Abstract
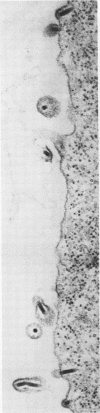
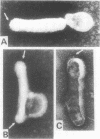
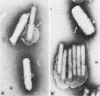
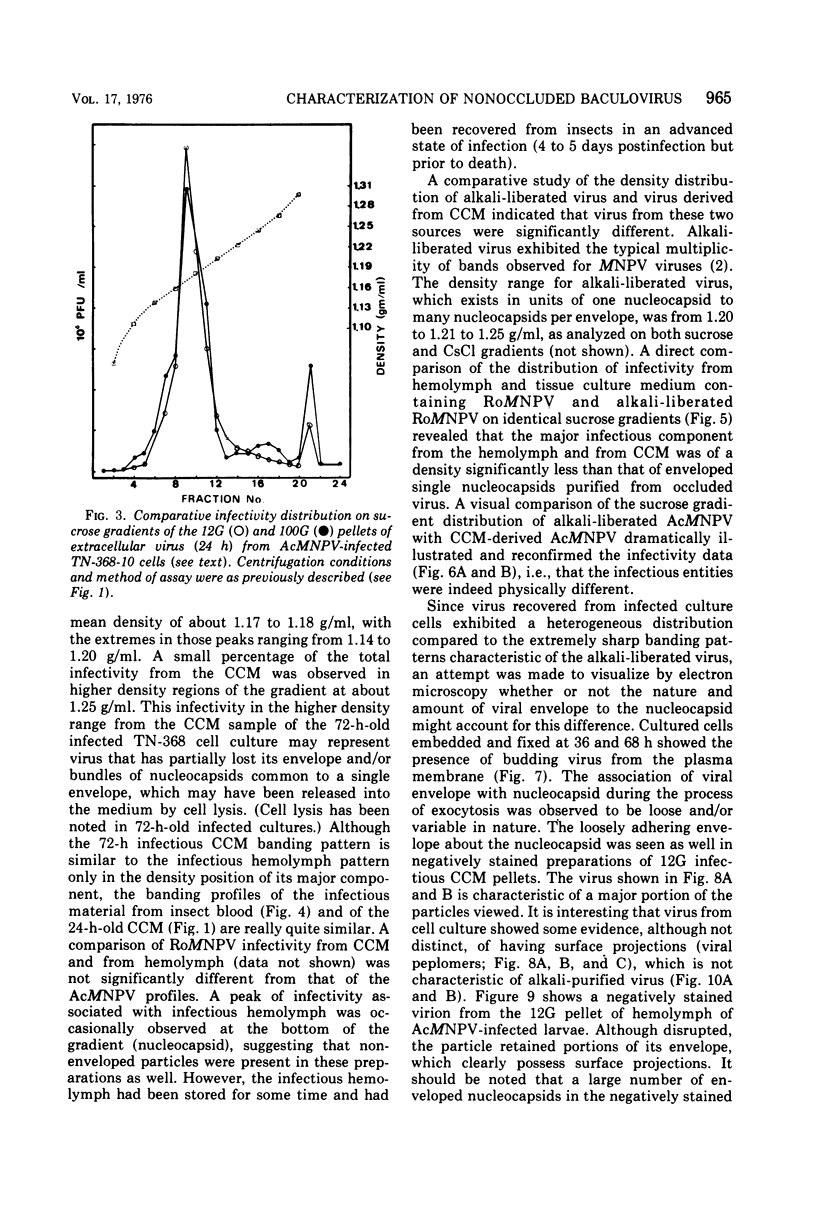
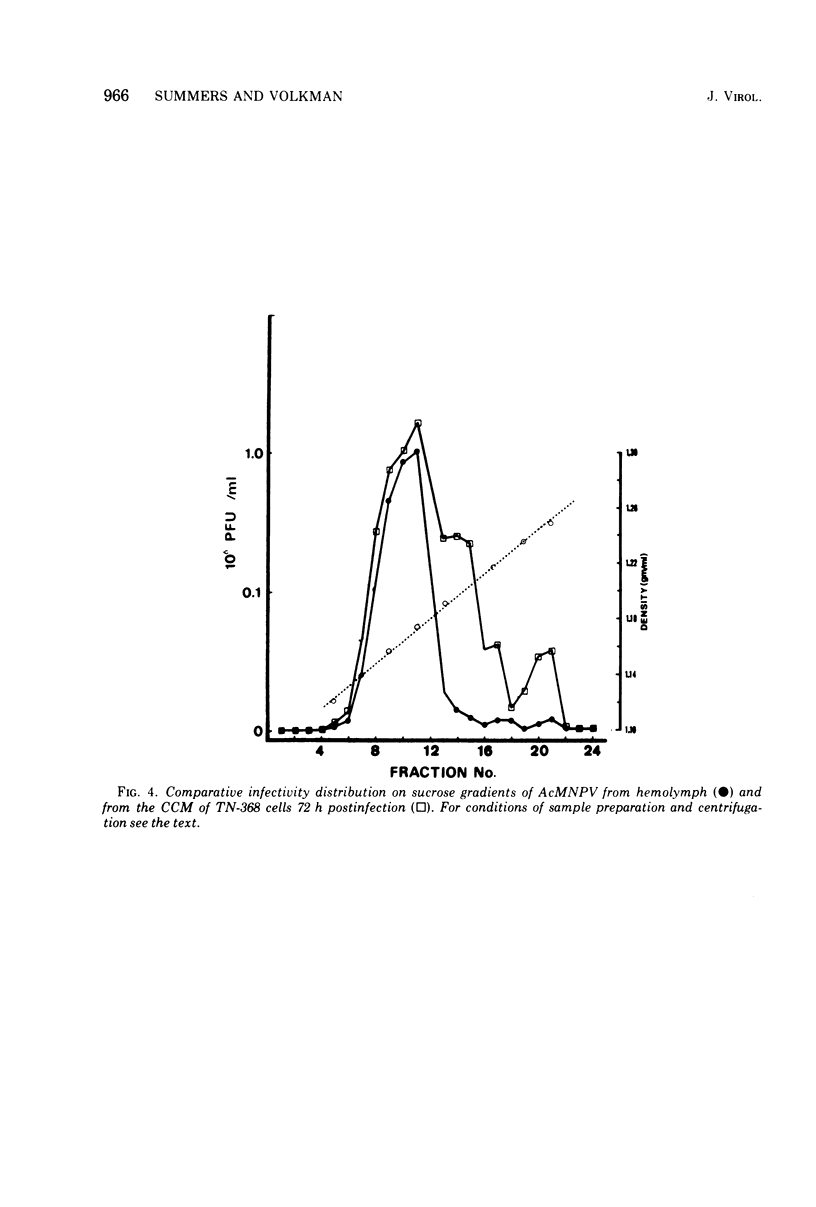
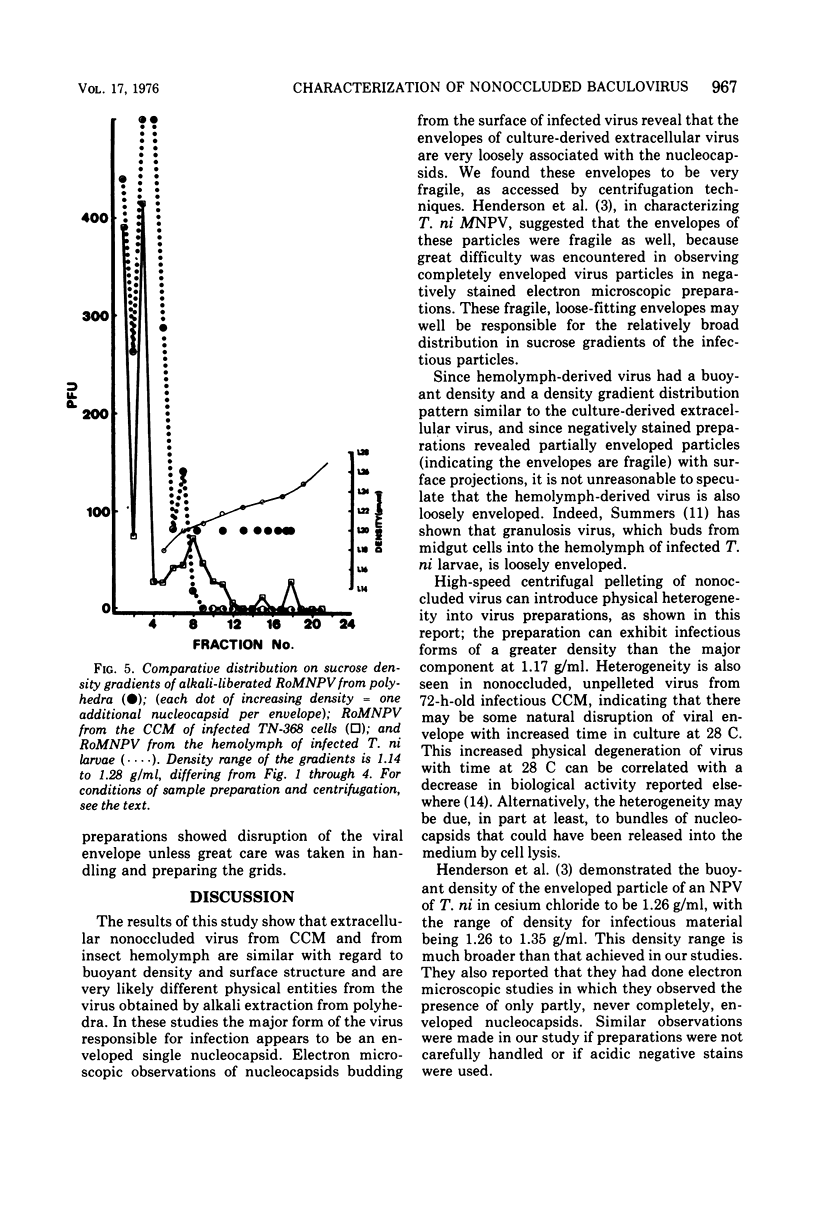
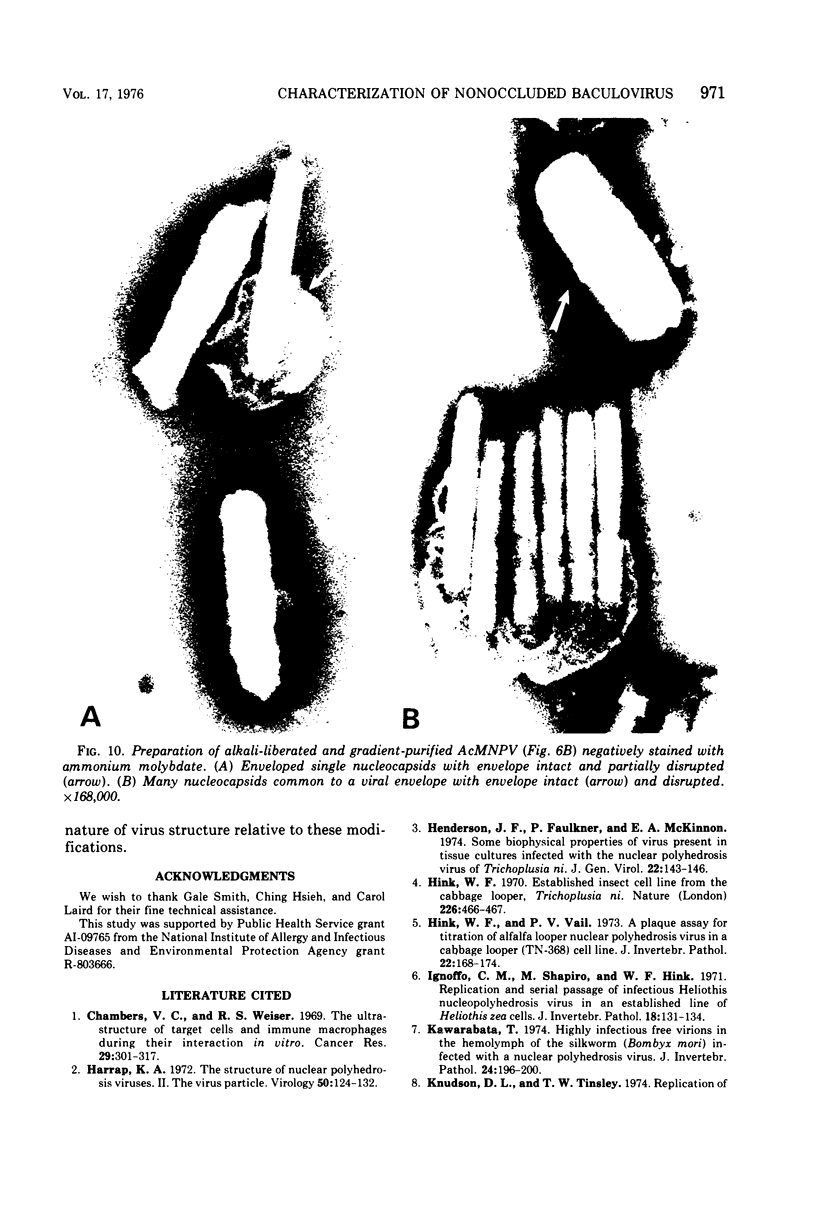
Electron microscopic examination and buoyant density profiles of nonoccluded Rachiplusia ou and Autographa californica nuclear polyhedrosis viruses purified from both infectious insect hemolymph and cell culture medium revealed that the viruses are enveloped, single nucleocapsids. The envelopes exhibited variation in the amount and degree of fit with regard to the nucleocapsids. This was determined by: (i) electron microscopic observations of virus budding from the surface of infected cells; (ii) electron microscopic observations of negatively stained preparations of pelleted, highly purified, nonoccluded enveloped particles; and (iii) the resolution and density distributions of nonoccluded virus in sucrose gradients after centrifugation to equilibrium; all were compared with virus extracted from polyhedra. Peplomers, ovserved on the surface of enveloped