1016/j.jad.2015.09.076. [DOI] [PubMed] [Google Scholar]
- First M.B. et, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Biometric Research Department; New York: 1997. [Google Scholar]
- Geoffroy PA, Boudebesse C, Bellivier F, Lajnef M, Henry C, Leboyer M, Scott J, Etain B. Sleep in remitted bipolar disorder: A naturalistic case-control study using actigraphy. J. Affect. Disord. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, Leboyer M, Bellivier F, Etain B. Sleep in patients with remitted bipolar disorders: a meta-analysis of actigraphy studies. Acta Psychiatr. Scand. 2015a;131:89–99. doi: 10.1111/acps.12367. [DOI] [PubMed] [Google Scholar]
- Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, Leboyer M, Bellivier F, Etain B. Reply: Sleep in Patients with Remitted Bipolar Disorders: Analyses stratified on actigraphy devices, age and gender. Acta Psychiatr. Scand. 2015b;131:400. doi: 10.1111/acps.12400. [DOI] [PubMed] [Google Scholar]
- Gershon A, Thompson WK, Eidelman P, McGlinchey EL, Kaplan KA, Harvey AG. Restless Pillow, Ruffled Mind: Sleep and Affect Coupling in Interepisode Bipolar Disorder. J. Abnorm. Psychol. 2012;121:863–873. doi: 10.1037/a0028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. Am. J. Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am. J. Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA. Guideline watch: Practice guideline for the treatment of patients with bipolar disorder. 2nd edition American Psychiatric Association; Arlington, VA: 2005. [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O'Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Heal. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J. Affect. Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Jones SH, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: A pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006;8:362–372. doi: 10.1111/j.1399-5618.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- Kanady JC, Soehnera AM, Harvey AG. A Retrospective Examination of Sleep Disturbance across the Course of Bipolar Disorder. Adv. Psychiatr. Treat. 2015;30:1000193. doi: 10.4172/2167-0277.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. a, Talbot LS, Gruber J, Harvey AG. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord. 2012;14:870–879. doi: 10.1111/bdi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten J, Hartman CA, Ormel J, Nolen WA, Penninx BWJH. Subthreshold depression based on functional impairment better defined by symptom severity than by number of DSM-IV symptoms. J. Affect. Disord. 2010;123:230–237. doi: 10.1016/j.jad.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol. Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, Denicoff KD, Suppes T, Altshuler LL, Kupka R, Kramlinger KG, Post RM. The Stanley Foundation Bipolar Treatment Outcome Network: I. Longitudinal methodology. J. Affect. Disord. 2001;67:33–44. doi: 10.1016/s0165-0327(01)00430-x. [DOI] [PubMed] [Google Scholar]
- Milhiet V, Etain B, Boudebesse C, Bellivier F. Circadian biomarkers, circadian genes and bipolar disorders. J. Physiol. Paris. 2011;105:183–189. doi: 10.1016/j.jphysparis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Millar A, Espie C. a., Scott J. The sleep of remitted bipolar outpatients: A controlled naturalistic study using actigraphy. J. Affect. Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Ng TH, Chung KF, Ho FYY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med. Rev. 2014;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Adkins S, Lahiri DK, Mayeda A, Hu K, Lewy A, Miller A, Bowman ES, Miller MJ, Rau L, Smiley C, Davis-Singh D. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch. Gen. Psychiatry. 2000;57:572–579. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- Pagani L, St. Clair P. a., Teshiba TM, Service SK, Fears SC, Araya C, Araya X, Bejarano J, Ramirez M, Castrillón G, Gomez-Makhinson J, Lopez MC, Montoya G, Montoya CP, Aldana I, Navarro L, Freimer DG, Safaie B, Keung L-W, Greenspan K, Chou K, Escobar JI, Ospina-Duque J, Kremeyer B, Ruiz-Linares A, Cantor RM, Lopez-Jaramillo C, Macaya G, Molina J, Reus VI, Sabatti C, Bearden CE, Takahashi JS, Freimer NB. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc. Natl. Acad. Sci. 2016;113:E754–E761. doi: 10.1073/pnas.1513525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce CA. Comprehensive Meta-analysis. Organ. Res. Methods. 2008;11:188–191. [Google Scholar]
- R Development Core Team, R. R: A Language and Environment for Statistical Computing. 2014 [Google Scholar]
- Rocha PMB, Neves FS, Corrêa H. Significant sleep disturbances in euthymic bipolar patients. Compr. Psychiatry. 2013;54:1003–1008. doi: 10.1016/j.comppsych.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz P. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. Int. J. Methods Psychiatr. Res. 2000;9:45–59. [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, Baldessarini RJ. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Seleem MA, Merranko JA, Goldstein TR, Goldstein BI, Axelson DA, Brent D. a, Nimgaonkar VL, Diler RS, Sakolsky DJ, Kupfer DJ, Birmaher B. The longitudinal course of sleep timing and circadian preferences in adults with bipolar disorder. Bipolar Disord. 2015;17:392–402. doi: 10.1111/bdi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- St-Amand J, Provencher MD, Bélanger L, Morin CM. Sleep disturbances in bipolar disorder during remission. J. Affect. Disord. 2013;146:112–119. doi: 10.1016/j.jad.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, McElroy SL, Rush AJ, Kupka R, Frye MA, Bickel M, Post RM. The Stanley Foundation Bipolar Treatment Outcome Network - II. Demographics and illness characteristics of the first 261 patients. J. Affect. Disord. 2001;67:45–59. doi: 10.1016/s0165-0327(01)00432-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.