nucleocapsids of nonoccluded virus, are not associated with polyhedra-derived virus. Density gradient analysis indicated that virus from insect hemolymph and culture medium exhibited similar densities of approximately 1.17 to 1.18 g/ml. This is significantly different from the buoyant density of an alkali-liberated, enveloped single nucleocapsid (1.20 g/ml). Results of this study show that the nonoccluded forms of two nuclear polyhedrosis viruses from two different sources, hemolymph and cell culture, are similar with regard to several morphological and biophysical characteristics but are quite different from the alkali-liberated, polyhedra-derived form of the virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers V. C., Weiser R. S. The ultrastructure of target cells and immune macrophages during their interaction in vitro. Cancer Res. 1969 Feb;29(2):301–317. [PubMed] [Google Scholar]
- Harrap K. A. The structure of nuclear polyhedrosis viruses. II. The virus particle. Virology. 1972 Oct;50(1):124–132. doi: 10.1016/0042-6822(72)90352-2. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Faulkner P., MacKinnon E. A. Some biophysical properties of virus present in tissue cultures infected with the nuclear polyhedrosis virus of Trichoplusia ni. J Gen Virol. 1974 Jan;22(1):143–146. doi: 10.1099/0022-1317-22-1-143. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Ignoffo C. M., Shapiro M., Hink W. F. Replication and serial passage of infectious Heliothis nucleopolyhedrosis virus in an established line of Heliothis zea cells. J Invertebr Pathol. 1971 Jul;18(1):131–134. doi: 10.1016/0022-2011(91)90021-h. [DOI] [PubMed] [Google Scholar]
- Kawarabata T. Highly infectious free virions in the hemolymph of the silkworm (Bombyx mori) infected with a nuclear polyhedrosis virus. J Invertebr Pathol. 1974 Sep;24(2):196–200. doi: 10.1016/0022-2011(74)90011-1. [DOI] [PubMed] [Google Scholar]
- Raghow R., Grace T. D. Studies on a nuclear polyhedrosis virus in Bombyx mori cells in vitro. 1. Multiplication kinetics and ultrastructural studies. J Ultrastruct Res. 1974 Jun;47(3):384–399. doi: 10.1016/s0022-5320(74)90016-1. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Arnott H. J. Ultrastructural studies on inclusion formation and virus occlusion in nuclear polyhedrosis and granulosis virus-infected cells of Trichoplusia ni (Hübner). J Ultrastruct Res. 1969 Sep;28(5):462–480. doi: 10.1016/s0022-5320(69)80034-1. [DOI] [PubMed] [Google Scholar]
- Summers M. D. Electron microscopic observations on granulosis virus entry, uncoating and replication processes during infection of the midgut cells of Trichoplusia ni. J Ultrastruct Res. 1971 Jun;35(5):606–625. doi: 10.1016/s0022-5320(71)80014-x. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Trichoplusia ni granulosis virus granulin: a phenol-soluble, phosphorylated protein. J Virol. 1975 Nov;16(5):1108–1116. doi: 10.1128/jvi.16.5.1108-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D. Nuclear polyhedrosis virus detection: relative capabilities of clones developed from Trichoplusia ni ovarian cell line TN-368 to serve as indicator cells in a plaque assay. J Virol. 1975 Dec;16(6):1630–1637. doi: 10.1128/jvi.16.6.1630-1637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zherebtsova E. N., Strokovskaya L. I., Gudz-Gorban A. P. Subviral infectivity in nuclear polyhedrosis of the great wax moth (Galleria mellonella L.). Acta Virol. 1972 Sep;16(5):427–431. [PubMed] [Google Scholar]